Abstract
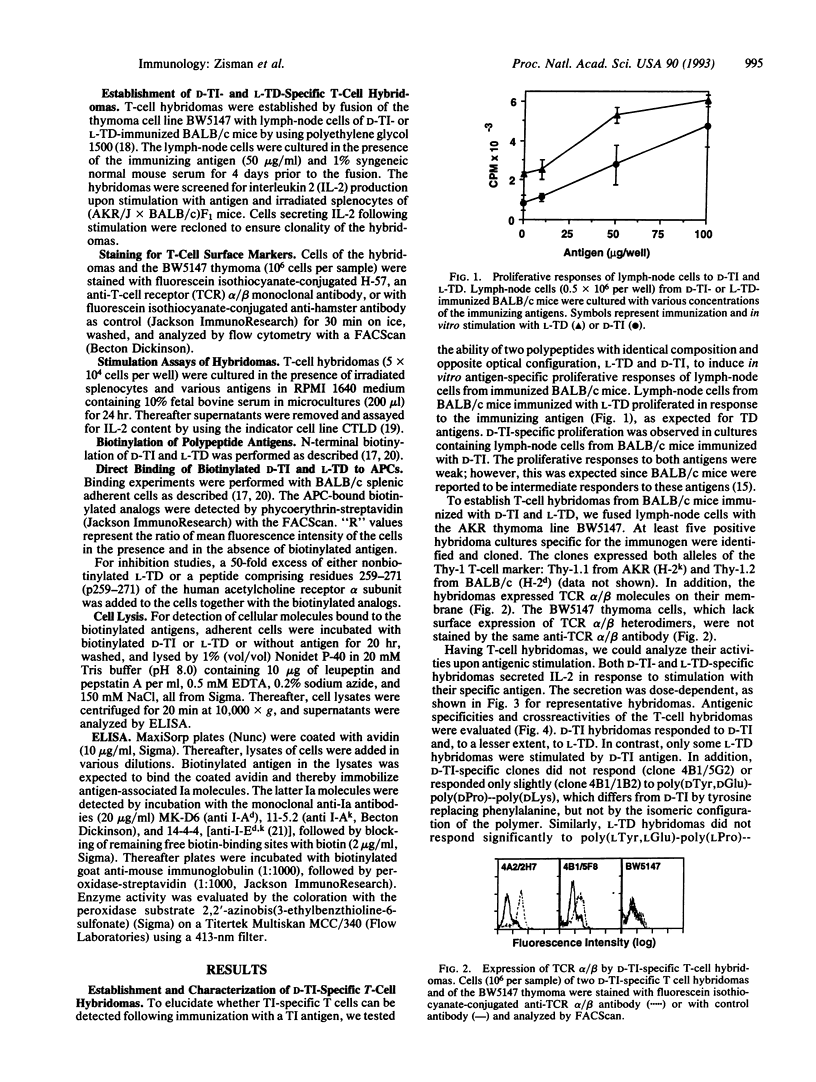
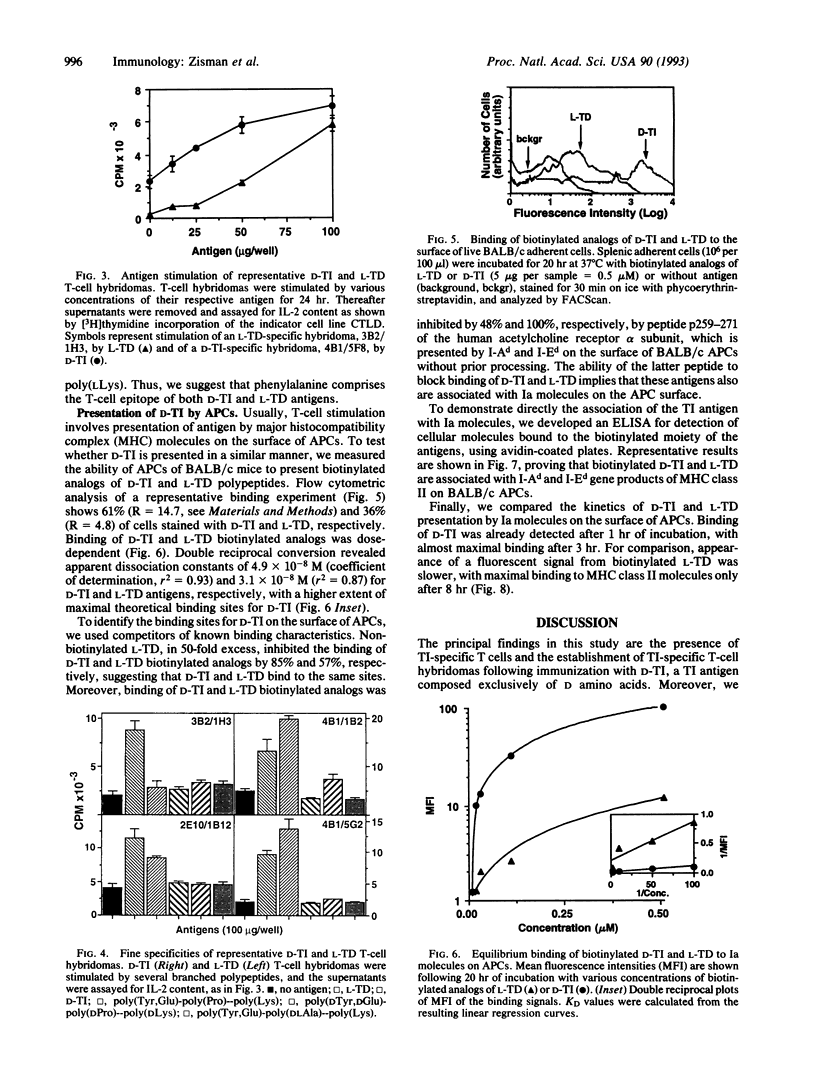
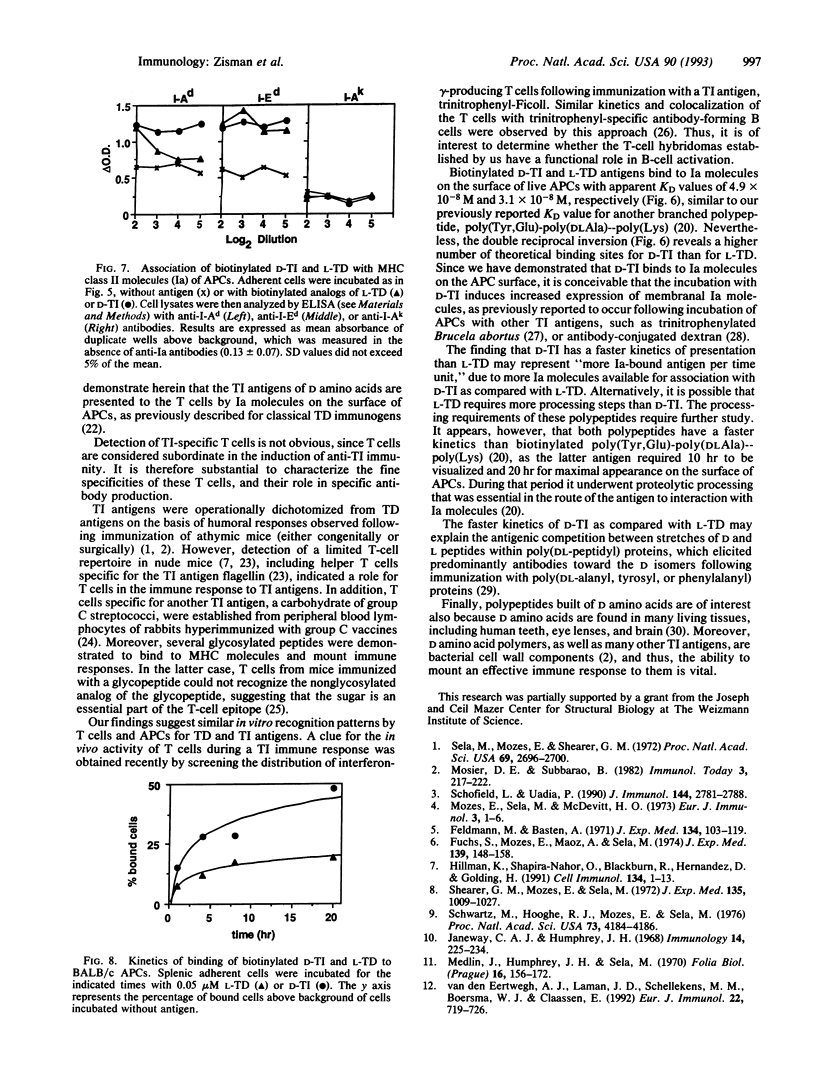
Synthetic polypeptide antigens of L amino acids, although bearing repeating sequences, are thymus-dependent (L-TD), whereas the same polymers composed of D amino acids are thymus-independent (D-TI), probably due to a slower rate of metabolism. Yet we found that lymph-node cells of BALB/c mice immunized with D-TI proliferate in response to it in vitro. To follow T-cell activation by D-TI, we established T-cell hybridomas to D-TI and to its analog composed of L isomers, L-TD, for comparison. The T-cell hybridomas express membrane alpha/beta T-cell receptors and secrete interleukin 2 upon stimulation with the respective antigen. In addition, D-TI-specific hybridomas are stimulated, to a lesser extent, by the L-TD antigen, whereas only some L-TD-specific hybridomas recognize D-TI. Moreover, biotinylated analogs of D-TI and L-TD bind to splenic antigen-presenting cells (APCs) from BALB/c mice. Binding is inhibited by an excess of nonbiotinylated L-TD, and by an excess of a peptide comprising residues 259-271 of the human acetylcholine receptor alpha subunit, which binds to I-Ad and I-Ed molecules without prior processing. Analysis of APC lysates following incubation of the APCs with biotinylated D-TI and L-TD reveals that the biotinylated antigen moiety is associated with Ia molecules. D-TI and L-TD bind to Ia molecules on intact APCs with similar KD values, 5 x 10(-8) M and 3 x 10(-8) M, respectively. However, D-TI has faster kinetics of binding than L-TD, probably due to different processing requirements. Hence, we have demonstrated a major histocompatibility complex class II-mediated T-cell response to a thymus-independent antigen.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell J. D., Schwartz R. H. T-cell recognition of antigen and the Ia molecule as a ternary complex. Nature. 1986 Mar 13;320(6058):176–179. doi: 10.1038/320176a0. [DOI] [PubMed] [Google Scholar]
- Axelrod O., Mozes E. Analysis of the biological functions and fine specificity of (T,G)-A--L specific T cell clones. Immunobiology. 1986 Aug;172(1-2):99–109. doi: 10.1016/S0171-2985(86)80056-0. [DOI] [PubMed] [Google Scholar]
