Abstract
Introduction
Vesicoureteral reflux appears in 20–50% of pediatric patients with recurrent urinary tract infections. The most common method of diagnosing this disease is voiding cystourethrography. However, contemporary pediatric radiology does not favor this method due to exposure to X-radiation.
Aim
The aim of this study was to assess the usefulness of 2D/3D/4D contrast-enhanced voiding urosonography in the diagnosis and treatment monitoring of vesicoureteral reflux in children and the possibility of using contrast-enhanced voiding urosonography to replace voiding cystourethrography.
Material and methods
Voiding cystourethrography and contrast-enhanced voiding urosonography were conducted in 80 pediatric patients in order to assess sensitivity, specificity, positive and negative predictive values as well as the number of vesicoureteral refluxes detected by each of the two methods. The second stage of the study involved performing voiding urosonography in an extended protocol in 58 children in order to determine the usefulness of three-dimensional (3D/4D) examinations in the assessment of vesicoureteral reflux and the ability to assess the urethra.
Results
The concordance between the two methods was 86.95%. The sensitivity of voiding urosonography was 84.51%, specificity – 90.99%, positive predictive value – 85.71% and negative predictive value – 90.17%. A 3D/4D assessment of the urinary bladder and transperineal 2D morphological assessment of the urethra were possible in all patients (100%). Assessment of the urethra during micturition with the use of 2D/3D/4D techniques was possible in all patients in whom voiding was elicited (95.83%), and 3D/4D assessment of vesicoureteral reflux was possible in all patients with reflux (100%). Although the application of 3D/4D techniques allowed accurate specification of the grade of reflux in all cases (100%), it appeared particularly useful in differentiating between grades II and III (70.97%).
Conclusions
Contrast-enhanced voiding urosonography allows the diagnosis and monitoring of treatment of vesicoureteral reflux in pediatric patients as well as assessment of the urethra in both girls and boys. The method is characterized by high sensitivity and specificity. Moreover, it is safe, relatively inexpensive and can replace voiding cystourethrography.
Keywords: contrast-enhanced voiding urosonography, vesicoureteral reflux, SonoVue®, urethra, 3D/4D ultrasound
Abstract
Wstęp
Odpływy pęcherzowo-moczowodowe występują u 20–50% dzieci z nawracającymi zakażeniami układu moczowego. Najczęściej wykorzystywaną metodą w diagnostyce tego schorzenia jest cystouretrografia mikcyjna, od której nowoczesna radiologia pediatryczna stara się odchodzić, z powodu narażenia na promieniowanie rentgenowskie.
Cel pracy
Celem pracy była ocena przydatności sonocystografii mikcyjnej w diagnostyce i monitorowaniu leczenia odpływów pęcherzowo-moczowych u dzieci i możliwości zastąpienia omawianą metodą dotychczas stosowanej cystouretrografii mikcyjnej.
Materiał i metoda
U 80 dzieci wykonano cystouretrografię mikcyjną oraz sonocystografię mikcyjną w celu określenia czułości, swoistości, wartości predykcyjnej dodatniej i ujemnej sonocystografii mikcyjnej oraz ilości wykrywanych odpływów pęcherzowo-moczowych przez każdą z metod. W drugim etapie u 58 dzieci wykonano sonocystografię mikcyjną w rozszerzonym protokole w celu określenia przydatności badania trójwymiarowego (3D/4D) w ocenie odpływów pęcherzowo-moczowych oraz możliwości oceny cewki moczowej.
Wyniki
Zgodność obu metod wynosiła 86,95%. Czułość sonocystografii mikcyjnej wynosiła 84,51%, swoistość 90,99%, wartość predykcyjna dodatnia 85,71%, ujemna 90,17%. Ocena 3D/4D pęcherza moczowego oraz ocena morfologiczna 2D cewki moczowej z dostępu przezkroczowego możliwa była u wszystkich pacjentów (100%). Ocena cewki moczowej w trakcie mikcji techniką 2D/3D/4D możliwa była u wszystkich pacjentów, u których uzyskano mikcję (95,83%), a ocena 3D/4D OPM – u wszystkich pacjentów z odpływem (100%). Zastosowanie technik 3D/4D pozwoliło na precyzyjne określenie stopnia odpływów we wszystkich przypadkach (100%), jednakże szczególnie przydatne okazało się w różnicowaniu pomiędzy stopniem II a III (70,97%).
Wnioski
Sonocystografia mikcyjna jest metodą umożliwiającą diagnostykę i monitorowanie leczenia odpływów pęcherzowo-moczowodowych u dzieci oraz ocenę cewki moczowej u dziewcząt i chłopców. Metodę charakteryzuje wysoka czułość i swoistość – jest bezpieczna, stosunkowo niedroga i może zastąpić cystouretrografię mikcyjną.
Introduction
Vesicoureteral reflux (VUR) occurs in approximately 1% of the pediatric population in the United States and Europe. It appears in 20–50% of pediatric patients with recurrent urinary tract infections, thus constituting an extremely complex diagnostic and therapeutic problem in pediatrics(1, 2). Currently, three imaging modalities are used in the diagnostic process of VUR: X-ray based voiding cystourethrography (VCUG), isotope cystography and contrast-enhanced voiding urosonography (ce-VUS). The most common method VCUG is based on ionizing radiation, but this is no longer favored by contemporary pediatric radiology due to the considerable radiation exposure involved. VUR diagnosis and monitoring of treatment usually require multiple X-ray examinations. Unfortunately, the gonads are always exposed to radiation during the examination, which is of concern given that they are one of the most radiosensitive organs in the human organism. Other limitations of VCUG are: the static character of the examination, the two-dimensional (“flat”) image, exposure to potential adverse reactions to iodine contrast agents, and the lack of intimacy during the examination, as frequently mentioned by parents and older children. ce-VUS is an alternative to VCUG. However, to date, the most frequently mentioned limitation of this method is the inability to assess the urethra, especially in boys. Nevertheless, recent reports indicate that ce-VUS with the use of SonoVue® is a highly sensitive and specific method in the diagnosis and treatment monitoring of VUR, which, in addition, enables assessment of the urethra in both girls and boys(3–6).
