Abstract
Tripartite motif-containing 29 (TRIM29) is a member of the tripartite motif (TRIM) protein family. TRIM29 has been reported to be deregulated in a number of cancer types, suggesting the oncogenic function of TRIM29. However, its clinical significance in non-small cell lung cancer (NSCLC) has not been fully elucidated. In the present study, the TRIM29 expression status was investigated by immunohistochemical analysis in paraffin-embedded specimens obtained from 320 patients with surgically resected NSCLC, treated between 2000 and 2007. High TRIM29 expression was significantly associated with smoking (P=0.012), T stage (P=0.015) and M stage (P=0.003). Furthermore, elevated TRIM29 expression level was correlated with reduced overall (OS) and disease-free survival. In addition, high TRIM29 expression was an independent prognostic factor for OS [P=0.003, hazard ratio (HR)=2.102, 95% confidence interval (CI), 1.069–3.193]. In conclusion, these results suggest that TRIM29 may be a useful prognostic marker in NSCLC patients and a potential molecular target for NSCLC treatment.
Keywords: TRIM29, non-small cell lung cancer, prognosis, biomarker
Introduction
Non-small cell lung cancer (NSCLC) accounts for ~80% of all lung cancer cases (1) and is the most common cause of cancer-associated mortality worldwide (2). Globally, the annual diagnosis rate of new NSCLC cases is ~1.6 million, with the rate increasing over the last decades (3). A previous study demonstrated the importance of molecular characterization of these tumors and the identification of potential molecular targets for treatment (3). However, the prognosis in these patients remains poor as the overall 5-year survival is <15% (4). These unsatisfactory clinical outcomes indicate the requirement for more reliable predictors of survival and novel therapeutic targets (3).
The tripartite motif (TRIM) family members are involved in numerous biological processes and, when altered, are implicated in a number of pathological conditions (5). TRIM29, which is also known as ataxia-telangiectasia group D complementing protein (ATDC), is a member of the TRIM family proteins (6). TRIM29 is highly expressed in gastric cancer and is involved in the differentiation, proliferation and progression of gastric cancer cells (7). In addition, TRIM29 was found to be overexpressed in bladder cancer, ovarian serous papillary tumors and endometrial neoplasms (8,9). However, the clinical significance and prognostic value of TRIM29 expression in NSCLC patients remain unclear. The present study aimed to investigate the association between TRIM29 expression and the clinicopathological features of NSCLC, as well as examine the potential role of TRIM29 as a prognostic factor in NSCLC patients.
Materials and methods
Patients and histological evaluation
NSCLC patients who were diagnosed, treated and followed-up at the Department of Thoracic Surgery (Shandong Qianfoshan Hospital, Jinan, China) between January 2000 and January 2007 were recruited in this study. The inclusion criteria were as follows: Patients with surgically resected and pathologically confirmed primary NSCLC, complete medical records and available paraffin-embedded specimens were included. In total, 320 patients with NSCLC were enrolled, including 221 patients treated with lobectomy and 99 patients treated with pneumonectomy. Clinical variables were retrieved from the patients' medical records, including age, gender, and survival or disease progression (Table I). Tumor diagnosis and histological classification were based on a new multidisciplinary classification of lung cancer proposed by the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society (10). Tumors were staged according to the 7th edition of the TNM Classification of Malignant Tumors (11,12). Normal bronchial epithelium obtained from noncancerous lung tissue of the NSCLC patients was used as the control. The study was approved by the Institutional Review Board at the Shandong Qianfoshan Hospital. Written informed consent was obtained from all patients.
Table I.
