Abstract
Background
Pulse pressure variation (PPV) and stroke volume variation (SVV) are frequently used to assess fluid responsiveness in critically ill patients on mechanical ventilation (MV). There are many factors, in addition to preload that influence the magnitude of these cyclic variations. We sought to investigate the effect of tidal volume (VT) on PPV and SVV, and prediction of fluid responsiveness in a model of intra-abdominal hypertension (IAH).
Methods
Twelve anesthetized and mechanically ventilated piglets on continuous pulse contour cardiac output monitoring. Hypovolemia was ruled out with 2 consecutive fluid boluses after instrumentation. IAH was induced by intraperitoneal instillation of colloid solution with a goal of reducing respiratory system compliance by 50 %. Subjects were classified as fluid responders if stroke volume increased >15 % after each fluid challenge. SVV and PPV were recorded with tidal volumes (VT) of 6, 12 and 18 ml/kg before IAH after IAH induction and after a fluid challenge during IAH.
Results
VT influenced PPV and SVV at baseline and during IAH, being significantly larger with higher VT. These differences were attenuated after fluid administration in both conditions. After IAH induction, there was a significant increase in SVV with the three-tested VT, but the magnitude of that change was larger with high VT: with 6 ml/kg from 3 % (3, 4) to 5 % (4, 6.25) (p = 0.05), with 12 ml/kg from 5 % (4, 6) to 11 % (8.75, 17) (p = 0.02) and 18 ml/kg from 5 % (4,7.5) to 15 % (8.75, 19.5) (p = 0.02). Similarly, PPV increased with all the tested VT after IAH induction, being this increase larger with high VT: with 6 ml/kg from 3 % (2, 4.25) to 6 % (4.75, 7) (p = 0.05), with 12 ml/kg from 5 % (4, 6) to 13.5 % (10.25, 15.5) (p = 0.02) and 18 ml/kg from 7 % (5.5, 8.5) to 24 % (13.5, 30.25) (p = 0.02). One third of subjects responded to fluid administration after IAH, but neither SVV nor PPV were able to identify the fluid responders with the tested VT.
Conclusion
IAH induction in non-hypovolemic subjects significantly increased SVV and PPV with the three tested VT, but the magnitude of that change was higher with larger VT. This observation reveals the dependence of functional hemodynamic markers on intrathoracic as well intra-abdominal pressures, in addition to volemic status. Also, PPV and SVV were unable to predict fluid responsiveness after IAH induction. Future studies should take into consideration these findings when exploring relationships between dynamic preload indicators and fluid responsiveness during IAH.
Electronic supplementary material
The online version of this article (doi:10.1186/s12871-015-0105-x) contains supplementary material, which is available to authorized users.
Background
Functional hemodynamic monitoring has been demonstrated to be a powerful tool in critically ill patients. Careful selection of patients that will respond to fluid administration may help avoid fluid overload. Functional hemodynamic monitoring including stroke volume variation (SVV) and pulse pressure variation (PPV), have been shown to be more accurate in predicting fluid responsiveness than classically used static parameters (central venous pressure (CVP) and pulmonary artery occlusion pressure) in mechanically ventilated patients without spontaneous breathing [1–3].
These dynamic indexes are based on cyclic transmission of airway pressure to the pleural and pericardial spaces, which induces changes in venous return and preload. Due to the complex relationship between intrathoracic structures (heart-lung interactions) numerous studies have demonstrated that functional hemodynamic monitoring parameters do not only depend on preload and ventricular interdependence. Mechanical ventilation (MV) settings like tidal volume (VT), PEEP, driving pressure and respiratory rate (RR) as well as lung compliance are potential factors that may decrease the ability of functional hemodynamic monitoring parameters to predict fluid responsiveness [4–6].
Abdominal Compartment Syndrome (ACS) represents the final stage of a pathologic process caused by an increase in intra-abdominal pressure (IAP) to a degree that can compromise regional blood flow. It has significant morbidity, related to the ongoing inflammation due to ischemia and organ dysfunction, ultimately causing death [7, 8]. Judicious fluid administration plays a key role in the management of ACS. Early fluid therapy is fundamental during the initial resuscitation phase, but its liberal use is a known risk factor for ACS and excessive fluid administration may worsen IAP [7–12]. Therefore functional hemodynamic monitoring has been proposed to guide fluid therapy in patients at risk of ACS and during increased IAP [10, 13, 14].
Cyclic changes in SV and PP induced by positive pressure ventilation can be affected by extra thoracic modifications in compliance, such as ACS or contained laparotomy [15, 16]. Current literature has yielded conflicting data regarding the ability of PPV and SVV to predict fluid responsiveness in subjects with increased IAP [17–22]. This shows the complexity of the relationship between IAP and intrathoracic pressure; as well as the effect of pressure transmitted to intrathoracic vascular structures during MV [5, 7, 8, 23]. In addition protective ventilatory strategies [24, 25], specifically small VT, have been shown to modify functional hemodynamic monitoring parameters when lung compliance is reduced, like ARDS, due to low transmission of pressure from airway to pleura [4–6, 26, 27]. However the effect of different VT on functional hemodynamic monitoring in conditions of reduced respiratory system compliance due to extrapulmonary causes has not been studied. We sought to investigate the effect of different VT on PPV and SVV in a model of intra-abdominal hypertension. We hypothesize that acute increase in IAP increases SVV and PPV readings in euvolemic animals, and this increase is proportional to the size of VT.
Methods
The experimental protocol was approved by Facultad de Medicina Clínica Alemana-Universidad del Desarrollo Ethics Committee and the CONICYT (Comisión Nacional de Investigación Científica y Tecnológica) Bioethics Advisory Committee. All of the experimental procedures were consistent with the Guiding Principles in the Care and Use of Laboratory Animals adopted by the American Physiological Society.
