Currently, T cells dedicated to mediating immunosuppression and homeostasis are called regulatory T (Treg) cells. Treg cells are closely associated with infection, allergy, autoimmunity, tumor immunity, fetal–maternal tolerance, and organ transplantation. Treg cells exert their immunosuppressive functions by directly killing or inhibiting T cells and antigen-presenting cells (dendritic cells, macrophages, and B cells) and by producing suppressive cytokines such as TGF-β, IL-10, IL-35, and galectin-1. However, the classification and phenotypes of Treg cell subsets remain largely unclear. The discovery of tissue-resident Treg cells with transcriptional, phenotypic, and functional heterogeneity renders Treg cell subsets more enigmatic. Research regarding tissue-resident Treg cells has become a hot topic. Due to the particular distribution, distinct developmental process and antigenic specificity of various tissue-resident Treg cells, their roles also go beyond the canonical functions of the immune system in health and disease1,2. The best example is the visceral adipose tissue Treg (VAT-Treg) cell subset, which prevents obesity-associated inflammation and preserves insulin sensitivity and glucose tolerance; thus, this subset of cells is closely associated with obesity, type 2 diabetes and metabolic cardiovascular disease3,4. In a recent issue of Nature Immunology, Vasanthakumar et al. revealed a critical role and mechanism of the IL-33/ST2 axis in the development and maintenance of VAT-Treg cells5.
The classification and phenotypes of Treg cell subsets are extremely complex and confusing. Listing all of the subsets that have been discussed in the literature is difficult. To recognize VAT-Treg cells better, the following basic information regarding how to group Treg cell subsets that have frequently appeared in published papers must be understood (Table 1).
Table 1. Enigmatic classification and phenotypic heterogeneity of regulatory T cell subsets.
| Treg cell subset | Development | Major phenotype | Effector |
|---|---|---|---|
| Foxp3+/Foxp3− Treg | Variety | Foxp3+… or Foxp3−… | Variety |
| CD4+/CD8+ Treg7 | Variety | CD4+Foxp3+/−… or CD8+Foxp3+/−… | Variety |
| NKTreg and γδ-Treg8 | Variety | Markers of NKT or γδ-T cells | IL-10/TGF-β |
| nTreg/iTreg9 | |||
| nTreg (natural Treg) | In thymus | CD4+Foxp3+CD25int/hi, CD127loCTLA4+GITR+ | IL-10/TGF-βDirect contactGranzyme B |
| iTreg (induced Treg) | |||
| Tr1 | In periphery | CD4+Foxp3−CD25−, CD25lo-varCD45RBlo | IL-10 |
| Th3 | In periphery | CD4+Foxp3+CD25+, CD25lo-varCD45RBlo | TGF-β |
| tTreg/pTreg/iTreg10 | |||
| tTreg (thymus-derived) | In thymus | CD4+Foxp3+… | Variety |
| pTreg (peripherally derived) | In periphery | CD4+Foxp3+, from Foxp3− T-conventional cells | Variety |
| iTreg (induced in cell culture) | In cell culture | CD4+Foxp3+… | Variety |
| TFR (follicular Treg)11 | In germinal center | CD4+Foxp3+Bcl-6+CXCR5+ICOS+CD19−, PD-1+GITR+ | Direct contactIL-10/TGF-β |
| cTreg/eTreg/tissue-resident Treg12 | |||
| cTreg (central Treg) | Located in circulation and secondary lymphoid organs | CD4+Foxp3+Blimp-1−CD62LhiCCR7+, CD45RAhiCD25lo | Variety |
| eTreg (effector Treg) | Located in circulation, secondary lymphoid organs, various tissues | CD4+Foxp3+Blimp-1+CD62LloCCR7lo, D44hiCD103+KLRG1+, CD45RAloCD25hi | Variety |
| Tissue-resident Treg1,2 | Located in various tissues | Phenotype of eTreg … | Variety |
Foxp3+/Foxp3−Treg cells. Although Foxp3 (the transcription factor forkhead box P3) is also expressed by activated conventional T cells, Foxp3 is generally considered the most important marker of Treg cells and plays acritical role in the development and function of Treg cells. However, the presence of Foxp3− Treg cells such as Foxp3− type 1 regulatory T (Tr1) cells is also well known6.