Association between TRIM29 expression and clinicopathological features.
| TRIM29 expression | |||
|---|---|---|---|
| Characteristic | High | Low | P-value |
| Patients, n (%) | 79 (24.7) | 241 (75.3) | |
| Gender, n (%) | 0.092 | ||
| Male | 65 (82.3) | 214 (88.8) | |
| Female | 14 (17.7) | 27 (11.2) | |
| Mean age ± SD, years | 57.32±11.8 | 59.26±9.77 | 0.113 |
| Smoking status, n (%) | 0.012 | ||
| Smoker | 50 (63.3) | 200 (83) | |
| Non-smoker | 29 (36.7) | 41 (17) | |
| Histology, n (%) | 0.821 | ||
| SCC | 31 (39.2) | 184 (76.3) | |
| ADC | 48 (60.8) | 57 (23.7) | |
| Pathological stage, n (%) | 0.210 | ||
| Stage I | 28 (35.4) | 110 (45.6) | |
| Stage II | 26 (32.9) | 72 (29.9) | |
| Stage III | 21 (26.6) | 50 (20.7) | |
| Stage IV | 4 (5.1) | 9 (3.7) | |
| T stage, n (%) | 0.015 | ||
| T1 | 23 (29.1) | 30 (12.4) | |
| T2 | 40 (50.6) | 163 (67.6) | |
| T3 | 12 (15.2) | 20 (8.3) | |
| T4 | 4 (5.1) | 28 (11.7) | |
| N stage, n (%) | 0.920 | ||
| N0 | 48 (60.8) | 147 (61) | |
| N1 | 15 (19) | 51 (21.2) | |
| N2 | 16 (20.2) | 43 (17.8) | |
| M stage, n (%) | 0.003 | ||
| M0 | 29 (36.7) | 205 (85.1) | |
| M1 | 50 (63.3) | 36 (14.9) | |
TRIM29, tripartite motif-containing 29; SD, standard deviation; SCC, squamous cell carcinoma; ADC, adenocarcinoma; T stage, tumor size; N stage, lymph nodes; M stage, distant metastases.
Follow-up
Standardized follow-up was conducted every 3 months for the first 2 years after surgery, every 6 months for the 3rd year and yearly thereafter. Follow-up included physical examination, complete blood count, chest computed tomography scans, brain magnetic resonance imaging scans and abdominal ultrasound. The median follow-up period for surviving patients was 31.5 months (6–72 months). Disease-free survival (DFS) was defined as the time from the initial surgery until a documented relapse, including locoregional recurrence and distant metastasis. The overall survival (OS) was defined from the date of the initial surgery until mortality or the end of follow-up.
Immunohistochemical analysis
Tissue sections (6 µm) were deparaffinized in xylene, rehydrated and heated at 100°C in citrate buffer (pH 6.0) for 5 min for nonenzymatic antigen retrieval. The sections were then incubated with monoclonal mouse anti-human TRIM29 antibody (cat. no. H00023650-B01; dilution, 1:100; Novus Biologicals, LLC, Littleton, CO, USA) for 60 min at room temperature, followed by incubation with a goat anti-mouse immunoglobulin G antibody (cat. no. AI-9200; dilution, 1:1,000; Vector Laboratories, Inc., Burlingame, CA, USA) for 1 h at room temperature. Staining was performed with 3,3′-diaminobenzidine chromogen for 5 min, followed by counterstaining using hematoxylin (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) for 5 min, as previously described (13). Rabbit IgG (cat. no. NP-001; Epitomics Inc., Burlingame, CA, USA) was used as a negative control. TRIM29 expression was scored as follows: 0, no staining; 1+, <10% of tumor cells expressing TRIM29; 2+, 10-50% of tumor cells expressing TRIM29; and 3+, >50% of tumor cells expressing TRIM29. Scores of 0 and 1+ were classified as low TRIM29 expression, whereas scores of 2+ and 3+ were classified as high TRIM29 expression. The scoring is a modification of a previously described classification (14). Two pathologists blinded to the patients' clinical data interpreted all the slides and agreed on the classification of the sections into the low or high TRIM29 expression groups.
Statistical analysis
In order to determine any possible associations between qualitative clinicopathological variables and TRIM29 expression, the χ2 test or two-tailed Fisher's exact test was applied. Survival differences among the high- and low-expression groups were calculated using the Kaplan-Meier method along with the log-rank test. The Cox proportional hazards model was used for multivariate analyses of OS and DFS. SPSS version 16.0 statistical software (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. P<0.05 was considered to indicate a statistically significant difference.