Animal preparation and anesthesia
12 anesthetized Large-White piglets (4.9 ± 0.05 kg) were used in this study. Animals were premedicated with intramuscular acepromazine (1.1 mg/kg) and ketamine (20 mg/kg). The trachea was cannulated with a 3.5-mm (internal diameter) cuffed tracheostomy tube (Mallinckrodt Shiley, St. Louis, MO), the left jugular vein with a 4 F double lumen catheter (Arrow, Reading, PA, USA) and the right femoral artery with a 4 F thermistor-tipped catheter (PV2014L08; Pulsion Medical Systems, Munich, Germany), all via cut down. A peritoneal dialysis catheter was inserted with Seldinger Technique under aseptic conditions. Animals were ventilated in a volume control mode (EVITA XL®, Dräger Medical, Lübeck, Germany). Initial settings were VT 10 ml/kg, PEEP 5 cmH2O, respiratory rate (RR) 20 breaths per minute (bpm), inspiratory time 0.75 s, and FiO2 0.5. Anesthesia and neuromuscular blockade were maintained by continuous infusion of propofol (10 mg/kg/h), fentanyl (4 μg/kg/h), and pancuronium (0.2 mg/kg/h) throughout the experiment. Hydration was maintained with a continuous infusion of normal saline at 5 ml/kg/h. The temperature was maintained at 37.2 ± 0.4 °C by conventional convective methods.
Experimental protocol
Subjects were exposed to three different VT 6, 12 and 18 ml/kg in random order during the following experimental steps: baseline, after a 20 ml/kg normal saline (NS) fluid bolus (Fluid Bolus 1), after a second 20 ml/kg NS fluid bolus (Fluid Bolus 2), after intra-abdominal hypertension (IAH) induction and after a fluid bolus of 20 ml/kg during IAH (Fluid Bolus 3) (Fig. 1). Each VT was applied from 2 to 5 min allowing at least 45 s of steady reading of SVV and PPV in PiCCO® plus display (Pulsion Medical Systems, München, Germany). After testing the 3 different VT, subjects were placed on VT 10 ml/kg until next randomization. After each fluid challenge PiCCO® plus device was re-calibrated and all measurements were obtained after 15 min of stable hemodynamic conditions.
Fig. 1.
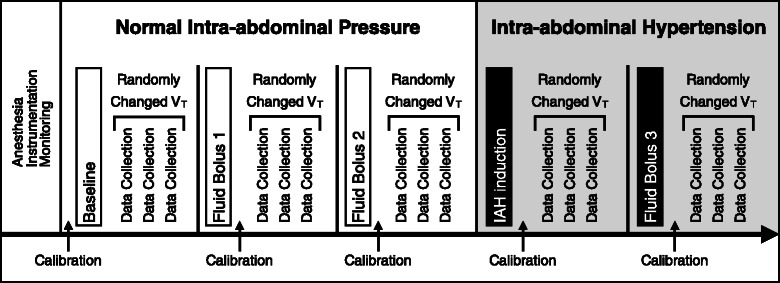
Diagram of the experimental protocol. Calibration refers to transpulmonary thermodilution for calibration of pulse contour–derived cardiac output and assessment of stroke volume variation and pulse pressure variation. Randomly changed VT refers to tidal volume 6, 12 or 18 ml/kg applied in random order. (VT: Tidal volume, IAH, intra-abdominal hypertension)
IAH was induced by intraperitoneal instillation of Voluven® 6 % (Fresenius-Kabi, Bad Homburg, Germany) in successive aliquots of 100 ml through the peritoneal catheter. Target IAP was defined as the IAP necessary to decrease the static respiratory system compliance (CRS) by 50 %. We chose this target to ensure that the experimentally induced IAP was high enough to induce significant extra-abdominal organ dysfunction. At the end of the experiment the animals were euthanized.
Measurements
Hemodynamic and respiratory measurements were performed at baseline and at the end of the experiment.
Pulmonary measurements: Arterial blood gases were determined with i-STAT EG6+ Cartridges (Abbott, Princeton, NJ, USA) from blood samples drawn from the arterial catheter. Oxygenation was assessed with the PaO2/FiO2 ratio. CRS was calculated as VT/(Ppl - PEEPTOT), where Ppl is the plateau pressure measured after an inspiratory hold of 4 s, and PEEPTOT is the total end-expiratory airway pressure measured after an expiratory hold of 4 s. These variables were recorded from the ventilator display.
Hemodynamic measurements: Heart rate (HR), mean arterial pressure (MAP), and central venous pressure were monitored with an Infinity® Delta XL monitor (Dräger Medical, Lübeck, Germany). Zero pressure was set at the midaxillary line. Cardiac index (CI) was measured with a commercially available device PiCCO® plus. According to manufacturer´s instructions, single thermal indicator transpulmonary thermodilution was performed in triplicate by injection of a 5-ml bolus of iced normal saline solution into the superior cava vein through the jugular catheter. The CI was internally computed from an analysis of the thermodilution curve with a modified Stewart-Hamilton algorithm [19]. The body surface area of the piglets was calculated as K/weight (in kg)2/3, where K = 0.112 for pigs.
-
Functional Hemodynamic measurements: In the PiCCO® plus device, SVV was calculated from the mean values of four minimum and maximum stroke volumes averaged during the last 30 s (SVmean):PPV was calculated during the same time interval:
Fluid responsiveness: At the normal intra-abdominal stage, and after IAH induction, subjects were classified as fluid responders if SVI increased greater than 15 % after the fluid bolus [5, 28].
Statistical analysis
Data are presented as median and 25th and 75th percentile range (P25–75), unless stated. Related-Samples Wilcoxon Signed Rank test was used to assess differences between baseline data and measurements at the end of the study and Independent-Samples Mann–Whitney U test was used to compare data between groups. Spearman rank correlations were performed between functional hemodynamic markers and changes in SVI. Significant outliers were defined as any value more than 1.5 × interquartile range and they were excluded of the analysis.
Receiver-operating characteristic (ROC) curves were constructed to evaluate the capacity of PPV and SVV to predict fluid responsiveness under the three tested VT [29].
Differences with P <0.05 were considered statistically significant. All statistical analyses were performed with the SPSS 20.0 software program (SPSS, Chicago, IL, USA).
Results
All the animals completed the experimental protocol. The hemodynamic data during the experiment are presented in Table 1. Respiratory parameters with normal intra-abdominal pressure and after IAH induction are summarized in supplemental material. (Additional file 1)
Table 1.
