Abstract
Anal fistula is a benign inflammatory disease with unclear etiology which develops in approximately 10 in 100 000 adult patients. Surgical treatment of fistulae is associated with a risk of damaging anal sphincters. This usually happens in treating high fistulae, branched fistulae, and anterior ones in females. In preoperative diagnosis of anal fistulae, endosonography and magnetic resonance imaging play a significant role in planning the surgical technique. The majority of fistulae are diagnosed in endosonography, but magnetic resonance is performed when the presence of high fistulae, particularly branched ones, and recurrent is suspected.
The aim of this paper
The aim of this paper was to compare the roles of the two examinations in preoperative assessment of high anal fistulae.
Material and methods
The results of endosonographic and magnetic resonance examinations performed in 2011–2012 in 14 patients (4 women and 10 men) with high anal fistulae diagnosed intraoperatively were subject to a retrospective analysis. The patients were aged from 23 to 66 (mean 47). The endosonographic examinations were performed with the use of a BK Medical Pro Focus system with endorectal 3D transducers with the frequency of 16 MHz. The magnetic resonance scans were performed using a Siemens Avanto 1.5 T scanner with a surface coil in T1, T1FS, FLAIR, T2 sequences and in T1 following contrast medium administration. The sensitivity and specificity of endosonography and magnetic resonance imaging were analyzed. A surgical treatment served as a method for verification. The agreement of each method with the surgery and the agreement of endosonography and magnetic resonance imaging were compared in terms of the assessment of the fistula type, localization of its internal opening and branches. The agreement level was determined based on the percentage of consistent assessments and Cohen's coefficient of agreement, κ. The integrity of the anal sphincters was assessed in each case.
Results
In determining the fistula type, magnetic resonance imaging agreed with intraoperative assessment in 79% of cases, and endosonography in 64% of cases. Endosonography agreed with magnetic resonance in 57% of cases. In the assessment of internal opening, the agreement between endosonography and intraoperative assessment was 65%, between magnetic resonance and intraoperative assessment – 41% and between endosonography and magnetic resonance – 53%. In the assessment of fistula branches, endosonography agreed with intraoperative assessment in 67% of cases, magnetic resonance in 87% of cases, and the agreement between the two methods tested was 67%.
Conclusions
Magnetic resonance is a more accurate method than endosonography in determining the type of high fistulae and the presence of branches. In assessing the internal opening, endosonography proved more accurate. The agreement between the two methods ranges from 53–67%; the highest level of agreement was noted for the assessment of branching.
Keywords: rectal fistula, anal fistula, endosonography, magnetic resonance imaging, fecal incontinence
Abstract
Przetoka odbytu jest łagodną chorobą zapalną o niejasnej etiologii, która występuje u około 10 na 100 000 osób populacji dorosłej. Leczenie operacyjne przetoki odbytu wiąże się z ryzykiem uszkodzenia zwieraczy odbytu. Najczęściej dochodzi do tego w przypadku leczenia przetok wysokich, rozgałęzionych oraz przetok przednich u kobiet. W przedoperacyjnej diagnostyce przetok odbytu ważne miejsce, pod kątem planowania techniki zabiegu operacyjnego, zajmują endosonografia oraz rezonans magnetyczny. Większość przetok diagnozowana jest w endosonografii, zaś rezonans wykonuje się w przypadku klinicznego podejrzenia przetoki wysokiej, zwłaszcza rozgałęzionej i nawrotowej.
Cel pracy
Celem pracy było porównanie obydwu badań w przedoperacyjnej ocenie wysokich przetok odbytu.
Materiał i metoda
Retrospektywnie przeanalizowano wyniki badań endosonograficznych i rezonansu magnetycznego wykonanych w latach 2011–2012 u 14 pacjentów (4 kobiety, 10 mężczyzn) w wieku 23–66 lat (średnia 47) ze śródoperacyjnym rozpoznaniem wysokiej przetoki odbytu. Badania endosonograficzne wykonano aparatem BK Medical Pro Focus, głowicą endorektalną 3D o częstotliwości 16 MHz. Badania rezonansu przeprowadzono z wykorzystaniem aparatu Siemens Avanto 1,5 T z cewką powierzchniową, przed podaniem środka kontrastowego w sekwencjach T1, T1FS, FLAIR, T2 i po podaniu. Oceniono czułość i swoistość endosonografii oraz rezonansu magnetycznego. Metodą weryfikującą był zabieg operacyjny. Porównano zgodność każdej metody z operacją oraz zgodność endosonografii i rezonansu magnetycznego w zakresie oceny typu przetoki, lokalizacji ujścia wewnętrznego oraz rozgałęzień. Poziom zgodności określano na podstawie odsetka ocen zgodnych oraz współczynnika zgodności κ Cohena. W każdym przypadku oceniano ciągłość zwieraczy odbytu.