Results
Association between TRIM29 expression and clinicopathological features of NSCLC patients
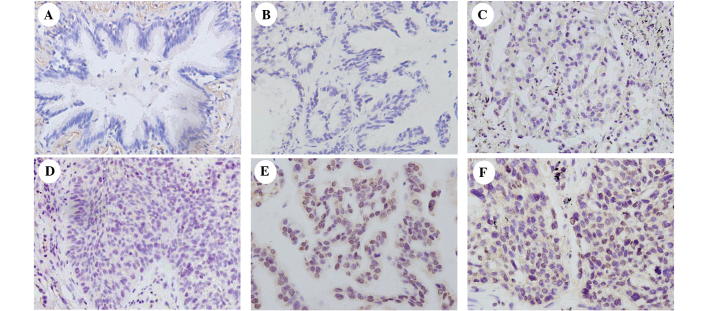
Various patient characteristics and their correlation with TRIM29 expression are listed in Table I. The mean age at diagnosis was 58.9±10.7 years (range, 34–82 years). A total of 138 patients (43.1%) were diagnosed with stage I, 98 (30.6%) with stage II, 71 (22.2%) with stage III and 13 (4.1%) with stage IV disease. TRIM29 expression was predominantly localized in the nuclear compartments of the tumor cells, while the normal bronchial epithelia exhibited negative or low staining (Fig. 1).
Figure 1.
Immunohistochemical staining of TRIM29 in lung cancer tissue specimens. (A) Negative staining in normal bronchial epithelium of noncancerous lung tissue (control). (B) Negative control using rabbit immunoglobulin. (C) Weak TRIM29 staining in lung adenocarcinoma. (D) Weak TRIM29 staining in a case of squamous cell carcinoma. (E) Positive TRIM29 staining in a case of lung adenocarcinoma. (F) Positive TRIM29 staining in a case of squamous cell carcinoma. TRIM29, tripartite motif-containing 29.
The association between total TRIM29 expression and various clinical parameters was investigated. As shown in Table I, a high TRIM29 expression was significantly associated with smoking (P=0.012), a higher T stage (P=0.015) and a higher M stage (P=0.003). However, no statistically significant correlation was observed between TRIM29 expression and other clinical features.
Association between TRIM29 expression and OS or DFS
The prognostic relevance of TRIM29 expression and other clinicopathological parameters with regard to OS and DFS in patients with NSCLC was investigated (Table II; Fig. 2A and B). Patients with high TRIM29 expression presented shorter OS and DFS rates compared with patients with low TRIM29 expression (P=0.007 and P=0.014, respectively). In addition, pathological stage (I/II vs. III/IV) and TNM stage were demonstrated to be independent prognostic factors affecting OS and DFS (P<0.001; Table II). Furhtermore, high TRIM29 expression was demonstrated to be an independent prognostic factor affecting OS (P=0.003; hazard ratio, 2.102; 95% confidence interval, 1.069–3.193) using multivariate analyses (Table III).
Table II.
Univariate analyses of overall survival and disease-free survival in 320 patients with non-small cell lung cancer.
| Overall survival | Disease-free survival | |||
|---|---|---|---|---|
| Characteristic | Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value |
| TRIM29 expression | 0.007 | 0.014 | ||
| Low vs. high | 1.52 (1.120–2.061) | 1.51 (1.089–2.119) | ||
| Gender | 0.134 | 0.982 | ||
| Female vs. male | 0.56 (0.267–1.049) | 0.97 (0.670–1.427) | ||
| Smoking status | 0.025 | 0.190 | ||
| No vs. yes | 1.48 (1.049–2.138) | 0.80 (0.570–1.121) | ||
| Age, years | 0.079 | 0.447 | ||
| ≤60 vs. >60 | 1.43 (1.036–1.901) | 0.82 (0.642–1.213) | ||
| Histology | 0.814 | 0.625 | ||
| SCC vs. ADC | 1.07 (0.766–1.403) | 1.34 (0.979–1.858) | ||
| Stage | <0.001 | <0.001 | ||
| I/II vs. III/IV | 2.69 (1.933–3.550) | 1.86 (1.314–2.648) | ||
| T stage | <0.001 | <0.001 | ||
| T1/T2 vs. T3/T4 | 2.84 (2.082–3.995) | 2.35 (1.621–3.429) | ||
| N stage | <0.001 | <0.001 | ||
| N0 vs. N1/N2 | 2.30 (1.725–3.120) | 1.96 (1.419–2.695) | ||
| M stage | <0.001 | <0.001 | ||
| M0 vs. M1 | 2.54 (1.894–3.725) | 2.02 (1.711–3.502) |
CI, confidence interval; SCC, squamous cell carcinoma; ADC, adenocarcinoma; T stage, tumor size; N stage, lymph nodes; M stage, distant metastases.