Aim of the study
The aim of this study was to assess the usefulness of ce-VUS in the diagnosis and treatment monitoring of VUR in children and to determine whether this method could replace the currently used VCUG.
The study consisted of two stages. Bearing in mind the main aim of the study, in the first stage, the authors attempted to determine:
The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of ce-VUS compared with X-ray VCUG as a reference method.
-
The number of VURs detected by each of these methods.
In the second stage, the protocol of ce-VUS was extended to include three-dimensional options (3D/4D) and urethra examination. Attempts were made to determine the following:
Usefulness of three-dimensional (3D/4D) examination in VUR assessment.
Ability to examine the urethra in transperineal 2D/3D/4D ce-VUS before and during micturition.
Material and methods
The examinations were carried out as part of the Polish National Science Centre Project No. NN403456137. The study was approved by the Ethics Committee at the Medical University in Lublin. The parents of all patients were informed about the aim of the study, the advantages and disadvantages of the method, and the off-label use of the contrast agent (SonoVue®, Bracco, Milan, Italy) in patients below 18 years of age. They gave written consent for such examinations in their children. The study was conducted in two stages in two subgroups. The study group comprised 138 pediatric patients [277 ureteral units (UUs)] including 106 girls and 32 boys aged from 3 months to 17 years and 3 months; the mean age was 4 years and 1 month. The patients were examined from December 2010 to October 2013.
Stage I
In the first stage (subgroup 1), all patients underwent VCUG as a reference method and ce-VUS with the use of SonoVue® with a maximum time interval between the examinations of 3 months. The aim was to specify the sensitivity, specificity, PPV and NPV as well as the number of VURs detected by each method.
Subgroup 1 consisted of 80 pediatric patients (161 UUs, 62 girls and 18 boys) aged from 3 months to 17 years and 3 months; the mean age was 4 years and 8 months. The patients were examined from December 2010 to October 2012. Of the examined patients, 45 children (91 UUs) were already being treated for VUR and were referred to the examination in order to monitor the effects of therapy. Thirty-five children (70 UUs), however, were patients with recurrent urinary tract infections in whom VUR was suspected. Urinary tract infections were diagnosed based on the clinical picture (loss of appetite, no weight gain, abdominal pain, raised body temperature, pain and burning sensation during urination, pollakiuria, urine retention etc.) as well as changes in urinalysis and positive urine culture test with a count of above 105/ml. The detected pathogens included: Escherichia coli, Proteus species, Pseudomonas aeruginosa, Staphylococcus species and Klebsiella pneumoniae. The examinations were performed no sooner than 2 weeks after the last urinary tract infection had been treated(7).
Contrast-enhanced voiding urosonography (ce-VUS)
The examinations were performed using a Phillips IU22 ultrasound system with a convex probe (2–5 MHz) or GE Voluson 730 Expert with a convex probe (2–5 MHz). The protocol included:
transabdominal assessment of the kidneys and urinary bladder in B-mode, color Doppler and tissue harmonic imaging (THI);
catheterization of the patient (6F or 8F);
ultrasound-guided slow administration of the solution of heated saline with ultrasound contrast agent SonoVue®(8) [assessing the volume of the bladder according to the formula: volume (ml) = (age + 2) × 30] with alternate assessment of both kidneys during the administration of the solution until the urinary bladder was full (in younger children – under ultrasound monitoring of the bladder volume; in older children – until strong urinary urgency);
removal of the catheter;
during micturition, alternate transabdominal examination of both kidneys using 2D technique;
transabdominal urinary tract examination (kidneys, urinary bladder) after voiding with residual urine assessment (after micturition and voiding of the pelvicalyceal systems).
The subsequent stages of the examination were recorded as image files (JPG, 2D/3D/4D DICOM) and films (AVI). The five-grade classification of Darge and Troeger(9) was used to assess the grade of reflux in ce-VUS.
Voiding cystourethrography (VCUG)
VCUG was performed by drip infusion of the heated saline with a non-ionic contrast agent Omipaque 300 (GE Healthcare) or Iomeron 300 (Bracco, Italy). The methodology of the study and classification used to grade VUR were consistent with the international system of VUR radiographic grading(10).
Stage II
The second stage of the study (subgroup 2) involved performing ce-VUS with SonoVue® in an extended protocol in all patients. The aim was to determine the usefulness of three-dimensional (3D/4D) examinations in the assessment of VUR and the ability to assess the urethra in transperineal 2D/3D/4D ce-VUS. In subgroup 2, ce-VUS was the only diagnostic method used for the identification of VUR.
Subgroup 2 consisted of 58 patients (116 UUs; 44 girls and 14 boys) aged from 4 months to 10 years and 2 months; the mean age was 2 years and 8 months. The patients were examined from January to October 2013. All children were referred to the examination due to recurrent urinary tract infections in order to confirm or rule out VUR. Urinary tract infections were diagnosed based on clinical picture, changes in urinalysis and positive urine culture test (indicated by a count of above 105/ml). The detected pathogens included: Escherichia coli, Proteus species, Pseudomonas aeruginosa, Staphylococcus species, Citrobacter freundii and Kluyvera ascorbata. The examinations were performed no sooner than 2 weeks after the last urinary tract infection had been treated.