Wyniki
W określaniu typu przetoki zgodność badania rezonansu magnetycznego z oceną śródoperacyjną stwierdzono w 79% przypadków, endosonografii z oceną śródoperacyjną w 64% przypadków, a endosonografii z rezonansem w 57%. W ocenie ujścia wewnętrznego zgodność endosonografii z oceną śródoperacyjną wyniosła 65%, rezonansu z oceną śródoperacyjną 41%, a endosonografii z rezonansem 53%. W ocenie rozgałęzień przetoki zgodność endosonografii z oceną śródoperacyjną wyniosła 67%, rezonansu z oceną śródoperacyjną 87%, a zgodność pomiędzy obiema metodami 67%.
Wnioski
Rezonans magnetyczny dokładniej niż endosonografia określa typ przetoki wysokiej i obecność rozgałęzień. W ocenie ujścia wewnętrznego metodą dokładniejszą jest endosonografia. Zgodność między metodami waha się w zakresie 53–67%; najwyższa jest w ocenie rozgałęzień.
Introduction
Anal fistula is a benign inflammatory disease which develops in approximately 10 in 100 000 adult patients, particularly in men (men-women ratio is 4:1), usually in the fourth decade of life(1, 2). The etiopathogenesis of the disease has not been entirely explained. The inflammatory factor is frequently believed to play a role, i.e. inflammation of anal glands and crypts that in 60% of patients, leads to the development of an abscess which transforms into a chronic form with a fistula. Treating fistulae may be associated with a severe risk of damaging the anal sphincters which manifests as the lack of control over passing gas and stool (incontinence). This happens in as many as 64% of patients following surgeries(3–5). The highest risk of incontinence concerns patients with high fistulae i.e. fistulae which run on more than 1/3 of the external sphincter's length what concerns trans-sphincteric and intersphincteric fistulae and all suprasphincteric and extrasphincteric ones. Also surgery for complex fistulae, i.e. branched ones, carries a high risk of incontinency due to a high probability that the surgery will not be complete and another operation will be necessary (i.e. sphincterotomy)(2). The data on the prevalence of individual types of fistulae are discrepant. High trans-and intersphincteric fistulae account for 21–30% of all anal fistulae, and suprasphincteric and extrasphincteric ones constitute 3–31% of all types(6). The only way to reduce the occurrence of the complications mentioned above is to apply a surgical method adjusted individually to the type of a fistula, also considering its position, presence of branching, recurrent character, presence or absence of internal opening, patient's sex (particularly in the case of problematic anterior fistulae in women) as well as morphological and functional condition of the sphincters.
The preoperative diagnosis of anal fistulae involves using two imaging methods, i.e. endosonography (anorectal endosonography, AES) and magnetic resonance imaging (MRI). The majority of fistulae are diagnosed in AES, but MRI, due to its high cost, is used practically solely when high, recurrent, or particularly branched fistulae. Another indication for MRI are inconclusive results of the AES or inconsistent with the clinical assessment. Such a procedure is also followed in our reference center. MRI is superior to AES in the fact that it enables differentiation of tissues, including identification of active inflammatory lesions and their differentiation from scars (recurrent fistulae and posttraumatic lesions) as well as in its ability of multiplanar imaging.
In the available literature, AES and MRI have been compared in terms of their accuracy to differentiate between individual types of fistulae as well as the ability to visualize their branches and internal openings(7–11), whereas what seems to be the most important for planning surgical procedures and estimating the risk of postoperative incontinence is the height of fistula. The aim of this paper was to compare AES and MRI in preoperative assessment of the height of fistulae, as verified in a surgical picture.