Hemodynamic variables at baseline, after fluid loading and after Intra-abdominal Hypertension. Presented as median and 25th and 75th percentile range
| Baseline | FB1 | FB2 | IAH | FB3 | |
|---|---|---|---|---|---|
| IAP | 4 (3,4) | 22 (19,24)a | |||
| CI | 4.30 (3.91,5.23) | 4.66 (4.23,5.61)a | 5.18 (4.31,5.86)a | 4.28 (3.88,4.52)b | 4.85 (4.04,5.16)b |
| SVI | 32.0 (30.0,33.8) | 39.5 (37.0,42.5)a | 40.5 (36.0,42.5)a | 37.5 (32.8,44.5)a | 40.5 (39.3,47.0)a |
| HR | 139 (120,180) | 129 (118,155)a | 131 (125,150)a | 125 (118,129)a,b | 128 (113,136)a,b |
| MAP | 69 (61,72) | 72 (68,82)a | 73 (70,82)a | 78 (75,95)a | 90 (77,98)a,b |
| CVP | 6 (6,7) | 10 (8,11)a | 11 (10,12)a | 11 (10,12)a | 16 (14,17)a,b,c |
| SVRI | 1152 (1007,1399) | 1041 (888,1276) | 935 (795,1234) | 1152 (1083,1408)b | 1216 (953,1479)b |
(FB fluid bolus, IAH intra-abdominal hypertension, IAP intra-abdominal pressure, mmHg, CI cardiac index, l/min/m2, SVI stroke volume index, mL/m2, HR heart rate, bpm, MAP mean arterial pressure, mmHg, CVP central venous pressure, mmHg, SVRI systemic vascular resistance index, dynes-sec/cm−5/m2)
aP <0.05 Respect to baseline
bP <0.05 Respect to fluid bolus 2 (FB2) (PRE-IAH)
cP <0.05 Respect to post-IAH
Before IAH induction, each successive fluid bolus increased CI, SVI, and CVP, and decreased HR (Table 1). Before fluid administration SVV and PPV were significant higher with VT 12 and 18 ml/kg versus 6 ml/kg. These differences were attenuated after fluid loading (Figs. 2 and 3).
Fig. 2.
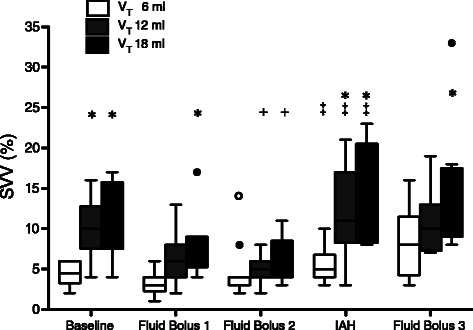
Boxplot graph of Stroke Volume Variation (SVV, %) with 6, 12 and 18 ml/kg at different steps of the experiment. Closed circles show outliers. Open circle shows outlier not included in the analysis. BL, baseline; FB1, First Fluid Bolus; FB2, Second Fluid Bolus; IAH, Intra-abdominal Hypertension; FB3, Fluid Bolus 3. *P <0.05 respect to VT 6 mL/kg (in the same step). +P <0.05 respect to BL (with equal VT). ‡P <0.05 respect to FB2 (normovolemic animals pre-HIA)
Fig. 3.
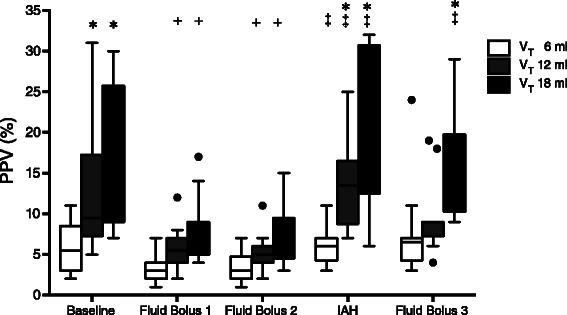
Boxplot graph of Pulse Pressure Variation (PPV, %) with 6, 12 and 18 ml/kg at different steps of the experiment. Closed circles show outliers. BL, baseline; FB1, First Fluid Bolus; FB2, Second Fluid Bolus; IAH, Intra-abdominal Hypertension; FB3, Fluid Bolus 3. *P <0.05 respect to VT 6 mL/kg (in the same step). +P <0.05 respect to BL (with equal VT). ‡P <0.05 respect to FB2 (normovolemic animals pre-HIA)
Fluid challenge resulted in 15 % or greater increase in SVI in 42 % of trials before the induction of IAH, 8 subjects after FB1 and 2 subjects after FB2 (Table 2A). Fluid responders had lower initial SVI and CVP, but there was no significant difference in CI. PPV was significantly higher in fluid responders across the range of VT. Of note is that this difference was more pronounced with VT 18 ml/kg. SVV was significantly higher with VT 12 and 18 ml/kg in fluid responders compared to non-responders, but not with VT 6 ml/kg (Table 2A). There was direct correlation between SVV and the increase in SVI after fluid challenge (ΔSVI) with VT 6 ml/kg (ρ = 0.4, p = 0.048), VT 12 ml/kg (ρ = 0.57, p = 0.004) and 18 ml/kg (ρ = 0.64, p = 0.001). Also a positive correlation was found between ΔSVI and PPV with VT 6 ml/kg (ρ = 0.59, p = 0.003), VT 12 ml/kg (ρ = 0.69, p <0.001) and 18 ml/kg (ρ = 0.69, p <0.001). Scatter plot of these correlations are provided as supplemental material (Additional files 2 and Additional file 3).
Table 2.
