Abstract
The pathologies of tendon and ligament attachments are called enthesopathies. Enthesitis is one of enthesopathies and it is considered a characteristic sign of rheumatic diseases from the spondyloarthritis group, including peripheral spondyloarthritis. Therefore, enthesitis has been included in a number of clinical classifications for diagnosing these diseases. Clinical diagnosis of enthesitis is based on rather non-specific clinical signs and results of laboratory tests. It is believed that imaging examinations might improve diagnosis, particularly because numerous papers prove that differentiating enthesitis from other enthesopathic processes is possible. On the other hand, a number of authors report the lack of specific signs in imaging as well as typical histological and immunological features that would enable confirmation of clinical diagnosis of enthesitis. The first part of the publication presented theories on the etiopathogenesis of enthesitis (inflammatory, mechanical, autoimmune and associated with the synovio-entheseal complex) as well as on the formation of enthesophytes (inflammatory, molecular and mechanical). This paper – the second part of the article, is a review of the state-of-the-art on the ability of imaging examinations to diagnose enthesitis. It turns out that none of the enthesitis criteria used in imaging examinations is specific for inflammation. As enthesitis may be the only symptom of early spondyloarthritis (particularly in patients with absent HLA-B27 antigen), the lack of its unambiguous picture in ultrasound and magnetic resonance imaging prompts the search for other signs characteristic of spondyloarthritis and more specific features in imaging in order to make a diagnosis as early as possible.
Keywords: enthesopathy, enthesitis, spondyloarthritis, rheumatic diseases, imaging
Abstract
Patologie przyczepów ścięgien i więzadeł są określane mianem entezopatii. Jednym z rodzajów entezopatii jest zapalenie (enthesitis). Uznaje się je za charakterystyczny objaw chorób reumatycznych z grupy spondyloartropatii (spondyloarthritis), w tym głównie spondyloartropatii obwodowych. Z tego powodu enthesitis włączono do szeregu klasyfikacji klinicznych, służących m.in. do rozpoznawania tych chorób. Klinicyści diagnozują enthesitis na podstawie mało specyficznych objawów oraz wyników badań laboratoryjnych. Duże nadzieje na poprawę możliwości diagnostycznych są wiązane z badaniami obrazowymi. Niektóre prace naukowe dowodzą możliwości różnicowania zapalenia entez z innymi procesami entezopatycznymi. Z drugiej strony szereg doniesień wskazuje na brak specyficznych zmian w badaniach obrazowych oraz typowych cech histologicznych i immunologicznych pozwalających na potwierdzenie klinicznego rozpoznania enthesitis. W pierwszej części publikacji przedstawiono teorie etiopatogenezy entezopatii (teorię zapalną, mechaniczną, kompleksu entezy i autoimmunologiczną) oraz koncepcje powstawania entezofitów (zapalną, molekularną i mechaniczną). W niniejszej, drugiej części zaprezentowano zaś przegląd wiedzy na temat możliwości badań obrazowych w rozpoznawaniu enthesitis. Jak się okazuje, żadne z kryteriów enthesitis stosowanych w badaniach obrazowych nie jest specyficzne dla zapalenia. Zważywszy na to, że enthesitis bywa jedynym objawem spondyloartropatii w początkowym okresie (zwłaszcza u chorych z nieobecnym antygenem HLA-B27), brak jednoznacznego obrazu w badaniach ultrasonograficznych i rezonansu magnetycznego wymaga poszukiwania innych objawów charakterystycznych dla spondyloartropatii i bardziej specyficznych markerów w badaniach obrazowych w celu jak najszybszego ustalenia rozpoznania.
Introduction
Enthesitis, an inflammation of the attachments of tendons, ligaments, fasciae or capsule, is considered to be the key sign of one of the most common groups of rheumatic diseases – spondyloarthritis (SpA). Clinical diagnosis of enthesitis is based on rather non-specific clinical signs and symptoms, i.e. medical history and physical examination(1, 2). More and more often, in order to confirm the initial diagnosis, rheumatologists refer patients to an ultrasound examination (or perform it themselves) in which the diagnosis of enthesitis is established on the basis of features that are frequently observed in other enthesopathies as well. This is rather questionable since a number of studies on histopathology, anatomopathology and immunology do not confirm the existence of enthesitis – or this area is not yet sufficiently explored(3).
Enthesopathic lesions in imaging
The imaging features of enthesopathic lesions (also interpreted as enthesitis) are: delaminated tears, presence of vessels, scars after damage to the fibrous part of an enthesis and its adjacent tendon zone, as well as erosions and cysts of its fibrocartilaginous and bony parts(4). Due to the presence of delamination and disordered structure of the collagen fibers, enthesopathically altered entheses are thickened, contain vessels (that take part in the inflammatory-repair and subsequently repair processes), and granulation tissue(5).