Contrast-enhanced voiding urosonography (ce-VUS)
The examinations were performed with the use of a GE Voluson 730 Expert ultrasound system with a convex probe (2–5 MHz) and a pediatric micro-convex 4D transducer (RNA 5–9 MHz). The protocol included:
transabdominal assessment of the kidneys and urinary bladder in B-mode, color Doppler and tissue harmonic imaging (THI);
transabdominal static three-dimensional urinary bladder assessment (3D) and real time assessment (4D) – so-called virtual cystoscopy;
a 2D transperineal morphological assessment of the urethra;
catheterization of the patient (6F or 8F);
slow administration of heated saline with ultrasound contrast agent SonoVue®(8);
removal of the catheter;
during micturition, transperineal examination of the urethra and alternate transabdominal assessment of both kidneys using 2D, 3D and 4D techniques;
transabdominal urinary tract examination (kidneys, urinary bladder) after voiding with residual urine assessment (after micturition and voiding of the pelvicalyceal systems).
transperineal examination of the urethra following micturition.
As in the procedure followed in subgroup 1, the subsequent stages of the examination were recorded as image files (JPG, 2D/3D/4D DICOM) and films (AVI). The five-grade classification of Darge and Troeger(9) was used to assess the grade of reflux in ce-VUS.
Results
Stage I
Data were obtained from 80 patients (161 UUs, 100%). Unilateral complete ureteral and pelvicalyceal duplication was detected in one patient. The concordance between the two methods, i.e. VCUG and ce-VUS, was 86.95%. With VCUG as a reference method, the sensitivity of ce-VUS was 84.51%, specificity – 90.99%, PPV – 85.71% and NPV – 90.17%.
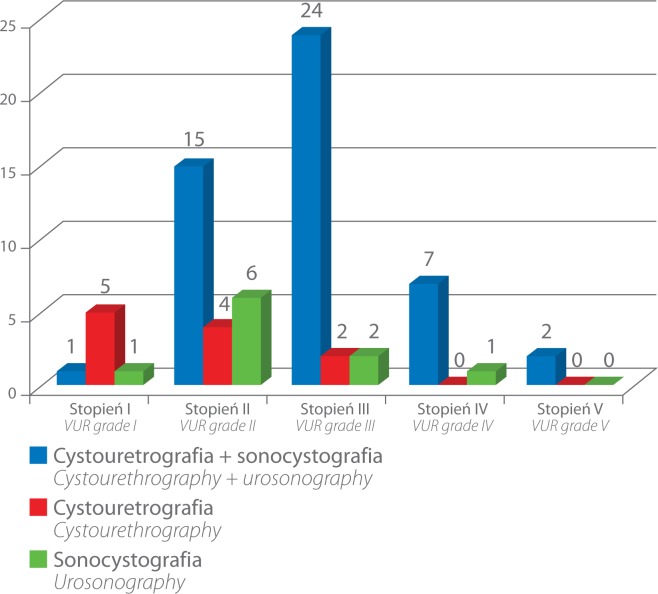
VCUG d emonstrated t he presence o f V UR i n 6 0 out o f 161 UUs (37.26%) and ce-VUS detected it in 59 out of 161 UUs (36.64%). VUR was detected in 49 of the 60 UUs (81.67%) by both methods, i.e. VCUG and ce-VUS (fig. 1). In 11 out of 60 UUs (18.33%), VUR was detected only with the help of VCUG (five cases – grade I, four – grade II and two – grade III). However, in 10 out of 59 UUs (16.94%), VUR was detected only with the help of ce-VUS (one case – grade I, six – grade II, two – grade III and one – grade IV) (fig. 2).
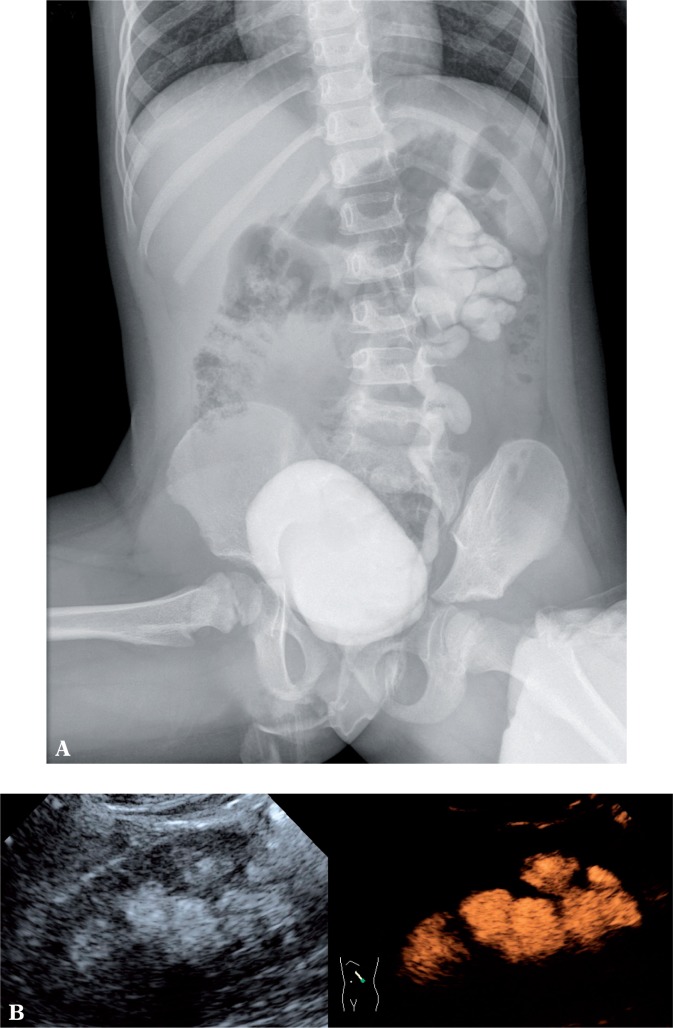
