Abstract
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) has a lifetime prevalence of 14% and is the most common urological diagnosis for men under the age of 50, yet it is the least understood and studied chronic pelvic pain disorder. A significant subset of patients with chronic pelvic pain report having experienced early life stress or abuse, which can markedly affect the functioning and regulation of the hypothalamic-pituitary-adrenal (HPA) axis. Mast cell activation, which has been shown to be increased in both urine and expressed prostatic secretions of CP/CPPS patients, is partially regulated by downstream activation of the HPA axis. Neonatal maternal separation (NMS) has been used for over two decades to study the outcomes of early life stress in rodent models, including changes in the HPA axis and visceral sensitivity. Here we provide a detailed protocol for using NMS as a preclinical model of CP/CPPS in male C57BL/6 mice. We describe the methodology for performing NMS, assessing perigenital mechanical allodynia, and histological evidence of mast cell activation. We also provide evidence that early psychological stress can have long-lasting effects on the male urogenital system in mice.
Keywords: Medicine, Issue 102, Stress, chronic prostatitis, chronic pelvic pain, mice, mast cell, allodynia, hypothalamic-pituitary-adrenal axis
Introduction
Chronic pelvic pain is not in itself a disease, but rather a term associated with the ongoing spontaneous and/or evoked pain experienced by patients diagnosed with irritable bowel syndrome (IBS), interstitial cystitis/painful bladder syndrome (IC/PBS), vulvodynia, or chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS). These syndromes are often comorbid and share many characteristics in that they have no associated pathology or identified underlying etiology, although dysfunction within the immune system, central nervous system, and peripheral nervous system has been shown to contribute towards the maintenance and progression of these disorders1-3. Patients with chronic pelvic pain are more likely to present with symptoms of additional, non-pelvic-related functional pain disorders and mood disorders, including anxiety, depression, and panic disorder4-6, which has been associated with altered functioning of the hypothalamic-pituitary-adrenal (HPA) axis7-10. Exposure to early life stress or trauma is a significant risk factor for developing HPA abnormalities and associated chronic pain syndromes10,11 and, as such, a significant subset of patients with functional pelvic pain disorders report having experienced adverse childhood events such as abuse or neglect12-14.
Rodent models of neonatal maternal separation (NMS), which involves removing the pups from their dam for a set period of time during the preweaning period, have been used for the past two decades to study the outcomes of early life stress. In general, NMS has been shown to increase HPA axis activation, and resultant anxiety-like behaviors, by directly affecting gene expression within the hypothalamus, as well as disrupting downstream regulation from limbic structures15-18. Disruption of proper HPA axis functioning has been shown to contribute towards increased colorectal19-22 and vaginal16 sensitivity displayed by rodent NMS models, but despite extensive characterization of the long-term effect of postnatal bladder inflammation23-25, the impact of early life stress has largely gone unstudied in the urogenital organs. Therefore, the following study will describe how to perform NMS in mice and later evaluate perigenital mechanical sensitivity and mast cell infiltration/activation in the prostate to validate the use of male NMS mice as a preclinical model for CP/CPPS.
Of all of the diagnosed chronic pelvic pain disorders, CP/CPPS is perhaps the least well-recognized and characterized syndrome, despite having a lifetime prevalence of approximately 14% 26 and annual patient costs estimated at $4,400 (twice that of low back pain or rheumatoid arthritis27). Patients with CP/CPPS report pain in the perineum, rectum, prostate, penis, testicles, and/or abdomen28, experience a higher degree of psychological stress than control patients29, and commonly present with symptoms of or are diagnosed with comorbid chronic pelvic pain or mood disorders5,29-31. Recurrent infection, leaky epithelium, neurogenic inflammation, and autoimmunity have all been surmised as potential underlying causes of CP/CPPS2,32,33, as well as mast cell activation and degranulation34. Expressed prostatic secretions from men with CP/CPPS had increased mast cell tryptase and nerve growth factor (NGF) levels34, and a later study confirmed that tryptase and carboxypeptidase A (CPA3), a marker of mast cell activation, were also increased in the urine of CP/CPPS patients35. The potential role for mast cells in the onset and maintenance of CP/CPPS has been a major focus of animal research on this syndrome thus far. The most commonly employed rodent model used to study CP/CPPS is an experimental autoimmune prostatitis (EAP) model generated by subcutaneous injection of prostate antigen in Complete Freund’s adjuvant, which results in varied degrees of prostatic inflammation depending on species and strain used34,36-39. Mast cell infiltration and activation/degranulation has been shown to increase following induction of EAP34,35,40. Transgenic mice deficient in either mast cells34 or the tryptase receptor PAR235 do not develop prostatic tactic sensitivity following EAP, unlike wildtype EAP mice. While this preclinical model replicates many of the characteristics of human CP/CPPS, the induction protocol is not indicative of the human condition, which has a diverse etiology and often does not involve direct inflammation, infection, or injury of the prostate.