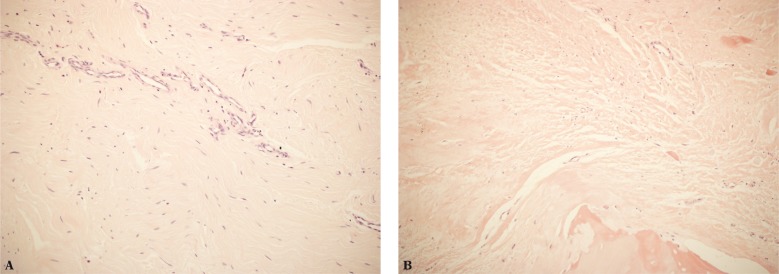
In such cases, histopathological examinations show collagen fiber damage, scars, degenerative changes (including hypocellular areas, hyalinization, lipid infiltration) as well as ruptures and features of repair processes(4, 6) (Fig. 1). Rufai et al.(7) demonstrated the presence of degenerative changes at all three localizations of the fibrocartilage in histopathological examinations of enthesopathically altered Achilles tendon entheses in elderly patients. They observed enthesophytes (in US examination in our practice they are called mineralized scars following past damage) and delamination of the sesamoid, periosteal and ligament FC.
Fig. 1.
A. Fibrous inflammatory granulation tissue in the tendon encompasses the fibrous connective tissue: fibrosis, scarce fibroblasts and slight blood vessels; B. degenerative changes within the tendon at the attachment site with a focal necrosis (lower right corner). H&E stain, 200x
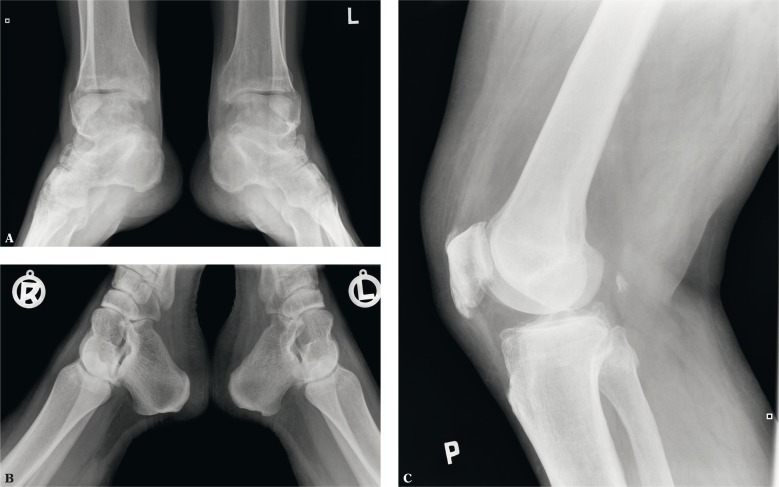
In the initial phase of enthesopathies, radiographs shows osteoporosis of the entheseal bony component and thickening of the soft tissues. In advanced stadia, we observe subchondral sclerosis, enthesophytes and erosions (Fig. 2).
Fig. 2.
X-ray: A. mineralized scar (enthesophyte, so-called lower spur) on the lower surface of the calcaneal tuberosity at the flexor digitorum brevis enthesis, erosions on the medial malleoli with concomitant ossification reactions; B. erosion in the bony part of the Achilles tendon enthesis on the left side; C. mineralized scars in the patellar entheses of the quadriceps femoris tendon and patellar ligament
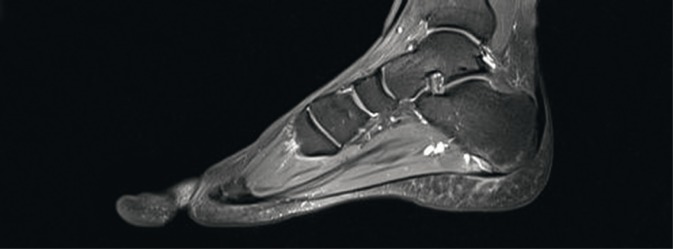
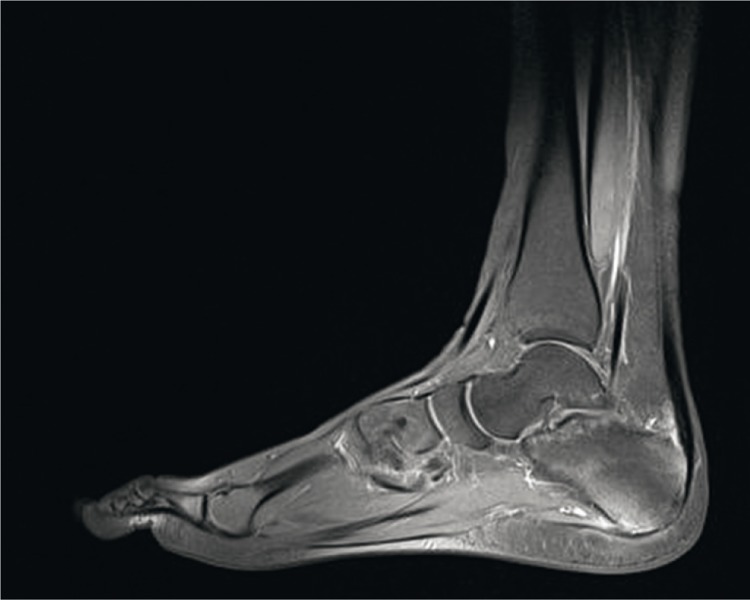
In MRI, the fibrous part of entheses is poorly visible due to low water content. More evident are changes in the tissues adjacent to an enthesis (e.g. those localized in the bursae or adipose tissue). MRI is the only imaging modality that enables identification of bone marrow edema (BME) in the subchondral tissue(8) (Fig. 3).