Figure 2.
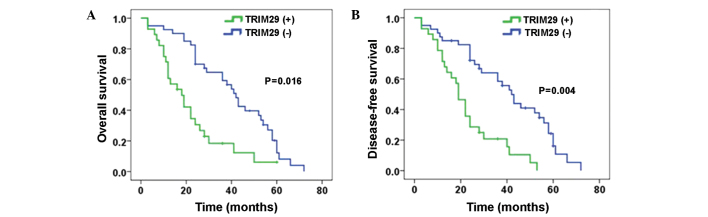
Kaplan-Meier curves for (A) overall survival and (B) disease-free survival in all non-small-cell lung cancer patients. TRIM29, tripartite motif-containing 29; (+), positive expression; (-), negative expression.
Table III.
Multivariate analyses of overall survival and disease-free survival in 320 patients with non-small cell lung cancer.
| Overall survival | Disease-free survival | |||
|---|---|---|---|---|
| Characteristic | Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value |
| TRIM29 expression | 0.003 | 0.064 | ||
| Low vs. high | 2.102 (1.069–3.193) | 1.384 (0.982–1.952) | ||
| Gender | 0.036 | 0.331 | ||
| Female vs. male | 1.921 (1.044–3.535) | 1.327 (0.750–2.347) | ||
| Smoking status | 0.312 | 0.406 | ||
| No vs. yes | 1.277 (0.795–2.052) | 0.813 (0.499–1.325) | ||
| Age, years | 0.001 | 0.692 | ||
| ≤60 vs. >60 | 1.752 (1.274–2.410) | 1.070 (0.766–1.495) | ||
| Histology | 0.007 | 0.040 | ||
| SCC vs. ADC | 1.611 (1.141–2.275) | 1.506 (1.020–2.225) | ||
| Stage | 0.415 | 0.649 | ||
| I/II vs. III/IV | 1.196 (0.778–1.838) | 0.891 (0.543–1.463) | ||
| T stage | <0.001 | <0.001 | ||
| T1/T2 vs. T3/T4 | 2.050 (1.371–3.065) | 2.246 (1.427–3.537) | ||
| N stage | <0.001 | <0.001 | ||
| N0 vs. N1/N2 | 2.053 (1.592–3.413) | 1.817 (1.100–2.713) | ||
| M stage | <0.001 | 0.001 | ||
| M0 vs. M1 | 2.316 (1.618–3.313) | 1.952 (1.320–2.888) |
CI, confidence interval; SCC, squamous cell carcinoma; ADC, adenocarcinoma; T stage, tumor size; N stage, lymph nodes; M stage, distant metastases.
Discussion
The aim of the present study was to determine the prognostic significance of TRIM29 protein expression in NSCLC. Based on the findings of the current study, TRIM29 overexpression appears to be associated with aggressive tumor behavior and ultimately influences patients' clinical outcomes. In addition, a high TRIM29 expression was prevalent in smokers and was found to be an unfavorable clinical factor in NSCLC.
