Abstract
The application of 3D imaging in pediatric rheumatology helps to make the assessment of inflammatory changes more objective and to estimate accurately their volume and the actual response to treatment in the course of follow-up examinations. Additional interesting opportunities are opened up by the vascularity analysis with the help of power Doppler and color Doppler in 3D imaging. Contrast-enhanced ultrasound examinations enable a more sensitive assessment of the vascularity of inflamed structures of the locomotor system, and a more accurate analysis of treatment's effect on changes in vascularity, and thereby the inflammation process activity, as compared to the classical options of power and color Doppler. The equipment required, time limitations, as well as the high price in the case of contrast-enhanced ultrasound, contribute to the fact that the 3D analysis of inflammatory changes and contrast-enhanced ultrasound examinations are not routinely applied for pediatric patients.
Keywords: ultrasound, 3D imaging, ultrasound contrast agents, juvenile idiopathic arthritis
Abstract
Zastosowanie obrazowania trójwymiarowego w reumatologii dziecięcej pozwala na zobiektywizowanie oceny zmian zapalnych i dokładne oszacowanie ich objętości oraz realną ocenę odpowiedzi na leczenie w badaniach kontrolnych. Dodatkowe interesujące możliwości otwiera analiza unaczynienia dopplerem mocy i kolorowym dopplerem w obrazowaniu trójwymiarowym. Badania kontrastujące z wykorzystaniem ultrasonograficznych środków kontrastujących umożliwiają czulszą ocenę unaczynienia zapalnie zmienionych struktur narządu ruchu oraz dokładniejszą analizę wpływu leczenia na zmiany unaczynienia, a tym samym aktywności procesu zapalnego, w porównaniu z klasycznymi opcjami dopplera mocy i kolorowego dopplera. Wymagania sprzętowe oraz ograniczenia czasowe, zaś w przypadku badań z wykorzystaniem ultrasonograficznych środków kontrastujących wysoka cena sprawiają, że trójwymiarowa analiza zmian zapalnych oraz badania kontrastowe z wykorzystaniem ultrasonograficznych środków kontrastujących nie są rutynowo stosowane w badaniach ultrasonograficznych u dzieci.
This study is aimed at presenting the advantages and disadvantages of 3D imaging in pediatric rheumatology. It also discusses the potential for using ultrasound contrast agents (UCA) in patients with juvenile idiopathic arthritis (JIA).
Introduction
Ultrasound examinations have become the routine technique for the evaluation of the inflammatory changes in the musculoskeletal system in children in the course of systemic inflammatory conditions such as JIA(1–3). They allow to identify the inflamed structures of the locomotor system such as e.g. joints, bursae, and tendon sheaths, and to determine the type of the inflammatory changes (the presence of fluid and/or synovial hypertrophy) and their severity. However, even though where there are no inflammatory changes within the examined area, the classical ultrasound examination rules them out with a probability nearing 100%, where they are present, an accurate evaluation of their severity is subjective and tends to depend on numerous factors, such as the examiner's experience, the position of the limb in the case of peripheral joints, or the device and the transducer used. In pediatric patients the considerable variations in the size of the joints depending on the child's age, and the presence, especially in the youngest children, of large quantities of hypoechogenic cartilage tissue easily mistaken for inflammatory changes, make up for additional difficulties. In compliance with the ultrasound examination standards, the severity of inflammatory changes is determined in a semi-quantitative 3-degree scale, describing them as slight, moderate and severe. Some authors score the fluid and the synovial membrane jointly, whereas others score them separately. This may lead to substantial variations between researches in the scoring of the fluid and the synovium, to the effect of different assessment of the effectiveness of the undertaken treatment. This is where the application of 3D imaging option, routinely used e.g. for fetus assessment, becomes helpful. 3D imaging may facilitate an objective evaluation of the severity of the inflammatory changes, and their quantitative presentation. This paper discusses the suitability and the limitations of 3D imaging for the sake of ultrasound examinations of the musculoskeletal system in pediatric patients with rheumatologic conditions.