Hemodynamic and functional hemodynamic markers in fluid responders and non-responders before (A) and after (B) intra-abdominal hypertension induction
| A. | ∆SVI >15 % | P-value | ||
|---|---|---|---|---|
| ALL (n = 24) | YES (n = 10) | NO (n = 14) | ||
| CI (l/min/m2) | 4.5 (4.0, 5.5) | 4.5 (4.1, 5.5) | 4.4 (3.9, 5.4) | 0.79 |
| ∆CI (%) | 5.7 (1.1, 15.1) | 14.9 (3.7, 24.6) | 4.7 (-8.3, 8.2) | 0.106 |
| ∆SVI (%) | 10.9 (2.6, 21.2) | 23.2 (18.1, 33.3) | 5.0 (-8.1, 9.4) | <0.001 |
| SVI (ml/m2) | 36.5 (32, 40) | 31 (30, 39) | 37 (35, 40) | 0.001 |
| SVV (%) | ||||
| VT 6 ml/kg | 4 (3, 6) | 4 (3.8, 6) | 3 (2, 5) | 0.12 |
| VT 12 ml/kg | 8 (6, 11) | 10 (9, 12) | 6.5 (4, 8) | 0.011 |
| VT 18 ml/kg | 9 (6, 12) | 12 (9, 16) | 6 (5, 9) | 0.011 |
| PPV (%) | ||||
| VT 6 ml/kg | 4 (3, 6) | 5 (3, 7) | 3 (2, 4) | 0.019 |
| VT 12 ml/kg | 7 (5, 11) | 11 (8, 19) | 6 (5, 7) | <0.001 |
| VT 18 ml/kg | 9 (7, 15) | 14 (9, 27) | 8 (5, 9) | 0.001 |
| HR (bpm) | 129 (117, 163) | 139 (119, 191) | 129 (114, 155) | 0.045 |
| MAP (mmHg) | 69 (63, 80) | 67 (63,71) | 73 (64, 83) | 0.214 |
| CVP (mmHg) | 7 (6, 10) | 7 (6, 8) | 8 (7, 11) | 0.016 |
| SVRI | 1070 (895,1408) | 1144 (880,1189) | 1041 (913,1475) | 0.837 |
| B. | ∆SVI > 15 % | P-value | ||
| ALL (n = 12) | YES (n = 4) | NO (n = 8) | ||
| CI (l/min/m2) | 4.3 (3.8, 4.6) | 3.8 (3.4, 4.1) | 4.4 (4.2, 5.4) | 0.073 |
| ∆CI (%) | 8.8 (0, 19) | 20.2 (13.2, 32.6) | −5.1 (-8.7, 6.4) | 0.028 |
| ∆SVI (%) | 6.4 (-6.5, 19.7) | 26.0 (19.6, 32.0) | −4.0 (-11.4, 6.4) | 0.004 |
| SVI (ml/m2) | 38 (33, 45) | 35 (30, 40) | 41 (35, 48) | 0.283 |
| SVV (%) | ||||
| VT 6 ml/kg | 5 (4, 7) | 6 (5, 7) | 5 (4, 8) | 0.461 |
| VT 12 ml/kg | 11 (9, 17) | 17 (13, 19) | 10 (7, 13) | 0.154 |
| VT 18 ml/kg | 15 (9, 20) | 20 (18, 22) | 10 (8, 16) | 0.073 |
| PPV (%) | ||||
| VT 6 ml/kg | 6 (5, 7) | 7 (6, 9) | 6 (4, 7) | 0.368 |
| VT 12 ml/kg | 14 (10, 16) | 16 (14, 20) | 12 (8, 15) | 0.154 |
| VT 18 ml/kg | 24 (13, 31) | 28 (22, 32) | 19 (11, 28) | 0.214 |
| HR (bpm) | 125 (118, 131) | 118 (103, 118) | 128 (125, 137) | 0.028 |
| MAP (mmHg) | 81 (71, 87) | 80 (75, 86) | 81 (68, 94) | 1 |
| CVP (mmHg) | 11 (10, 13) | 11 (10, 13) | 11 (10, 16) | 0.808 |
| SVRI | 1231 (1044,1495) | 1382 (1150,1657) | 1160 (985,1368) | 0.368 |
Presented as median and 25th and 75th percentile range. (CI Cardiac index, l/min/m2; SVI stroke volume index, ml/m2; SVV stroke volume variation, %, PPV pulse pressure variation, %, VT Tidal Volume, ml/kg, HR Heart rate, bpm, MAP mean arterial pressure, mmHg, CVP central venous pressure, mmHg SVRI systemic vascular resistance index, dynes-sec/cm−5/m2)
ROC analysis showed that PPV was a good predictor of fluid responsiveness with all VT. SVV had lower performance than PPV with all tested VT. The best cutpoint changed according the VT applied, being higher with larger VT. (Table 3A)
Table 3.
Receiver operating characteristics (ROC) curve statistic for predicting changes > 15 % in SVI (fluid responders) at normal intra-abdominal pressure (A) and during intra-abdominal hypertension (B)
| A | ||||||
|---|---|---|---|---|---|---|
| AUC | 95 % CI | p-value | Best cut point | Sensitivity | Specificity | |
| VT 6 ml/kg | ||||||
| SVV | 70 % | 49–92 | 0.111 | 3.5 | 75 % | 56 % |
| PPV | 79 % | 61–98 | 0.022 | 4.5 | 75 % | 81 % |
| VT 12 ml/kg | ||||||
| SVV | 82 % | 65–99 | 0.012 | 8.5 | 88 % | 75 % |
| PPV | 95 % | 87–100 | <0.001 | 9.5 | 75 % | 94 % |
| VT 18 ml/kg | ||||||
| SVV | 82 % | 65–99 | 0.013 | 8.5 | 88 % | 63 % |
| PPV | 89 % | 76–100 | 0.002 | 10 | 88 % | 81 % |
| B | ||||||
| VT 6 ml/kg | ||||||
| SVV | 64 % | 33–95 | 0.445 | 5.5 | 50 % | 63 % |
| PPV | 67 % | 35–99 | 0.35 | 5.5 | 75 % | 38 % |
| VT 12 ml/kg | ||||||
| SVV | 78 % | 48–100 | 0.126 | 15 | 75 % | 88 % |
| PPV | 78 % | 51–100 | 0.126 | 14.5 | 75 % | 75 % |
| VT 18 ml/kg | ||||||
| SVV | 84 % | 61–100 | 0.062 | 18.5 | 50 % | 75 % |
| PPV | 73 % | 44–100 | 0.203 | 24 | 75 % | 63 % |
(VT, Tidal Volume, SVV stroke volume variation, PPV Pulse pressure variation, AUC area under the curve)
IAP was 4 (3, 4) mmHg at baseline. The IAP of 22 (19, 23) mmHg (p <0.01 respect to baseline IAP) was achieved after intraperitoneal infusion of 2.5 (2.1, 2.7) l of colloid solution over 35 (30, 40) min. As planned in the experimental design, the rise of IAP caused a decrease in CRS from 1.27 (1.06, 1.41) to 0.55 (0.49, 0.75) ml/cmH2O/kg (p <0.01) and PaO2/FiO2 ratio from 412 (387, 424) to 330 (290, 351) (p <0.01). After IAH induction there was a significant decrease in CI with no changes in CVP and HR (Table 1). Interestingly in 5 animals SVI increased (range 6 to 25 %). There was a significant increase in SVV with the three-tested VT, but the magnitude of that change was larger with high VT: with 6 ml/kg from 3 % (3, 4) to 5 % (4, 6.25) (p = 0.049), with 12 ml/kg from 5 % (4, 6) to 11 % (8.75, 17) (p = 0.02) and 18 ml/kg from 5 % (4, 7.5) to 15 % (8.75, 19.5) (p = 0.02) (Fig. 2). Similarly, PPV increased with all the tested VT, being this increase larger with high VT: with 6 ml/kg from 3 % (2, 4.25) to 6 % (4.75, 7) (p = 0.047), with 12 ml/kg from 5 % (4, 6) to 13.5 % (10.25, 15.5) (p = 0.02) and 18 ml/kg from 7 % (5.5, 8.5) to 24 % (13.5, 30.25) (p = 0.02) (Fig. 3).