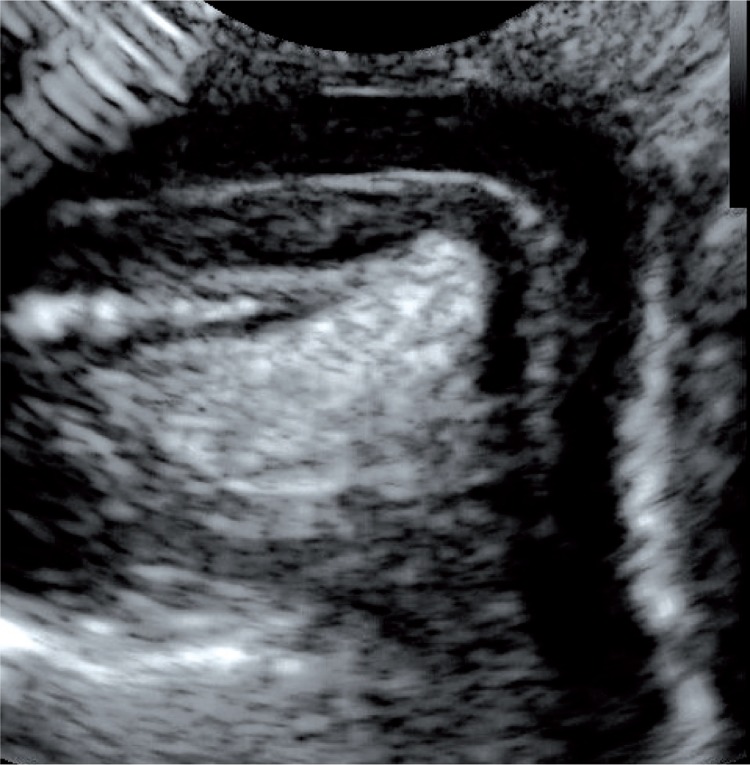
Fig. 1.
Grade V VUR on the left side detected by both methods, i.e. voiding cystourethrography (A) and 2D voiding urosonography (B) (grey scale and contrast-enhanced image)
Fig. 2.
Number of VUR detected by the two methods, i.e. in VCUG and ce-VUS, and by each method separately with division into VUR grades
Stage II
Out of the 58 (100%) examined patients, the presence of VUR was ruled out in 35 cases (60.34%, 70 UUs) and diagnosed in 23 children (39.65%), including eight bilateral cases and 15 unilateral cases (in total – 31 ureteral units). Of the 31 (100%) visualized cases of VUR, 11 (35.48%) were assessed as grade II, 11 (35.48%) as grade III, eight (25.80%) as grade IV and one (3.22%) as grade V.
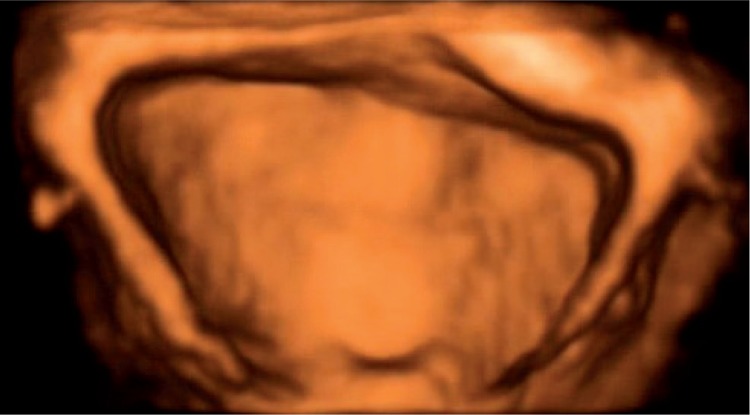
Transabdominal static three-dimensional urinary bladder assessment (3D) and real time (4D) assessment, known as virtual cystoscopy, was possible in all patients (n = 58, 100%). In one case (1.72%), virtual cystoscopy visualized the atypical localization of the ureteral orifice beyond the triagone of the bladder, which had not been seen in a previous 2D B-mode examination. In the remaining 57 patients (98.28%), the image of the urinary bladder was normal (fig. 3).
Fig. 3.
Normal presentation of the urinary bladder in real time (4D) examination – so-called virtual cystoscopy
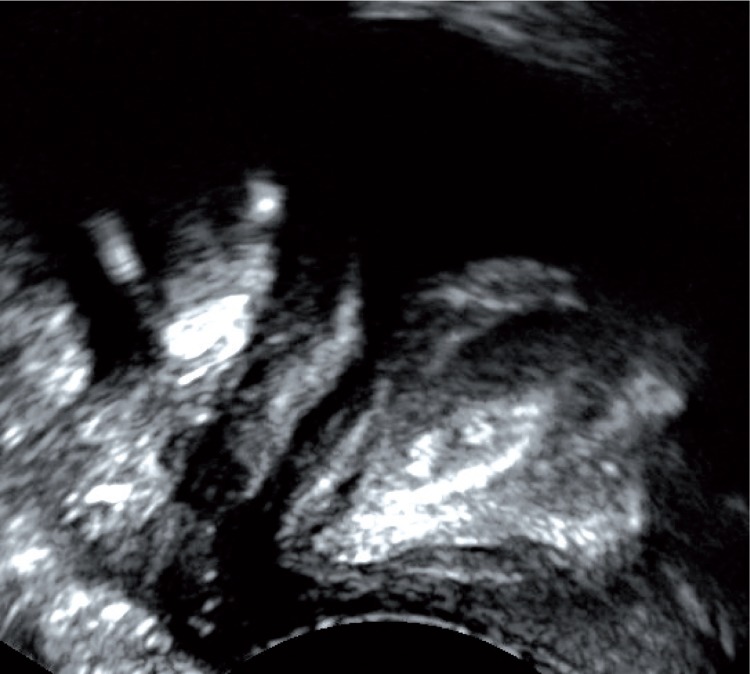
Transperineal morphological assessment of the urethra using the 2D method was possible in all cases (n = 58, 100%). In girls, the examination was conducted via the vaginal orifice, which allowed the entire course of the urethra from the neck of the urinary bladder to its external opening to be visualized with one application of the transducer (fig. 4). In boys, assessment of the bladder neck and the proximal fragment of the urethra was conducted via the suprapubic access and via the perineum/scrotum. Assessment of the distal part of the urethra was conducted by placing the transducer directly on the distal part of the penis (fig. 5). In infant boys, the entire urethra could be visualized with one application of the transducer. In older boys, the assessment was made by moving the probe in order to visualize the entire urethra. In all subjects, the morphology of the urethra in a 2D examination was judged to be normal.