CD4+/CD8+Treg cells. Treg cells can be divided into CD4+ and CD8+subsets based on their expression. CD4+Foxp3+ Treg cells have been dominantly investigated; CD8+ Treg cells are currently being addressed7.
Regulatory natural killer T (NKTreg) cells and γδ Treg cells. NKTreg cells and Treg cells with γδ TCRs can exert suppressive functions in certain settings8.
Natural Treg (nTreg) and induced Treg (iTreg) cells. Compared to nTreg (thymically derived or naturally occurring Treg) cells, iTreg cells are induced peripherally via post-thymic maturation. Both Foxp3− Tr1 cells and Foxp3+ Th3 cells are included in iTreg cells9.
Thymus-derived Treg (tTreg), peripherally derived Treg (pTreg), and induced Treg cells in cell culture (iTreg)10. tTreg and pTreg cells partly overlap with the above-described nTreg and iTreg cells.
Follicular Treg (TFR) cells. CD4+Foxp3−Bcl-6+CXCR5+ICOS+CD19−and PD-1+GITR– follicular helper T (TFH) cells are essential for germinal center formation and B-cell activation/differentiation into plasma cells. In contrast, the CD4+Foxp3+Bcl-6+CXCR5+ICOS+CD19−and PD-1+GITR+ TFR cells within the germinal center can inhibit TFH cell function and naïve T- and B-cell activation11.
Central Treg (cTreg), effector Treg (eTreg), and tissue-resident Treg cells. Drs. Liston and Gray classified the peripheral CD4+Foxp3+Treg cells into cTreg (also referring to as ‘resting' or ‘naive' Treg), eTreg (or activated Treg), and tissue-resident Treg cells12. The majority of Treg cells in the circulation and secondary lymphoid organs belong to the cTreg cell subset that expresses CD62LhighCCR7+ or CD45RAhighCD25low, while a minor fraction of the Treg cells are eTreg cells that express CD62LlowCCR7lowCD44highCD103+KLRG1+ (killer cell lectin-like receptor subfamily G member 1-positive) or CD45RAlowCD25high. The transcription factor Blimp-1 (B lymphocyte-induced maturation protein-1) is a key player in late B-cell and conventional T-cell differentiation. Blimp-1 is also expressed on all eTreg cells and is required for IL-10 production. The phenotypes of Blimp-1+CD62LlowCCR7lowand Blimp-1−CD62LhighCCR7+ are thereby recognized as markers for distinguishing eTreg from cTreg cells5,13, whereas Blimp-1+ eTreg cells are derived from Blimp-1− cTreg cells in response to specific stimuli13. Treg cells that reside in non-lymphoid tissues for long-term are characterized as tissue-resident Treg cells. Most tissue-resident Treg cells dominantly express the phenotypes of eTreg cells1,5. However, tissue-specific factors that promote the development of specialized tissue-resident eTreg cells from cTreg cells in each tissue need to be further determined.