Material and methods
Retrospectively, the results of AES and MRI examinations performed in 14 patients (4 women and 10 men) in 2011–2012 with high anal fistulae diagnosed intraoperatively were compared. The patients’ age ranged from 23 to 66 (mean 47 years of age). In 9 patients, fistulae were of cryptoglandular origin, and in 5 cases, they developed in the course of Crohn's disease.
The AES examinations were performed with the use of a BK Medical Pro Focus system with endorectal 3D transducers with the frequency of 16 MHz. The MR scans were performed using a Siemens Avanto 1.5 T scanner with a surface coil in T1, T1FS, FLAIR, T2 and T1 after administration of a contrast medium sequences in transverse, sagittal and coronal planes.
The levels of sensitivity and specificity were calculated for each of the methods. A surgical treatment served as a method for verification. The agreement of each method with the intraoperative assessment and the agreement of AES with MRI were compared in terms of the assessment of the fistula type according to Parks classification(12), localization of its internal opening and presence of branching. The agreement of MRI and AES with intraoperative assessment as well as the agreement of MRI with AES was determined by calculating the rate of agreement and Cohen's coefficient of agreement, κ. The values of κ coefficient were interpreted in the fashion suggested by Fleiss et al. (13), where κ < 0.40 means poor agreement, the values of 0.40–0.75 indicate moderate or good agreement and the values >0.75 indicate high agreement.
All patients gave written consent to the participation in the study.
Results
Comparative assessment of AES and MRI in determining the fistula type
Intraoperative assessment confirmed the presence of 10 suprasphincteric and 4 high trans-sphincteric fistulae. Nine of 14 were correctly diagnosed in AES examination (64%) and 11/14 were correctly diagnosed in MRI (79%) (tabs. 1, 2); the sensitivity of AES was 0.64 and MRI – 0.79. Disagreements in differentiation occurred between suprasphincteric and high trans-sphincteric types, and in one case, between a high trans-sphincteric fistula and a high intersphincteric one. The agreement between AES and MRI in determining the fistula type was observed in 8 cases (57%). The Cohen's coefficient of agreement, κ, was <0.
Tab. 1.
Sensitivity and specificity of AES and MRI in anal fistula diagnosis
| AES | MRI | |||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| Fistula type | 64% | -* | 79% | -* |
| Localization of internal opening | 63% | 67% | 50% | 33% |
| Branching | 75% | 57% | 100% | 71% |
Determining the value of specificity is impossible due to the absence of false negative cases.
Tab. 2.
Comparison of AES and MRI in anal fistula diagnosis
| Agreement between AES and intraoperative assessment | Agreement between MRI and intraoperative assessment | Agreement between AES and MRI | ||||
|---|---|---|---|---|---|---|
| Agreement rate | κ | Agreement rate | κ | Agreement rate | κ | |
| Fistula type | 64% | 0* | 79% | 0* | 57% | <0 |
| Localization of internal opening | 65% | 0,29 | 41% | <0 | 53% | 0,07 |
| Branching | 67% | 0,32 | 87% | 0,74 | 67% | 0,29 |
κ coefficient is not determined in a correct way due to the absence of negative cases.