The influence of early life stress on the development of CP/CPPS in humans has largely gone uninvestigated; however, a study by Hu, et al.41, demonstrated that men who reported a history of childhood physical, emotional, and/or sexual abuse were significantly more likely to experience symptoms suggestive of CP/CPPS. Furthermore, they showed that both pain and urinary scores were increased in patients with a history of physical and emotional abuse. We have previously demonstrated that the same NMS paradigm in female C57BL/6 mice produces vaginal hypersensitivity and abnormal gene expression in both the vagina and bladder suggestive of dysfunctional HPA axis output16. This evidence combined with the high prevalence of CP/CPPS patients presenting with other comorbidities42, including IC/PBS and mood disorders, that have more clearly been shown to be linked with early life stress exposure12-14, provides the rationale for using an NMS model to investigate CP/CPPS in mice.
Protocol
All experiments described in this protocol conform to NIH guidelines in accordance with the guidelines specified by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
1. Neonatal Maternal Separation (NMS)
Monitor pregnant dams daily for litter births. Note: The day the litter is born is considered P0.
On P1, remove the dam from the home cage and place into a clean secondary container.
Rub a handful of bedding between clean-gloved hands to maintain scent of the home cage.
Add a depth of 1 - 2 cm of the home cage bedding into a clean, appropriately labeled 2 L glass beaker. Add approximately half of any additional enrichment bedding material that is present in the home cage, e.g., nestlet, crinkle paper, to the glass beaker.
Gently place all of the pups from a single litter, individually, in the same beaker.
Immediately place the beaker in an incubator held at 33 oC and 50% humidity for 180 min.
Remove the dam from the secondary container and return her to the home cage. Return the home cage to its appropriate housing location within the vivarium.
Repeat this procedure for each litter undergoing NMS, using clean gloves and secondary containers for each litter/dam.
At the end of the 180 min separation period, return the litters to their home cages in the same order as they were removed.
Retrieve the home cage of the first litter that was separated earlier in the day. Remove the dam from the home cage and place into a clean secondary container.
Remove the first beaker from the incubator and rub a handful of bedding between clean gloved hands to maintain scent of the home cage while handling the pups.
Gently move the pups, individually, from the beaker to the home cage.
Return any enrichment and bedding remaining in the beaker to the home cage.
Return the dam from the secondary container to the home cage and return it to its appropriate housing location within the vivarium.
Rinse the beaker with water and return it to the incubator. Use the same beaker for each litter throughout the separation procedure.
Repeat this procedure for each litter undergoing NMS in the same order as they were placed in the incubator.
Repeat this entire procedure for each litter undergoing NMS every day through P21.
Wean NMS and naïve pups on P22 by placing littermates of the same sex together in clean cages complete with food and water. Naïve pups should be born and housed under the same conditions as NMS mice, but undergo no additional handling outside of normal animal husbandry tasks.
2. Perigenital Mechanical Sensitivity
- von Frey Apparatus Setup
- Bring the mice to the testing room and allow them to acclimate for 30 min while remaining in their home cages.
- Prepare the testing area by placing an absorbent underpad beneath an elevated wire mesh-top table (79 cm x 28 cm) that provides enough space to allow the investigator to approach from the underside without startling the mice, approximately 55 cm in height.
- Place up to 12 mice, individually, under clear, perforated plastic chambers (11 cm x 5 cm x 3.5 cm) on top of the wire mesh table. Place a heavy object on top of the chambers to prevent mice from escaping.
- Acclimate the mice for an additional 30 min on the screen prior to withdrawal threshold assessment.
- Perigenital Mechanical Sensitivity Assessment
- Perform the up-down method using a standard set of graded von Frey monofilaments43. Use the following monofilaments: 1.65 g, 2.36 g, 2.83 g, 3.22 g, 3.61 g, 4.08 g, 4.31 g, and 4.74 g.
- Hold the base of the 3.22 g monofilament and expose the retracted monofilament. Note: Some models may not have a retracted monofilament.