Recent evidence has suggested that TRIM29 promotes cancer cell proliferation via inhibiting the nuclear activities of p53, which is a major tumor suppressor gene involved in the determination of proliferation or growth arrest at the cellular level (15,16). TRIM29 binds p53 and represses the expression of p53-regulated genes, including p21 and NOXA (15,17). In addition, TRIM29 is selectively expressed in basal cells of the normal prostate gland. In a previous study, immunohistochemical staining with anti-TRIM29 antibody revealed the same expression pattern as that observed for staining with 34βE12 in prostate cancer and its benign mimics, indicating that TRIM29 may be useful for distinguishing prostate cancer from benign tissues (18). Furthermore, younger females with early-stage breast cancer who were not administered adjuvant systemic therapy had a significantly lower risk of relapse when their tumor exhibited a high TRIM29 expression (19). This finding suggests that loss of TRIM29 expression in normal breast luminal cells can contribute to malignant transformation and lead to progression of breast cancer in premenopausal women (19). The expression of TRIM29 was also significantly associated with progression to muscle-invasive bladder cancer and was identified as an independent prognostic marker (20). In addition, TRIM29 has been reported to upregulate matrix metalloproteinase 9 to promote lung cancer cell invasion by activating the extracellular signal-regulated kinase and c-Jun N-terminal kinase signaling pathways (21). Previous results have identified a DNA repair pathway leading from MAPK-activated protein kinase-2 and ataxia telangiectasia mutated to TRIM29 (22). Therefore, as TRIM29 has been used as a therapeutic target to radiosensitize pancreatic ductal adenocarcinoma and improve the efficacy of DNA-damaging treatment in pancreatic cancer (22), the use of TRIM29 as a potential therapeutic target for the treatment of NSCLC was investigated in the present study.
In conclusion, in the present study, TRIM29 expression was investigated by immunohistochemical analysis in paraffin-embedded specimens obtained from 320 patients with surgically resected NSCLC, treated between 2000 and 2007. High TRIM29 expression was significantly associated with smoking, T stage and M stage. Furthermore, elevated TRIM29 expression level was correlated with reduced OS and DFS and was an independent prognostic factor for OS in NSCLC. Therefore, TRIM29 may be a useful prognostic marker in NSCLC patients and a potential molecular target for NSCLC treatment.
References
- 1.Dempke WC, Suto T, Reck M. Targeted therapies for non-small cell lung cancer. Lung Cancer. 2010;67:257–274. doi: 10.1016/j.lungcan.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics. CA Cancer J Clin. 2011;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 3.Gainor JF, Shaw AT. Novel targets in non-small cell lung cancer: ROS1 and RET fusions. Oncologist. 2013;18:865–875. doi: 10.1634/theoncologist.2013-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villarreal Garza, de la Mata D, Zavala DG, Macedo-Perez EO, Arrieta O. Aggressive treatment of primary tumor in patients with non-small-cell lung cancer and exclusively brain metastases. Clin Lung Cancer. 2013;14:6–13. doi: 10.1016/j.cllc.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Napolitano LM, Meroni G. TRIM family: Pleiotropy and diversification through homomultimer and heteromultimer formation. IUBMB Life. 2012;64:64–71. doi: 10.1002/iub.580. [DOI] [PubMed] [Google Scholar]
- 6.Hatakeyama S. TRIM proteins and cancer. Nat Rev Cancer. 2011;11:792–804. doi: 10.1038/nrc3139. [DOI] [PubMed] [Google Scholar]
- 7.Kosaka Y, Inoue H, Ohmachi T, Yokoe T, Matsumoto T, Mimori K, Tanaka F, Watanabe M, Mori M. Tripartite motif-containing 29 (TRIM29) is a novel marker for lymph node metastasis in gastric cancer. Ann Surg Oncol. 2007;14:2543–2549. doi: 10.1245/s10434-007-9461-1. [DOI] [PubMed] [Google Scholar]