Comparative assessment of AES and MRI in identifying the internal opening of a fistula
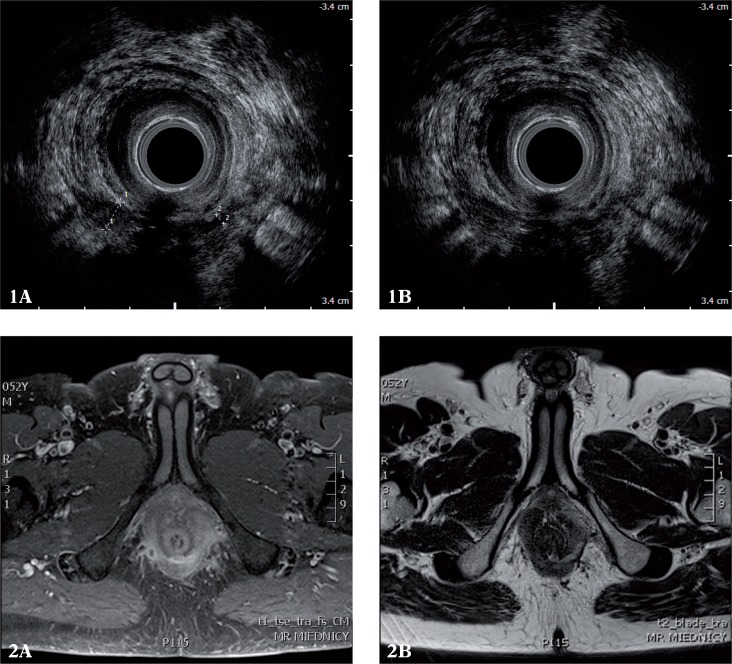
In this study, 17 internal openings were analyzed, including 3 additional ones in patients with suspected complex fistulae having more than one internal opening. The surgery revealed 8 internal openings. In 6 cases, fistulae were blind and the presence of 3 fistulae with multiple openings was not confirmed. The agreement between AES and the surgery was observed in 11 cases (65%), and no agreement was seen in 6 cases including 3 false positive and 3 false negative diagnoses (sensitivity 63%, specificity 67%, κ = 0.29) (tabs. 1, 2). The agreement between MRI and the surgery was observed in 7 cases (41%); no agreement was seen in 10 cases including 4 false negative and 6 false positive diagnoses (sensitivity 50%, specificity 33%, κ < 0). The agreement between AES and MRI in the diagnosis of internal openings was observed in 9 cases (53%); κ = 0.07 (figs. 1, 2).
Figs. 1, 2.
Posterior high trans-sphincteric fistula branching to the right and left of the apex of the ischiorectal fossa observable in AES and MRI (1 A, 2 A), with its internal opening localized medially and posteriorly in AES and MRI (1 B, 2 B)
Comparative analysis of AES and MRI in the assessment of fistula branches
The analysis involved 15 branches, including single branching in 14 patients and 2 branches of a high trans-sphincteric fistula in one patient. During the surgery, 8 branched fistulae (the single case with multiple branches was not confirmed) and 6 simple ones were identified. The 6 branches diagnosed intraoperatively were visualized in AES and 8 in MRI.
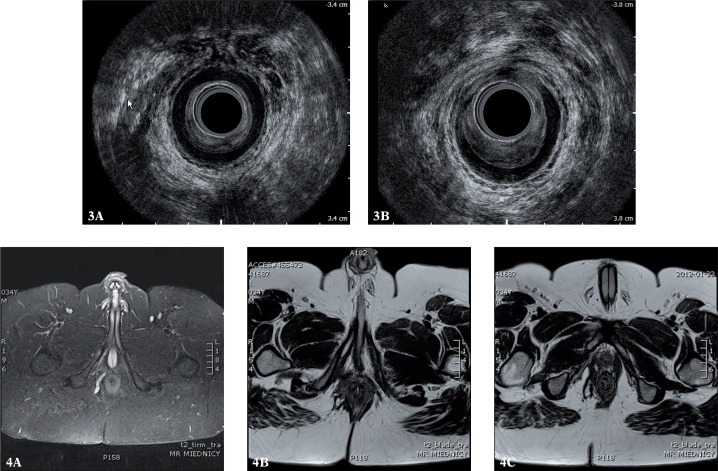
AES erroneously diagnosed 3 additional tracks and MRI erroneously diagnosed 2. AES failed to diagnose 2 branches, whereas in MRI, no false negative cases were observed (figs. 3, 4). The agreement between AES and the surgery was 67% (sensitivity 75%, specificity 57%, κ = 0.32) (tabs. 1, 2). The agreement between MRI and intraoperative findings was 87% (sensitivity 100%, specificity 71%, κ = 0.74). The agreement between MRI and AES in diagnosing branched fistulae was observed in 10 cases (67%); κ = 0.29.
Figs. 3, 4.