Fig. 3.
MRI of the foot: bone marrow edema at the bony part of the plantar aponeurosis enthesis, the enthesis is thickened and its adjacent soft tissues are swollen
Enthesopathies in US
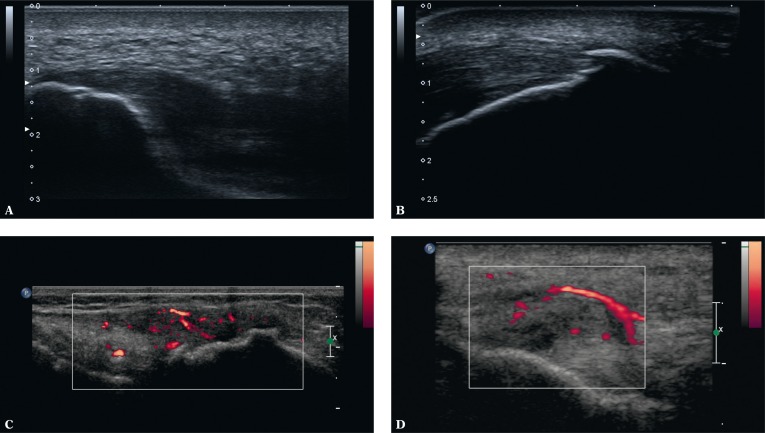
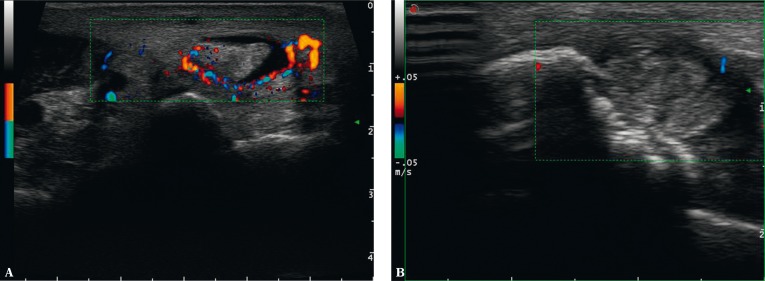
US is the modality of choice in assessing entheses(9–12). The US features that are considered signs of enthesitis in the literature are: reduced echogenicity of an enthesis (due to disordered fibrillar structure), enthesis thickening (due to damage to the collagen fibers), presence of structural lesions, i.e. multidirectional injuries, and scars following such injuries (ranging from those similar in their structure to a tendon or ligament, to hyalinizing, mineralized or ossified ones, i.e. enthesophytes) as well as erosions in the bony component of an enthesis and increased vascularity seen in a Doppler examination(9, 11, 12) (Fig. 4).
Fig. 4.
Enthesopathic lesions in US: A. thickened, hypoechoic enthesis of the plantar fascia with a minor geode in the bony part; B. hypoechoic Achilles tendon enthesis with mineralized scars (enthesophytes) in the fibrous and fibrocartilaginous parts and with scars following delamination damage; C. thickened and hypoechoic tibial enthesis of the iliotibial band due to intratendinous damage with inflammatory-repair process vessels; uneven bony part; D. thickened and hypoechoic humeral enthesis of the common extensor tendon due to intratendinous damage with inflammatory-repair process vessels
The presence of vessels in entheses is considered a sign specific for SpA that confirms enthesitis(10, 13–16). D'Agostino et al.(16) examined 164 patients with SpA and 64 in a control group: 34 with mechanical back pain (MBP) and 30 with rheumatoid arthritis (RA). Vascularized entheses were identified only in SpA patients (81%). Unfortunately, the authors did not specify which entheses were vascularized, i.a. whether vessels were present in the plantar fascia and Achilles tendon entheses, which has not been observed in our practice(17). Nevertheless, they noted signs of bursitis of the Achilles tendon and considered them non-specific for SpA. They did not observe vascularity of the tendon(16), which we did observe in our own studies concerning the Achilles tendon – secondary to bursitis or inflammation of the Kager's fat pad as well as damage to the enthesis and its adjacent tendon zone. In fact, these are the only cases of abnormal vascularity noted in our study in patients with clinically diagnosed calcaneal tuberosity enthesitis(17).
Based on the aforementioned US features in entheses (regarding as enthesitis), the sensitivity and specificity of the US in diagnosing enthesitis were as follows(15): Lehtinen – 37.5% and 92.7%, Balint et al. – 22.5% and 79.7% and D'Agostino – 41% and 65% respectively. The value of the above mentioned criteria has been analyzed by various researchers(18), but the results cannot be compared due to lack of standarised methods. For the majority of authors, the sole presence of enthesophytes (mineralized or ossified scars; bony spurs) is the diagnostic sign of enthesitis. There are few studies which indicate the need for the presence of enthesis’ vascularity as an absolute proof of inflammation. Despite numerous inconsistencies, the outcomes of the studies led to establishing several semi-quantitative (scoring) systems for enthesitis that include, among others, the result of power Doppler US examination (PDUS). Currently, these systems are being validated, although they include the elements of the image of enthesopathically altered entheses that occur in enthesis micro- and partial damage with the same frequency.
