Abstract
The primary aim of this paper was to assess the relevance of high-frequency ultra-sound examination in qualifying patients for either surgical or conservative treatment of peripheral entrapment neuropathies. The study was conducted in a group of 55 patients aged 7–83 (mean age 43.6), including 28 males and 27 females, who in 2009–2011 were referred to an ultrasound examination due to a clinical suspicion of entrapment neuropathies. For the purposes of the analysis, the patients were divided into four groups: carpal tunnel syndrome (1), ulnar nerve entrapment (2) (cubital tunnel syndrome and Guyon's canal syndrome), posterior interosseous nerve syndrome (3) and other entrapment neuropathies (4). The cases of isolated idiopathic carpal tunnel syndrome were excluded from the analysis. All patients underwent the interview, physical examination and ultrasound examination. Ultrasound examinations were performed with Esaote MyLab 50 and MyLab 60 systems using high-frequency broadband linear transducers: 6–18 MHz. Sixty-seven percent of patients (37 persons) underwent a neurophysiological test. Nerve echostructure, its hyperemia as well as nerve cross-sectional area or, in the case of small nerves, diameter were assessed in all patients. Furthermore, the following were assessed in individual groups: notch sign in group 1, nerve instability in a dynamic ultrasound examination in group 2, nerve angulation in a dynamic ultrasound examination and tenderness on nerve compression at the site of the visualized pathology in group 3. The analyses of the collected material were performed by means of descriptive statistics. The results of clinical and surgical verification were consistent with ultrasound findings in 96.4%. The results indicate that high-frequency ultrasonography is a valuable method in qualifying patients for various types of treatment of peripheral neuropathies resulting from compression.
Keywords: ultrasonography, peripheral nerves, neuropathies, carpal tunnel syndrome, ulnar nerve entrapment
Abstract
Podstawowym celem pracy była ocena przydatności badania ultrasonograficznego z zastosowaniem głowic wysokiej częstotliwości w kwalifikowaniu do leczenia operacyjnego albo zachowawczego neuropatii obwodowych o charakterze uciskowym. Materiał pracy stanowiła grupa 55 osób w wieku 7–83 lata (średnia wieku 43,6 roku), w tym 28 mężczyzn i 27 kobiet, kierowanych w latach 2009–2011 na badanie ultrasonograficzne z klinicznym podejrzeniem neuropatii uciskowych, na potrzeby analizy podzielonych na cztery grupy: zespół kanału nadgarstka (1), zespół ucisku nerwu łokciowego (2) (ucisk na poziomie rowka nerwu łokciowego oraz ucisk na poziomie kanału Guyona), zespół ucisku nerwu międzykostnego tylnego (3), inne neuropatie uciskowe (4). Z analizowanej grupy wyłączono przypadki izolowanego idiopatycznego zespołu kanału nadgarstka. U wszystkich pacjentów przeprowadzono badanie podmiotowe, przedmiotowe oraz badanie ultrasonograficzne. Badania ultrasonograficzne przeprowadzono aparatami Esaote MyLab 50 oraz MyLab 60 z zastosowaniem szerokopasmowych głowic liniowych o wysokich częstotliwościach: 6–18 MHz. U 67% pacjentów (37 osób) wykonano badanie neurofizjologiczne. U wszystkich pacjentów oceniano echostrukturę nerwu i jego przekrwienie oraz określano pole powierzchni przekroju nerwu albo – w przypadku drobnych nerwów – średnicę. Dodatkowo w grupie 1 oceniano objaw wcięcia, w grupie 2 – niestabilność nerwu w dynamicznym badaniu ultrasonograficznym, w grupie 3 – zaginanie kątowe nerwu w dynamicznym badaniu ultrasonograficznym oraz tkliwość w czasie ucisku nerwu w miejscu uwidocznionej patologii. Analizy zebranego materiału dokonano za pomocą statystyki opisowej. W odniesieniu do weryfikacji klinicznej i operacyjnej zgodność z rozpoznaniem ultrasonograficznym osiągnięto w 96,4% przypadków. Uzyskane wyniki wskazują, że badanie ultrasonograficzne z zastosowaniem głowic wysokiej częstotliwości jest cenną metodą w kwalifikowaniu do rodzaju leczenia neuropatii obwodowych o charakterze uciskowym.
Introduction
The first reports on the application of ultrasonography in peripheral nerve assessment appeared in the 1990s and concerned carpal tunnel syndrome, i.e. compression of the median nerve(1, 2). In the past decade, following the introduction of high-frequency transducers, ultrasonography has become an integral element in assessing patients with suspected peripheral neuropathies thus complementing routine diagnostic tests, such as clinical examination and electromyography (EMG)(3–7). Due to the possibility to assess only short fragments of peripheral nerves, lower resolution (compared to a US examination), limited availability and high cost, high-field magnetic resonance is rarely used(8–11). Peripheral neuropathies include those resulting from entrapment, trauma, peripheral nerve tumors and surgeries.
Entrapment neuropathies result from chronic compression of a peripheral nerve trunk, usually by fibrous bands and ligaments at the level of osseous and fibrous canals where the nerve runs. Another cause of these neuropathies may be soft tissue tumors (lipoma or fibroma), vascular changes (aneurysm, anatomic variants) or osseous changes, bony spurs. Compression may cause perfusion disorders and edema-like processes, and subsequently lead to the degeneration of the nervous tissue. A later consequence is function impairment, i.e. disorder in impulse conduction distally to the compression site.
The aim of the US examinations was to determine the level and cause of compression and assess the nerve trunk image at the site of pathology.
The analysis was based on the most common entrapment neuropathies excluding idiopathic carpal tunnel syndrome (CTS), which is extensively discussed in the literature.
Specific objectives were:
to specify ultrasound features of peripheral nerve pathologies in terms of their qualification for surgical or conservative treatment;
to determine the diagnostic value of ultrasonography in assessment of nerve pathologies with respect to clinical and surgical verification as well as results of functional examinations.
The article is the first part of a series of publications prepared on the basis of the author's doctoral dissertation entitled: Usefulness of ultrasonography with high-frequency transducers in the diagnosis of peripheral neuropathies (supervised by: Prof. Iwona Sudoł-Szopińska MD, PhD, defended on November 4, 2014 in Warsaw).
Material and methods
The study was conducted in a group of 55 patients aged 7–83 (mean age 43.6) who in 2009–2011 were referred to ultrasound examinations due to a clinical suspicion of peripheral entrapment neuropathies. The group consisted of 28 females and 27 males. Patients were referred to a US examination by orthopedists, neurologists and physiotherapists. All patients gave a written consent to the participation in the study. The examinations were conducted in two health care facilities in Cracow: Intermed and TLK Med.
The US examinations were performed with Esaote MyLab 50 and MyLab 60 systems using high-frequency broadband linear transducers: 6–18 MHz (mainly of 12–18 MHz).
The US images were analyzed with respect to clinical signs (all patients) and EMG results (37 patients).
The analyses of the collected material were performed by means of descriptive statistics. Mean values of crosssectional areas and diameters for individual pathology groups were calculated. The ultrasound features of the peripheral nerves evaluated in the study, such as echostructure, notch sign and hyperemia, were divided into subgroups to determine the most common features of a US image of the nerves analyzed in the individual groups. Moreover, the frequency of occurrence of pain on compression with a transducer and instability of the ulnar nerve as well as angulation of the posterior interosseous nerve in a dynamic examination was calculated. The consistency of the US examinations with EMG tests, clinical examination and surgical verification was also checked. The values obtained were presented in the figures.
Results
In the patients with symptoms of entrapment neuropathies, 55 pathologies were diagnosed in a US examination, which were then divided into four groups:
carpal tunnel syndrome (CTS) – 19 patients;
ulnar nerve entrapment (cubital tunnel syndrome – CubTS and Guyon's canal syndrome) – 19 patients;
posterior interosseous nerve syndrome – 11 patients;
other entrapment neuropathies – 6 patients.
The cases of isolated idiopathic CTS were excluded from analysis due to the fact that there are numerous papers devoted to this type of neuropathy(1, 4, 12–26). Only rare cases of CTS were included, such as CTS caused by a fracture of a forearm bone (3 patients), a case of bilateral CTS in a 7-year-old boy, a case of acute bilateral CTS symptoms and CTS caused by a thrombus in the persistent median artery. Moreover, the study also included the cases of CTS that accompanied other pathologies which were operated simultaneously (12 patients).
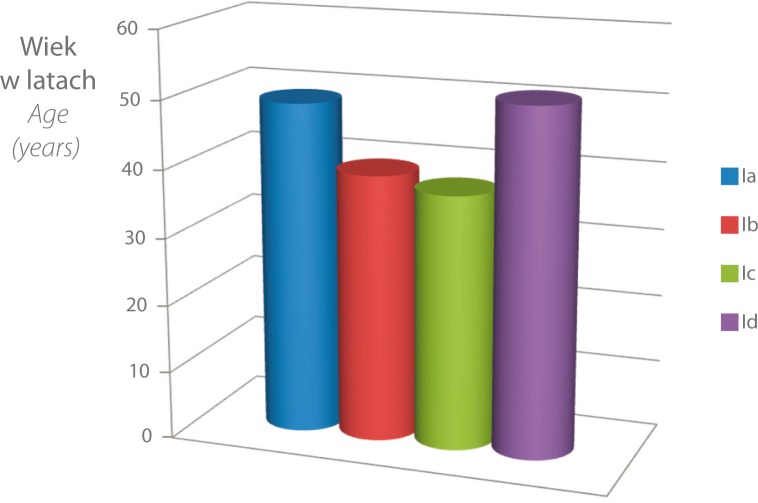
The mean age of patients in the groups was: 49 in Group 1 (CTS), 39.4 in Group 2 (CubTS), 37.5 in Group 3 (posterior interosseous nerve syndrome) and 51 in Group 4 (other entrapment neuropathies) (fig. 1).