Fig. 4.
Transperineal assessment of the morphology of the urethra in a girl
Fig. 5.
Assessment of the morphology of the urethra in an infant boy performed with the transducer placed in the suprapubic region and on the anterior aspect of the penis
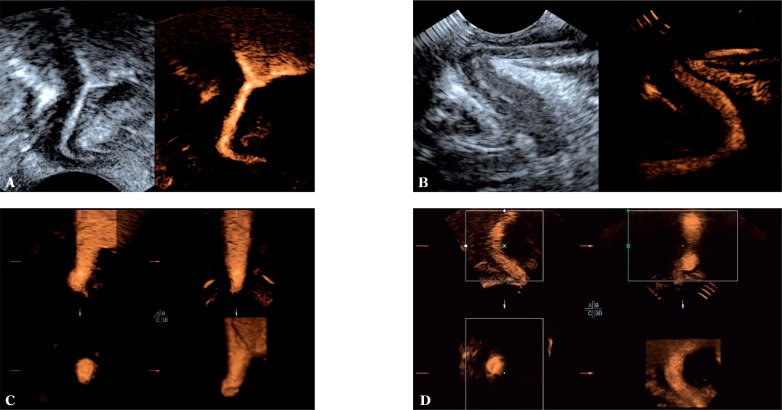
The 2D, 3D and 4D assessment of the urethra during micturition was possible in all patients in whom voiding was elicited during the examination, i.e. in 55 out of 58 (95.83%) patients. Micturition was not elicited in three patients (5.17%) due to the lack of cooperation. The 2D technique enabled assessment of the bladder neck and the lumen of the urethra. The three-dimensional techniques (3D and 4D), however, allowed for an additional spatial assessment of the studied anatomical structures (fig. 6). These 2D, 3D and 4D examinations of the urethra during micturition did not demonstrate any pathological changes in any of the 55 patients.
Fig. 6.
Contrast-enhanced examination during voiding. 2D examination of the female (A) and male (B) urethra. Three-dimensional (3D/4D) imaging of the female urethra (C) and male urethra on its proximal aspect (D)
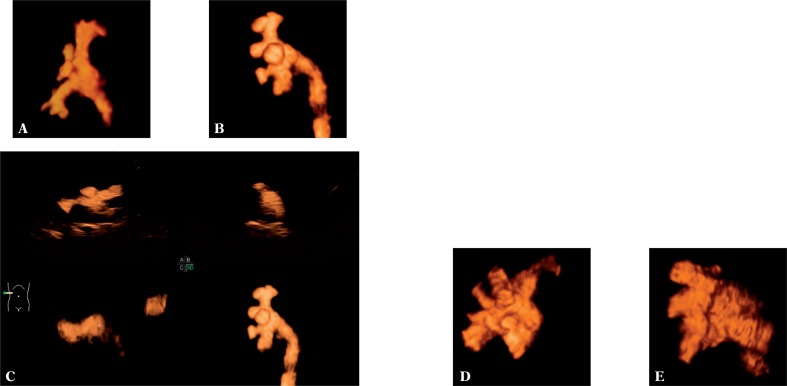
The 3D and 4D assessment of VUR was possible in all patients in whom VUR was diagnosed, i.e. in 23 children (31 UUs) (100%). The application of the three-dimensional (3D/4D) techniques enabled accurate specification of a VUR grade in all ureteral units (31 UUs, 100%) (fig. 7). Nevertheless, they proved the most useful in differentiating between grade II and III (in total 22 UUs, 70.97%). In this case, a three-dimensional examination facilitated the assessment of the renal calyces in visualized VUR to a considerable extent.
Fig. 7.
Visualization of VUR grade II (A), grade III (B, C), grade IV (D) and grade V (E) using the three-dimensional technique (A, B, D, E) and real-time three-dimensional examination, so-called 4D (C)
Discussion
In 1997, the American Urological Association (AUA) reported the need to search for “less traumatic” diagnostic methods that would allow the detection of VUR as well as techniques of VCUG that would reduce exposure to ionizing radiation(11). Ce-VUS, which meets both these criteria, appears to be one of the most promising methods. It has been used in the diagnosis and treatment monitoring of VUR in children for approximately 15 years. As early as in 2001, certain publications proved that the application of ce-VUS would reduce by over a half the number of pediatric patients exposed to ionizing radiation during the diagnosis and treatment of VUR(12). Initially, the examinations were performed with the use of the second-generation ultrasound contrast agent Levovist (Schering, Berlin, Germany). Ce-VUS with Levovist was used successfully from 1999 in numerous European countries and in Australia. It was proven that when administered intravesically, Levovist, being a solution of galactose-based, palmitic acid microbubbles, enables the visualization of the urinary tract in children and possibly also the identification of VUR (sensitivity 57–100%, specificity 85–100%, positive predictive value 58–100%, negative predictive value 87–100%, diagnostic accuracy 78–96%)(13–15). In 2001, a third-generation ultrasound contrast agent – SonoVue® – was introduced. In vitro comparative studies of Levovist (5% volume) and SonoVue® demonstrated that enhancement observed with SonoVue® lasted seven times longer when an 80% lower dose, compared to Levovist, was used(16). SonoVue®, more modern than its predecessor, does not require the usage of Doppler techniques, has a parenchymal enhancement phase and enables considerably longer enhancement, started to gradually replace Levovist, which soon after was withdrawn from the market. Currently, SonoVue®, which is a suspension of sulfur hexafluoride microbubbles with a diameter smaller than that of capillaries, is a recognized agent both in Poland and abroad. It is used with success in the diagnosis and monitoring of focal lesions in numerous organs, most frequently in the diagnosis of hepatic focal lesions(17, 18).