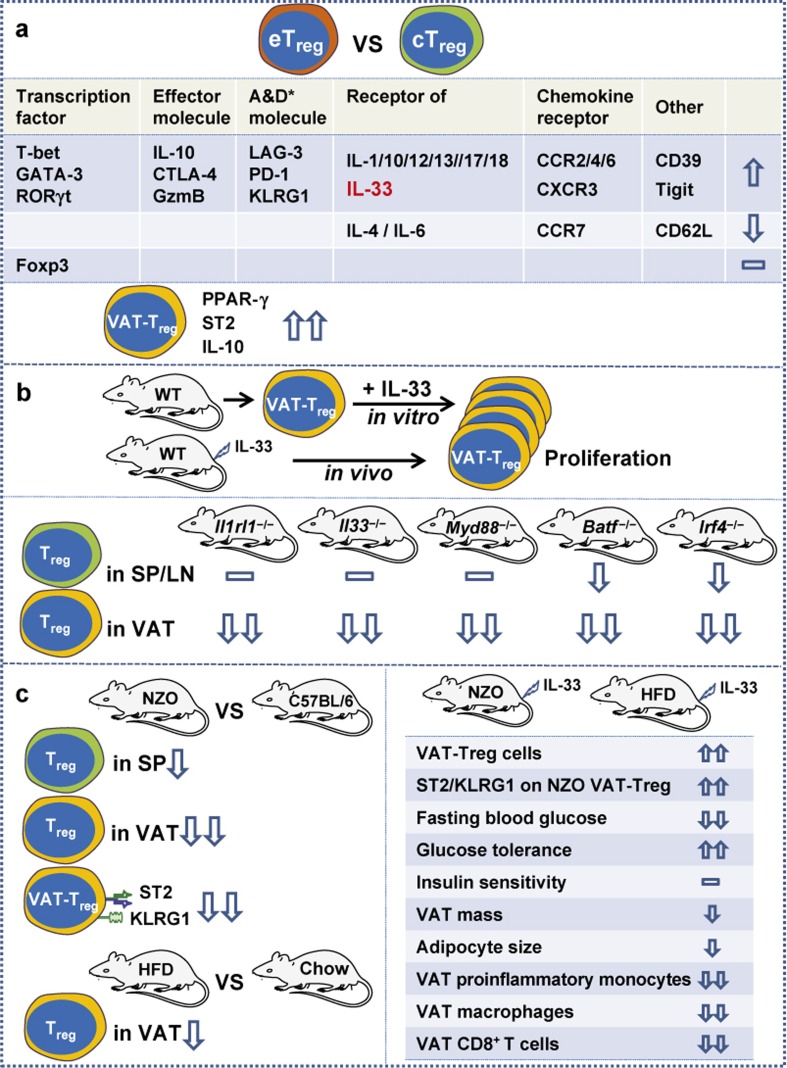
IL-33 plays a crucial role in inflammatory, allergic, infectious, and autoimmune diseases. IL-33 binding to its receptor, which is composed of IL-33Rα (ST2) and IL-33Rβ (IL-1RAcP), is involved in the differentiation and functions of various immunocytes including Th2 cells, CD8+ T cells, group 2 innate lymphoid cells (ILC2), NK cells, mast cells, basophils, and eosinophils. Recently, IL-33 was shown to be essential for VAT-Treg cell development and maintenance5, thereby linking IL-33 to obesity and associated diseases. As outlined in Figure 1, CD4+Foxp3+Treg cells were grouped into three subsets of cTreg, eTreg, and tissue-resident Treg cells, and how the IL-33/ST2 axis specifically controls VAT-Treg cell development was revealed via three major approaches5.
Figure 1.
The major experimental approaches revealing the specific requirement of the IL-33/ST2 axis for VAT-Treg cell development. Cells: cTreg, central Treg; eTreg, effector Treg; VAT-Treg, visceral adipose tissue Treg. Mice: WT, wild-type; Il1rl1−/−, ST2-deficient; Il33−/−, IL-33-deficient; Myd88−/−, Myd88-deficient; Batf−/−, BATF-deficient; Irf4−/−, IRF4-deficient; NZO; New Zealand obese; Chow, fed with normal chow; HFD, high fat diet-induced obese mouse. *A&D molecules, activation- and differentiation-associated molecules; GzmB, granzyme B; SP, spleen; LN, lymph nodes; CD39, ectonucleotidase; Tigit, co-inhibitory molecule.
In the first approach (Figure 1a), RNA sequencing (RNA-seq) confirmed that the transcriptional profiles of cTreg and eTreg cells from spleen and lymph nodes of Blimp1GFP reporter mice are distinct from each other. The differential expression of some crucial molecules on eTreg and VAT-Treg cells was also verified by flow cytometric analyses. Typically, PPAR-γ (the known transcription factor essential for VAT-Treg cell development)14, ST2 and IL-10 are markedly expressed by VAT-Treg cells.