Fig. 1.
Mean age in individual categories of entrapment neuropathies
Analyzed ultrasound features of the investigated neuropathies
1. Echostructure of the peripheral nerves
For the purposes of the analysis, the pathologically altered nerves were divided into four groups in terms of their echostructure:
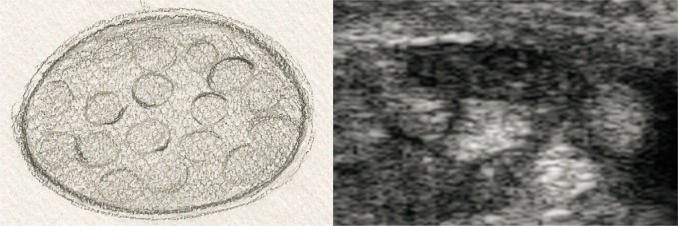
nerves with normal echostructure, i.e. with retained bundle structure, visible perineurium layer of individual bundles and epineurium of the nerve trunk (fig. 2A, B);
nerves with disordered (blurred) echostructure, i.e. with visible bundles but blurred perineurium outline (fig. 3A, B);
nerves with partially absent echostructure – next to normal bundle structure, pathological tissue is visible (fig. 4A, B);
nerves with completely absent bundle echostructure – advanced, long-lasting entrapment neuropathies (fig. 5A, B).
Fig. 2A, B.
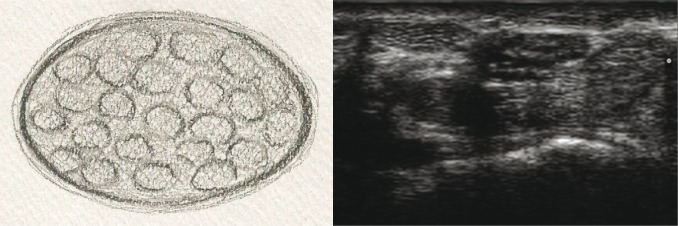
Cross-section of a peripheral nerve with normal echostructure: A. diagram; B. US image
Fig. 3A, B.
Cross-section of a peripheral nerve with blurred echostructure: A. diagram; B. US image
Fig. 4A, B.
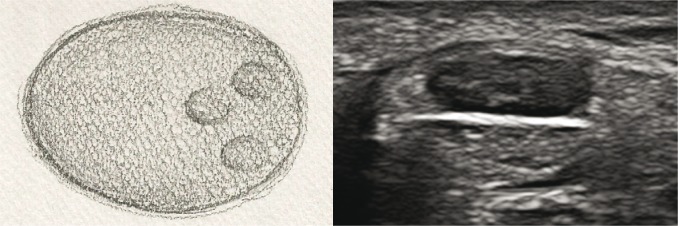
Cross-section of a peripheral nerve with partially absent echostructure: A. diagram; B. US image
Fig. 5A, B.
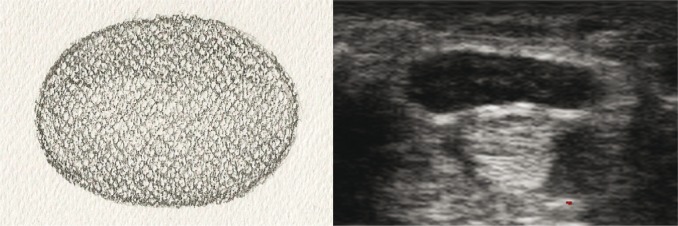
Cross-section of a peripheral nerve with completely absent echostructure: A. diagram; B. US image
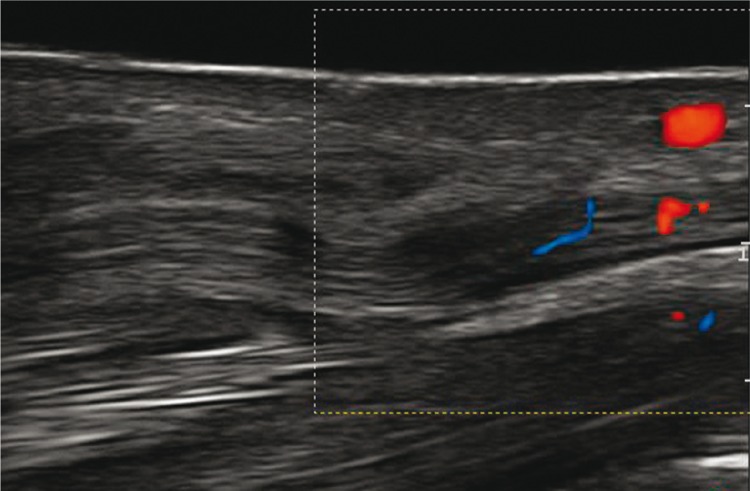
2. Vascularization of the peripheral nerves
Normal nerves showed no signs of vascularization in a power Doppler examination. The presence of vessels was considered a sensitive indicator of neuropathy. Blood vessels were visible within the perineurium or epineurium (fig. 6).
Fig. 6.
Ultrasound image of the median nerve with hyperemia
3. Measurements
In large nerve trunks, the cross-sectional area was measured with the use of the manual contour tracing method. When examining small nerves, the diameters of their crosssections were measured. Due to the risk of errors resulting from trembling hands, three measurements were taken and the mean value was determined. The measurement lines were each time drawn at the level of the epineurium.
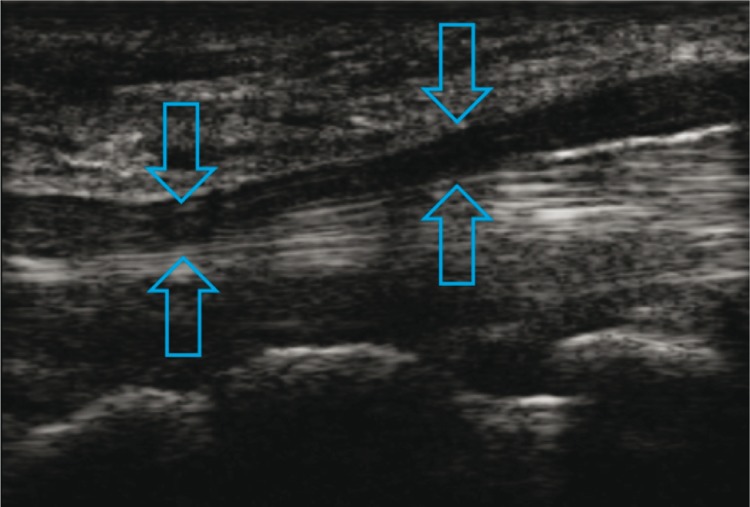
4. Notch sign in the nerve
The presence of an hourglass-like narrowing in the nerve trunk was seen in certain patients from Group 1, 2 and 3. This sign was visible when the transducer's footprint was applied longitudinally and when the nerve trunk was modelled by a fibrous structure running transversely to the nerve, i.e. transverse carpal ligament (fig. 7), arcuate ligament at the level where the ulnar nerve enters between the heads of the flexor carpi ulnaris (fig. 8) or the arcade of Frohse (fig. 9).
Fig. 7.
Notch sign in the median nerve (arrows) at the level of the transverse carpal ligament
Fig. 8.
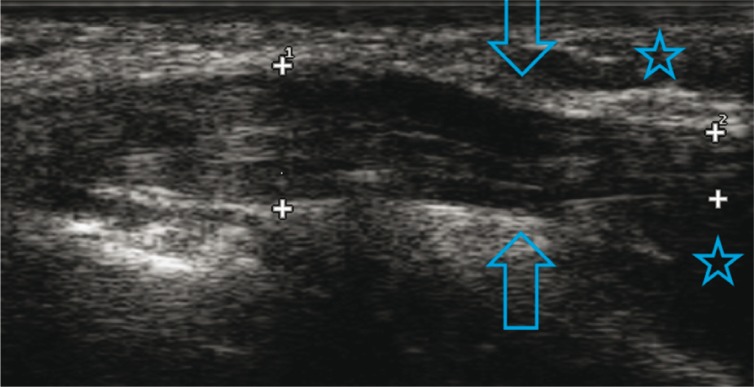
Notch sign in the ulnar nerve (arrows); humeral and cubital aspects of the flexor carpi ulnaris (asterisks)
Fig. 9.