- Brunswick M., June C. H., Finkelman F. D., Dintzis H. M., Inman J. K., Mond J. J. Surface immunoglobulin-mediated B-cell activation in the absence of detectable elevations in intracellular ionized calcium: a model for T-cell-independent B-cell activation. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6724–6728. doi: 10.1073/pnas.86.17.6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshhar Z., Apte R. N., Löwy I., Ben-Neriah Y., Givol D., Mozes E. T-cell hybridoma bearing heavy chain variable region determinants producing (T,G)-A--L-specific helper factor. Nature. 1980 Jul 17;286(5770):270–272. doi: 10.1038/286270a0. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Easten A. The relationship between antigenic structure and the requirement for thymus-derived cells in the immune response. J Exp Med. 1971 Jul 1;134(1):103–119. doi: 10.1084/jem.134.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher G. H., D'Aniello A., Vetere A., Padula L., Cusano G. P., Man E. H. Free D-aspartate and D-alanine in normal and Alzheimer brain. Brain Res Bull. 1991 Jun;26(6):983–985. doi: 10.1016/0361-9230(91)90266-m. [DOI] [PubMed] [Google Scholar]
- Fuchs S., Mozes E., Maoz A., Sela M. Thymus independence of a collagen-like synthetic polypeptide and of collagen, and the need for thymus and bone marrow-cell cooperation in the immune response to gelatin. J Exp Med. 1974 Jan 1;139(1):148–158. doi: 10.1084/jem.139.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman K., Shapira-Nahor O., Blackburn R., Hernandez D., Golding H. A polymer containing a repeating peptide sequence can stimulate T-cell-independent IgG antibody production in vivo. Cell Immunol. 1991 Apr 15;134(1):1–13. doi: 10.1016/0008-8749(91)90326-7. [DOI] [PubMed] [Google Scholar]
- Ishioka G. Y., Lamont A. G., Thomson D., Bulbow N., Gaeta F. C., Sette A., Grey H. M. MHC interaction and T cell recognition of carbohydrates and glycopeptides. J Immunol. 1992 Apr 15;148(8):2446–2451. [PubMed] [Google Scholar]
- Jackson S., Folks T. M., Wetterskog D. L., Kindt T. J. A rabbit helper T cell clone reactive against group-specific streptococcal carbohydrate. J Immunol. 1984 Sep;133(3):1553–1557. [PubMed] [Google Scholar]
- Janeway C. A., Jr, Humphrey J. H. Synthetic antigens composed exclusively of L- or D- amino acids. II. Effect of optical configuration on the metabolism and fate of synthetic polypeptide antigens in mice. Immunology. 1968 Feb;14(2):225–234. [PMC free article] [PubMed] [Google Scholar]