Unfortunately, to date, ce-VUS has not been introduced as a routine procedure in the diagnostic algorithm in Poland. Therefore, we attempted to assess the relevance of ce-VUS in the diagnosis and treatment monitoring of VUR in children and the possibility of replacing the currently used VCUG.
One of the most important limitations to popularizing the method is the fact that to date, SonoVue® has not been registered for use in patients below the age of 18. Even so, it is commonly used for VUR diagnosis in children both in Europe and the United States. This was confirmed in a survey conducted in 2011 which indicated that in European children, this agent had been used intravesically in over 4000 examinations(19, 20). However, the lack of registration for use in pediatric patients a ssociated w ith the lack of clinical t rials conducted among a sufficiently large population refers to its intravenous use. The manufacturer makes no mention of the issue of its use in the cavities despite the common belief that such use is safe and does not cause adverse reactions in children. Both the European Society of Paediatric Radiology (ESPR) and the European Society of Urogenital Radiology (ESUR) – Paediatric Working Group claim that f rom medical a nd scientific points of view, the use of ultrasound contrast agents in children should be promoted and propagated. At the same time, appropriate trials allowing for the official legalization and registration of such agents in pediatrics should be conducted(19). In their diagnostic algorithms, the aforementioned Societies recommend ce-VUS as the method of choice for diagnosing VUR in girls with recurrent urinary tract infections and for monitoring the treatment of VUR in girls and boys. Moreover, this method is recommended in girls with prenatally diagnosed hydronephrosis, in girls and boys with the suspicion of obstruction u ropathy, for d iagnosing congenital defects of the region of urogenital hiatus, in cases of undifferentiated sex, in abscess drainage as well as for fistula diagnosis and others(19).
Despite the fact that this agent is not registered for use in patients below the age of 18, there are numerous reports worldwide describing ce-VUS in pediatric patients with the intravesical administration of SonoVue®. In 2009, Papadopoulou et al. published a paper i n which, based on studies conducted with SonoVue® among 228 pediatric patients with VUR, they indicate that ce-VUS is superior to VCUG in terms of sensitivity and that it enabled the visualization of refluxes in 37% of patients more than the conventional method(21). In 2012, Ključevšek et al. obtained similar results following the analysis of 132 children. They showed that the X-ray-based method visualized refluxes in 12% of patients whereas the ultrasound-based method visualized the pathology in 32% of patients(22). In 2011, Otukesh et al. published the outcomes of their studies in which the sensitivity of ce-VUS was 88% and specificity was 94% when isotope cystography was used as the reference method(23). Numerous publications indicate that ce-VUS with the use of the third-generation contrast agent SonoVue®, is ideal for diagnosing and monitoring the course of treatment of VUR and that in many cases, it may replace X-ray-based VCUG(8, 24). The superiority of ce-VUS compared to VCUG, has also been confirmed in other publications that attribute the larger number of refluxes detected in ce-VUS to the more dynamic and longer examination as well as the ability to detect additional anatomical defects thanks to the transperineal access(3, 25). The results obtained in this paper are consistent with the outcomes of the aforementioned publications. The authors demonstrated high concordance between VCUG and ce-VUS (86.95%) as well as the high sensitivity, specificity, positive and negative predictive values of ce-VUS (84.51%, 90.99%, 85.71% and 90.17% respectively). In certain patients, VUR was visualized by only one of the methods. In the case of VUR diagnosed solely by means of VCUG, the great majority of such instances (9 of 11 UUs, 81.81%) were low-grade refluxes, i.e. grade I and II. However, in the case of VUR diagnosed by means of ce-VUS only, these were mainly refluxes assessed as grade II or higher (9 of 10 UUs, 90%). The authors also demonstrated that in ce-VUS, a reliable assessment of the urethra is possible in both girls and boys. Undoubtedly, however, further studies of a larger group of patients and with the use of a reference method need to be conducted.
Besides the fact that the sensitivity and specificity of this method are non-inferior to those of VCUG, the lack of exposure to ionizing radiation is one of the most essential arguments attesting to the superiority of ce-VUS with respect to VCUG. This is particularly important in pediatric patients whose organs and tissues are more radiosensitive than tissues of adults and thus, more susceptible to the negative effects of ionizing radiation, both threshold and stochastic ones. One should bear in mind that during conventional VCUG, the gonads are exposed to radiation, which is a particular concern given that they are the most radiosensitive organs of the human body. Moreover, repeated exposure increases the risk of carcinogenesis and of genetic anomalies in the offspring(26).