Ultrasound examinations are crucial for the vascularity assessment of inflammatory changes detected in the structures of the locomotor system. The vascularity is an indicator of the inflammatory process activity(4). Rheumatologic patients are evaluated with the help of power Doppler and color Doppler options. These, however, are not always sufficiently sensitive to detect synovial vascularity, which prompted search for other ways of determining the flow within the inflamed structures. The introduction of third generation UCA, which are readily mobile within the vascular bed and reach the vascularized synovium with the blood stream, while at the same time being resistant to the pressure in the left heart ventricle(5), has facilitated more sensitive detection of the flow within the synovial membrane. Even though the role of UCA for rheumatology has not yet been fully determined, they hardly seem to find routine application in future clinical practice(6).
3D imaging
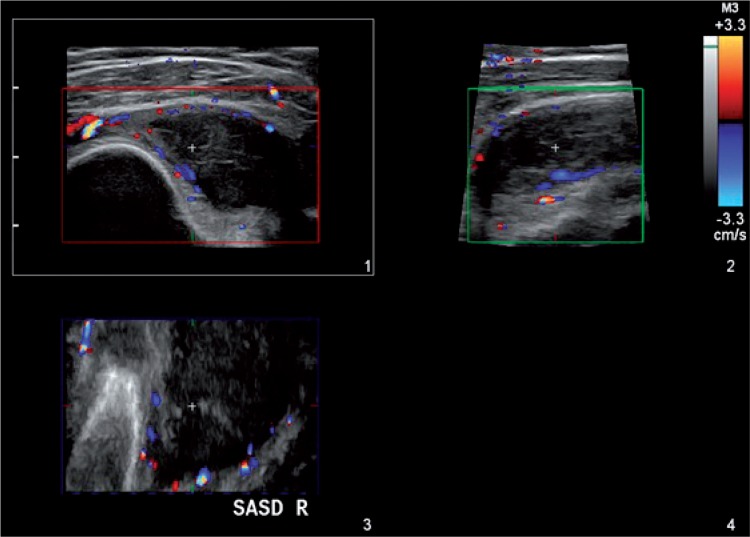
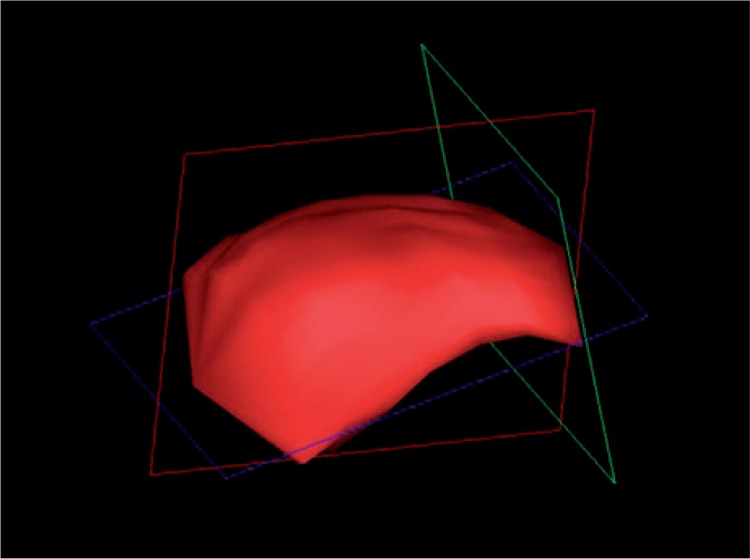
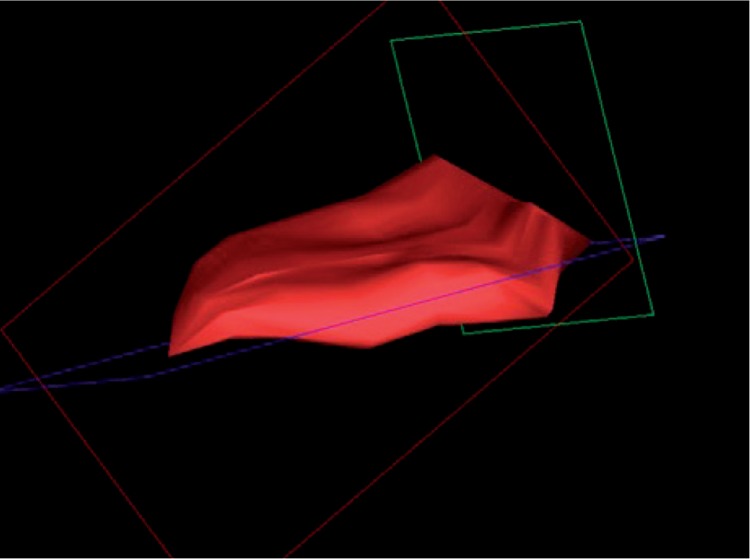
The role of 3D imaging is to present the examined region in a spatial way, thereby facilitating evaluation which is accurate and as objective as possible. The examined structures are frequently recorded in the form of “tissue blocks” which are processed and assessed after the examination has been completed, allowing a thorough, comprehensive analysis of the examined region, and calculation of various parameters whose assessment in the course of a routine, gray-scale ultrasound is difficult, if not impossible altogether. Additional volumetric transducers (for the locomotor system ultrasound, a linear one) facilitating data acquisition, as well as suitable software for further processing are required to carry out a 3D examination. The acquisition of 3D data takes place while the transducer is statically placed over the assessed area which is then automatically swept by the ultrasound beam. The collected data are presented in three planes: x, y and z (fig. 1). The 3D option allows imaging in a plane parallel to the front-face of the transducer – z or c plane (also known as “bird's eye view”), inaccessible in typical 2D imaging. Moreover, the data may be presented in other forms, e.g. surface rendering. A valuable 3D option in ultrasound examinations in rheumatology is the presentation of the revealed inflammatory