Rheumatology publications lacking the term “enthesitis” are extremely rare. They are represented by the study of Spadaro et al.(9) based on large material. The authors examined 432 different entheses, both clinically symptomatic and asymptomatic, in patients with AS (ankylosing spondylitis). The most common findings were enthesophytes (mineralized scars) and calcifications as well as thickened entheses. Erosions were detected in 9.7% of the subjects and hyperemia of entheses – merely in 6%. As for the Achilles tendon enthesis, the most common abnormalities were enthesophytes (31.9%), thickened enthesis (27.7%), its vascularity (8.3%) and tendon's bursitis (13.9%). In the plantar fascia enthesis, the most common signs were its thickening (13.9%) and calcifications (the authors distinguish enthesophytes from calcifications – the former being probable ossifications, the latter – mineralized scars localized in a certain distance from the attachment) as well as reduced echogenicity (9.7%)(17). In the majority of patients with suspected enthesitis, no abnormalities were detected: 63.1% of the Achilles tendon entheses and 60% of the plantar fascia entheses were found normal. Of the remaining entheses altered in a B-mode examination, enhanced vascularity was found in only one calcaneal enthesis of the Achilles tendon (5.3%). Such vascularity was not observed in any case of the plantar aponeurosis.
Saibani et al., Sebes et al. and Genc et al. draw even more direct conclusions(19). They demonstrated that enthesopathic lesions in the lower extremities, such as thickened entheses and erosions, especially enthesophytes, do not enable differentiation between RA and AS and are as often found in healthy individuals. The number of such lesions increases with age as a result of chronic damage and improper remodeling leading to enthesis degeneration.
Similar conclusions were drawn by researchers who analyzed enthesis vascularity which is considered a specific sign of enthesitis in rheumatic diseases(10). PDUS and MRI, conducted in 2011 by Feydy et al.(20), demonstrated that enthesopathic lesions of the Achilles tendon and plantar aponeurosis were not specific for SpA and as often occurred in the the control group, i.e. those with mechanical back pain. This had been previously published by other authors(20). Vessels of the repair process that appear in the course of damage healing process are identical to those in rheumatic diseases. Feydy et al.(20) demonstrated hypervascularity of the Achilles tendon enthesis in merely 5% of patients with SpA and in 6% of controls. As Spadaro et al.(9), they did not find vessels in the plantar fascia enthesis. Vessels in the Achilles tendon enthesis were observed in patients with erosions in the entheseal bony part.
Enthesopathies in MRI
The MRI features of enthesitis are bone marrow edema (BME) of the entheseal bony part as well as edema of entheseal soft tissues and adjacent tissues (Fig. 5). Authors of several studies correlated the MR image with histopathological and immunological examinations of the sacroiliac and spinal joints in the course of axial SpA. At the sites of edema, infiltrates of macrophages as well as B and T lymphocytes were observed(21–23).
Fig. 5.
Bone marrow edema in the bony part of the plantar fascia enthesis and edema of the adjacent soft tissues. Bone marrow edema in the bony part of the Achilles tendon enthesis, increased signal of the soft tissues of the Achilles tendon bursa (bursitis); at the bursal level, the tendon shows intratendinous damage
As for peripheral SpA, older publications indicated that BME was the factor that differentiated spondyloarthritis from other pathologies. McGonagle et al.(25), in their knee joint MRI examinations conducted in 10 patients with SpA and in 10 patients with RA, detected BME in all patients with SpA and in none of the patients with RA. The authors assessed attachments of ligaments and tendons of the knee extensor complex, collateral and cruciate ligaments, joint capsule as well as semitendinosus, semimembranosus and biceps femoris tendons and iliotibial band. In all RA patients, edema of the soft tissues surrounding the enthesis was found, which was the case in less than a half of SpA patients.
Subsequent studies did not confirm that BME is a specific element of enthesitis(24, 26). This sign is observed in the course of posttraumatic, degenerative, neoplastic as well as inflammatory changes, not only associated with SpA(8). Eshed et al.(24) observed BME both in patients with SpA and with RA, however in SpA patients edema was more extensive. A correlation between the changes and the presence of HLAB27 antigen was noticed. Benjamin et al.(26) report that BME occurs in approximately 50% of cases of enthesitis.
Bursitis rather than enthesitis?