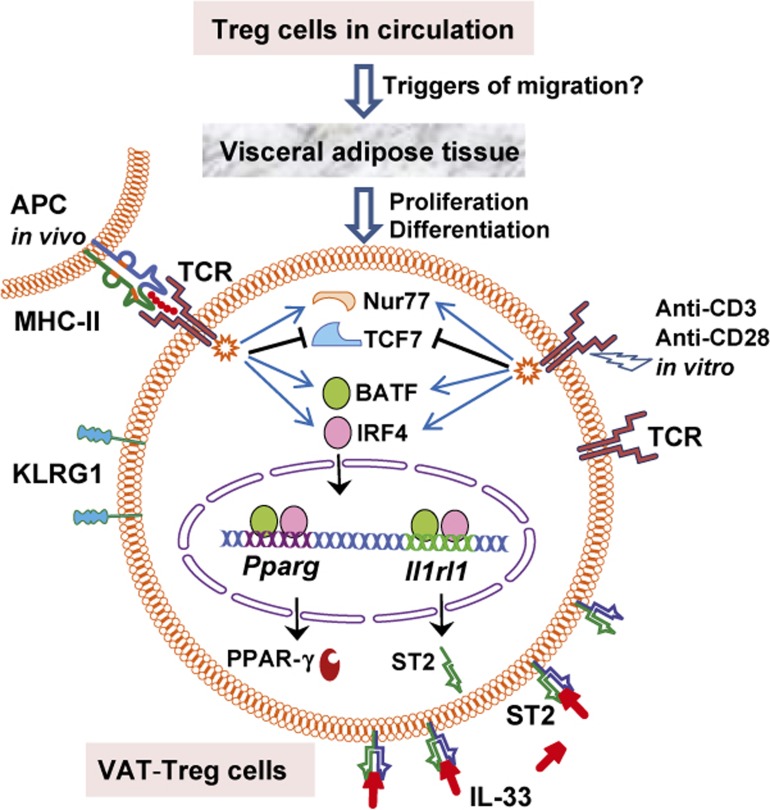
In the second approach (Figure 1b), wild-type (WT), ST2-deficient (Il1rl1−/−), IL-33-deficient (Il33−/−), Myd88-deficient (Myd88−/−), BATF-deficient (Batf−/−) and IRF4-deficient (Irf4−/−) mice were used to reveal the crucial roles of these molecules in VAT-Treg cell development and how PPAR-γ and ST2 expression was upregulated. IL-33 is known to signal through the adaptor protein MyD88, while IRF4 and BATF are T cell-associated transcriptional regulators. Compared to WT mice, the deficiencies of ST2, IL-33, Myd88, BATE, and IRF4 greatly reduced VAT-Treg cells, while the numbers of cTreg or eTreg cells in spleen, lymph nodes, and some other tissues were moderately affected or unaffected. Sufficient and convincing data have demonstrated that (i) the IL-33/ST2 axis is specifically required for VAT-Treg cell proliferation and differentiation both in vitro and in vivo, (ii) VAT-Treg cell differentiation requires MyD88-mediated signaling downstream of IL-33, and (iii) BATF and IRF4 are indispensable transcriptional regulators for VAT-Treg cells to express PPAR-γ and ST2. As outlined in Figure 2, TCR stimulation via a specific antigen in vivo or via anti-CD3/anti-CD28 in vitro induced BATF and IRF4 expression, which triggered PPAR-γ and ST2 expression by simultaneously binding Pparg (encoding PPAR-γ) and Il1rl1 (encoding ST2) loci. The requirement for TCR signalling in VAT-Treg cell development was also confirmed by detecting the expression of Nur77 and TCF7, two transcription factors that are up- and downregulated, respectively, in response to TCR signaling.
Figure 2.
Crucial roles of TCR signals, the transcriptional regulators BATF and IRF4, and the IL-33/ST2 axis in VAT-Treg cell proliferation and differentiation from cTreg or eTreg cells. APC, antigen-presenting cells; Pparg and Il1rl1, encoding PPAR-γ and ST2, respectively; Nur77 and TCF7, transcription factors.