changes in the form of a solid (fig. 2). This gives the possibility to calculate accurately the volume of the involved structures, such as joints or bursae(7). Due to their irregular shape, their volume should not be estimated with the aid of standard algorithms used for volumetric evaluation, employing three dimensions or one dimension and the area of the change. The unquestionable advantage of calculating the volume of an inflamed structure presented in 3D consists in the feasibility to objectively assess both the severity of the inflammation process, and the changes potentially occurring in response to treatment (fig. 3). This also results in a more unanimous assessment of the ultrasound by various examiners(8).
Fig. 1.
3D imaging of the subacromial-subdeltoid bursa (SASD) also revealing the vascularity of the bursa walls in color Doppler option
Fig. 2.
3D imaging. SASD bursa volume calculation
Fig. 3.
3D imaging. Fig. 2 SASD volume, 6 weeks after topical administration of glucocorticoids
This type of imaging, however, has numerous limitations. It is impossible to present as one solid some structures that are larger and longer (e.g. some involved tendon sheaths), and which exceed the size swept by the ultrasound beam. Also, there is the problem of assessing larger joints through osseous structures, impermeable to the ultrasound beam. Cartilage elements of poor echogenicity present in children's skeleton structures can be mistaken for inflammatory changes, making the examination more difficult. In 2D imaging, dynamic examination aids such cases, helping to successfully distinguish inflammatory changes and cartilage structures. The small size of the examined structures in younger children causes some problems too, and so does poor adherence of the transducer to the examined region e.g. the ankle. Moreover, 3D investigations are sensitive to motion artifacts, therefore the examined region should be stabilized and still upon data acquisition, which is naturally more challenging in pediatric patients than in adults, of tentimes requiring multiple attempts. It also seems that 3D evaluation should be applied for structures in which the inflammation process occurs mainly in the form of synovial membrane hypertrophy, since inflammatory changes visible predominantly in the form of effusion, which is prone to pressure and readily mobile, may render varying images during a single examination.