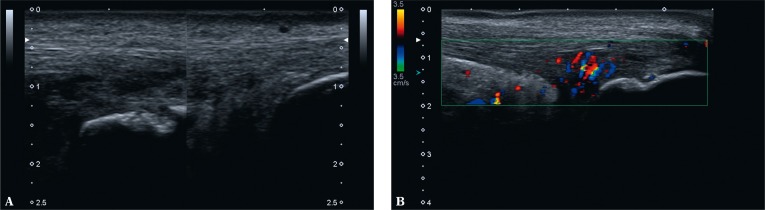
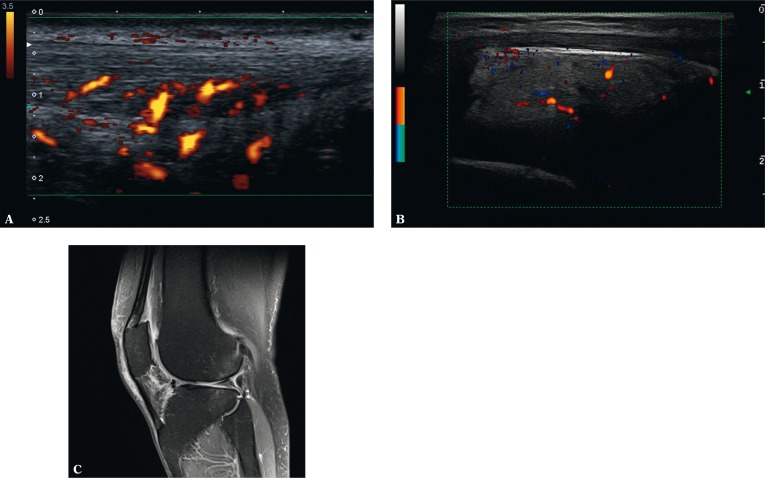
Enthesitis-like lesions in the course of rheumatic diseases are usually found in the calcaneal tuberosity. Based on the clinical examination, it is difficult to determine the origin of pain in the plantar side (i.a. attachment of the plantar aponeurosis, tendon of the flexor digitorum brevis, plantar fat pad rupture or medial plantar nerve irritation) or its posterosuperior aspect. In the latter case, a frequent cause of clinically painful enthesis of the Achilles tendon is the tendon's bursitis, which occurs in patients with RA, SpA, crystalopathies and those with overload changes in the joint and tendon(27). In such situations, we observe effusion in the bursa as well as thickening and increased vascularity of the synovium(27). Additionally, in the context of Achilles tendon bursitis, one may detect erosions in the osseous wall of the bursa and inflammatory vessels infiltrating the tendon zone adjacent to the enthesis (Fig. 6). Equal changes may be found in the other entheses and tendons adjacent to bursae (Fig. 7).
Fig. 6.
Bursitis of the left Achilles tendon: A. B-mode examination: on the left: effusion, thickened bursal synovium and erosions in the bony wall of the bursa (bursitis), intratendinous damage to the Achilles tendon; on the right: normal image; B. the same case in color Doppler examination: inflammatory-repair vessels permeate within the tendon; enhanced vascularity of the Kager's adipose tissue indicates an inflammatory reaction
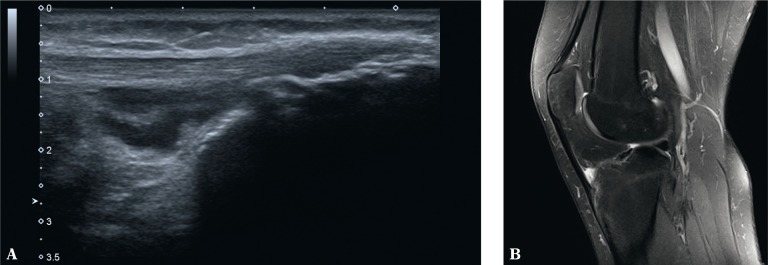
Fig. 7.
US (A) and MRI (B) of the knee joint: A. effusion and thickened synovium of the deep infrapatellar bursa with erosions in the bony wall of the bursa and in the bony part of the tendon enthesis; B. MRI additionally shows bone marrow edema in the bony part of the enthesis
According to the theories on the etiopathogenesis of SpA(1), US and MRI examinations confirmed that the inflammatory process may begin beyond entheses, i.e. in the synovium of the neighboring bursa (bursitis) where it is triggered (according to the mechanical theory) by enthesis microdamage (fig. 8), in the synovium of the tendon sheath (tenosynovitis, tendovaginitis) or in the peritendineum (according to the functional enthesis theory) as a response to a strain, e.g. pressing the tibialis posterior muscle on the medial malleolus(1, 25) (Fig. 9). In MRI, Olivieri et al.(28) observed features of achillobursitis calcanei (inflammation of the bursa and Achilles tendon) in 74% of patients with SpA.
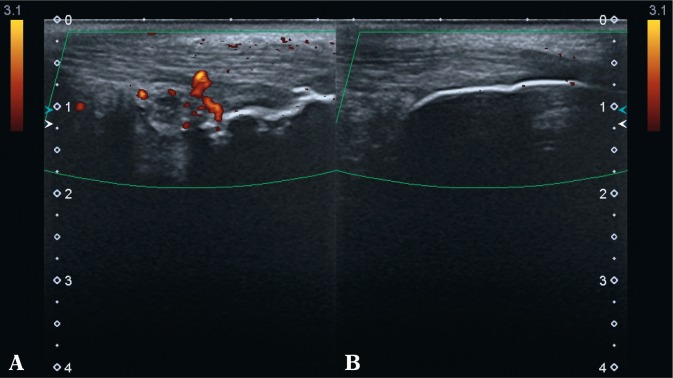
Fig. 8.