Another advantage of ce-VUS is its dynamic character, unlike VCUG, in which four X-ray pictures capture selected, millisecond stages of voiding. Therefore, it is possible that the precise moment that would enable a diagnosis might not be captured by the latter method. This is particularly significant in refluxes, which may be visible solely at the start of voiding when the pressure in the urinary bladder is the greatest. Voiding urosonography, as a dynamic examination, enables the observation of the entire voiding process and the recording of films and images for their thorough assessment following the examination(21, 22).
Furthermore, VCUG is performed with the use of iodine contrast agents, which induce adverse reactions more frequently than ultrasound contrast agents, the latter being the safest contrast media available in medical imaging, particularly when administered intravesically. Over the last 15 years, thousands of voiding urosonography examinations have been conducted in children and no serious adverse reactions have been noted(19, 27).
In VCUG, we obtain anteroposterior flat images in which the image comprises numerous structures overlaid on one another, i.e. both the kidneys and the organs located on their anterior or posterior sides. This frequently results in various artefacts caused by intestinal gas and other internal organs. Such artefacts hinder or prevent the correct interpretation of the results and carry the risk of making a potential diagnostic error. In addition, the obtained flat image of reflux to the pelvicalyceal system, particularly in the case of anomalous kidney rotation or ectopic kidneys, may be erroneously interpreted in terms of the reflux grade. The present study demonstrates the usefulness of three-dimensional techniques (3D/4D) both for more precise reflux grading, particularly in the differentiation between grade II and III, as well as for the assessment of the bladder neck and urethra.
To conclude, the advantages of two-dimensional (2D) voiding urosonography include: a) the lack of exposure to ionizing radiation; b) the dynamic character of the examination; c) the lack of risk associated with the administration of ionizing contrast agents; d) greater intimacy compared to voiding cystourethrography and e) the ability to store the examination in its dynamic form (films), which facilitates retrospective analysis, enables consultations with other specialists and may be used for didactic purposes. If the protocol of the examination is extended to include three-dimensional static or real-time (3D/4D) examinations, additional advantages arise, such as: accurate assessment of the interior of the urinary bladder (so-called virtual cystoscopy), more precise reflux grading, a more thorough assessment of the bladder and urethra in multiple planes that is impossible to obtain in 2D examinations, the possibility of postprocessing, the option to compare 3D ultrasound findings with the results of magnetic resonance imaging and computed tomography, and the creation of an excellent didactic tool that allows repeated “virtual” examination of the patient.
The limitations of ce-VUS include: a) lack of registration of the contrast agent in pediatrics; b) the longer duration of the examination compared to VCUG; c) the need to directly engage a radiologist; d) more difficult assessment of grade I refluxes; e) dependence on the examiner's experience and f) higher cost of the contrast agent in comparison with iodine agents.
Conclusions
Ce-VUS enables diagnosis and treatment monitoring of VUR in children and allows assessment of the urethra in both girls and boys. This method is characterized by high sensitivity and specificity. Moreover, it is safe, relatively inexpensive and should be conducted on a routine basis. Moreover, ce-VUS is more sensitive than VCUG due to the properties of the microbubbles, the dynamic character of the examination, the lack of exposure to radiation and iodine contrast agents as well as the potential for three-dimensional assessment. This technique may replace VCUG, the most commonly used method to date.
Conflict of interest
This study was funded by a research grant from the National Science Center, No. NN403456137. The authors do not report any financial or personal links with other persons or organizations that might negatively affect the content of this publication and claim authorship rights to this publication.
Piśmiennictwo
- 1.Galloy MA, Mandry D, Pecastaings M, Mainard-Simard L, Claudon M. [Sonocystography: a new method for the diagnosis and follow-up of vesico-ureteric reflux in children] J Radiol. 2003;84:2055–2061. [PubMed] [Google Scholar]
- 2.Routh JC, Bogaert GA, Kaefer M, Manzoni G, Park JM, Retik AB, et al. Vesicoureteral reflux: current trends in diagnosis, screening, and treatment. Eur Urol. 2012;61:773–782. doi: 10.1016/j.eururo.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Duran C, del Riego J, Riera L. [Serial voiding urosonography: a safe technique for the study of the entire urinary tract in children] Radiologia. 2013;55:160–166. doi: 10.1016/j.rx.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Deng J, Zhou L, Zeng S, Zhang C, Zeng G, Wang J, et al. [Voiding urosonography with SonoVue and fluoroscopic voiding cystourethrography in evaluation of vesicoureteral reflux: a comparative study] Nan Fang Yi Ke Da Xue Xue Bao. 2013;33:1467–1470. [PubMed] [Google Scholar]
- 5.Duran C, del Riego J, Riera L, Martin C, Serrano C, Palaña P. Voiding urosonography including urethrosonography: high-quality examinations with an optimised procedure using a second-generation US contrast agent. Pediatr Radiol. 2012;42:660–667. doi: 10.1007/s00247-012-2360-z. [DOI] [PubMed] [Google Scholar]