Suprasphincteric fistula on the right wall in AES and MRI (3 A, 4 A) with the internal opening localized medially and anteriorly (3 B, 4 B). AES failed to identify a high track within the right branch of the puborectalis muscle (4 C). The defect of 1/3 of the anterior circumference of the internal sphincter in the medial part of the canal is better visible in AES than in MRI (3 B)
Discussion
The most common cause of fistulae is a developing infection of the anal crypt. Fistulae may also develop in the course of Crohn's disease, neoplasms, tuberculosis, actinomycosis, after radiotherapy, chemotherapy and in immunodeficiency disorders. Another cause of fistulae are injuries, both iatrogenic and associated with introducing a foreign body to the anus(14). The diagnostic process begins with a conventional anorectal examination. Frequently, it also includes an abdominal US examination, which is successfully used by some examiners in assessing bowel pathologies, including Crohn's disease(15–17). In the centers specializing in treating anal fistulae, the standard additional examination, performed to qualify patients to surgical treatment, is currently AES. This method is fully satisfactory in diagnosing low fistulae, which constitute the majority of all anal fistulae (50–70%) and rarely require performing supplemental MRI scans. In high fistulae, particularly branched or recurring ones, ultrasound images are sometimes difficult to interpret irrespective of the fistula type and its etiology (however, the images are particularly problematic in Crohn's disease). This results from lesions extending through several anatomic compartments as well as from the presence of other tracks (branches), including high ones, and scars (in recurrent fistulae), the echogenicity of which is identical to or resembling that of fistulae. In such cases, an MRI scan is indicated(18–20).
In the recent years, many reports on anal fistula diagnosis have been published, also comparing AES to MRI(7–9, 21–23). The sensitivity of these methods have been found comparable, but the specificity was higher for MRI due to the application of various sequences and contrast enhancement. Our studies demonstrated that MRI is superior to AES in assessing the type and branching of fistulae, and that AES is superior to MRI in diagnosing internal openings. In assessing the fistula type, the sensitivity of AES was 64% and of MRI – 79%. In assessing branches, the sensitivity and specificity of AES amounted to 75% and 57%, and in the case of MRI the values were 100% and 71%, respectively. In assessing the internal opening of fistulae, the sensitivity and specificity of AES were 63% and 67%, and in the case of MRI the values were 50% and 33%, respectively.
In the studies conducted by Maier et al. (7), the sensitivity of MRI and AES in identifying the type of fistulae amounted to 84% and 60% respectively, and the respective values for specificity were 68% and 21%. The number of false positive cases was three times higher in AES than in MRI. Similarly, in the study of Hussain et al. (8), the accuracy of MRI was superior to AES (64% vs. 36%). Lunniss et al. (24) also demonstrated the superiority of MRI (85% vs. 65%), but the difference was not statistically significant. On the other hand, Orsoni et al. (25) showed a considerably higher effectiveness of AES compared to MRI (82% vs. 50%). In the authors’ own material, the differentiation of high trans-sphincteric fistulae from suprasphincteric ones was problematic, presumable due to the oblique course of the puborectalis muscle in relation to the transverse plane of imaging. Maruyama et al. (26) suggested a similar explanation. From the practical point of view, an error of this type has no serious clinical implications since the technique of surgical treatment applied in all these fistula types is identical.
One of the basic factors causing a fistula to recur is the failure to find and, consequently, to remove its inner opening(27, 28). Identifying internal openings is problematic both in AES and in MRI as lesions in the layer of the mucous membrane(29–31). The results of studies on the relevance of endosonography in localizing internal openings are highly discrepant and the percentage of agreement ranges from 11% to 87.5%. In the authors’ own study, more openings were diagnosed by means of AES. A large number of false diagnoses, including 3 false positive and 3 false negative cases in AES and 4 false negative and as many as 6 false positive cases in MRI, are alarming. It indicates that this significant element of the image cannot be assessed in a reliable way either in AES or in MRI, and the intraoperative assessment is the conclusive one.
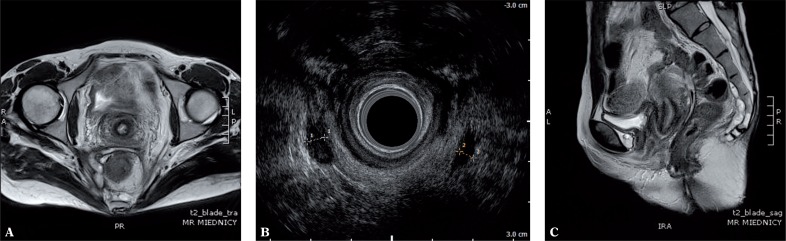
In the authors’ study, MRI proved more accurate in visualizing branches. As in internal openings, a fistula may recur when its branches are not found and left untreated during the surgery and consequently, another surgical procedure is needed. However, determining the genuine value of the assessment of branches in AES and MRI is problematic. Apart from the fact that MRI is more accurate in such an assessment (fig. 5), there are reasons to assume that not all branches visible in AES and MRI are identified during surgeries (in particular supralevator branches), which was also noted by other authors(32–34). In this study, the intraoperative assessment failed to confirm the presence of 3 branches visible in AES and 2 tracks observed in MRI scans.