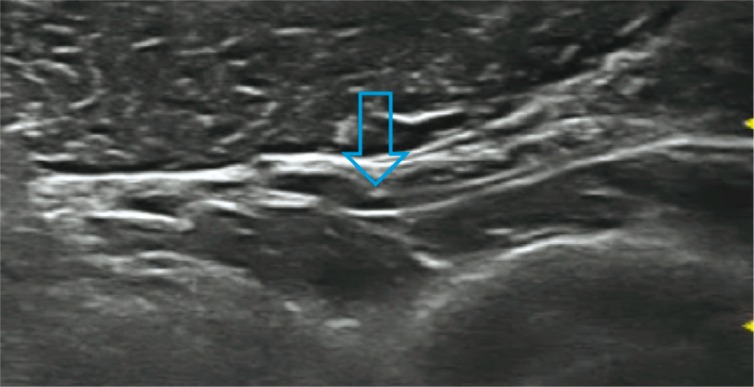
Notch sign in the posterior interosseous nerve (arrow)
5. Tenderness on palpation
When pathologies have been visualized in Groups 3 and 4, a compression with a transducer was applied to the altered nerve segment to see whether it provoked pain or paresthesia.
In multiple cases, these sites were consistent with the points used by clinicians in neuropathy diagnosis, analogously to the Tinel sign.
6. Stability of the ulnar nerve at the level of the humeral groove
During a dynamic examination, which consisted in flexion and extension of the arm at the elbow joint, it was observed whether the ulnar nerve was stable and remained in the groove, or whether it manifested features of instability by e.g. dislocation to the anterior surface of the medial epicondyle of the humerus (nerve dislocation) or no further than to the apex of the epicondyle (subluxation). Both these conditions were treated as a manifestation of instability. The examination involved transverse footprint application at the level of the ulnar nerve groove.
Based on clinical and US examinations, patients were qualified for either surgical or conservative treatment. Fifty-two patients were operated on, and 3 received conservative treatment.
In the case of chronic entrapment neuropathies, US images usually revealed edema and hyperemia of the nerve proximally to the compression site. In the longitudinal view, an hourglass-like narrowing of the nerve was observed, and in the transverse view, in a so-called “lift technique,” a segmental change of shape was observed – from oval to round, loss of echogenicity corresponding to this site and blurred bundle structure, which attested to the presence of edema. The tissues adjacent to the nerve were assessed in terms of possible pathologies that affected the nerve by socalled “mass effect,” e.g. joint effusion, benign proliferative lesions or bony spurs (osteophytes). In situations of doubt, a contralateral extremity was scanned for comparison provided that there were no pathologies in the reference area. Certain indirect information on the condition of the nerve was also obtained by scanning the muscles innervated by this nerve.
Comparison of EMG and US findings
Sixty-seven percent of patients underwent an EMG examination. The greatest number of such tests was conducted in Group 2 (100%), and the lowest number – in Group 3 (chronic posterior interosseous nerve entrapment, 36.36%) and in Group 4 (chronic entrapment neuropathies, 16.6%).
A small number of examinations of the posterior and anterior interosseous nerves was a result of technical difficulties and a large number of false negative results in assessing less advanced changes. The RSNR was not assessed in the EMG test due to a too unambiguous clinical assessment (the nerve is purely sensory, and clinical and US examinations were diagnostic for the surgeon).
The EMG examination did not confirm the features of neuropathy detected in ultrasonography in 20% of cases (11 patients). The greatest number of errors in EMG was noted in the CubTS group (27.27%).
Discussion
Entrapment neuropathies of the peripheral nerves are a numerous group of pathologies bordering neurology, orthopedics and traumatology. Patients with such conditions also report to rehabilitation clinics. For many years, the clinical assessment was the basis for diagnosing entrapment neuropathies of the peripheral nerves. In the 1940s, the diagnosis of peripheral nerve pathologies was expanded to include functional electromyographic tests(27–29). Since the 1990s, ultrasonography has been gaining popularity. In the past decade, this examination has become the leading method for diagnosis of these pathologies and monitoring of their treatment.
In the author's own study, the median nerve manifested the following ultrasound features in the course of CTS (tab. 1):
Tab. 1.
Frequency in which individual ultrasound elements occurred in patients with CTS symptoms
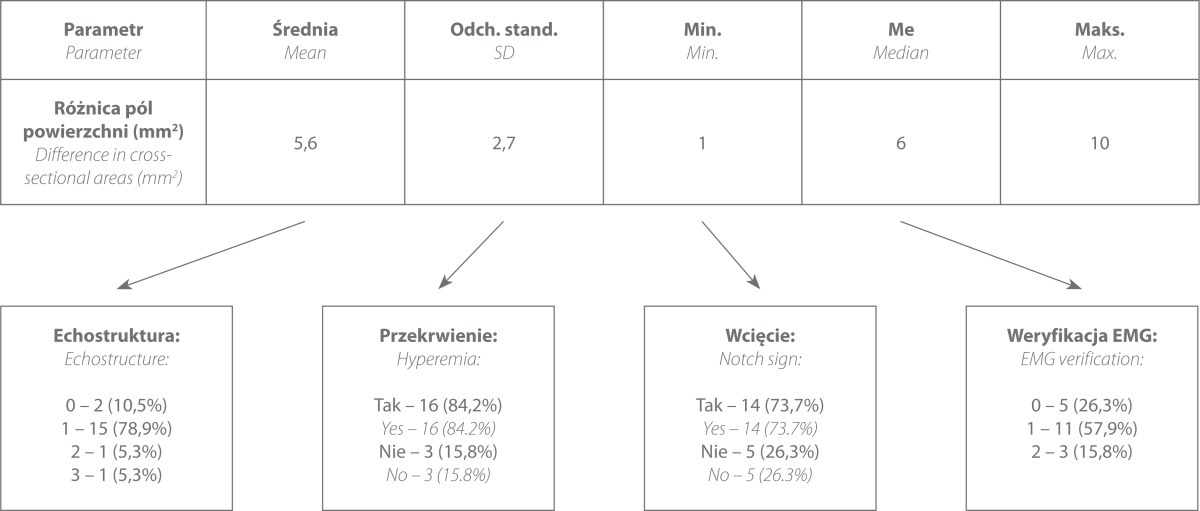
Carpal tunnel syndrome (19 patients)
the mean value of the nerve cross-sectional area CSAc – 13.7 mm2, CSAp – 8.6 mm2
An EMG examination was conducted in 14 patients and correlated with the US findings only in 11 cases, which is 57.9%. In 3 patients, the EMG result showed no abnormalities while the US revealed changes verified intraoperatively. Five patients did not undergo a neurophysiological examination.
In the world literature, the most common diagnostic criterion of CTS is the measurement of the cross-sectional area of the median nerve at two levels: at the site where it enters the carpal tunnel (CSAc) and at the level of the distal aspect of the pronator quadratus muscle (CSAp), or the measurement of its transverse dimensions at the level of the carpal tunnel(1, 18–22, 24–26, 30–32). Investigators also mention nerve echogenicity and vascularity assessment at the proximal level of the transverse carpal ligament(4, 12, 16, 18, 23). Some authors indicate the usefulness of measuring the thickness of this ligament(23) or assessment of the nerve shape in the transverse plane(14).
In the author's own study, the main diagnostic criterion was the difference inbetween CSAc and CSAp values – this criterion was fulfilled by all the nerves examined. This median nerve assessment method was introduced by Klauser et al. in 2009(30). They examined 68 patients with CTS symptoms (100 wrists) and 58 healthy volunteers.
In patients with CTS, the mean CSAc was 16.8 mm2 and CSAp – 9.5 mm2. The authors assumed that a 2 mm2 difference in the cross-sectional values at these levels was diagnostic for CTS. Using this criterion, they diagnosed CTS with the sensitivity of 99% and specificity of 100%. The results obtained by the aforementioned investigators are similar to the findings of the author in her own study. However, the CSAp and CSAc difference was the only parameter tested by Klauser et al. whereas the author of this study also included other criteria, as mentioned above.
Two years later, another article by Klauser et al. appeared regarding diagnostic criteria of the bifid median nerve neuropathies(31). In such cases, the authors decided that a diagnostic difference between CSAp and a total of the cross-sectional areas of both branches of the nerve as they entered the carpal tunnel was 4 mm2. The sensitivity and specificity of the US examination in identifying CTS for this value was 92.5% and 94.6% respectively.
In the author's own study, the bifid nerve was observed in 4 patients: in 3 persons, it manifested features of neuropathy, and in 1 – the ultrasound image was normal. The differences between CSAp and the sum of CSAc of both branches of the nerve measured in the 3 patients were 4 mm2, 5 mm2 and 8 mm2 respectively.
The difference values of CSAp and CSAc in Group 1a (CTS) were similar to those obtained by Klauser et al.(30, 31), and in the author's own study amounted to 8.6 mm2 and 13.7 mm2. In the material of Klauser et al., CSAp of patients with less advanced changes (41% of examinations) amounted to 9.1 mm2 and CSAc was 14.4 mm2; in those with highly advanced changes (59%), CSAp was 9.7 mm2 and CSAc – 18.5 mm2. In the author's own study, only 6 of 19 patients manifested clinical signs and had EMG results that could indicate considerable advancement of neuropathy (31.7%).
Until Klauser et al. published their two papers(30, 31), the leading CTS diagnostic criterion was the measurement of CSAc only, the values of which ranged from 9–14 mm2 and the sensitivity and specificity ranges were 82–94% and 65–97% respectively(18–23). In the studies conducted in the Polish center, published in 2013, the CSAc values amounted to 17.6 mm2(28). In the papers published earlier, the results varied considerably as well.