Once IAH was established, SVV and PPV were significantly higher with VT 12 and 18 ml/kg compared with 6 ml/kg. The differences between 6 and 12 ml/kg disappeared after the fluid bolus, but between 6 and 18 ml/kg were still significant (Figs. 2 and 3). One third of fluid bolus trials (FB3) resulted in an increase of SVI greater than 15 % during IAH. Fluid responders had lower CI and SVI, higher SVV and PPV, but these differences were not significant (Table 2B). There was not a significant correlation between ΔSVI and SVV once IAH was established with any of the tested VT: 6 ml/kg ρ = 0.23, p = 0.46; 12 ml/kg ρ = 0.46, p = 0.13; and 18 ml/kg (ρ = 0.56, p = 0.064) (Additional file 2: Figure S1B). As shown in Additional file 2: Figure S1B, there was not a significant correlation between ΔSVI and PPV after IAH induction with any of the applied VT: 6 ml/kg (ρ = 0.29, p = 0.36), VT 12 ml/kg (ρ = 0.45, p = 0.13) and 18 ml/kg (ρ = 0.38, p = 0.22) (Additional file 2: Figure S1B). Scatter plot of these correlations are provided in supplemental material, Additional file 2: Figure S1. AUC showed that none of the studied variables were good predictor of fluid responsiveness during IAH (Table 3B), even adjusting for higher cutoff points.
Discussion
The main findings of this experimental study are as follows:
-
i.
IAH increased PPV and SVV compared to normal IAP with the three-tested VT, but the magnitude of these changes were larger with higher VT.
-
ii.
PPV and SVV were influenced by VT during IAH, being significant larger with high VT. These differences were attenuated after fluid administration.
-
iii.
During IAH, neither SVV nor PPV predicted response to fluids with the three tested VT.
We observed significant differences in PPV and SVV with the studied VT at baseline. These differences reflect the transmission of swings in the airway pressure to the vascular compartment, with greater transmission occurring at higher VT. After fluid challenge, CI, SVI, and static markers of intravascular fluid status increased. PPV and SVV significantly decreased with respect to baseline only under VT 12 and 18 ml/kg. This observation is in accordance with previous studies that suggested that in conditions of hypovolemia fluctuations in pulse pressure and stroke volume are related to transmission of airway pressure to the vascular compartment and not the VT itself [27, 30, 31]. Our findings suggest that the effect of the airway pressurization on the cyclic changes was less significant in conditions of euvolemia or hypervolemia, after the fluid administration. Low VT produced variations in PP and SV, but they were very small. As shown in Fig. 1 there was a significant variation on SVV and PPV at the beginning of the experiment when high VT was applied. The attenuation of this dispersion after the first and second fluid bolus also supports the change in hemodynamic condition of the subjects to a more flat portion of the Frank-Starling curve, explaining the trend towards homogeneity. We think that our experimental model may explain this, because we did not actively induce hypovolemia, animals fasted the night before the experiment, but had access to water ad libitum.
Forty two percent of subjects were fluid responders before IAH. Both PPV and SVV were higher in fluid responders compared to non-responders, but these differences were smaller with lower VT. It is important to note that when VT 6 ml/kg was applied, all but one subject had PPV and SVV readings under the usual threshold to define fluid responders, leading to non-useful clinical differences, like SVV 4 (3.8, 5.3) v/s 3 (2, 5) and PPV 5 (3, 7) v/s 3 (2, 4) for responders and non-responders respectively. In accordance to previous observations, airway pressurization was not enough to demonstrate preload dependency with low VT in mechanically ventilated healthy piglets [4, 6, 32, 33]. On the other hand, ROC analysis showed that PPV and SVV were good predictors of fluid response with VT 12 and 18 ml/kg. PPV had better performance predicting fluid responsiveness with all the tested VT, being better with 12 ml/kg with a cut off of 9.5, strikingly similar to previous studies [12, 34]. Also IAH is a common problem in the intensive care unit and is responsible for significant morbidity in critically ill patients [7, 8]. Usual definitions for IAH is a sustained or repeated pathologic elevation of IAP ≥12 mmHg and ACS a sustained IAP >20 mmHg that is associated with new organ dysfunction or failure in the intensive care unit setting [7]. An important methodological difference of our experimental protocol versus previous studies was the definition of IAH. Our target was not a fixed IAP, but a significant effect on lung compliance. Surprisingly this target was achieved in accordance with current definitions of ACS, with IAP slightly greater than 20 mmHg. It has been previously reported that there is a high risk of irreversible tissue damage in with IAP >20 mmHg [7, 8].