- Letvin N. L., Benacerraf B., Germain R. N. B-lymphocyte responses to trinitrophenyl-conjugated Ficoll: requirement for T lymphocytes and Ia-bearing adherent cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5113–5117. doi: 10.1073/pnas.78.8.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlín J., Humphrey J. H., Sela M. Studies on synthetic polypeptide antigens derived from multichain polyproline. II. Metabolism and localization. Folia Biol (Praha) 1970 Jun;16(3):156–172. [PubMed] [Google Scholar]
- Mozes E., Dayan M., Zisman E., Brocke S., Licht A., Pecht I. Direct binding of a myasthenia gravis related epitope to MHC class II molecules on living murine antigen-presenting cells. EMBO J. 1989 Dec 20;8(13):4049–4052. doi: 10.1002/j.1460-2075.1989.tb08588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozes E., McDevitt H. O., Jaton J. C., Sela M. The nature of the antigenic determinant in a genetic control of the antibody response. J Exp Med. 1969 Sep 1;130(3):493–504. doi: 10.1084/jem.130.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozes E., Sela M., McDevitt H. O. Genetic control of immune response in mice to derivatives of multichain polyproline differing in the optical configuration of component amino acids. Eur J Immunol. 1973 Jan;3(1):1–6. doi: 10.1002/eji.1830030102. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- SELA M., FUCHS S., ARNON R. Studies on the chemical basis of the antigenicity of proteins. 5. Synthesis, characterization and immunogenicity of some multichain and linear polypeptides containing tyrosine. Biochem J. 1962 Oct;85:223–235. doi: 10.1042/bj0850223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I., Sela M. Preferential formation of antibodies specific toward D-amino acid residues upon immunization with poly-DL-peptidyl proteins. Biochemistry. 1967 Mar;6(3):897–905. doi: 10.1021/bi00855a034. [DOI] [PubMed] [Google Scholar]
- Schofield L., Uadia P. Lack of Ir gene control in the immune response to malaria. I. A thymus-independent antibody response to the repetitive surface protein of sporozoites. J Immunol. 1990 Apr 1;144(7):2781–2788. [PubMed] [Google Scholar]