The pioneer reports regarding ultrasonography in CTS diagnosis appeared in 1992(1). The Austrian authors presented the results of US examinations performed with the use of 7 MHz transducers in 18 patients with CTS symptoms (in the author's own study the transducer frequency was 18 MHz). They measured two transverse dimensions of the nerve trunk and its cross-sectional areas at three levels: at the DRUJ joint (distal radioulnar joint which is currently referred to as CSAp both in the literature and in the author's own study), the pisiform bone (CSAc) and the hook of the hamate bone. The diagnosis was made on the basis of three criteria: increase in the cross-sectional area of the median nerve at one of the three levels, flattening of the nerve trunk in the longitudinal section and the presence of palmar bulge of the transverse carpal ligament. The mean cross-sectional area of the median nerve at the level of the pisiform bone was 8.1 mm2 (it was lower than in the author's own study and in the contemporary articles presented above), nerve flattening – 2:7 (the ratio of the AP dimension to the lateral one), and palmar bulge exceeded 2.1 mm. The last two criteria are not currently used due to their low sensitivity and specificity.
Another study conducted in the 1990s resulted in different outcomes than those mentioned above(21). Duncan et al., in their study conducted in a group of 68 patients with median nerve neuropathy symptoms, assumed that CSAc diagnostic value was 9 mm2. The measurements were made at the level of the pisiform bone, and the examinations were conducted with probes with the frequency of 7–10 MHz.
In the same year (1999), Lee et al. decided that CSAc of 15 mm2 should be considered a diagnostic values (this value is comparable with the one used in this study and by Klauser et al.)(29). The reason for such discrepancies between the values obtained in the aforementioned studies could, apart from technical aspects (low frequency of the probe compared to contemporary studies), lie in a different method of taking the measurements. Only few publications indicate that the nerve contour tracing should encompass the line of the epineurium (i.e. not its internal or external aspects)(30).
In 2000, Sarría et al. assumed that CSAc of 11 mm2 should be the diagnostic value of CTS(32). They conducted ultrasound examinations of 64 wrists with symptoms of the median nerve compression confirmed in neurophysiological tests. The sensitivity of this criterion reached only 60%. The sensitivity of the flexor retinaculum bulging above 2.5 mm was also low (69.2%). Moreover, the Spanish authors failed to confirm the diagnostic value of nerve flattening. Since such a conclusion is consistent with the author's own observations, this element of the US median nerve image was not used in this study.
In 2004, Altinok et al. decided that the diagnostic value of the median nerve cross-sectional area which is a reliable indicator of CTS should be 9 mm2(25). This was based on US examinations conducted in 26 patients with CTS symptoms, including 14 bilateral cases. The value assumed by the authors was similar to the one proposed several years before by Duncan et al.(21) The authors measured the median nerve cross-sectional area at three levels (DRUJ, pisiform bone and the hook of the hamate bone). Moreover, they also analyzed the carpal transverse ligament bulging and flattening of the nerve in the longitudinal section. The most sensitive diagnostic parameter for CTS (sensitivity of 72.5%) was nerve edema calculated as the ratio of the cross-sectional area at the distal level to the proximal one (DRUJ and the hook of the hamate bone). The sensitivity of the cross-sectional area exceeding 9 mm2 measured at the level of the pisiform bone was 65%, and the sensitivity of bulging above 2.5 mm – 62.5%. Although the authors did not present the measuring methods of this criterion in detail, the only explanation of such a low sensitivity of the cross-sectional area may be inadequate measuring method (along the internal outline of the epineurium).
In 2004, a Polish publication based on 52 patients and 44 controls appeared(12). The cross-sectional area and echogenicity of the median nerve were assessed at the level of the pisiform bone. For the cross-sectional area above 9.3 mm2, the sensitivity of ultrasonography in CTS diagnosis was 92.1%, and the sensitivity of the parameter associated with nerve trunk thickness reduction in the longitudinal projection was 92.1% as well. In the author's own studies, the cross-sectional area was larger and amounted to 13.7 mm2. In this case, the reason for the differences was probably the lower frequency of the transducer used by the authors – 6–11 MHz. Lower echogenicity was demonstrated in 82.9% of patients, which is similar to the percentage obtained in the author's own study (78.9%).
Furthermore, the discrepancies between the results of the next paper and author's own outcomes should be also explained with the lower frequency of the US probe used. Having analyzed 221 patients (319 wrists) in 2005, Koyuncuoglu et al. assumed that CSAc value of 8.83 mm2 is the diagnostic value (sensitivity 89%, specificity 94.7%)(33). The authors used the transducers with the frequency of 5–12 MHz, and the measurements were taken at the level of the pisiform bone.
In 2009, Polish authors(13) obtained a slightly higher value of the median nerve cross-sectional area, but it was still lower than the one obtained in the author's own and Klauser's studies(30). The median nerve cross-sectional area that indicated CTS was higher than 10 mm2 (CSAc) (sensitivity 74.5%, specificity 88%). Furthermore, the authors, as the first ones in the literature, drew attention to radial “sliding” of the nerve, which was observed in 68% of patients. This sign was neither observed by other investigators nor in the author's own study.
In 2011, another Polish team analyzed the results obtained in 139 examinations conducted in 76 patients with features of CTS confirmed in electrophysiological tests and in 25 examinations conducted in 14 healthy volunteers(14). The authors evaluated the parameter that was not included in the author's own study, i.e. the shape of the cross-section of the nerve at the level of the carpal tunnel. In patients with abnormal results of electrodiagnostic tests, a triangular cross-section was visualized in 13 of 118 cases (11%), whereas in patients with normal electrodiagnostic result, such a shape was observed in only 2 of 21 cases (9.5%). Such a deformation was not observed in any of the healthy volunteers. In the author's own opinion and according to other investigators, the relevance of this criterion seems dubious since the shape of the median nerve at the level of the carpal tunnel undergoes natural changes depending on the flexion angle of each finger (in metacarpophalangeal joints as well as proximal and distal interphalangeal joints), flexion angle of the wrist, supination degree and even on the flexion angle of the elbow joint (since it affects the pressure in the carpal tunnel). Therefore, determining the standard position of the upper extremity for the examination seems problematic and difficult to reproduce. Moreover, the assessment of the nerve's shape is subjective, and distinguishing oval from triangular shapes is frequently not feasible.
In 2013, the authors from Cracow, Poland, published a review on the ultrasound assessment of the median nerve(17). They presented criteria for pre- and postoperative assessment and used the same criteria as in this study. In the same year, a research team from Szczecin, Poland, conducted a study that included 113 patients(28). The mean cross-sectional area of the median nerve at the forearm was 9.9 mm2 and at the level of the entry to the carpal tunnel – 17.8 mm2. The values were similar to those obtained in the author's own study. The authors drew attention to the fact that the relevance of ultrasonography largely depends on the examiner, the frequency of the transducer and assumed diagnostic criteria (the level and methods of taking the measurements as well as the assumed limit values).
In the author's own material, 14 patients (73.4% of all patients from the Group 1a) reported for a US examination with a result of a previously conducted EMG test. In 11 cases, the abnormal result of the functional examination correlated with the US image and intraoperative picture, which constitutes 57.9% of all patients who underwent the EMG examination. In 3 persons (15.8%), however, the EMG result was normal despite the presence of clinical symptoms, ultrasound signs and intraoperative picture. A similar comparative analysis was conducted by Altinok et al. and demonstrated that the ultrasound image was abnormal in as many as 85–100% of patients with conduction disorders, and in the case of normal EMG reading – in 30–55% of cases, i.e. more frequently than in the author's own study(25).
A high correlation of EMG and US examinations in the context of CTS was also proven by other authors(26, 34–35), who noted(35), however, that ultrasonography does not allow conclusions about advancement of changes to be drawn. A year before, the authors from a different center questioned the relevance of ultrasonography, not only in correlation with electrophysiological testing, but also as a diagnostic tool in CTS(36).
Furthermore, in a very interesting article published in 2005, Turkish authors analyzed a group of 221 patients (319 wrists) with clinical symptoms of carpal tunnel syndrome(33). Although the neurophysiological tests were normal in 49 patients (59 wrists, 22.2%), almost 30% manifested CTS signs in a US examination. The authors concluded that in early stages of CTS when EMG results are normal, ultrasonography is a valuable diagnostic tool. A difference between the author's own results and those quoted above may be associated with too few patients in the early stage of the disease (only 3 cases) in the author's own study. Moreover, discrepant opinions on the correlation of US and EMG can be also found in several Polish publications(14, 28).
In this study, the result of the US examination was false negative in one patient. The patient reported complex pain symptoms and sensation disorders associated with the metacarpus injury sustained in the past with a power saw. The injury resulted in damage to the digital nerves, tendons and metacarpal bone fracture. Despite five repair and revision surgeries, the symptoms exacerbated. Based on US findings, the patient was qualified for a procedure to manage neuromas of the digital nerves. A US examination determined the localization and size of the neuromas, which was confirmed intraoperatively. One of the neuromas was dislocated to the radial surface of the first lumbrical muscle. The result of the US examination provided important information for the operator. The median nerve was normal in the US image, but intraoperatively the surgeon detected compression at the level of the carpal tunnel and performed transverse carpal ligament reconstruction. After the procedure, pain symptoms subsided temporarily but subsequently recurred. It is hard to assess whether this was the result of the surgical procedure of the neuromas or of the carpal tunnel.