In the third approach (Figure 1c), the crucial role of IL-33 in VAT-Treg cell development and maintenance was further tested by IL-33 administration into New Zealand obese (NZO, genetically obese) mice and high fat diet-induced obese mice (HFD mice). Compared to control C57BL/6 mice, NZO mice displayed early onset of obesity and hyperglycemia, as well as severely reduced numbers of VAT-Treg cells14,15. The VAT-Treg cells from NZO mice expressed extremely low levels of ST2 and KLRG1. Compared to control mice fed with normal chow, HFD mice also displayed a reduced number of VAT-Treg cells and abnormal metabolic parameters. IL-33 administration in NZO and HFD mice rescued VAT-Treg cell numbers, restored ST2 and KLRG1 expression on NZO VAT-Treg cells, improved metabolic parameters including fasting blood glucose concentration and glucose tolerance, decreased VAT mass and adipocyte size, and suppressed the obesity-associated inflammation in VAT with reduced VAT proinflammatory monocytes, macrophages, and CD8+ T cells. However, IL-33 administration did not affect insulin sensitivity in NZO and HFD mice.
Taken together, the three approaches outlined above demonstrate that the IL-33/ST2 axis plays an essential role in the development of VAT-Treg cells in both the mouse and human. These findings provide new insight into the differential mechanism of specialized Treg cell subsets. Thus, IL-33 has potential future uses in treating obesity and obesity-associated metabolic cardiovascular diseases. Further studies are required to determine the triggers of cTreg or eTreg cell migration into adipose tissue. The antigenic specificity of VAT-Treg cells remains to be further investigated. In addition, clarifying the causality between obesity and the reduced Treg cell number in adipose tissue and uncovering the underlying mechanisms are important. Whether obesity-associated inflammation results in the reduced VAT-Treg cell number in adipose tissue or whether the reduction of VAT-Treg cell number causes the obesity-associated inflammation remains to be determined.
References
- 1Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol 2013; 14: 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Zhou X, Tang J, Cao H, Fan H, Li B. Tissue resident regulatory T cells: novel therapeutic targets for human disease. Cell Mol Immunol 2015; 12: 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Ryba-Stanislawowska M, Stanislawowski M, Mysliwska J. Effector and regulatory T cell subsets in diabetes-associated inflammation. Is there a connection with ST2/IL-33 axis? Perspective. Autoimmunity 2014; 47: 361–371. [DOI] [PubMed] [Google Scholar]
- 4Cipolletta D, Kolodin D, Benoist C, Mathis D. Tissular Tregs: a unique population of adipose-tissue-resident Foxp3+CD4+ T cells that impacts organismal metabolism. Semin Immunol 2011; 23: 431–437. [DOI] [PubMed] [Google Scholar]
- 5Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol 2015; 16: 276–285. [DOI] [PubMed] [Google Scholar]
- 6Zeng H, Zhang R, Jin B, Chen L. Type 1 regulatory T cells: a new mechanism for the peripheral immune tolerance. Cell Mol Immunol 2015; 12: 566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T-helper cells by CD8+ regulatory T cells is essential for self tolerance. Nature 2010; 467: 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Peterson RA. Regulatory T-cells: diverse phenotypes integral to immune homeostasis and suppression. Toxicol Pathol 2012; 40: 186–204. [DOI] [PubMed] [Google Scholar]
- 9Jonuleit H, Schmitt E. The regulatory T cell family: distinct subsets and their interrelations. J Immunol 2003; 171: 6323–6327. [DOI] [PubMed] [Google Scholar]
- 10Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev 2014; 259: 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol 2013; 14: 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Liston A, Gray DH. Homeostatic control of regulatory T cell diversity. Nat Rev Immunol 2014; 14: 154–165. [DOI] [PubMed] [Google Scholar]
- 13Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol 2011; 12: 304–311. [DOI] [PubMed] [Google Scholar]
- 14Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE et al. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 2012; 486: 549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009; 15: 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]