- Situate the probe so that the monofilament is vertically oriented and when the mouse is alert, immobile, and its body weight is distributed evenly between the hind paws apply it to either the left or right side of the scrotum, avoiding the midline, until a slight bend is observed in the filament.
- Hold the monofilament in place for 10 sec or until the animal shows a positive response. Note: A positive response is considered to be a brisk jerk or jump in response to monofilament application or licking or biting behavior directed towards the monofilament. If the mouse moves during the 10 sec monofilament application without displaying these behaviors, the monofilament must be retested following a minimum 1 min-long rest period. If a mouse neither moves nor exhibits any of above listed behaviors during the 10 sec monofilament application, it is considered a negative response.
- Record the response, either negative or positive, in a lab notebook or laptop and repeat this procedure on all of the remaining mice using the 3.22 g monofilament.
- Test all of the mice again using the next appropriate monofilament. Note: If the mouse exhibited a negative response to the 3.22 g monofilament, apply the 3.61 g monofilament in the same manner and record the response. If the mouse exhibited a positive response to the 3.22 g monofilament, apply the 2.83 g monofilament and record the response.
- Continue testing each mouse using the next larger or smaller monofilament, as appropriate, for an additional 4 applications after the first positive response is observed.
- Return mice to home cages.
- Use the value of the final von Frey monofilament applied in the trial series in log units to calculate a 50% g withdrawal threshold as described in Chaplan et al.43.
3. Acidified Toluidine Blue Mast Cell Staining
- Tissue Processing
- Dissect prostate tissue44 from mice that have been intracardially perfused with ice-cold 4% paraformaldehyde45. Postfix the tissue in 4% paraformaldehyde for 1 hr at RT and then cryoprotect in 30% sucrose in 1x PBS at 4 °C O/N.
- Prepare a small (30 ml) bath of heptane and place it on dry ice.
- Rinse the prostate tissue in 1x PBS and dry using a light duty wipe.
- Insert the prostate tissue into the cold heptane until it is frozen.
- Immediately place the frozen prostate tissue in a cryomold containing mounting media and place on dry ice until frozen.
- Store cryomolds at -20 °C.
- Transversely cut the prostate tissue into 7 µm-thick cryosections using a cryostat.
- Place 8 - 12 non-serial cryosections that span the length of the tissue, onto positively-charged microscope slides.
- Store finished slides at -20 °C until further processing.
- Preparing Acidified Toluidine Blue
- Add 1 g of Toluidine Blue O to 100 ml 70% ethanol and vortex until in solution to prepare a 1% stock solution. The stock solution can be stored at RT for up to three weeks.
- Add 1 g NaCl to 100 ml water in a beaker placed on top of a stir plate.
- Adjust the pH of the NaCl solution using 12 M HCl until a pH range of 0.5 - 1.0 is achieved.
- Prepare the 0.25% Toluidine Blue working solution by combining 8 ml of the Toluidine Blue stock solution and 32 ml of the 1% NaCl solution. Pipette up and down to ensure total incorporation of the Toluidine Blue stock solution and mix using a vortex. The working solution must be made fresh and discarded after using.
- Staining Prostate Cryosections
- Remove the slides to be processed from the freezer and allow to equilibrate to RT for approximately 30 min.
- Wash the tissue sections by individually dipping the slides for approximately 1 sec into a 50 ml conical tube containing a sufficient volume of 1x PBS to ensure that the slide-mounted tissue sections will be completely submerged. Allow slides to air dry on a paper towel.
- Place slides in a glass staining jar containing enough 0.25% working Toluidine Blue solution to ensure that the slide-mounted tissue sections will be completely submerged and incubate for 10 - 15 min while on a platform rocker set at 15 rpm.
- Dip slides in 1x PBS for approximately 1 sec to wash out excess Toluidine Blue.
- Place slides in a slide box or rack in such a way that the slides are on their sides to allow for drainage.
- Dry the slides in an oven at 37 °C for a minimum of 2 hrs or O/N at RT.
- Dehydrate slides quickly using alcohol, allowing the slides to completely dry between alcohol exposures. Dry slides by draining on a paper towel and wiping backs and edges with a light duty wipe.
- Immerse the slides in 95% EtOH for approximately 1 sec and allow to completely dry. Repeat this procedure 1 - 10 times until tissue dries more blue than purple.
- Immerse the slides in 100% EtOH for approximately 1 sec and allow to completely dry. Repeat this procedure 1 - 10 times until tissue is dark to medium blue, but not purple.