In one case, an acute thrombus of the persistent median artery was identified as the cause of CTS. During the US examination, the nerve was thickened, presented a blurred bundle echostructure, showed features of hyperemia and modelling by the transverse carpal ligament. No flow was seen in the persistent median artery. A wait-and-see attitude was assumed, and the treatment with low-molecular- weightheparin, anti-inflammatory drugs and anti-edema medications was instituted. A complete recanalization of the artery was not achieved, but neuropathic symptoms subsided after 3 months of treatment, and a US image of the median nerve improved. A similar case was also reported in the literature(15).
Ulnar nerve compression syndromes (19 patients)
In the group of analyzed entrapment neuropathies, chronic compression of the ulnar nerve was observed in 19 patients: in 14 cases the compression occurred at the level of the arcuate ligament, in 2 patients – at the level of the inter- muscular septum, in further 2 patients the accessory anconeus muscle was found, and in 1 patient the accessory abductor digiti minimi was observed and compression at the Guyon's canal was identified. Of 19 cases, 4 patients manifested instability of the nerve at the ulnar groove on dynamic US examination.
In one patient, the pathology occurred at two levels, i.e. compression occurred at the level of the humeral groove and at the Guyon's canal. The cause of entrapment at both levels was the presence of accessory muscles (accessory anconeus muscle and accessory abductor digiti minimi). The EMG test revealed abnormality only at the level of the groove.
In one case of ulnar nerve edema at the level of the humeral groove, the referring physician decided about ultrasound-guided iniection of 1 ml of anti-inflammatory steroid drug (Diprophos) to the area of the nerve. The neuropathic symptoms subsided. The author has not found a report of a similar procedure in the literature.
In the ultrasound assessment, the mean cross-sectional area of the ulnar nerve at the level of the groove amounted to 12.6 mm2 (range: 6–22 mm2), and hyperemia at the site of the visualized thickening was present in 15 patients (78.9%). Disordered bundle echostructure, as a sign of edema and degenerative changes of the bundles, was observed in 17 patients (89.5%), and its absence was identified in 2 cases (10.5%) (tab. 2).
Tab. 2.
Frequency in which individual ultrasound elements occurred in patients with CubTS symptoms
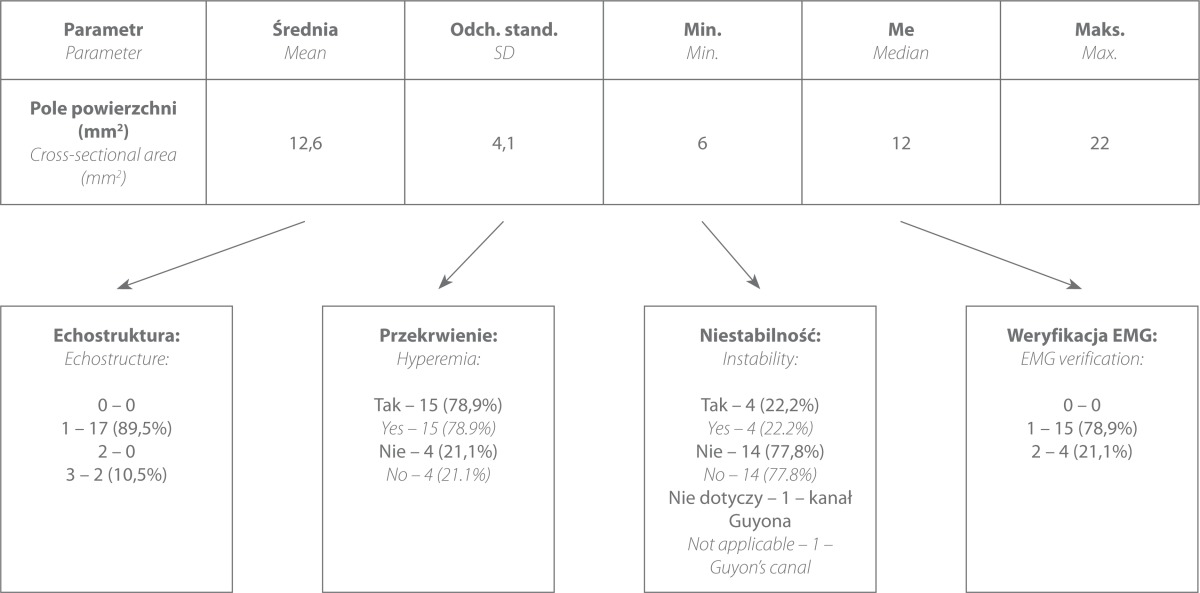
EMG tests were conducted in all patients. In 15 cases, the tests confirmed the pathology (78.9%), and in 4 cases, EMG revealed normal conduction (21.1%) despite the presence of clinical symptoms and abnormal US image. The US image was verified intraoperatively in each case.
In one of the first studies on this syndrome (14 symptomatic patients and 10 controls) published in 1988, the normal cross-sectional area of the ulnar nerve at the level of the groove was 6.8 mm2, and proximally (at the forearm) – 5.4 mm2(37). The criterion for CubTS diagnosis was the value of 7.5 mm2 (measured at the level of the groove) – it was much lower than the value used in the author's own study. Perhaps, the reason for such discrepancies was the lower frequency of the probe used by the authors of the study (7.5 MHz). Nonetheless, the authors drew attention to the presence of physiological dilation of the nerve outlines at the level of the groove.
Moreover, in another paper published in 2004, the mean cross-sectional area of the normal ulnar nerve at the level of the condyle of the humerus was lower than in the author's own study and amounted to 7.9 ± 3.1 mm2(38). The investigators also drew attention to sex-related differences in cross-sectional areas, particularly in 20–40 age range. In the author's own assessment, the mean crosssectional area in 13 men was 12.5 mm2 and in the group of 6 women, it was slightly higher and amounted to 12.8 mm2.
Wiesler et al., in the study published in 2006 and conducted in the group of 14 patients (15 elbow joints) with symptomatic ulnar nerve neuropathy at the level of the groove and 60 asymptomatic individuals, demonstrated that the cross-sectional area was 6.5 mm2 in the asymptomatic group and 19 mm2 in the symptomatic group(39). The pathological value obtained by the authors places itself in the upper range of the values obtained in the author's own study (range 6–22 mm2), which could result from more advanced neuropathy. According to the authors, the limit value for a cross-sectional area was 10 mm2; the sensitivity in CubTS diagnosis was 93%, and specificity – 98%.
Two years later, Yoon et al. evaluated 26 patients and 30 controls(40). They determined the ratio of the cross-sectional area of the ulnar nerve at the level of the groove to the area measured proximally to the groove. An abnormal value, that diagnosed CubTS, was 1.5/1 ratio and cross-sectional area of the ulnar nerve at the level of the groove of 8.3 mm2 (sensitivity 100%). The mean cross-sectional area of the ulnar nerve at the level of the medial condyle of the humerus was 18.6 mm2, which considerably exceeded the value determined in the author's own study. As with the previous report, this could result from the fact that 50% of Yoon's et al. patients were in an advanced stadium of the neuropathy (in the author's own paper this percentage was only 31.6%).
In 2009, Italian investigators demonstrated that the cross-sectional area of 11.1 mm2 was the basis to diagnose mild neuropathy (in 36% of patients), 15.8 mm2 – to diagnose moderate neuropathy (38% of patients) and 18.3 mm2 – to identify advanced neuropathy (26% of patients)(41). They used transducers with the frequency of 12 MHz. The sensitivity and specificity of ultrasonography in CubTS diagnosis were 88%. These results are consistent with those obtained in the author's own study and may indicate that, apart from the specificity of the examined group (stadium of the disease), the frequency of the US transducers, available in the past several years, had a significant influence on the results.
The prevalence of the accessory anconeus muscle in the population varies from 3 to 28% depending on the source(42). The opinions concerning the influence of this muscle on CubTS symptoms at the level of the groove are divergent. It is generally believed that its presence does not condition neuropathy but merely predisposes to it. Single cases of compression syndromes associated with the presence of the accessory muscle were published in 2012 and 2013(43, 44). In the author's own paper, two cases of the accessory anconeus muscle were identified. In one of them the belly of this muscle was considerably thickened, and the ulnar nerve at the level of the groove showed the features of edema, hyperemia and disordered echostructure. Moreover, at the level of the Guyon's canal, the accessory abductor digiti minimi was found as well.
Instability of the ulnar nerve was found in 22.2% of cases (in 4 of 19 patients). The degree of luxation (i.e. subluxation to complete dislocation) was not differentiated since it was assumed that in both situations the irritation of the nerve was sufficient for a neuropathy to develop. One of the first papers in the world literature concerning dynamic US examination conducted in two patients with ulnar nerve subluxation appeared in 2003(45). Seven years later, a broader review was presented by Filippou et al.(46) Their results were based on 91 patients with symptoms of ulnar nerve neuropathy at the elbow joint. They demonstrated that ulnar nerve subluxation occurred in 18.7% of patients, dislocation to the anterior surface of the epicondyle – in 9.9% and the accessory muscle was present in 8.8%. Ulnar nerve subluxation must be carefully differentiated from snapping of the medial head of the triceps over the epicondyle. Clinically, both conditions are similar – there is a palpable dislocation of the structure in the medial cubital compartment on its flexion. The instability of the ulnar nerve in the “snapping elbow” context was discussed by Okamoto et al. in 2000 and Jacobson et al. in 2001(47, 48). In the former paper, the authors performed US examinations of 200 elbows of healthy volunteers and found that nerve subluxation (dislocation towards the apex of the epicondyle) occurred in 27% of cases, and a full dislocation (towards the anterior surface of the epicondyle) occurred in 20% of cases. These results are considerably different from the author's own observations and investigations of other authors, which may result from demographic differences in the Japanese population. It seems less likely that the difference could be associated with the lower frequency of the transducer used in the US examinations (7.5 MHz).