- 8.Santin AD, Zhan F, Bellone S, Palmieri M, Cane S, Bignotti E, Anfossi S, Gokden M, Dunn D, Roman JJ, et al. Gene expression profiles in primary ovarian serous papillary tumors and normal ovarian epithelium: Identification of candidate molecular markers for ovarian cancer diagnosis and therapy. Int J Cancer. 2004;112:14–25. doi: 10.1002/ijc.20408. [DOI] [PubMed] [Google Scholar]
- 9.Dyrskjøt L, Kruhøffer M, Thykjaer T, Marcussen N, Jensen JL, Møller K, Ørntoft TF. Gene expression in the urinary bladder: A common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res. 2004;64:4040–4048. doi: 10.1158/0008-5472.CAN-03-3620. [DOI] [PubMed] [Google Scholar]
- 10.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstraw P, Crowley J, Chansky K, et al. International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions: The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 12.Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: Validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of Malignant Tumours. J Thorac Oncol. 2007;2:694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 13.Kwon MJ, Park S, Choi JY, Oh E, Kim YJ, Park YH, Cho EY, Kwon MJ, Nam SJ, Im YH, et al. Clinical significance of CD151 overexpression in subtypes of invasive breast cancer. Br J Cancer. 2012;106:923–930. doi: 10.1038/bjc.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki S, Miyazaki T, Tanaka N, Sakai M, Sano A, Inose T, Sohda M, Nakajima M, Kato H, Kuwano H. Prognostic significance of CD151 expression in esophageal squamous cell carcinoma with aggressive cell proliferation and invasiveness. Ann Surg Oncol. 2011;18:888–893. doi: 10.1245/s10434-010-1387-3. [DOI] [PubMed] [Google Scholar]
- 15.Yuan Z, Villagra A, Peng L, Coppola D, Glozak M, Sotomayor EM, Chen J, Lane WS, Seto E. The ATDC (TRIM29) protein binds p53 and antagonizes p53-mediated functions. Mol Cell Biol. 2010;30:3004–3015. doi: 10.1128/MCB.01023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sho T, Tsukiyama T, Sato T, Kondo T, Cheng J, Saku T, Asaka M, Hatakeyama S. TRIM29 negatively regulates p53 via inhibition of Tip60. Biochim Biophys Acta. 2011;1813:1245–1253. doi: 10.1016/j.bbamcr.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Shibata MA, Yoshidome K, Shibata E, Jorcyk CL, Green JE. Suppression of mammary carcinoma growth in vitro and in vivo by inducible expression of the Cdk inhibitor p21. Cancer Gene Ther. 2001;8:23–35. doi: 10.1038/sj.cgt.7700275. [DOI] [PubMed] [Google Scholar]
- 18.Kanno Y, Watanabe M, Kimura T, Nonomura K, Tanaka S, Hatakeyama S. TRIM29 as a novel prostate basal cell marker for diagnosis of prostate cancer. Acta Histochem. 2014;116:708–712. doi: 10.1016/j.acthis.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Welm B, Boucher KM, Ebbert MT, Bernard PS. TRIM29 functions as a tumor suppressor in nontumorigenic breast cells and invasive ER+breast cancer. Am J Pathol. 2012;180:839–847. doi: 10.1016/j.ajpath.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fristrup N, Birkenkamp-Demtröder K, Reinert T, Sanchez-Carbayo M, Segersten U, Malmström PU, Palou J, Alvarez-Múgica M, Pan CC, Ulhøi BP, et al. Multicenter validation of cyclin D1, MCM7, TRIM29 and UBE2C as prognostic protein markers in non-muscle-invasive bladder cancer. Am J Pathol. 2013;182:339–349. doi: 10.1016/j.ajpath.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Tang ZP, Cui QZ, Dong QZ, Xu K, Wang EH. Ataxia-telangiectasia group D complementing gene (ATDC) upregulates matrix metalloproteinase 9 (MMP-9) to promote lung cancer cell invasion by activating ERK and JNK pathways. Tumour Biol. 2013;34:2835–2842. doi: 10.1007/s13277-013-0843-7. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Yang H, Palmbos PL, Ney G, Detzler TA, Coleman D, Leflein J, Davis M, Zhang M, Tang W, et al. ATDC/TRIM29 phosphorylation by ATM/MAPKAP kinase 2 mediates radioresistance in pancreatic cancer cells. Cancer Res. 2014;74:1778–1788. doi: 10.1158/0008-5472.CAN-13-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]