- Fix the stain by incubating the slides in 100% xylene for 3 min. Allow to dry.
- Coverslip the slides with glycerol, PBS, or xylene-based permanent mounting media. Drain excess media and store at RT.
- Quantifying Mast Cells
- Visualize the toluidine blue-stained tissue sections at 20X magnification using light microscopy (Figure 2).
- Count the total number of mast cells present within the visualized area and differentiate between non-degranulated and degranulated mast cells. 40X magnification may be necessary to confirm granulation status in some mast cells. Note: Non-degranulated mast cells exhibit dense metachromasia with no or faint nuclear outline and/or no granular extrusion around the cell. Degranulated mast cells exhibit less intense metachromasia and have an obvious clear outline of the nucleus and/or free granules within the cytoplasm and outside of the cell border.
- Continue to assess the total number of non-degranulated and degranulated mast cells in the entirety of the current tissue section. Repeat this procedure on at least 7 additional separate sections, spanning the length each tissue.
- Calculate the percentage of degranulated (DG) to total mast cells for each tissue/mouse according to the following equation: (DG mast cells / Total mast cells) x 100.
Representative Results
Mice that have undergone NMS showed behavioral evidence indicative of CP/CPPS. When tested with a graded series of von Frey monofilaments, 8 week-old NMS mice (n = 4) displayed perigenital mechanical allodynia when compared to naïve counterparts (n = 5, Figure 2). This is evidenced as a significant reduction (p < 0.01; Student’s t-test) in the mechanical withdrawal threshold that elicited a positive behavioral response, recorded as a brisk jerk or jump in response to monofilament application or licking or biting behavior directed towards the monofilament.
Mice that were exposed to NMS also displayed histological evidence of CP/CPPS. Cryostat sections of prostate tissue were stained with acidified o-Toluidine Blue to observe tryptase granules housed within mast cells (Figure 3A-D). The percentage of mast cells that showed evidence of activation, e.g., diffuse metachromasia, granules present outside of the cell border (Figure 3D), was significantly increased in prostate tissue from 8 week-old NMS mice, compared to naïve (p < 0.0001, Student’s t-test, Figure 3E). The total number of infiltrated mast cells, regardless of granulation status, was not significantly different between naïve (99.5 ± 15.2 cells/section) and NMS mice (108.2 ± 22.5 cells/section).
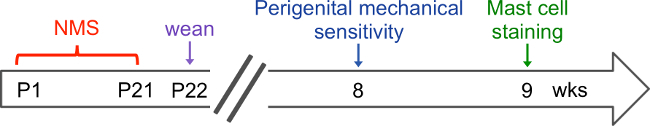 Figure 1. Procedural time line. The schematic outlines a recommended time line for performing the described methodology. Neonatal maternal separation (NMS) is performed daily from postnatal day (P) 1 until P21 and mice are weaned on P22. Mice remain undisturbed outside of normal animal husbandry until 8 weeks of age when they are tested for perigenital mechanical sensitivity. If the same mice are going to be assessed for mast cell staining, one week should be allowed to elapse following behavioral testing to allow for any residual stress effects to resolve.
Figure 1. Procedural time line. The schematic outlines a recommended time line for performing the described methodology. Neonatal maternal separation (NMS) is performed daily from postnatal day (P) 1 until P21 and mice are weaned on P22. Mice remain undisturbed outside of normal animal husbandry until 8 weeks of age when they are tested for perigenital mechanical sensitivity. If the same mice are going to be assessed for mast cell staining, one week should be allowed to elapse following behavioral testing to allow for any residual stress effects to resolve.
 Figure 2. Perigenital mechanical sensitivity. Perigenital mechanical sensitivity was measured using von Frey monofilament application. Male mice that underwent neonatal maternal separation (NMS) displayed a significant reduction in withdrawal threshold, compared to naïve mice, indicative of mechanical allodynia. Data represents mean SEM, n = 4 for each group. *p < 0.05, Student’s t-test.
Figure 2. Perigenital mechanical sensitivity. Perigenital mechanical sensitivity was measured using von Frey monofilament application. Male mice that underwent neonatal maternal separation (NMS) displayed a significant reduction in withdrawal threshold, compared to naïve mice, indicative of mechanical allodynia. Data represents mean SEM, n = 4 for each group. *p < 0.05, Student’s t-test.