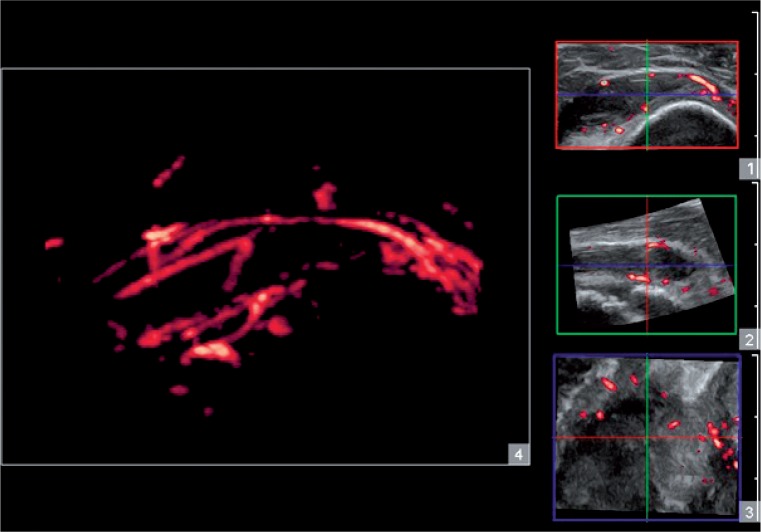
A 3D examination may also be combined with the Doppler option, as in 3D power Doppler ultrasound (3D PDU). It facilitates three-dimensional imaging of the distribution of vascular segments within an involved structure, t hu s enabli ng to a ssess t he va scu la r a rch itect u re of a given change (fig. 4). The additional benefit is a quantitative assessment of the vascularity of the region of interest, e.g. the hypertrophic synovium in the joint or in the bursa, and presenting it in the form of parameters such as the vascular index (VI), the flow index (FI) or an index combining both preceding parameters, the vascular-flow index (VFI). Determining these parameters for specific inflamed synovial structures allows to evaluate treatment effectiveness accurately, and to detect potential remission in the course of follow-up examinations.
Fig. 4.
3D imaging. Assessment of the SASD vascular architecture visible in examination 1
All things considered, 3D imaging is an interesting option for the sake of assessment of inflammatory changes visible in an ultrasound examination of patients suffering with rheumatologic conditions. It makes the examinations more objective and provides new parameters valuable for the analysis of the vascularity of the changes, and thereby of the inflammation process activity. Hence, it aids follow-up examinations and helps to evaluate remission in response to treatment. It is, nonetheless, considerably limited by factors such as the necessary equipment (specialized transducer and software), anatomy, and the time required for the examination (a technically valid examination and its further processing are substantially more time-consuming than the classical ultrasound method). As a result, the application of 3D examinations in rheumatology and pediatric rheumatology is limited.
Contrast-enhanced ultrasound
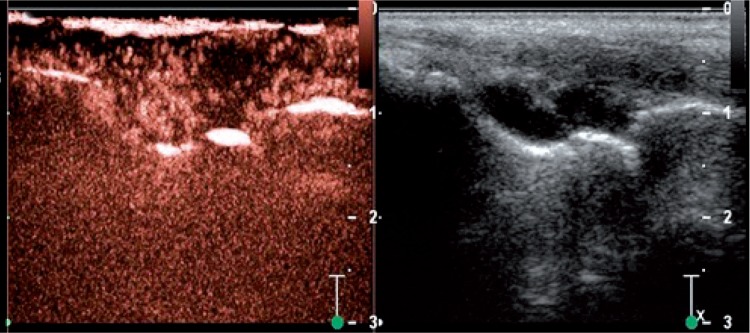
UCA are gas-filled microbubbles administered intravascularly to obtain blood flow image. Currently, third generation UCA are used in clinical practice, such as SonoVue® from Bracco, that is sulphur hexafluoride stabilized by a phospholipid shell(5). The investigation uses a suspension of gas-filled microbubbles of a diameter <10 µm allowing the agent to move easily within the vascular tree, without the risk of occluding the microcirculation. With the blood flow, the bubbles reach also inflamed regions such as the thickened and hypertrophic synovium present in JIA, thereby finding use in rheumatology diagnostics. Contrast-enhanced ultrasound examinations were first employed for rheumatology, including JIA patients, in the beginning of the last decade(9). Yet, originally, the agents were used to enhance Doppler signal in vessels, hence facilitating vessel detection in the classical Doppler options like contrast-enhanced power/ color Doppler (CEPD/CECD). These days, the flow of UCA in blood vessels is rendered as gray-scale image with low mechanical index (MI), known as contrast-enhanced ultrasound (CEUS) (fig. 5)(10), and is carried out with special software. Even in the beginning, it was noted that regardless of the used method, UCA in rheumatology increase the sensitivity of vascular detection in the synovium as compared to the classical Doppler options(11). The agents help to detect vessels of a diameter <40 µm, invisible in classical Doppler options, bringing us closer to evaluation of flow at tissue level.