Relationships between thoracic and abdominal compartments are complex and both have significant independent as well as interdependent influences on cardiovascular physiology. As expected, we observed a reduction in CI after induction of IAH, but without significant changes in blood pressure [33, 35]. The variable and unpredictable individual response of SVI to IAH is a practical example of the complex relationship between intra-abdominal and intrathoracic compartments. It has been described in experimental studies that moderate or small increases in IAP can increase intrathoracic blood volume, left ventricular end-diastolic area, and transmural left ventricular end-diastolic pressure, suggesting an auto-transfusion effect in euvolemic subjects, but not during hypovolemia [20, 33, 36, 37]. In our model some subjects might have been hypervolemic, that can explain that even facing IAP >20 mmHg SVI did not significantly decrease.
We found that after IAH induction, PPV and SVV increased with the 3 tested VT. Previous studies showed that cyclic changes in PP and SV during IAH are dependent on the pressure of abdominal compartment [5, 20, 21, 28]. Interestingly with low VT (6 ml/kg) changes in SVV and PPV after IAH induction were very small and most animals were below the usual threshold described to predict fluid responsiveness. On the other hand, high VT produced a large increase in SVV and PPV, showing that during IAH in addition to the pressure of the abdominal compartment, PP and SV are dependent on the airway pressure swings, and not the applied VT. This supports the clinical observation of Muller et al. who found that lack of significant variation of airway pressure, expressed as PIP-Ppl, in patient on mechanical ventilation with low VT might limit the use of PPV to predict fluid responsiveness [27].
Fluid administration during IAH increased SVI greater than 15 % in 1/3 of the subjects. There was a non-significant trend towards higher PPV and SVV in these fluid responsive patients. ROC analysis showed that PPV and SVV were not able to discriminate between fluid responders and non-responders during IAH with the tested VT. It is important to note that AUC might look similar between pre-IAH and after IAH induction, but the wide confidence interval (crossing 50 %) as well as the large p-value observed, reflect that SVV and PPV did not discriminate between animals that increased their SVI greater than 15 % after the third fluid challenge. Other studies have found that SVV and PPV were useful to predict fluid responsiveness, but with different thresholds [14, 17, 19]. We think that the discrepancy of our findings is related to the experimental design. We were trying to demonstrate the complex relationship between intrathoracic and IAP and this experimental model was not designed to study hypovolemic conditions. Subjects at this stage of the experiment were euvolemic or even hypervolemic after the initial fluid challenges; surprisingly we observed a significant number of fluid responders, but SVV and PPV failed to identify them. Furthermore, in contrast to previous studies, the significant variations in SV and PP with VT 12 and 18 ml/kg even in the absence of changes in SVI during fluid challenge can be explained by the large variation in intrathoracic pressures due to decreased CRS in accordance with our definition of IAH.
The present study should not be understood as challenging the ability of PPV or SVV to predict fluid responsiveness. With our initial hypothesis and the obtained results we intend to point out the relationship between abdominal and thoracic compartment, considering the VT, airway pressurization and IAP when interpreting functional hemodynamic monitoring parameters to decide fluid administration.
Our study has some limitations. First, in addition to the interspecies physiologic differences (i.e. chest wall compliance, thorax shape and relation of intrathoracic structures [38]), we studied piglets with normal respiratory and cardiovascular function. We must be cautious extrapolating these results to critically ill patients where multiorgan involvement is frequently observed. Second, a limitation of our model is that IAH was induced over a short period of time. As most of experimental models, this one mimics acute IAH rather than the usual subacute IAH observed in the clinical setting. Our target IAP was different from previous studies, being compatible with severe ACS during critical illness and not with usual increase of IAP of pneumoperitoneum during laparoscopic surgery. Also IAH was induced after fluid challenge, limiting generalization of these observations in under-resuscitated, hypovolemic, or actively bleeding patients. Third we did not include chest wall or abdominal wall compliance measurements in our experiment, preventing the analysis of the isolated components that might have influenced SVV and PPV. Fourth, we used PiCCO® plus system to measure PPV and SVV. Although we used frequent calibration, it is not the gold standard for these measurements. Finally, the small number of subjects as well the scattered response of fluids after IAP does not allow robust receiver operating characteristic curve analysis.
Conclusion
We found significant differences in PPV and SVV with different VT at baseline and after IAH induction. These differences were attenuated after fluid administration. IAH induction in non-hypovolemic subjects significantly increased SVV and PPV with the three tested VT, but the magnitude of that change was higher with larger VT. This observation reveals the dependence of functional hemodynamic markers on intrathoracic as well intra-abdominal pressures, in addition to volemic status. In agreement with this physiological description, we found that PPV and SVV were good predictors of fluid responsiveness at baseline, but not after IAH induction, showing the complex relationship of the thoracic and abdominal compartments. Future studies should take into consideration these findings when exploring relationships between functional hemodynamic monitoring and fluid responsiveness in IAH.
Also our observations need to be studied in other hemodynamic conditions, like hypovolemia, low cardiac output and sepsis, and should include measurements of pleural pressure, chest and abdominal wall compliance, for better understanding of determinants of the dynamic markers of fluid responsiveness.
Acknowledgements
The Authors wish to thank Dr. Nancy M. Tofil and Dr. Priya Prabhakaran, from Division of Critical, Department of Pediatrics, University of Alabama at Birmingham, USA, for scientific editing of this manuscript.
Funding
This work was supported in part by grant 2008009 from SOCHIPE (Sociedad Chilena de Pediatría) to F. Díaz.