 Figure 3. Mast cell activation. Acidified toluidine blue was used to visualize tryptase granules and calculate the percentage of activated/degranulated mast cells in cryostat sections of prostate tissue. Representative photomicrographs are shown of toluidine blue-stained sections from naïve (A) and NMS (B) prostate with arrows indicating intact (non-degranulated) and arrowheads indicating activated (degranulated) mast cells. Higher magnification images from naïve (C) and NMS (D) bladder are shown to illustrate histological differences from non-degranulated (C) and degranulated (D) mast cells. A significantly higher percentage of degranulated mast cells were observed in prostate sections from NMS mice when compared to naïve mice (E). Data represents mean SEM, n = 4 for each group. ****p < 0.0001, Student’s t-test. Please click here to view a larger version of this figure.
Figure 3. Mast cell activation. Acidified toluidine blue was used to visualize tryptase granules and calculate the percentage of activated/degranulated mast cells in cryostat sections of prostate tissue. Representative photomicrographs are shown of toluidine blue-stained sections from naïve (A) and NMS (B) prostate with arrows indicating intact (non-degranulated) and arrowheads indicating activated (degranulated) mast cells. Higher magnification images from naïve (C) and NMS (D) bladder are shown to illustrate histological differences from non-degranulated (C) and degranulated (D) mast cells. A significantly higher percentage of degranulated mast cells were observed in prostate sections from NMS mice when compared to naïve mice (E). Data represents mean SEM, n = 4 for each group. ****p < 0.0001, Student’s t-test. Please click here to view a larger version of this figure.
Discussion
This protocol provides methodology for using neonatal stress, in the form of NMS, to induce symptomology indicative of CP/CPPS in adult male mice. As adults, the NMS mice display significant perigenital mechanical allodynia, as well as evidence of mast cell degranulation in prostate tissue. The use of NMS is a novel approach to developing a preclinical model of CP/CPPS in that it replicates the early life stress that is often reported by patients with chronic pelvic pain12-14, as well as incorporating a non-invasive induction, as opposed to more commonly used, chemically- and immunologically-induced inflammation of the prostate34,36-39. Furthermore, NMS in mice has been shown to produce comorbid symptomology, including altered anxiety-like and anhedonic behaviors, increased micturition rates, and hindpaw hypersensitivity (16; data not shown), similar to the comorbid somatic, mood, and visceral disorders exhibited by CP/CPPS patients4-6.
The main advantage of using NMS to induce CP/CPPS in mice is that it uses a psychological intervention to initiate physiological changes. Patients with CP/CPPS have variant etiology that largely does not involve previous infection of or direct injury to the prostate1-3. Despite this fact, published models of CP/CPPS have incorporated direct or indirect inflammation of the prostate to induce hypersensitivity of the perigenital region and/or mast cell activation. A significant proportion of CP/CPPS and chronic pelvic pain patients in general, report having experienced early life adversity, which has largely gone unstudied in rodent models of urogenital pain syndromes. Previous work from our lab has shown that NMS induces vaginal hypersensitivity and associated disruption of proper functioning of the HPA axis16. Future work using this model will investigate the mechanisms that are driven by early life stress to produce the altered phenotype in adulthood, as well as to explore potential pharmacological or lifestyle interventions that could be applied in a preclinical or clinical setting.
Achieving optimal and repeatable results from this protocol depends on observing several critical steps that may need to be optimized depending on the environment and equipment used. Considering that NMS dramatically affects the functioning of the HPA axis, it is important to control for outside stimuli and environmental stress during both the neonatal and adult periods. Age-matched, non-handled naïve mice should be born and housed in concordance with each cohort of NMS mice to control for external stressors present in the environment that may affect the development of the HPA axis. It is important to determine the number of mice needed to perform the intended experiment prior to initiating breeding or ordering pregnant females into the animal facility. While performing NMS, the greatest concern is rejection of the pups by the dam, therefore it is imperative to maintain the scent of the home cage throughout the NMS protocol. To minimize the effects of diurnal rhythms, the suggested separation time for NMS is during the middle of the light cycle (from 11AM to 2PM). Perigenital sensitivity measurements should be obtained in fully mature adult male mice, approximately 8 weeks of age, and taken at the same time of day for each group, preferably early in the light cycle to coincide with the trough in diurnal rhythms. To reduce the occurrence of non-evoked movements during this procedure, assessments may be performed in a temperature-controlled, sound proof room with white noise playing (20 - 20,000 Hz). The same investigator should assess all withdrawal thresholds throughout the study to maintain consistency in the observance of positive and negative responses. As alternatives to measuring the 50% mechanical threshold, the withdrawal frequency to a single monofilament could be assessed or an electronic von Frey apparatus46 could be utilized. For mast cell visualization, all slides that are to be analyzed should be processed together to ensure equal staining of mast cells, as staining intensity can vary between experiments. A pH below 1.0 is necessary for proper contrast between the deep purple mast cells and the light blue background. Too many rinses in alcohol can wash out the stain from the mast cells and negatively affect the contrast. Finally, superimposing a grid onto the tissue can be helpful in counting cells throughout the whole tissue section.