Fig. 5.
Anterior intersphincteric branch of suprasphincteric fistula (A, B); the anterior course of the branch towards the intestinal loop to the depth of 14 cm from the anal verge was visible only in MRI (C)
In the study of Lunniss et al. (24) that compared AES and MRI in terms of differentiating simple from complex fistulae, MRI proved to be more accurate (100%). AES failed to identify 75% of supralevator and 50% of ischiorectal fistula branches. Buchanan et al. (18) demonstrated the superiority of MRI over AES in determining the type of fistula and in visualizing its branches, but AES occurred to indicate the localization of the internal opening more accurately (the same observation was made in the authors’ own study). Toyanaga et al. (35) obtained very good results for AES – the accuracy of this method amounted to 88.8% in identifying the type of fistula, 85.7% in assessing horseshoe fistulae and 89.5% in determining the localization of internal openings.
In the authors’ own study, interobserver variability was not assessed. All examinations were analyzed by two radiologists simultaneously (IS-S, AW). The fact that the person who performed the AES examinations also interpreted the MRI scans and compared the results is a strong aspect of this study. The result of the MRI examination is, then, an added value. Despite this, it was not always consistent with the intraoperative assessment. The discrepancies between the findings of AES and MRI and the intraoperative verification resulted from the limitations of the method, particularly in terms of the identification of the fistula type and internal opening. Since it is not possible to verify all diagnoses intraoperatively, the genuine value of these methods in identifying fistula branches may, in fact, be higher. Performing another MRI scan or further follow-up of patients in terms of the recurrence might be a good solution.
Conclusions
Magnetic resonance is a more accurate method than endosonography in determining the type of high fistulae and allows a greater number of branches to be detected.
In assessing the internal opening, endosonography proved more accurate.
The agreement between the two methods ranges from 53 to 67%; the highest level of agreement was noted for the assessment of branching.
Conflict of interest
Authors do not report any financial or personal links with other persons or organizations, which might affect negatively the content of this publication and/or claim authorship rights to this publication.
References
- 1.Morris J, Spencer JA, Ambrose NS. MR imaging classification of perianal fistulas and its implications for patient management. Radiographics. 2000;20:623–635. doi: 10.1148/radiographics.20.3.g00mc15623. [DOI] [PubMed] [Google Scholar]
- 2.Lunniss PJ, Jenkins PJ, Besser GM, Perry LA, Phillips RK. Gender differences in incidence of idiopathic fistula-in-ano are not explained by circulating sex hormones. Int J Colorectal Dis. 1995;10:25–28. doi: 10.1007/BF00337582. [DOI] [PubMed] [Google Scholar]
- 3.Ommer A, Wenger FA, Rolfs T, Walz MK. Continence disorders after anal surgery – a relevant problem? Int J Colorectal Dis. 2008;23:1023–1031. doi: 10.1007/s00384-008-0524-y. [DOI] [PubMed] [Google Scholar]
- 4.Sygut A, Zajdel R, Kedzia-Budziewska R, Trzciński R, Dziki A. Late results of treatment of anal fistulas. Colorectal Dis. 2007;9:151–158. doi: 10.1111/j.1463-1318.2006.01036.x. [DOI] [PubMed] [Google Scholar]