PDUS: A. on the left – active inflammatory changes in the bursa of the Achilles tendon with erosions, including the active ones in the bony wall of the bursa; mineralized scar in the Achilles tendon enthesis; B. on the right – minor effusion in the bursa and rounded apex of the bursal fat fold
Fig. 9.
A, B. Color Doppler US (CDUS) in a patient with RA: inflammation of the tendon and sheath of the tibialis posterior tendon with erosions of the medial malleolus
Taking into account the range of tissues involved in the course of SpA, Benjamin and McGonagle proposed that the definition of an enthesis should be extended to a synovio- entheseal complex (SEC)(29). The concept of SEC assumes that entheses, adjacent synovial membrane and adipose tissue form a functional unit. The authors claim that this concept may concern approximately 82% of entheses since they are all adjacent to synovia and fat pads(30). Inflammation is probably triggered by enthesis microdamage: by activating the immune system, it initiates an inflammatory reaction in the synovium of the bursal walls or in the overlaying adipose tissue(9, 30). The SEC theory seems to match with the biomechanical enthesis theory(1) and concerns numerous entheses that are subject to considerable mechanical stress, which is indicated by the presence of fibrocartilage. The SEC concept may also explain inflammation of numerous joints involved in SpA, such as sacroiliac, sternocostal, sternoclavicular, acromioclavicular and temporomandibular, covered with fibrocartilage and being subject to considerable mechanical shear stress.
Benjamin M et al.(30) performed histopathological examinations of 49 entheses of 60 cadavers. The clinical data were unavailable. The authors detected features of degeneration in 76% of cases. Synovial inflammatory changes of SECs were noted in 85% of cases (including 57% of Achilles tendon entheses). Enthesitis of the Achilles tendon was found in 73% of cases with prevalent lymphocyte and macrophage infiltration and vessels within a dense fibrous part of entheses, in the endotendon and endoligament (i.e. within loose connective tissue between fiber bundles), sometimes in the sesamoid or periosteal FC as well as in the adipose tissue in the vicinity of entheses (e.g. Hoffa's fat pad and in the region of the flexor hallucis longus tendon)(30). Vessels penetrated the tendon from the side of the bursal synovium. Moreover, the authors identified the presence of vessels, probably originating from the bone marrow, in entheses that do not fulfill the criteria of SEC (i.e. are not adjacent to bursae)(1, 30). They also demonstrated the presence of inflammatory cells (macrophages) in normal entheses. In the case of damage, they probably can adopt an alternative phenotype – anti-inflammatory one, and participate in tissue repair processes(30).
Benjamin and McGonagle are not the only authors who emphasize endocrine and paracrine relevance of adipose tissue associated among others with entheses. This is relevant since adipocytes secrete growth factors and proinflammatory cytokines to the joint cavity(31, 32). Due to rich vascularity and innervation, adipose tissue is susceptible to inflammation, which might develop secondary to enthesis damage. This is manifested by pain(14, 33) which in turn leads rheumatologist to diagnose enthesitis. In the case of the Achilles tendon, nociceptors may be stimulated by effusion in the bursa that compresses against the Kager's fat fold. Inflamed adipose tissue has increased signal in T2-weighted or FLASH sequences in MRI. In US, such tissues are characterized by increased echogenicity and sometimes can be observed dilated vessels resulting from the inflammatory process (Fig. 10).
Fig. 10.
PDUS (A), CDUS (B), MRI (C). A. edema and hyperemia of the Kager's adipose tissue whose inflammatory-repair vessels take part in the repair process of the damaged Achilles tendon; B. edema and hyperemia of the Hoffa's fat pad; C. edema of the Hoffa's fat pad. No edema in the patellar entheses
Some authors accept the SEC theory, others do not. The latter refer to the definition of an enthesis which evidently separates enthesopathic lesions from tendinopathy, bursitis or inflammation of both intra-articular and peri-articular adipose tissues(8).
Conclusion
Gandjbakhch et al.(34) analyzed the literature concerning enthesitis from the period of 1985–2010 available in the PubMed and Embase. The most common criteria of enthesitis in the US examinations were: thickened entheses, its hypoechogenicity, presence of enthesophytes, irregularity of bony part of the entheses and erosions as well as bursitis adjacent to tendons. None of these criteria is specific for inflammation and they may originate from chronic damage and degeneration of entheses, and what is more bursitis is a different pathology. In 2003, increased vascularity of entheses started to be considered as a specific sign of enthesitis, which was confirmed by other authors(34). At the same time, inflammatory vessels were also detected in bursae and tendons. It was proved that the presence of vessels in entheses differentiated enthesitis from enthesis degeneration or mechanical damage and that it was specific to SpA, but numerous authors questioned the specificity of this criterion(10).
Based on the aforementioned features of the US image, clinical indices and scoring systems have been created and used mainly to monitor the efficacy of treatment: Madrid Sonographic Enthesis Index – MASEI, Glasgow Ultrasound Enthesitis Scoring System – GUESS and others(1). It seems, however, that they should be wisely used since they are based on criteria that are non-specific to enthesitis.