Several considerations should be made prior to the use of NMS to induce CP/CPPS or related centralized pain disorders. First, the vast majority of publications that have incorporated early life stress have been done in rat models of NMS, using a truncated separation period of P1 - P14. Many groups have shown that this paradigm generates an IBS-like symptomology in rats and mice15; however, in our previous study a P1 - P14 separation period in C57BL/6 mice did not generate a significant increase in vaginal sensitivity16, nor did it generate colorectal hypersensitivity (data not shown). Therefore, the species and length of NMS may determine the specific outcome in adulthood, including the severity of symptomology and the specific organ(s) that is affected. Second, the behavioral and molecular changes resulting from NMS are largely due to dysregulation of the HPA axis15-18, suggesting that the effect is centrally-mediated and could have comorbid results, including changes in anxiety- and/or depression-like behaviors and increased sensitivity in other pelvic organs or more distant locations. This comorbid phenotype is indicative of what is commonly seen clinically and may be more representative of CP/CPPS patients as a whole5,28-31,42. Third, based on these changes occurring as a result of HPA axis dysregulation, the impact of stress should be of utmost concern during both the NMS procedure and later behavioral testing and in vitro analysis. Output from the HPA axis is diurnal, with greater output during the more active dark cycle and lesser output during the more quiescent light cycle, which could affect the outcome of behavioral or in vitro analysis depending on the time of the testing/sacrifice47. Likewise, exposure to stressors, including undergoing perigenital mechanical sensitivity testing, can transiently effect functioning of the HPA axis18 and potentially alter gene expression within associated brain regions and downstream peripheral tissues.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors would like to thank Janelle Ryals, Rachel Supple, and Frank Wang for technical assistance. This work was supported by NIH grants R03 DK080182 (JAC), R01 DK099611 (JAC), R01 DK103872 (JAC), Center of Biomedical Research Excellence (COBRE) grant P20 GM104936 (JAC), start-up funds and core support from the Kansas Institutional Development Award (IDeA) P20 GM103418, core support from the Kansas IDDRC P30 HD002528, and The Madison and Lila Self Fellowship Program (ANP).
References
- Mehik A, Leskinen MJ, Hellstrom P. Mechanisms of pain in chronic pelvic pain syndrome: influence of prostatic inflammation. World journal of urology. 2003;21:90–94. doi: 10.1007/s00345-003-0334-3. [DOI] [PubMed] [Google Scholar]
- Schaeffer AJ. Epidemiology and evaluation of chronic pelvic pain syndrome in men. International journal of antimicrobial agents. 2008;31(1):S108–S111. doi: 10.1016/j.ijantimicag.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Wesselmann U. Neurogenic inflammation and chronic pelvic pain. World journal of urology. 2001;19:180–185. doi: 10.1007/s003450100201. [DOI] [PubMed] [Google Scholar]
- Arnold LD, Bachmann GA, Rosen R, Kelly S, Rhoads GG. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstetrics and gynecology. 2006;107:617–624. doi: 10.1097/01.AOG.0000199951.26822.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullones Rodriguez MA, et al. Evidence for overlap between urological and nonurological unexplained clinical conditions. The Journal of urology. 2013;189:S66–S74. doi: 10.1016/j.juro.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JW, van de Merwe JP, Nickel JC. Interstitial cystitis/bladder pain syndrome and nonbladder syndromes: facts and hypotheses. Urology. 2011;78:727–732. doi: 10.1016/j.urology.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. The American journal of psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Mayson BE, Teichman JM. The relationship between sexual abuse and interstitial cystitis/painful bladder syndrome. Current urology reports. 2009;10:441–447. doi: 10.1007/s11934-009-0070-3. [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biological psychiatry. 2008;64:521–526. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videlock EJ, et al. Childhood trauma is associated with hypothalamic-pituitary-adrenal axis responsiveness in irritable bowel syndrome. Gastroenterology. 