Jacobson reported three cases of “snapping elbow” identified in a US examination. In the author's own study, there were no cases of this condition – each patient with ulnar nerve instability at the cubital level was also evaluated for the presence of this pathology.
Posterior interosseous nerve syndrome (11 patients)
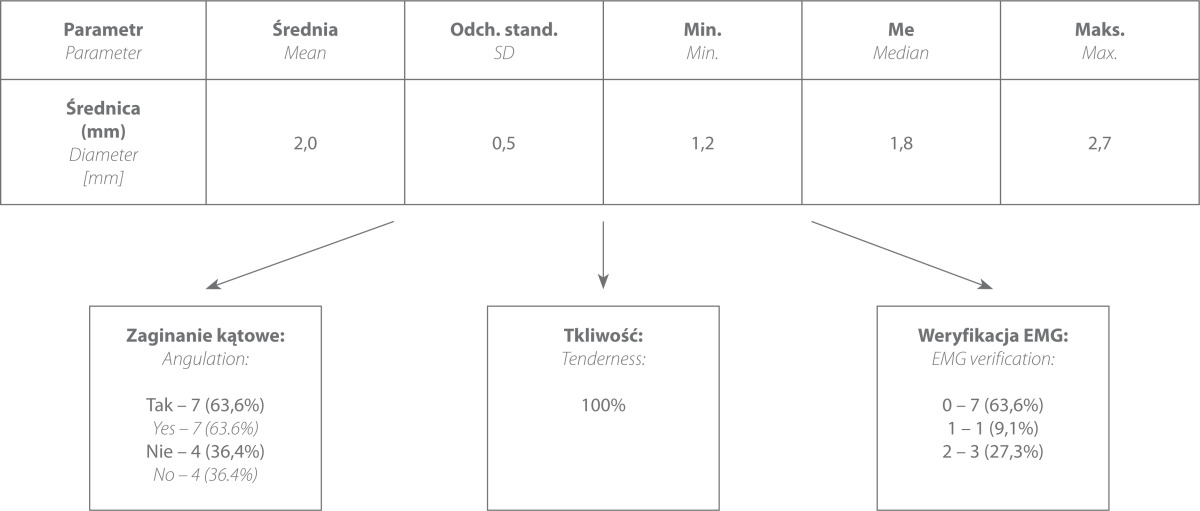
The group of patients with posterior interosseous nerve entrapment consisted of 11 patients, including 7 cases in which compression was caused by the bands of fascia and 4 cases in which entrapment was caused by the crossing vascular bundle. EMG tests were conducted in four patients, but the US findings were confirmed in merely one case; in the remaining cases the EMG was normal. In all patients, compression applied with the US transducer at the site of the visualized pathology induced characteristic severe pain. The diameter of the nerve at its entry to the supinator muscle ranged from 1.2 to 2.7 mm (mean 2.0 mm) (tab. 3). Due to the small size of the nerve, the assessment of its cross-sectional area and vascularity was not reliable.
Tab. 3.
Frequency in which individual ultrasound elements occurred in patients with symptoms of chronic PIN neuropathy
In the past years, posterior interosseous nerve entrapment has been discussed in several publications. One of the first papers was prepared by Bodner et al. in 2002(49). Having compared the US images of 4 patients with PIN symptoms and 10 healthy volunteers, the authors demonstrated an increase in the diameter of the nerve to approximately 3.3 mm (anteroposterior diameter) in patients with the symptoms of a entrapment neuropathy, whereas the diameter in healthy individuals amounted to 1.3 mm. All cases of PIN neuropathy observed in US examinations were verified intraoperatively and in neurophysiological tests. In the subsequent years, single reports appeared that confirmed the effectiveness of ultrasonography in preoperative PIN assessment(50, 51).
In the author's own paper, a group of patients with PIN neuropathy was larger compared to previous reports. The mean diameter of the nerve was 2.0 mm. Ten patients were operated, and the intraoperative picture verified the pathology visualized in the US examination. Rehabilitation was recommended in one case, with a good final outcome.
An evident difference between the diameters of the nerves measured in the author's own study and in certain papers quoted above may result from the fact that in the author's own study the measurements were performed along the epineurium line whereas in the remaining papers, this element of the methods was not specified. The fact that the measurements were made by marking the outer outline of the epineurium cannot be excluded. Moreover, the investigators conducted US examinations with the use of 12 MHz probes, whereas in the author's own study high- frequency probes were used (18 MHz), which is of particular significance in the case of slight structures, such as the PIN.
In 2010, the American investigators drew attention to the phenomenon of physiological change of shape of the nerve at the entry to the supinator muscle(52). They described 50 elbows in 47 asymptomatic patients and found that despite nerve flattening at its entry to the supinator muscle, its cross-sectional area does not change in a significant way. In the author's own opinion, measuring the cross-sectional area is burdened with a considerable statistical error due to minute size of this nerve.
In 2013, a paper concerning PIN entrapment appeared(53). It was based on 13 symptomatic patients. The results were compared with a group of 20 healthy volunteers. The diameters of the nerve at its entry under the supinator muscle were consistent with those observed in the author's own study (2 mm in symptomatic patients vs 1.1 mm in healthy volunteers).
The author of this study assessed PIN neuropathy in a static US examination and used also the elements of a dynamic test. The dynamic assessment of this condition (which is normally used for assessment of posttraumatic changes and in postoperative follow-up) in a preoperative diagnosis of entrapment neuropathies was a novelty considering previous reports. During pronation and supination movement, US showed nerve angulation at its entry to and exit from the supinator muscle or at the level of the neurovascular bundle. Angulation was observed in 7 patients (67.6%). To date, only Martinoli et al., in 2004, drew attention to the relationship of the shape of the nerve and the position of the forearm in which the nerve is assessed(52).
Moreover, it was attempted to induce pain by precise ultrasound-guided palpation in each patient. This element of assessment was also omitted in previous studies despite the fact that, according to the author of this paper, it contributed to diagnostic certainty (all neuropathies diagnosed were surgically verified).
Anterior interosseous nerve (AIN) syndrome
AIN entrapment neuropathy was identified in one case – in a 37-year-old woman who, based on US findings, was qualified to a two-level decompression procedure: of the median nerve at the carpal tunnel and the AIN in its initial segment. EMG tests conducted prior to the procedure indicated only the median nerve compression at the carpal tunnel. The US findings were verified intraoperatively.
The problem of ultrasound diagnosis of AIN entrapment syndrome was discussed in merely several publications. The first one appeared in 1999(54). The authors presented a case of a 44-year-old female who underwent a US and MRI examinations. In the authors’ opinion, the major diagnostic criterion was the mass and echogenicity of the muscles innervated by this nerve (flexor pollicis longus, pronator quadratus and flexor digitorum profundus of the second digit), compared in two extremities. The relevance of this criterion is also indicated by other authors(5, 7). Another interesting aspect of this paper was the assessment of muscle vascularity with the use of the Doppler mode while resting and following physical exertion(54). While resting, single vessels were visible whereas the examination conducted after physical exertion showed the absence of flow in the symptomatic extremity and hyperemia in the contralateral one. When comparing US findings with MRI results, the authors drew attention to an objective character of morphology assessment of the innervated muscles in MRI. Ultrasonography, in turn, enabled a simple and non-invasive functional assessment of muscle perfusion degree (i.e. while resting and after physical exertion). In the author's own study, vascularity of the muscles was not assessed. Nevertheless, this parameter seems to be an important diagnostic criterion which will broaden the author's methods in the future. The investigators decided that the direct assessment of the nerve trunk was not possible, which probably resulted from the unavailability of high-frequency transducers at that period. A similar situation occurred in another study(7). Thanks to using highfrequency transducers, the nerve morphology assessment was one of the criteria for its evaluation in the author's own study and in other contemporary studies(5).
Neuropathy of the superficial branch of the radial nerve (RSNR)
The features of Wartenberg's syndrome, i.e. RSNR neuropathy at the level where it crosses the first extensor compartment, was found in three patients. In one case, it was caused by a ganglion of the retinaculum of this compartment, and in two patients – by a thickened retinaculum in the course of de Quervain's disease.
In one case, the US result was false negative. No nerve pathology was observed, but the patient was operated due to local clinical signs and ineffectiveness of the previous conservative treatment. Intraoperatively, features of RSNR neuropathy were observed. Following the procedure, the symptoms did not subside, and the surgeon had probably misinterpreted the nerve thickening as pathology and not as an anatomic variant. After 11 months, a follow-up US examination showed no change of the RSNR compared to the preoperative image.