- 5.Toyonaga T, Matsushima M, Kiriu T, Sogawa N, Kanyama H, Matsumura N, et al. Factors affecting continence after fistulotomy for intersphincteric fistula-in-ano. Int J Colorectal Dis. 2007;22:1071–1075. doi: 10.1007/s00384-007-0277-z. [DOI] [PubMed] [Google Scholar]
- 6.Kołodziejczak M, Sudoł-Szopińska, editors. Diagnostyka i leczenie ropni i przetok odbytu. Warszawa: Borgis; 2008. [Google Scholar]
- 7.Maier AG, Funovics MA, Kreuzer SH, Herbst F, Wunderlich M, Teleky BK, et al. Evaluation of perianal sepsis: comparison of anal endosonography and magnetic resonance imaging. J Magn Reson Imaging. 2001;14:254–260. doi: 10.1002/jmri.1181. [DOI] [PubMed] [Google Scholar]
- 8.Hussain SM, Stoker J, Schouten WR, Hop WCJ, Laméris JS. Fistula in ano: endoanal sonography versus endoanal MR imaging in classification. Radiology. 1996;200:475–481. doi: 10.1148/radiology.200.2.8685344. [DOI] [PubMed] [Google Scholar]
- 9.Beets-Tan RG, Morren GL, Beets GL, Kessels AG, el Naggar K, Lemaire E, et al. Measurement of anal sphincter muscles: endoanal US, endoanal MR imaging, or phased-array MR imaging? A study with healthy volunteers. Radiology. 2001;220:81–89. doi: 10.1148/radiology.220.1.r01jn1481. [DOI] [PubMed] [Google Scholar]
- 10.Torkzad MR, Karlbom U. MRI for assessment of anal fistula. Insights Imaging. 2010;1:62–71. doi: 10.1007/s13244-010-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziech M, Felt-Bersma R, Stoker J. Imaging of perianal fistulas. Clin Gastroenterol Hepatol. 2009;7:1037–1045. doi: 10.1016/j.cgh.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 12.Kołodziejczak M, Sudoł-Szopińska I, Grochowicz P, Wagiel K. Utility of fistulography in diagnosing a post-traumatic rectal fistula – a case report. New Medicine. 2011;15:90–92. [Google Scholar]
- 13.Fleiss JL, Levin B, Paik MC. Statistical Methods for Raters and Proportions; New York: Wiley and Sons; 2003. [Google Scholar]
- 14.Smereczyński A, Starzyńska T, Kołaczyk K. Ultrasound of selected pathologies of the small intestine. J Ultrason. 2013;13:155–166. doi: 10.15557/JoU.2013.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodzisz A, Woźniak MM, Dudkiewicz E, Grabowski D, Stefaniak J, Wieczorek AP, et al. Ultrasound presentation of abdominal non-Hodgkin lymphomas in pediatric patients. J Ultrason. 2013;13:373–378. doi: 10.15557/JoU.2013.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smereczyński A, Kołaczyk K, Lubiński J, Bojko S, Gałdyńska M, Bernatowicz E. Sonographic imaging of Spigelian hernias. J Ultrason. 2012;12:269–275. doi: 10.15557/JoU.2012.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63:1–12. doi: 10.1002/bjs.1800630102. [DOI] [PubMed] [Google Scholar]
- 18.Buchanan GN, Halligan S, Bartram CI, Williams AB, Tarroni D, Cohen CR. Clinical examination, endosonography, and MR imaging in preoperative assessment of fistula in ano: comparison with outcome-based reference standard. Radiology. 2004;233:674–681. doi: 10.1148/radiol.2333031724. [DOI] [PubMed] [Google Scholar]
- 19.Hvas CL, Dahlerup JF, Jacobsen BA, Ljungmann K, Qvist N, Staun M, et al. Diagnosis and treatment of fistulising Crohn's disease. Dan Med Bull. 2011;58:C4338. [PubMed] [Google Scholar]
- 20.West RL, Zimmerman DD, Dwarkasing S, Hussain SM, Hop WC, Schouten WR, et al. Prospective comparison of hydrogen peroxide-enhanced three-dimensional endoanal ultrasonography and endoanal magnetic resonance imaging of perianal fistulas. Dis Colon Rectum. 2003;46:1407–1415. doi: 10.1007/s10350-004-6758-z. [DOI] [PubMed] [Google Scholar]