There is a possibility that enthesitis is a sign of peripheral SpA, which is demonstrated by the theories on the etiopathogenesis of enthesitis presented in the first part of the article, such as genetic or autoimmune ones(1). As enthesitis may be the only symptom of early SpA (particularly in HLA-B27 antigen-negative patients), the lack of its unambiguous picture in US and MRI still requires clinicians’ attention as well as continuing the search for other signs characteristic of SpA and more specific markers in imaging in order to make a diagnosis as early as possible.
The final diagnosis is significant for the selection of treatment method, which in case of enthesopathy is based on steroids. The presence of posttraumatic or degenerative changes is an indication for treatment with non-steroidal anti-inflammatory drugs. Administration of steroids, which combat repair processes, will bring short-lasting relief, but will also aggravate tissue damage processes, and consequently it will put patients at risk of severe damage to the entheseal region(4).
Conflict of interest
The authors do not report any financial or personal links with other persons or organizations, which might affect negatively the content of this publication or claim authorship rights to this publication.
References
- 1.Sudoł-Szopińska I, Kwiatkowska B, Prochorec-Sobieszek M, Maśliński W. Enthesopathies and enthesitis. Part 1. Etiopathogenesis. J Ultrason. 2015;15:72–84. doi: 10.15557/JoU.2015.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balint PV, Kane D, Wilson H, McInnes IB, Sturrock RD. Ultrasonography of entheseal insertions in the Lower limb in spondyloartropathy. Ann Rheum Dis. 2002;61:905–910. doi: 10.1136/ard.61.10.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldring SR. Osteoimmunology and Bone Homeostasis: Relevance to Spondyloarthritis. Curr Rheumatol Rep. 2013;15:342. doi: 10.1007/s11926-013-0342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czyrny Z. Sonographic and histological appearance of heel enthesopathy, what the „heel spurs” really are and what are their consequences. J Orthop Trauma Surg Rel Res. 2010;2:23–36. [Google Scholar]
- 5.Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: attachment sites („entheses”) in relation to exercise and/or mechanical load. J Anat. 2006;208:471–490. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin M, McGonagle D. The anatomical basis for disease localization in seronegative spondyloarthropathy at entheses and related sites. J Anat. 2001;199:503–526. doi: 10.1046/j.1469-7580.2001.19950503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments – an adaptation to compressive load. J Anat. 1998;193:481–494. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francois RJ, Braun J, Khan MA. Entheses and enthesitis: a histopathologic review and relevance to spondyloarthritides. Curr Opin Rheumatol. 2001;13:255–264. doi: 10.1097/00002281-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Spadaro A, Iagnocco A, Perrotta FM, Modesti M, Scarno A, Valesini G. Clinical and ultrasonography assessment of peripheral enthesitis in ankylosing spondylitis. Rheumatology. 2011;50:2080–2086. doi: 10.1093/rheumatology/ker284. [DOI] [PubMed] [Google Scholar]
- 10.Maffulli N, Kader D. Tendinopathy of tendo Achillis. Tendinopathy of tendo Achillis. J Bone Joint Surg. 2002;84:1–8. doi: 10.1302/0301-620x.84b1.12792. [DOI] [PubMed] [Google Scholar]
- 11.Czyrny Z. Diagnostic anatomy and diagnostics of enthesal pathologies of the rotator cuff. J Ultrason. 2012;12:178–187. doi: 10.15557/JoU.2012.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dębek A, Nowicki P, Czyrny Z. Ultrasonographic diagnostics of pain in the lateran cubital compartment and proximal forearm. J Ultrason. 2012;12:188–201. doi: 10.15557/JoU.2012.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Agostino MA. Enthesitis. Best Pract Res Clin Rheumatol. 2006;20:473–486. doi: 10.1016/j.berh.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 14.D'Agostino MA, Aegerter P, Bechara K, Salliot C, Judet O, Chimenti MS, et al. How to diagnose spondyloarthritis early? Accuracy of peripheral enthesitis detection by power doppler ultrasonography. Ann Rheum Dis. 2011;70:1433–1440. doi: 10.1136/ard.2010.138701. [DOI] [PubMed] [Google Scholar]
- 15.Balint PV, Kane D, Wilson H, McInnes IB, Sturrock RD. Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis. 2002;61:905–910. doi: 10.1136/ard.61.10.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Agostino MA, Said-Nahal R, Hacquard-Bouder C, Brasseur JL, Dougados M, Breban M. Assessment of peripheral enthesitis in the spondyloarthropathies by ultrasonogfraphy combined with power Doppler: a cross-sectional study. Arthritis Rheum. 2003;48:523–533. doi: 10.1002/art.10812. [DOI] [PubMed] [Google Scholar]