In the author's own study, the diameter of the nerve was measured instead of the cross-sectional area since the risk of error was too high with manual contour marking (as with the PIN). The mean diameter of the RSNR was 2.5 mm (0.7–4 mm) in patients with Wartenberg's syndrome. Moreover, all patients with RSNR neuropathy manifested a considerable tenderness to compression with a US transducer.
There are few publications concerning the US image of the RSNR. One of the first studies involved 20 asymptomatic patients; the cross-sectional area of the nerve was measured(55). In two patients, pathological lesions were observed (neuroma and schwannoma). The largest study in terms of the number of patients was presented by the investigators from India in 2010(56). They examined 60 forearms of fresh cadavers and conducted an ultrasound examination, dissection and histological analysis. During the US examinations, the following were assessed: the cross-sectional area of the nerve, number of bundles, nerve trunk vascularization, area of the local adipose tissue and sum of cross-sectional areas of individual bundles. The histological analyses revealed that the number of bundles visible in the US examinations was consistent with the actual situation (6–12). The authors noted that the amount of the adipose tissue in the nerve was greater in older subjects and in the proximal segments of the nerve. The assessment of the adipose tissue area and areas of individual bundles occurred to be unreliable. Due to the small size of nerve and a risk of error, the author, in her own study, did not assess vascularity or bundle structure of the nerve.
Conclusions
High-frequency ultrasonography is a valuable modality in qualifying patients to surgical procedures or conservative treatment of entrapment neuropathies of the peripheral nerves.
- The ultrasound features of peripheral nerve defects which in the author's own study occurred to be the most relevant in terms of qualifying patients for surgical or conservative neuropathy treatment were:
- diameter or cross-sectional area of the nerve at the site of the visualized pathology;
- nerve echostructure, hyperemia;
- presence of instability in a dynamic US examination;
- pain reaction to nerve compression with the transducer.
The results of clinical and surgical verification were consistent with the ultrasound findings in 98.6%. Inconsistency was observed in 2 patients.
Conflict of interests
The author does not report any financial or personal links with other persons or organizations, which might negatively affect the content of this publication and claim authorship rights to this publication.
References
- 1.Buchberger W, Judmaier W, Birbamer G, Lener M, Schmidauer C. Carpal tunnel syndrome: diagnosis with high-resolution sonography. AJR Am J Roentgenol. 1992;159:793–798. doi: 10.2214/ajr.159.4.1529845. [DOI] [PubMed] [Google Scholar]
- 2.Silvestri E, Martinoli C, Derchi LE, Bertolotto M, Chiaramondia M, Rosenberg I. Echotexture of peripheral nerves: correlation between US and histologic findings and criteria to differentiate tendons. Radiology. 1995;197:291–296. doi: 10.1148/radiology.197.1.7568840. [DOI] [PubMed] [Google Scholar]
- 3.Martinoli C. Imaging of the peripheral nerves. Semin Musculoskelet Radiol. 2010;14:461–462. doi: 10.1055/s-0030-1268395. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi S, Martinoli C. Warszawa: Medipage; 2009. Ultrasonografia układu mięśniowo-szkieletowego. Tom I; p. 97. [Google Scholar]
- 5.Jacobson JA, Fessell DP, da Gama Lobo L, Yang LJS. Entrapment neuropathies I: upper limb (carpal tunnel excluded) Semin Musculoskelet Radiol. 2010;14:473–486. doi: 10.1055/s-0030-1268068. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi S, Martinoli C. Warszawa: Medipage; 2009. Ultrasonografia układu mięśniowo-szkieletowego. Tom II. [Google Scholar]
- 7.Martinoli C, Bianchi S, Pugliese F, Bacigalupo L, Gauglio C, Valle M, et al. Sonography of entrapment neuropathies in the upper limb (wrist excluded) J Clin Ultrasound. 2004;32:438–450. doi: 10.1002/jcu.20067. [DOI] [PubMed] [Google Scholar]
- 8.Zanetti M, Ledermann T, Zollinger H, Hodler J. Efficacy of MR imaging in patients suspected of having Morton's neuroma. AJR Am J Roentgenol. 1997;168:529–532. doi: 10.2214/ajr.168.2.9016241. [DOI] [PubMed] [Google Scholar]
- 9.Woertler K. Soft tissue masses in the foot and ankle: characteristics on MR imaging. Semin Musculoskelet Radiol. 2005;9:227–242. doi: 10.1055/s-2005-921942. [DOI] [PubMed] [Google Scholar]
- 10.Rowland LP, editor. Wrocław: Elsevier Urban & Partner; 2008. Neurologia Merritta. Tom I. [Google Scholar]
- 11.Khachi G, Skirgaudes M, Lee WP, Wollstein R. The clinical applications of peripheral nerve imaging in the upper extremity. J Hand Surg Am. 2007;32:1600–1604. doi: 10.1016/j.jhsa.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Ciechomska A, Tomczykiewicz K, Bachta A, Tłustochowicz W. Ultrasonograficzna ocena nerwu pośrodkowego w zespole cieśni nadgarstka. Ultrasonografia. 2004;4:36–41. [Google Scholar]
- 13.Domanasiewicz A, Koszewicz M, Jabłecki J. Porównanie wartości diagnostycznej badań ultrasonograficznego i neurograficznego w zespole cieśni nadgarstka. Neurol Neurochir Pol. 2009;43:433–438. [PubMed] [Google Scholar]
- 14.Szopiński K, Mazurczak-Pluta T. Ultrasonograficzne rozpoznanie zespołu kanału nadgarstka – wartość objawu trójkątnego przekroju poprzecznego nerwu pośrodkowego. Neurol Neurochir Pol. 2011;45:556–560. doi: 10.1016/S0028-3843(14)60122-5. [DOI] [PubMed] [Google Scholar]
- 15.Rzepecka-Wejs L, Multan A, Konarzewska A. Thrombosis of the persistent median artery as a cause of carpal tunnel syndrome – case study. J Ultrason. 2012;12:487–492. doi: 10.15557/JoU.2012.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klauser AS, Faschingbauer R, Bauer T, Wick MC, Gabl M, Arora R, et al. Entrapment neuropathies II: carpal tunnel syndrome. Semin Musculoskelet Radiol. 2010;14:487–500. doi: 10.1055/s-0030-1268069. [DOI] [PubMed] [Google Scholar]
- 17.Kapuścińska K, Urbanik A. Ultrasonograficzna ocena nerwu pośrodkowego w zespole kanału nadgarstka. Przegl Lek. 2013;70:281–285. [PubMed] [Google Scholar]
- 18.Wong SM, Griffith JF, Hui AC, Lo SK, Fu M, Wong KS. Carpal tunnel syndrome: diagnostic usefulness of sonography. Radiology. 2004;232:93–99. doi: 10.1148/radiol.2321030071. [DOI] [PubMed] [Google Scholar]
- 19.Ziswiler HR, Reichenbach S, Vögelin E, Bachmann LM, Villiger PM, Jüni P. Diagnostic value of sonography in patients with suspected carpal tunnel syndrome: a prospective study. Arthritis Rheum. 2005;52:304–311. doi: 10.1002/art.20723. [DOI] [PubMed] [Google Scholar]
- 20.Yesildag A, Kutluhan S, Sengul N, Koyuncuoglu HR, Oyar O, Guler K, et al. The role of ultrasonographic measurements of the median nerve in the diagnosis of carpal tunnel syndrome. Clin Radiol. 2004;59:910–915. doi: 10.1016/j.crad.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Duncan I, Sullivan P, Lomas F. Sonography in the diagnosis of carpal tunnel syndrome. AJR Am J Roentgenol. 1999;173:681–684. doi: 10.2214/ajr.173.3.10470903. [DOI] [PubMed] [Google Scholar]
- 22.Hammer HB, Hovden IA, Haavardsholm EA, Kvien TK. Ultrasonography shows increased cross-sectional area of the median nerve in patients with arthritis and carpal tunnel syndrome. Rheumatology (Oxford) 2006;45:584–588. doi: 10.1093/rheumatology/kei218. [DOI] [PubMed] [Google Scholar]
- 23.Sernik RA, Abicalaf CA, Pimentel BF, Braga-Baiak A, Braga L, Cerri GG. Ultrasound features of carpal tunnel syndrome: a prospective case-control study. Skeletal Radiol. 2008;37:49–53. doi: 10.1007/s00256-007-0372-9. [DOI] [PubMed] [Google Scholar]