2009;137:1954–1962. doi: 10.1053/j.gastro.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, et al. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biological psychiatry. 2008;63:1147–1154. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. The American journal of gastroenterology. 2008;103:765–774. doi: 10.1111/j.1572-0241.2007.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GT, Power C, Macfarlane GJ. Adverse events in childhood and chronic widespread pain in adult life. Results from the 1958 British Birth Cohort. 2009;143:92–96. doi: 10.1016/j.pain.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Peters KM, Killinger KA, Ibrahim IA. Childhood symptoms and events in women with interstitial cystitis/painful bladder syndrome. Urology. 2009;73:258–262. doi: 10.1016/j.urology.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Mahony SM, Hyland NP, Dinan TG, Cryan JF. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology. 2011;214:71–88. doi: 10.1007/s00213-010-2010-9. [DOI] [PubMed] [Google Scholar]
- Pierce AN, Ryals JM, Wang R, Christianson JA. Vaginal hypersensitivity and hypothalamic-pituitary-adrenal axis dysfunction as a result of neonatal maternal separation in female mice. Neuroscience. 2014;263:216–230. doi: 10.1016/j.neuroscience.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biological psychiatry. 2004;55:367–375. doi: 10.1016/j.biopsych.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Malley D, Dinan TG, Cryan JF. Neonatal maternal separation in the rat impacts on the stress responsivity of central corticotropin-releasing factor receptors in adulthood. Psychopharmacology. 2011;214:221–229. doi: 10.1007/s00213-010-1885-9. [DOI] [PubMed] [Google Scholar]
- Chung EK, Zhang XJ, Xu HX, Sung JJ, Bian ZX. Visceral hyperalgesia induced by neonatal maternal separation is associated with nerve growth factor-mediated central neuronal plasticity in rat spinal cord. Neuroscience. 2007;149:685–695. doi: 10.1016/j.neuroscience.2007.07.055. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, et al. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307–G316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- Moloney RD, et al. Early-life stress induces visceral hypersensitivity in mice. Neuroscience letters. 2012;512:99–102. doi: 10.1016/j.neulet.2012.01.066. [DOI] [PubMed] [Google Scholar]
- Wijngaard RM, et al. Peripheral alpha-helical CRF (9-41) does not reverse stress-induced mast cell dependent visceral hypersensitivity in maternally separated rats. Neurogastroenterol Motil. 2012;24:274–282. doi: 10.1111/j.1365-2982.2011.01840.x. [DOI] [PubMed] [Google Scholar]
- DeBerry J, Ness TJ, Robbins MT, Randich A. Inflammation-induced enhancement of the visceromotor reflex to urinary bladder distention: modulation by endogenous opioids and the effects of early-in-life experience with bladder inflammation. J Pain. 2007;8:914–923. doi: 10.1016/j.jpain.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randich A, Uzzell T, DeBerry JJ, Ness TJ. Neonatal urinary bladder inflammation produces adult bladder hypersensitivity. J Pain. 2006;7:469–479. doi: 10.1016/j.jpain.2006.01.450. [DOI] [PubMed] [Google Scholar]
- Shaffer AD, Ball CL, Robbins MT, Ness TJ, Randich A. Effects of acute adult and early-in-life bladder inflammation on bladder neuropeptides in adult female rats. BMC urology. 2011;11:18. doi: 10.1186/1471-2490-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontari MA, Joyce GF, Wise M, McNaughton-Collins M. Urologic Diseases in America, P. Prostatitis. The Journal of urology. 2007;177:2050–2057. doi: 10.1016/j.juro.2007.01.128. [DOI] [PubMed] [Google Scholar]
- Calhoun EA, et al. The economic impact of chronic prostatitis. Archives of internal medicine. 2004;164:1231–1236. doi: 10.1001/archinte.164.11.1231. [DOI] [PubMed] [Google Scholar]
- Nickel JC, et al. Category III chronic prostatitis/chronic pelvic pain syndrome: insights from the National Institutes of Health Chronic Prostatitis Collaborative Research Network studies. Current urology reports. 2008;9:320–327. doi: 10.1007/s11934-008-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehik A, Hellstrom P, Sarpola A, Lukkarinen O, Jarvelin MR. Fears, sexual disturbances and personality features in men with prostatitis: a population-based cross-sectional study in Finland. BJU international. 2001;88:35–38. doi: 10.1046/j.1464-410x.2001.02259.x. [DOI] [PubMed] [Google Scholar]