- 17.Sudoł-Szopińska I, Zaniewicz-Kaniewska K, Kwiatkowska B. Spectrum of ultrasound pathologies of Achilles tendon, plantar aponeurosis and flexor digiti brevis entheses in patients with clinically suspected enthesitis. Pol J Radiol. 2014;79:402–408. doi: 10.12659/PJR.890803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Agostino MA, Palazzi C, Olivieri I. Entheseal involvement. Clin Exp Rheumatol. 2009;27(Suppl. 55):S50–S55. [PubMed] [Google Scholar]
- 19.Genc H, Cakit BD, Tuncbilek I, Erdem HR. Ultrasonographic evaluation of tendons and enthesal sites in rheumatoid arthritis: comparison with ankylosing spondylitis and healthy subjects. Clin Rheumatol. 2005;24:272–277. doi: 10.1007/s10067-004-0997-1. [DOI] [PubMed] [Google Scholar]
- 20.Feydy A, Lavie-Brion MC, Gossec L, Lavie F, Guerini H, Nguyen C, et al. Comparative study of MRI and power Doppler ultrasonography of the heel in patients with spondyloarthritis with and without heel pain and in controls. Ann Rheum Dis. 2012;71:498–503. doi: 10.1136/annrheumdis-2011-200336. [DOI] [PubMed] [Google Scholar]
- 21.Hermann KG, Baraliakos X, van der Heijde DM, Jurik AG, Landewé R, Marzo-Ortega H, et al. Descriptions of spinal MRI lesions and definition of a positive MRI of the spine in axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI study group. Ann Rheum Dis. 2012;71:1278–1288. doi: 10.1136/ard.2011.150680. [DOI] [PubMed] [Google Scholar]
- 22.Bollow M, Fischer T, Reisshauer H, Backhaus M, Sieper J, Hamm B, et al. Quantitative analyses of sacroiliac biopsies in spondyloarthropathies: T cells and macrophages predominate in early and active sacroiliitiscellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann Rheum Dis. 2000;59:135–140. doi: 10.1136/ard.59.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appel H, Loddenkemper C, Grozdanovic Z, Ebhardt H, Dreimann M, Hempfing A, et al. Correlation of histopathological findings and magnetic resonance imaging in the spine of patients with ankylosing spondylitis. Arthritis Res Ther. 2006;8:R143. doi: 10.1186/ar2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eshed I, Bollow M, McGonagle D, Tan AL, Althoff CE, Asbach P, et al. MRI of enthesitis of the appendicular skeleton in spondyloarthritis. Ann Rheum Dis. 2007;66:1553–1559. doi: 10.1136/ard.2007.070243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGonagle D, Gibbon W, O'Connor P, Green M, Pease C, Emery P. Characteristic magnetic resonance imaging entheseal changes of knee synovitis in spondyloarthropathy. Arthritis Rheum. 1998;41:694–700. doi: 10.1002/1529-0131(199804)41:4<694::AID-ART17>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Benjamin M, McGonagle D. Entheses, enthesitis and enthesopathy. ARC, Topical Reviews. 2009;4:1–6. [Google Scholar]
- 27.Pierre-Jerome C, Moncayo V, Terk M. MRI of the Achilles tendon: a comprehensive review of the anatomy, biomechanics, and imaging of overuse tendinopathies. Acta Radiol. 2010;51:438–454. doi: 10.3109/02841851003627809. [DOI] [PubMed] [Google Scholar]
- 28.Olivieri I, Barozzi L, Padula A, De Matteis M, Pierro A, Cantini F, et al. Retrocalcaneal bursitis in spondyloarthropathy: assessment with ultrasonography and magnetic resonance imaging. J Rheumatol. 1998;25:1352–1357. [PubMed] [Google Scholar]
- 29.Benjamin M, McGonagle D. The enthesis organ concept and its relevance to the spondyloarthropathies. In: Lopez-Larrea C, Diaz-Pena R, editors. Molecular mechanisms of spondyloarthropathies. New York: Springer-Science + Business Media LLC; 2009. pp. 57–70. [DOI] [PubMed] [Google Scholar]
- 30.Benjamin M, McGonagle D. Histopathologic changes at „synovio-entheseal complexes” suggesting a novel mechanizm for synovitis in osteoarthritis and spondylarthritis. Arthritis Rheum. 2007;56:3601–3609. doi: 10.1002/art.23078. [DOI] [PubMed] [Google Scholar]
- 31.Sudoł-Szopińska I, Kontny E, Zaniewicz-Kaniewska K, Prohorec-Sobieszek M, Saied F, Maśliński W. Role of inflammatory factors and adipose tissue in pathogenesis of rheumatoid arthritis and osteoarthritis. Part I: Rheumatoid adipose tissue. J Ultrason. 2013;13:192–201. doi: 10.15557/JoU.2013.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sudoł-Szopińska I, Hrycaj P, Prohorec-Sobieszek M. Role of inflammatory factors and adipose tissue in pathogenesis of rheumatoid arthritis and osteoarthritis. Part II: Inflammatory background of osteoarthritis. J Ultrason. 2013;13(54):319–328. doi: 10.15557/JoU.2013.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benjamin M, Redman S, Buttner A, Amin A, Moriggl B, Brenner E, et al. Adipose tissue at entheses: the rheumatological implications of its distribution. A potential site of pain and stress dissipation? Ann Rheum Dis. 2004;63:1549–1555. doi: 10.1136/ard.2003.019182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gandjbakhch F, Terslev L, Joshua F, Wakefield RJ, Naredo E, D'Agostino MA. Ultrasound in the evaluation of enthesis: status and perspectives. Arthritis Res Ther. 2011;13:R188. doi: 10.1186/ar3516. [DOI] [PMC free article] [PubMed] [Google Scholar]