- 24.Wiesler ER, Chloros GD, Cartwright MS, Smith BP, Rushing J, Walker FO. The use of diagnostic ultrasound in carpal tunnel syndrome. J Hand Surg Am. 2006;31:726–732. doi: 10.1016/j.jhsa.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 25.Altinok T, Baysal O, Karakas HM, Sigirci A, Alkan A, Kayhan A, et al. Ultrasonographic assessment of mild and moderate idiopathic carpal tunnel syndrome. Clin Radiol. 2004;59:916–925. doi: 10.1016/j.crad.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Pastare D, Therimadasamy AK, Lee E, Wilder-Smith EP. Sonography versus nerve conduction studies in patients referred with a clinical diagnosis of carpal tunnel syndrome. J Clin Ultrasound. 2009;37:389–393. doi: 10.1002/jcu.20601. [DOI] [PubMed] [Google Scholar]
- 27.Banach M, Bogucki A, editors. Kraków: Medycyna Praktyczna; 2003. Zespoły z ucisku – diagnostyka i leczenie. [Google Scholar]
- 28.Żyluk A, Walaszek I, Szlosser Z. No correlation between sonographic and electrophysiological parameters in carpal tunnel syndrome. J Hand Surg Eur Vol. 2014;39:161–166. doi: 10.1177/1753193413489046. [DOI] [PubMed] [Google Scholar]
- 29.Lee D, van Holsbeeck MT, Janevski PK, Ganos DL, Ditmars DM, Darian VB. Diagnosis of carpal tunnel syndrome. Ultrasound versus electromyography. Radiol Clin North Am. 1999;37:859–872. doi: 10.1016/s0033-8389(05)70132-9. [DOI] [PubMed] [Google Scholar]
- 30.Klauser AS, Halpern EJ, De Zordo T, Feuchtner GM, Arora R, Gruber J, et al. Carpal tunnel syndrome assessment with US: value of additional cross-sectional area measurements of the median nerve in patients versus healthy volunteers. Radiology. 2009;250:171–177. doi: 10.1148/radiol.2501080397. [DOI] [PubMed] [Google Scholar]
- 31.Klauser AS, Halpern EJ, Faschingbauer R, Guerra F, Martinoli C, Gabl MF, et al. Bifid median nerve in carpal tunnel syndrome: assessment with US cross-sectional area measurement. Radiology. 2011;259:808–815. doi: 10.1148/radiol.11101644. [DOI] [PubMed] [Google Scholar]
- 32.Sarría L, Cabada T, Cozcolluela R, Martínez-Berganza T, García S. Carpal tunnel syndrome: usefulness of sonography. Eur Radiol. 2000;10:1920–1925. doi: 10.1007/s003300000502. [DOI] [PubMed] [Google Scholar]
- 33.Koyuncuoglu HR, Kutluhan S, Yesildag A, Oyar O, Guler K, Ozden A. The value of ultrasonographic measurement in carpal tunnel syndrome in patients with negative electrodiagnostic tests. Eur J Radiol. 2005;56:365–369. doi: 10.1016/j.ejrad.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Visser LH, Smidt MH, Lee ML. High-resolution sonography versus EMG in the diagnosis of carpal tunnel syndrome. J Neurol Neurosurg Psychiatry. 2008;79:63–67. doi: 10.1136/jnnp.2007.115337. [DOI] [PubMed] [Google Scholar]
- 35.Moran L, Perez M, Esteban A, Bellon J, Arranz B, del Cerro M. Sonographic measurement of cross-sectional area of the median nerve in the diagnosis of carpal tunnel syndrome: correlation with nerve conduction studies. J Clin Ultrasound. 2009;37:125–131. doi: 10.1002/jcu.20551. [DOI] [PubMed] [Google Scholar]
- 36.Pinilla I, Martin-Hervas C, Sordo G, Santiago S. The usefulness of ultrasonography in the diagnosis of carpal tunnel syndrome. J Hand Surg Eur Vol. 2008;33:435–439. doi: 10.1177/1753193408090396. [DOI] [PubMed] [Google Scholar]
- 37.Chiou HJ, Chou YH, Cheng SP, Hsu CC, Chan RC, Tiu CM, et al. Cubital tunnel syndrome: diagnosis by high-resolution ultrasonography. J Ultrasound Med. 1998;17:643–648. doi: 10.7863/jum.1998.17.10.643. [DOI] [PubMed] [Google Scholar]
- 38.Jacob D, Creteur V, Courthaliac C, Bargoin R, Sassus B, Bacq C, et al. Sonoanatomy of the ulnar nerve in the cubital tunnel: a multicentre study by the GEL. Eur Radiol. 2004;14:1770–1773. doi: 10.1007/s00330-004-2401-6. [DOI] [PubMed] [Google Scholar]
- 39.Wiesler ER, Chloros GD, Cartwright MS, Shin HW, Walker FO. Ultrasound in the diagnosis of ulnar neuropathy at the cubital tunnel. J Hand Surg Am. 2006;31:1088–1093. doi: 10.1016/j.jhsa.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Yoon JS, Walker FO, Cartwright MS. Ultrasonographic swelling ratio in the diagnosis of ulnar neuropathy at the elbow. Muscle Nerve. 2008;38:1231–1235. doi: 10.1002/mus.21094. [DOI] [PubMed] [Google Scholar]
- 41.Volpe A, Rossato G, Bottanelli M, Marchetta A, Caramaschi P, Bambara LM, et al. Ultrasound evaluation of ulnar neuropathy at the elbow: correlation with electrophysiological studies. Rheumatology (Oxford) 2009;48:1098–1101. doi: 10.1093/rheumatology/kep167. [DOI] [PubMed] [Google Scholar]
- 42.O'Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. J Bone Joint Surg Br. 1991;73:613–617. doi: 10.1302/0301-620X.73B4.2071645. [DOI] [PubMed] [Google Scholar]
- 43.Yalcin E, Demir SO, Dizdar D, Buyukvural S, Akyuz M. Hypertrophic ancenous epitrochlearis muscle as a cause of ulnar neuropathy at elbow. J Back Musculoskelet Rehabil. 2013;26:155–157. doi: 10.3233/BMR-2012-00360. [DOI] [PubMed] [Google Scholar]
- 44.Dekelver I, Van Glabbeek F, Dijs H, Stassijns G. Bilateral ulnar nerve entrapment by the M. anconeus epitrochlearis. A case report and literature review. Clin Rheumatol. 2012;31:1139–1142. doi: 10.1007/s10067-012-1991-7. [DOI] [PubMed] [Google Scholar]
- 45.Grechenig W, Mayr J, Peicha G, Boldin C. Subluxation of the ulnar nerve in the elbow region – ultrasonographic evaluation. Acta Radiol. 2003;44:662–664. doi: 10.1080/02841850312331287789. [DOI] [PubMed] [Google Scholar]
- 46.Filippou G, Mondelli M, Greco G, Bertoldi I, Frediani B, Galeazzi M, et al. Ulnar neuropathy at the elbow: how frequent is the idiopathic form? An ultrasonographic study in a cohort of patients. Clin Exp Rheumatol. 2010;28:63–67. [PubMed] [Google Scholar]
- 47.Okamoto M, Abe M, Shirai H, Ueda N. Morphology and dynamics of the ulnar nerve in the cubital tunnel: observation by ultrasonography. J Hand Surg Eur Vol. 2000;25:85–89. doi: 10.1054/jhsb.1999.0317. [DOI] [PubMed] [Google Scholar]
- 48.Jacobson JA, Jebson PJL, Jeffers AW, Fessell DP, Hayes CW. Ulnar nerve dislocation and snapping triceps syndrome: diagnosis with dynamic sonography – report of three cases. Radiology. 2001;220:601–605. doi: 10.1148/radiol.2202001723. [DOI] [PubMed] [Google Scholar]
- 49.Bodner G, Harpf C, Meirer R, Gardetto A, Kovacs P, Gruber H. Ultrasonographic appearance of supinator syndrome. J Ultrasound Med. 2002;21:1289–1293. doi: 10.7863/jum.2002.21.11.1289. [DOI] [PubMed] [Google Scholar]
- 50.Chien AJ, Jamadar DA, Jacobson JA, Hayes CW, Louis DS. Sonography and MR imaging of posterior interosseous nerve syndrome with surgical correlation. AJR Am J Roentgenol. 2003;181:219–221. doi: 10.2214/ajr.181.1.1810219. [DOI] [PubMed] [Google Scholar]
- 51.Nakamichi K, Tachibana S. Ultrasonographic findings in isolated neuritis of the posterior interosseous nerve: comparison with normal findings. J Ultrasound Med. 2007;26:683–687. doi: 10.7863/jum.2007.26.5.683. [DOI] [PubMed] [Google Scholar]
- 52.Dong Q, Jamadar DA, Robertson BL, Jacobson JA, Caoili EM, Gest T, et al. Posterior interosseous nerve of the elbow: normal appearances simulating entrapment. J Ultrasound Med. 2010;29:691–696. doi: 10.7863/jum.2010.29.5.691. [DOI] [PubMed] [Google Scholar]
- 53.Djurdjevic T, Loizides A, Löscher W, Gruber H, Plaikner M, Peer S. High resolution ultrasound in posterior interosseous nerve syndrome. Muscle Nerve. 2014;49:35–39. doi: 10.1002/mus.23867. [DOI] [PubMed] [Google Scholar]
- 54.Hide IG, Grainger AJ, Naisby GP, Campbell RS. Sonographic findings in the anterior interosseous nerve syndrome. J Clin Ultrasound. 1999;27:459–464. doi: 10.1002/(sici)1097-0096(199910)27:8<459::aid-jcu7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 55.Visser LH. High-resolution sonography of the superficial radial nerve with two case reports. Muscle Nerve. 2009;39:392–395. doi: 10.1002/mus.21246. [DOI] [PubMed] [Google Scholar]
- 56.Marx SC, Kumar P, SD. Marx CA, Babu MS, Bhat KM. Histological and ultrasonographical study of the human superficial branch of the radial nerve at distal forearm and its clinical implications. Rom J Morphol Embryol. 2010;51:751–758. [PubMed] [Google Scholar]