Fig. 5.
Contrast-enhanced ultrasound of the wrist joints. On the left side, visible contrast-enhanced synovium within the midcarpal and the radiocarpal joint; synovial membrane enhancement in the radiocarpal joint is partial, with an area of unvascularized synovium. On the right side, view of the evaluated area in B-mode option
The test is carried out upon administering the UCA suspension intravenously, e.g. into the basilic vein. Then, the flow of the agent into the inflamed region, displaying hypertrophic and thickened synovial membrane, is observed in real time. Where there is active inflammation, contrast enhancement of the synovial membrane is visible. Fluid and inactive changes, such as “old,” fibrous synovium are not enhanced. Hence, contrast-enhanced ultrasound helps to distinguish active changes in need of treatment or treatment modification, from inactive ones found e.g. in the case of a long-established JIA with a persisting residual synovial hypertrophy. The effect may also be evaluated quantitatively by analyzing time intensity curve (TIC) and then estimating the area under curve (AUC)(12). It is not only peripheral joints that may be evaluated in this way, as some reports have suggested the usefulness of UCA e.g. for the assessment of sacroiliac joints(13).
The examination has numerous limitations, including the cost of the contrasting agent, and invasiveness (intravenous administration of the agents). UCA are safe substances which are quickly removed from the body, and rarely cause any side effects(14), yet so far they have not been registered for use in rheumatology or pediatric rheumatology. Oftentimes, the vascularity assessment performed with the aid of classical Doppler options is sufficient. The sensitivity of traditional examinations using power Doppler option is comparable to the sensitivity of postcontrast MRI(15). JIA cases with subclinical course of disease, where classical Doppler ultrasound does not reveal synovial inflammatory activity(6), would be where contrast agents should be employed to confirm the activity of the inflammation process. As of now, CEUS examinations remain a highly sensitive method of synovial vascularity assessment, yet one without practical indications.
Conclusion
3D imaging is an option enabling a more accurate assessment of inflamed structures such as joints or bursae, which moreover provides new parameters useful for the assessment. Also, it contributes to an increased comparability of assessment by various examiners, and helps to determine treatment effectiveness in follow-up ultrasound examinations. Due to limitations arising from equipment requirements and technical difficulties, the method is rarely used in clinical practice.
Contrast-enhanced ultrasound is the most sensitive ultrasound method available, allowing to detect the synovial vascularity in rheumatologic patients, and to confirm the vascular flow where it is impossible to determine by classic Doppler ultrasound. Due to the high cost of the contrast agents, the invasiveness of the method, and the fact that UCA have not yet been registered for pediatric rheumatology examinations, it has not found its way to routine medical practice.
Conflict of interest
Author does not report any financial or personal links with other persons or organizations, which might affect negatively the content of this publication and/or claim authorship rights to this publication.