Abbreviations
- ACS
Abdominal compartment syndrome
- CI
Cardiac index
- CRS
Static respiratory system compliance
- CVP
Central venous pressure
- IAP
Intra-abdominal pressure
- IAH
Intra-abdominal hypertension
- MV
Mechanical ventilation
- NS
Normal saline
- PEEPTOT
Total end-expiratory airway pressure
- PPV
Pulse pressure variation
- ROC
Receiver-operating characteristic
- SVV
Stroke volume variation
- VT
Tidal volume
Additional files
Respiratory parameters after instrumentation (normal intra-abdominal pressure) and after induction of intra-abdominal hypertension (IAH). Presented as median and 25th and 75th percentile range. (IAP: intra-abdominal pressure; VT: Tidal volume, ml/kg; PIP, peak inspiratory pressure, cmH2O; PEEP, positive end expiratory pressure, cmH2O; PPL: Plateau pressure, cmH2O; CRS: Static Respiratory System Compliance, ml/cmH2O/kg.) *p value < 0.05 respect to baseline. (DOCX 51 kb)
Scatterplot showing percentage changes in stroke volume index (ΔSVI, %) and functional hemodynamic markers, Stroke Volume Variation (SVV, %) Pulse Pressure Variation (PPV, %), with the three tested tidal volumes (VT), 6, 12 and 18 ml/kg with normal intra-abdominal pressure. Solid line shows regression line between variables. (PDF 53 kb)
Scatterplot showing percentage changes in stroke volume index (ΔSVI, %) and functional hemodynamic markers, Stroke Volume Variation (SVV, %) Pulse Pressure Variation (PPV, %), with the three tested tidal volumes (VT), 6, 12 and 18 ml/kg during intra-abdominal hypertension. Solid line shows regression line between variables. (PDF 56 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
FD designed the study, carried out the experiments, gathered experimental data, performed the statistical analysis and drafted the manuscript. BE designed the study and helped its coordination, carried out the experiments, gathered experimental data, performed the statistical analysis and revised the manuscript. AD conceived the study, carried out the experiments, gathered experimental data, and revised the manuscript. TS carried out the experiments, gathered experimental data and revised the manuscript. PC conceived and designed the study, carried out the experiments, gathered experimental data, analyzed the data and revised the manuscript. All authors read and approved the final manuscript.
Authors’ information
Not applicable.
Availability of data and materials
Not applicable.
Contributor Information
F. Díaz, Email: francodiazr@gmail.com
B. Erranz, Email: berranz@gmail.com
A. Donoso, Email: adonosofuentes@gmail.com
T. Salomon, Email: tattysalomon@hotmail.com
Pablo Cruces, Email: pcrucesr@gmail.com.
References
- 1.Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162:134–8. doi: 10.1164/ajrccm.162.1.9903035. [DOI] [PubMed] [Google Scholar]
- 2.Michard F, Teboul J-L. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121:2000–8. doi: 10.1378/chest.121.6.2000. [DOI] [PubMed] [Google Scholar]
- 3.Marx G, Cope T, McCrossan L, Swaraj S, Cowan C, Mostafa SM, et al. Assessing fluid responsiveness by stroke volume variation in mechanically ventilated patients with severe sepsis. Eur J Anaesthesiol. 2004;21:132–8. doi: 10.1097/00003643-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 4.De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent J-L. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31:517–23. doi: 10.1007/s00134-005-2586-4. [DOI] [PubMed] [Google Scholar]
- 5.Jardin F, Genevray B, Brun-Ney D, Bourdarias JP. Influence of lung and chest wall compliances on transmission of airway pressure to the pleural space in critically ill patients. Chest. 1985;88:653–8. doi: 10.1378/chest.88.5.653. [DOI] [PubMed] [Google Scholar]
- 6.De Backer D, Scolletta S. Why do pulse pressure variations fail to predict the response to fluids in acute respiratory distress syndrome patients ventilated with low tidal volume? Crit Care. 2011;15:150. doi: 10.1186/cc10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain MLNG, De Keulenaer B, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedenstierna G, Larsson A. Influence of abdominal pressure on respiratory and abdominal organ function. Curr Opin Crit Care. 2012;18:80–5. doi: 10.1097/MCC.0b013e32834e7c3a. [DOI] [PubMed] [Google Scholar]
- 9.Malbrain MLNG, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri VM, et al. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med. 2005;33:315–22. doi: 10.1097/01.CCM.0000153408.09806.1B. [DOI] [PubMed] [Google Scholar]
- 10.Cheatham ML, Malbrain MLNG, Kirkpatrick A, Sugrue M, Parr M, De Waele J, et al. Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. II. Recommendations. Intensive Care Med. 2007;33:951–62. doi: 10.1007/s00134-007-0592-4. [DOI] [PubMed] [Google Scholar]
- 11.Sümpelmann R, Schuerholz T, Marx G, Jesch NK, Osthaus WA, Ure BM. Hemodynamic changes during acute elevation of intra-abdominal pressure in rabbits. Paediatr Anaesth. 2006;16:1262–7. doi: 10.1111/j.1460-9592.2006.01987.x. [DOI] [PubMed] [Google Scholar]
- 12.Mutoh T, Lamm WJ, Embree LJ, Hildebrandt J, Albert RK. Volume infusion produces abdominal distension, lung compression, and chest wall stiffening in pigs. J Appl Physiol. 1992;72:575–82. doi: 10.1063/1.351835. [DOI] [PubMed] [Google Scholar]
- 13.Cheatham ML. Intraabdominal pressure monitoring during fluid resuscitation. Curr Opin Crit Care. 2008;14:327–33. doi: 10.1097/MCC.0b013e3282fce783. [DOI] [PubMed] [Google Scholar]
- 14.Malbrain MLNG, De Laet I. Functional haemodynamics during intra-abdominal hypertension: what to use and what not use. Acta Anaesthesiol Scand. 2008;52:576–7. doi: 10.1111/j.1399-6576.2007.01567.x. [DOI] [PubMed] [Google Scholar]
- 15.van Lavieren M, Veelenturf J, Hofhuizen C, van der Kolk M, van der Hoeven J, Pickkers P, et al. Dynamic preload indicators decrease when the abdomen is opened. BMC Anesthesiol. 2014;14:90. doi: 10.1186/1471-2253-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malbrain M, Nieuwendijk R, Verbrugghe W. Effect of intra-abdominal pressure on pleural and filling pressures. Intensive Care Med. 2003;29:S64–78. doi: 10.1007/s00134-003-1972-z. [DOI] [Google Scholar]