- Clemens JQ, Brown SO, Calhoun EA. Mental health diagnoses in patients with interstitial cystitis/painful bladder syndrome and chronic prostatitis/chronic pelvic pain syndrome: a case/control study. The Journal of urology. 2008;180:1378–1382. doi: 10.1016/j.juro.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez MA, et al. Evidence for overlap between urological and nonurological unexplained clinical conditions. The Journal of urology. 2009;182:2123–2131. doi: 10.1016/j.juro.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SF, Schaeffer AJ, Thumbikat P. Immune mediators of chronic pelvic pain syndrome. Nature reviews. Urology. 2014;11:259–269. doi: 10.1038/nrurol.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CL. The role of a leaky epithelium and potassium in the generation of bladder symptoms in interstitial cystitis/overactive bladder, urethral syndrome, prostatitis and gynaecological chronic pelvic pain. BJU international. 2011;107:370–375. doi: 10.1111/j.1464-410X.2010.09843.x. [DOI] [PubMed] [Google Scholar]
- Done JD, Rudick CN, Quick ML, Schaeffer AJ, Thumbikat P. Role of mast cells in male chronic pelvic pain. The Journal of urology. 2012;187:1473–1482. doi: 10.1016/j.juro.2011.11.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman K, Done JD, Schaeffer AJ, Murphy SF, Thumbikat P. Tryptase-PAR2 axis in experimental autoimmune prostatitis, a model for chronic pelvic pain syndrome. Pain. 2014;155:1328–1338. doi: 10.1016/j.pain.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadio AC, Depiante-Depaoli M. Inflammatory cells and MHC class II antigens expression in prostate during time-course experimental autoimmune prostatitis development. Clinical immunology and immunopathology. 1997;85:158–165. doi: 10.1006/clin.1997.4427. [DOI] [PubMed] [Google Scholar]
- Keetch DW, Humphrey P, Ratliff TL. Development of a mouse model for nonbacterial prostatitis. The Journal of urology. 1994;152:247–250. doi: 10.1016/s0022-5347(17)32871-9. [DOI] [PubMed] [Google Scholar]
- Rivero VE, Cailleau C, Depiante-Depaoli M, Riera CM, Carnaud C. Non-obese diabetic (NOD) mice are genetically susceptible to experimental autoimmune prostatitis (EAP) Journal of autoimmunity. 1998;11:603–610. doi: 10.1006/jaut.1998.0248. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Schaeffer AJ, Thumbikat P. Experimental autoimmune prostatitis induces chronic pelvic pain. American journal of physiology. Regulatory, integrative and comparative physiology. 2008;294:R1268–R1275. doi: 10.1152/ajpregu.00836.2007. [DOI] [PubMed] [Google Scholar]
- Rivero VE, Iribarren P, Riera CM. Mast cells in accessory glands of experimentally induced prostatitis in male Wistar rats. Clinical immunology and immunopathology. 1995;74:236–242. doi: 10.1006/clin.1995.1035. [DOI] [PubMed] [Google Scholar]
- Hu JC, Link CL, McNaughton-Collins M, Barry MJ, McKinlay JB. The association of abuse and symptoms suggestive of chronic prostatitis/chronic pelvic pain syndrome: results from the Boston Area Community Health survey. Journal of general internal. 2007;22:1532–1537. doi: 10.1007/s11606-007-0341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegel B, et al. Assessing psychological factors, social aspects and psychiatric co-morbidity associated with Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS) in men - A systematic review. Journal of psychosomatic research. 2014;77:333–350. doi: 10.1016/j.jpsychores.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Watkins SK, Zhu Z, Watkins KE, Hurwitz AA. Isolation of immune cells from primary tumors. Journal of visualized experiments : JoVE. 2012. p. e3791. [DOI] [PMC free article] [PubMed] [Retracted]
- Gage GJ, Kipke DR, Shain W. Whole animal perfusion fixation for rodents. Journal of visualized experiments : JoVE. 2012. [DOI] [PMC free article] [PubMed]
- Martinov T, Mack M, Sykes A, Chatterjea D. Measuring changes in tactile sensitivity in the hind paw of mice using an electronic von Frey apparatus. Journal of visualized experiments : JoVE. 2013. p. e51212. [DOI] [PMC free article] [PubMed]
- Lutgendorf SK, et al. Diurnal cortisol variations and symptoms in patients with interstitial cystitis. The Journal of urology. 2002;167:1338–1343. [PubMed] [Google Scholar]