References
- 1.Johnson K. Imaging of juvenile idiopathic arthritis. Pediatr Radiol. 2006;36:743–758. doi: 10.1007/s00247-006-0199-x. [DOI] [PubMed] [Google Scholar]
- 2.Tok F, Demirkaya E, Ozçakar L. Musculoskeletal ultrasound in pediatric rheumatology. Pediatr Rheumatol Online J. 2011;9:25. doi: 10.1186/1546-0096-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collado P, Jousse-Joulin S, Alcalde M, Naredo E, D'Agostino MA. Is ultrasound a validated imaging tool for the diagnosis and management of synovitis in juvenile idiopathic arthritis? A systematic literature review. Arthritis Care Res (Hoboken) 2012;64:1011–1019. doi: 10.1002/acr.21644. [DOI] [PubMed] [Google Scholar]
- 4.Koski JM, Saarakkala S, Helle M, Hakulinen U, Heikkinen JO, Hermunen H. Power Doppler ultrasonography and synovitis: correlating ultrasound imaging with histopathological findings and evaluating the performance of ultrasound equipments. Ann Rheum Dis. 2006;65:1590–1595. doi: 10.1136/ard.2005.051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosgrove D, Eckersley R. Contrast-enhanced ultrasound: basic physics and technology overview. In: Lencioni R, editor. Enhancing the Role of Ultrasound with Contrast Agents. Milan: Springer; 2006. pp. 3–14. [Google Scholar]
- 6.De Zordo T, Mlekusch SP, Feuchtner GM, Mur E, Schirmer M, Klauser AS. Value of contrast-enhanced ultrasound in rheumatoid arthritis. Eur J Radiol. 2007;64:222–230. doi: 10.1016/j.ejrad.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Klauser AS, Peetrons P. Developments in musculoskeletal ultrasound and clinical applications. Skeletal Radiol. 2010;39:1061–1071. doi: 10.1007/s00256-009-0782-y. [DOI] [PubMed] [Google Scholar]
- 8.Naredo E, Möller I, Acebes C, Batlle-Gualda E, Brito E, de Agustín JJ, et al. Three-dimensional volumetric ultrasonography. Does it improve reliability of musculoskeletal ultrasound? Clin Exp Rheumatol. 2010;28:79–82. [PubMed] [Google Scholar]
- 9.Doria AS, Kiss MH, Lotito AP, Molnar LJ, de Castro CC, Medeiros CC, et al. Juvenile rheumatoid arthritis of the knee: with contrastenhanced color Doppler ultrasound. Pediatr R adiol. 2001;31:524–531. doi: 10.1007/s002470100474. [DOI] [PubMed] [Google Scholar]
- 10.Klauser A, Demharter J, De Marchi A, Sureda D, Barile A, Masciocchi C, et al. Contrast enhanced gray-scale sonography in assessment of joint vascularity in rheumatoid arthritis: results from the IACUS study group. Eur Radiol. 2005;15:2404–2410. doi: 10.1007/s00330-005-2884-9. [DOI] [PubMed] [Google Scholar]
- 11.Schueller-Weidekamm C, Krestan C, Schueller G, Kapral T, Aletaha D, Kainberger F. Power Doppler sonography and pulse-inversion harmonic imaging in evaluation of rheumatoid arthritis synovitis. Am J Roentgenol. 2007;188:504–508. doi: 10.2214/AJR.05.2165. [DOI] [PubMed] [Google Scholar]
- 12.Platzgummer H, Schueller G, Grisar J, Weber M, Schueller-Weidekamm C. Quantification of synovitis in rheumatoid arthritis: do we really need quantitative measurement of contrast-enhanced ultrasound? Eur J Radiol. 2009;71:237–241. doi: 10.1016/j.ejrad.2009.03.044. [DOI] [PubMed] [Google Scholar]
- 13.Klauser AS, De Zordo T, Bellman-Weiler R, Feuchtner GM, Sailer-Höck M, Sögner P, et al. Feasibility of second-generation ultrasound contrast media in the detection of active sacroiliitis. Arthritis Rheum. 2009;61:909–916. doi: 10.1002/art.24648. [DOI] [PubMed] [Google Scholar]
- 14.Piscaglia F, Bolondi L. Italian Society for Ultrasound in Medicine and Biology (SIUMB) Study Group on Ultrasound Contrast Agents: The safety of SonoVue® in abdominal applications: ret rospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32:1369–1375. doi: 10.1016/j.ultrasmedbio.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 15.Laurell L, Court-Payen M, Nielsen S, Zak M, Boesen M, Fasth A. Comparison of ultrasonography with Doppler and MRI in assessment of disease activity in juvenile idiopathic arthritis: a pilot study. Pediatr Rheumatol Online J. 2012;10:23. doi: 10.1186/1546-0096-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]