- 6.Duran C, Valera A, Alguersuari A, Ballesteros E, Riera L, Martin C, et al. Voiding urosonography: the study of the urethra is no longer a limitation of the technique. Pediatr Radiol. 2009;39:124–131. doi: 10.1007/s00247-008-1050-3. [DOI] [PubMed] [Google Scholar]
- 7.Doganis D, Mavrikou M, Delis D, Stamoyannou L, Siafas K, Sinaniotis K. Timing of voiding cystourethrography in infants with first time urinary infection. Pediatr Nephrol. 2009;24:319–322. doi: 10.1007/s00467-008-1018-4. [DOI] [PubMed] [Google Scholar]
- 8.Riccabona M, Avni FE, Blickman JG, Dacher JN, Darge K, Lobo ML, et al. Imaging recommendations in paediatric uroradiology: minutes of the ESPR workgroup session on urinary tract infection, fetal hydronephrosis, urinary tract ultrasonography and voiding cystourethrography, Barcelona, Spain, June 2007. Pediatr Radiol. 2008;38:138–145. doi: 10.1007/s00247-007-0695-7. [DOI] [PubMed] [Google Scholar]
- 9.Darge K, Troeger J. Vesicoureteral reflux grading in contrast-enhanced voiding urosonography. Eur J Radiol. 2002;43:122–128. doi: 10.1016/s0720-048x(02)00114-6. [DOI] [PubMed] [Google Scholar]
- 10.Lebowitz RL, Olbing H, Parkkulainen KV, Smellie JM, Tamminen-Mobius TE. International system of radiographic grading of vesicoureteric reflux. International Reflux Study in Children. Pediatr Radiol. 1985;15:105–109. doi: 10.1007/BF02388714. [DOI] [PubMed] [Google Scholar]
- 11.Elder JS, Peters CA, Arant BS, Jr., Ewalt DH, Hawtrey CE, Hurwitz RS, et al. Pediatric Vesicoureteral Reflux Guidelines Panel summary report on the management of primary vesicoureteral reflux in children. J Urol. 1997;157:1846–1851. [PubMed] [Google Scholar]
- 12.Darge K, Ghods S, Zieger B, Rohrschneider W, Troeger J. Reduction in voiding cystourethrographies after the introduction of contrast enhanced sonographic reflux diagnosis. Pediatr Radiol. 2001;31:790–795. doi: 10.1007/s002470100556. [DOI] [PubMed] [Google Scholar]
- 13.Darge K, Moeller RT, Trusen A, Butter F, Gordjani N, Riedmiller H. Diagnosis of vesicoureteric reflux with low-dose contrast-enhanced harmonic ultrasound imaging. Pediatr Radiol. 2005;35:73–78. doi: 10.1007/s00247-004-1317-2. [DOI] [PubMed] [Google Scholar]
- 14.Darge K. Voiding urosonography with US contrast agents for the diagnosis of vesicoureteric reflux in children. II. Comparison with radiological examinations. Pediatr Radiol. 2008;38:54–63. doi: 10.1007/s00247-007-0528-8. quiz 126–127. [DOI] [PubMed] [Google Scholar]
- 15.Darge K. Voiding urosonography with ultrasound contrast agents for the diagnosis of vesicoureteric reflux in children. I. Procedure. Pediatr Radiol. 2008;38:40–53. doi: 10.1007/s00247-007-0529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robrecht J, Darge K. [In-vitro comparison of a 1st and a 2nd generation US contrast agent for reflux diagnosis] RöFo. 2007;179:818–825. doi: 10.1055/s-2007-963312. [DOI] [PubMed] [Google Scholar]
- 17.Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver – update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187–210. doi: 10.1016/j.ultrasmedbio.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33–59. doi: 10.1055/s-0031-1281676. [DOI] [PubMed] [Google Scholar]
- 19.Riccabona M, Avni FE, Damasio MB, Ording-Müller LS, Blickman JG, Darge K, et al. ESPR Uroradiology Task Force and ESUR Paediatric Working Group – Imaging recommendations in paediatric uroradiology, part V: childhood cystic kidney disease, childhood renal transplantation and contrast-enhanced ultrasonography in children. Pediatr Radiol. 2012;42:1275–1283. doi: 10.1007/s00247-012-2436-9. [DOI] [PubMed] [Google Scholar]
- 20.Riccabona M. Application of a second-generation US contrast agent in infants and children – a European questionnaire-based survey. Pediatr Radiol. 2012;42:1471–1480. doi: 10.1007/s00247-012-2472-5. [DOI] [PubMed] [Google Scholar]
- 21.Papadopoulou F, Anthopoulou A, Siomou E, Efremidis S, Tsamboulas C, Darge K. Harmonic voiding urosonography with a second-generation contrast agent for the diagnosis of vesicoureteral reflux. Pediatr Radiol. 2009;39:239–244. doi: 10.1007/s00247-008-1080-x. [DOI] [PubMed] [Google Scholar]
- 22.Ključevšek D, Battelino N, Tomažič M, Kersnik Levart T. A comparison of echo-enhanced voiding urosonography with X-ray voiding cystourethrography in the first year of life. Acta Paediatr. 2012;101:e235–e239. doi: 10.1111/j.1651-2227.2011.02588.x. [DOI] [PubMed] [Google Scholar]
- 23.Otukesh H, Hoseini R, Behzadi AH, Mehran M, Tabbaroki A, Khamesan B, et al. Accuracy of cystosonography in the diagnosis of vesicourethral reflux in children. Saudi J Kidney Dis Transpl. 2011;22:488–491. [PubMed] [Google Scholar]
- 24.Kis E, Nyitrai A, Várkonyi I, Máttyus I, Cseprekál O, Reusz G, et al. Voiding urosonography with second-generation contrast agent versus voiding cystourethrography. Pediatr Nephrol. 2010;25:2289–2293. doi: 10.1007/s00467-010-1618-7. [DOI] [PubMed] [Google Scholar]
- 25.Ascenti G, Zimbaro G, Mazziotti S, Chimenz R, Fede C, Visalli C, et al. Harmonic US imaging of vesicoureteric reflux in children: usefulness of a second generation US contrast agent. Pediatr Radiol. 2004;34:481–487. doi: 10.1007/s00247-004-1190-z. [DOI] [PubMed] [Google Scholar]
- 26.Perisinakis K, Raissaki M, Damilakis J, Stratakis J, Neratzoulakis J, Gourtsoyiannis N. Fluoroscopy-controlled voiding cystourethrography in infants and children: are the radiation risks trivial? Eur Radiol. 2006;16:846–851. doi: 10.1007/s00330-005-0072-6. [DOI] [PubMed] [Google Scholar]