- 21.Sun MR, Smith MP, Kane RA. Current techniques in imaging of fistula in ano: three-dimensional endoanal ultrasound and magnetic resonance imaging. Semin Ultrasound CT MR. 2008;29:454–471. doi: 10.1053/j.sult.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 22.George U, Sahota A, Rathore S. MRI in evaluation of perianal fistula. J Med Imaging Radiat Oncol. 2011;55:391–400. doi: 10.1111/j.1754-9485.2011.02268.x. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqui MR, Ashrafian H, Tozer P, Daulatzai N, Burling D, Hart A, et al. A diagnostic accuracy meta-analysis of endoanal ultrasound and MRI for perianal fistula assessment. Dis Colon Rectum. 2012;55:576–585. doi: 10.1097/DCR.0b013e318249d26c. [DOI] [PubMed] [Google Scholar]
- 24.Lunniss PJ, Barker PG, Sultan AH, Armstrong P, Reznek RH, Bartram CI, et al. Magnetic resonance imaging of fistula-in-ano. Dis Colon Rectum. 1994;37:708–718. doi: 10.1007/BF02054416. [DOI] [PubMed] [Google Scholar]
- 25.Orsoni P, Barthet M, Portier F, Panuel M, Desjeux A, Grimaud JC. Prospective comparison of endosonography, magnetic resonance imaging and surgical findings in anorectal fistula and abscess complicating Crohn's disease. Br J Surg. 1999;86:360–364. doi: 10.1046/j.1365-2168.1999.01020.x. [DOI] [PubMed] [Google Scholar]
- 26.Maruyama R, Noguchi T, Takano M, Takagi K, Morita N, Kikuchi R, et al. Usefulness of magnetic resonance imaging for diagnosing deep anorectal abscesses. Dis Colon Rectum. 2000;43:S2–S5. doi: 10.1007/BF02237218. [DOI] [PubMed] [Google Scholar]
- 27.Poon CM, Ng DC, Ho-Yin MC, Li RS, Leong HT. Recurrence pattern of fistula-in-ano in a Chinese population. J Gastrointestin Liver Dis. 2008;17:53–57. [PubMed] [Google Scholar]
- 28.Sainio P, Husa A. Fistula-in-ano. Clinical features and long-term results of surgery in 199 adults. Acta Chir Scand. 1985;151:169–176. [PubMed] [Google Scholar]
- 29.Spencer JA, Ward J, Beckingham IJ, Adams C, Ambrose NS. Dynamic contrast-enhanced MR imaging of perianal fistulas. AJR Am J Roentgenol. 1996;167:735–741. doi: 10.2214/ajr.167.3.8751692. [DOI] [PubMed] [Google Scholar]
- 30.Barker PG, Lunniss PJ, Armstrong P, Reznek RH, Cottam K, Phillips RK. Magnetic resonance imaging of fistula-in-ano: technique, interpretation and accuracy. Clin Radiol. 1994;49:7–13. doi: 10.1016/s0009-9260(05)82906-x. [DOI] [PubMed] [Google Scholar]
- 31.Scholefield JH, Berry DP, Armitage NC, Wastie ML. Magnetic resonance imaging in the management of fistula in ano. Int J Colorectal Dis. 1997;12:276–279. doi: 10.1007/s003840050105. [DOI] [PubMed] [Google Scholar]
- 32.Beckingham IJ, Spencer JA, Ward J, Dyke GW, Adams C, Ambrose NS. Prospective evaluation of dynamic contrast enhanced magnetic resonance imaging in the evaluation of fistula in ano. Br J Surg. 1996;83:1396–1398. doi: 10.1002/bjs.1800831022. [DOI] [PubMed] [Google Scholar]
- 33.Spencer JA, Chapple K, Wilson D, Ward J, Windsor AC, Ambrose NS. Outcome after surgery for perianal fistula: predictive value of MR imaging. AJR Am J Roentgenol. 1998;171:403–406. doi: 10.2214/ajr.171.2.9694464. [DOI] [PubMed] [Google Scholar]
- 34.Beets-Tan RG, Beets GL, van der Hoop AG, Kessels AG, Vliegen RF, Baeten CG, et al. Preoperative MR imaging of anal fistulas: does it really help the surgeon? Radiology. 2001;218:75–84. doi: 10.1148/radiology.218.1.r01dc0575. [DOI] [PubMed] [Google Scholar]
- 35.Toyonaga T, Tanaka Y, Song JF, Katori R, Sogawa N, Kanyama H, et al. Comparison of accuracy of physical examination and endoanal ultrasonography for preoperative assessment in patients with acute and chronic anal fistula. Tech Coloproctol. 2008;12:217–223. doi: 10.1007/s10151-008-0424-8. [DOI] [PubMed] [Google Scholar]