- Schwartz M., Geiger B., Hooghe R., Bar-Eli M., Gallily R., Mozes E., Sela M. The mode of interaction with macrophages of two ordered synthetic polypeptides which differ in their thymus dependency. Immunology. 1978 Nov;35(5):849–855. [PMC free article] [PubMed] [Google Scholar]
- Schwartz M., Hooghe R. J., Mozes E., Sela M. Role of antigenic structure in cell to cell cooperation. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4184–4186. doi: 10.1073/pnas.73.11.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela M., Mozes E., Shearer G. M. Thymus-independence of slowly metabolized immunogens. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2696–2700. doi: 10.1073/pnas.69.9.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer G. M., Mozes E., Sela M. Contribution of different cell types to the genetic control of immune responses as a function of the chemical nature of the polymeric side chains (poly-L-prolyl and poly-DL-alanyl) of synthetic immunogens. J Exp Med. 1972 May 1;135(5):1009–1027. doi: 10.1084/jem.135.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Bossie A., Fernandez-Botran R., Myers C. D., Oliver K. G., Sanders V. M., Stevens T. L. Interaction and activation of antigen-specific T and B cells. Immunol Rev. 1987 Oct;99:193–239. doi: 10.1111/j.1600-065x.1987.tb01178.x. [DOI] [PubMed] [Google Scholar]
- Zisman E., Sela M., Mozes E. Direct binding of a synthetic multichain polypeptide to class II major histocompatibility complex molecules on antigen-presenting cells and stimulation of a specific T-cell line require processing of the polypeptide. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9738–9742. doi: 10.1073/pnas.88.21.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Eertwegh A. J., Fasbender M. J., Schellekens M. M., van Oudenaren A., Boersma W. J., Claassen E. In vivo kinetics and characterization of IFN-gamma-producing cells during a thymus-independent immune response. J Immunol. 1991 Jul 15;147(2):439–446. [PubMed] [Google Scholar]
- van den Eertwegh A. J., Laman J. D., Schellekens M. M., Boersma W. J., Claassen E. Complement-mediated follicular localization of T-independent type-2 antigens: the role of marginal zone macrophages revisited. Eur J Immunol. 1992 Mar;22(3):719–726. doi: 10.1002/eji.1830220315. [DOI] [PubMed] [Google Scholar]