- 17.Malbrain MLNG, De Laet I. Functional hemodynamics and increased intra-abdominal pressure: Same thresholds for different conditions …? Crit Care Med. 2009;37:781–3. doi: 10.1097/CCM.0b013e318194c397. [DOI] [PubMed] [Google Scholar]
- 18.Renner J, Gruenewald M, Quaden R, Hanss R, Meybohm P, Steinfath M, et al. Influence of increased intra-abdominal pressure on fluid responsiveness predicted by pulse pressure variation and stroke volume variation in a porcine model. Crit Care Med. 2009;37:650–8. doi: 10.1097/CCM.0b013e3181959864. [DOI] [PubMed] [Google Scholar]
- 19.Jacques D, Bendjelid K, Duperret S, Colling J, Piriou V, Viale JP. Pulse pressure variation and stroke volume variation during increased intra-abdominal pressure: an experimental study. Crit Care. 2011;15:R33. doi: 10.1186/cc9980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duperret S, Lhuillier F, Piriou V, Vivier E, Metton O, Branche P, et al. Increased intra-abdominal pressure affects respiratory variations in arterial pressure in normovolaemic and hypovolaemic mechanically ventilated healthy pigs. Intensive Care Med. 2007;33:163–71. doi: 10.1007/s00134-006-0412-2. [DOI] [PubMed] [Google Scholar]
- 21.Bliacheriene F, Machado SB, Fonseca EB, Otsuke D, Auler JOC, Michard F. Pulse pressure variation as a tool to detect hypovolaemia during pneumoperitoneum. Acta Anaesthesiol Scand. 2007;51:1268–72. doi: 10.1111/j.1399-6576.2007.01432.x. [DOI] [PubMed] [Google Scholar]
- 22.Tournadre JP, Allaouchiche B, Cayrel V, Mathon L, Chassard D. Estimation of cardiac preload changes by systolic pressure variation in pigs undergoing pneumoperitoneum. Acta Anaesthesiol Scand. 2000;44:231–5. doi: 10.1034/j.1399-6576.2000.440303.x. [DOI] [PubMed] [Google Scholar]
- 23.Malbrain MLNG, Wilmer A. The polycompartment syndrome: towards an understanding of the interactions between different compartments! Intensive Care Med. 2007;33:1869–72. doi: 10.1007/s00134-007-0843-4. [DOI] [PubMed] [Google Scholar]
- 24.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 25.Wolthuis EK, Choi G, Dessing MC, Bresser P, Lutter R, Dzoljic M, et al. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents pulmonary inflammation in patients without preexisting lung injury. Anesthesiology. 2008;108:46–54. doi: 10.1097/01.anes.0000296068.80921.10. [DOI] [PubMed] [Google Scholar]
- 26.Lakhal K, Ehrmann S, Benzekri-Lefèvre D, Runge I, Legras A, Dequin P-F, et al. Respiratory pulse pressure variation fails to predict fluid responsiveness in acute respiratory distress syndrome. Crit Care. 2011;15:R85. doi: 10.1186/cc10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller L, Louart G, Bousquet PJ, Candela D, Zoric L, de La Coussaye JE, et al. The influence of the airway driving pressure on pulsed pressure variation as a predictor of fluid responsiveness. Intensive Care Med. 2010;36:496–503. doi: 10.1007/s00134-009-1686-y. [DOI] [PubMed] [Google Scholar]
- 28.Stetz CW, Miller RG, Kelly GE, Raffin TA. Reliability of the thermodilution method in the determination of cardiac output in clinical practice. Am Rev Respir Dis. 1982;126:1001–4. doi: 10.1164/arrd.1982.126.6.1001. [DOI] [PubMed] [Google Scholar]
- 29.Akobeng AK. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007;5:644–7. doi: 10.1111/j.1651-2227.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 30.Wauters J, Wilmer A, Valenza F. Abdomino- thoracic transmission during ACS: Facts and figures. Acta Clin Belg. 2007;62:S200–5. doi: 10.1179/acb.2007.62.s1.026. [DOI] [PubMed] [Google Scholar]
- 31.Wiklund CU, Morel DR, Orbring-Wiklund H, Romand JA, Piriou V, Teboul JL, et al. Influence of tidal volume on pulse pressure variations in hypovolemic ventilated pigs with acute respiratory distress-like syndrome. Anesthesiology. 2010;113:630–8. doi: 10.1097/ALN.0b013e3181e908f6. [DOI] [PubMed] [Google Scholar]
- 32.Vallée F, Richard J-CM, Mari A, Gallas T, Arsac E, Verlaan PS, et al. Pulse pressure variations adjusted by alveolar driving pressure to assess fluid responsiveness. Intensive Care Med. 2009;35:1004–10. doi: 10.1007/s00134-009-1478-4. [DOI] [PubMed] [Google Scholar]
- 33.Vivier E, Metton O, Piriou V, Lhuillier F, Cottet-Emard JM, Branche P, et al. Effects of increased intra-abdominal pressure on central circulation. Br J Anaesth. 2006;96:701–7. doi: 10.1093/bja/ael071. [DOI] [PubMed] [Google Scholar]
- 34.Renner J, Cavus E, Meybohm P, Gruenewald M, Steinfath M, Scholz J, et al. Pulse pressure variation and stroke volume variation during different loading conditions in a paediatric animal model. Acta Anaesthesiol Scand. 2008;52:374–80. doi: 10.1111/j.1399-6576.2007.01544.x. [DOI] [PubMed] [Google Scholar]
- 35.Ridings PC, Bloomfield GL, Blocher CR, Sugerman HJ. Cardiopulmonary effects of raised intra-abdominal pressure before and after intravascular volume expansion. J Trauma. 1995;39:1071–5. doi: 10.1097/00005373-199512000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Kitano Y, Takata M, Sasaki N, Zhang Q, Yamamoto S, Miyasaka K. Influence of increased abdominal pressure on steady-state cardiac performance. J Appl Physiol. 1999;86:1651–6. doi: 10.1152/jappl.1999.86.5.1651. [DOI] [PubMed] [Google Scholar]
- 37.Renner J, Gruenewald M, Meybohm P, Hedderich J, Steinfath M, Scholz J, et al. Effect of elevated PEEP on dynamic variables of fluid responsiveness in a pediatric animal model. Paediatric Anaesth. 2008;18:1170–7. doi: 10.1111/j.1460-9592.2008.02770.x. [DOI] [PubMed] [Google Scholar]
- 38.De Robertis E, Liu JM, Blomquist S, Dahm PL, Thörne J, Jonson B. Elastic properties of the lung and the chest wall in young and adult healthy pigs. Eur Respir J. 2001;4:703–11. doi: 10.1183/09031936.01.17407030. [DOI] [PubMed] [Google Scholar]


