Abstract
AIM: To investigate the clinical usefulness of early endoscopic ultrasonography (EUS) in the management of acute biliary pancreatitis (ABP).
METHODS: All consecutive patients entering the emergency department between January 2010 and December 2012 due to acute abdominal pain and showing biochemical and/or radiological findings consistent with possible ABP were prospectively enrolled. Patients were classified as having a low, moderate, or high probability of common bile duct (CBD) stones, according to the established risk stratification. Exclusion criteria were: gastrectomy or patient in whom the cause of biliary obstruction was already identified by ultrasonography. All enrolled patients underwent EUS within 48 h of their admission. Endoscopic retrograde cholangiopancreatography was performed immediately after EUS only in those cases with proven CBD stones or sludge. The following parameters were investigated: (1) clinical: age, sex, fever; (2) radiological: dilated CBD; and (3) biochemical: bilirubin, AST, ALT, gGT, ALP, amylase, lipasis, PCR. Association between presence of CBD stone at EUS and the individual predictors were assessed by univariate logistic regression. Predictors significantly associated with CBD stones (P < 0.05) were entered in a multivariate logistic regression model.
RESULTS: A total of 181 patients with pancreatitis were admitted to the emergency department between January 2010 and December 2012. After exclusion criteria a total of 71 patients (38 females, 53.5%, mean age 58 ± 20.12 years, range 27-89 years; 33 males, 46.5%, mean age 65 ± 11.86 years, range 41-91 years) were included in the present study. The probability of CBD stones was considered low in 21 cases (29%), moderate in 26 (37%), and high in the remaining 24 (34%). The 71 patients included in the study underwent EUS, which allowed for a complete evaluation of the target sites in all the cases. The procedure was completed in a mean time of 14.7 min (range 9-34 min), without any notable complications.The overall CBD stone frequency was 44% (31 of 71), with a significant increase from the group at low pretest probability to that at moderate (OR = 5.79, P = 0.01) and high (OR = 4.25, P = 0.03) pretest probability.
CONCLUSION: Early EUS in ABP allows, if appropriate, immediate endoscopic treatment and significant spare of unnecessary operative procedures thus reducing possible related complications.
Keywords: Acute biliary pancreatitis, Choledocolithiasis, Common bile duct stones, Endoscopic retrograde cholangiography, Endoscopic ultrasonography
Core tip: The decision to perform an endoscopic retrograde cholangiopancreatography (ERCP) in acute biliary pancreatitis (ABP) is often based on biochemical and radiological criteria despite they have been shown to be unreliable predictors of common bile duct stone presence. Endoscopic ultrasonography (EUS) has recently been proposed as the new gold standard in the diagnosis of choledocholithiasis. Accordingly, the present prospective pilot study was designed to investigate the clinical usefulness of early EUS in the management of ABP. Early EUS-guided ERCP is an accurate, safe and quick strategy as a first step in the management of ABP.
INTRODUCTION
Acute biliary pancreatitis (ABP) is a potentially life-threatening condition caused by common bile duct (CBD) stones or sludge, which requires prompt diagnosis and treatment by endoscopic removal of the material. Accurate detection of CBD stones is warranted to select patients for early therapeutic endoscopic retrograde cholangiopancreatography (ERCP). For many years, ERCP with endoscopic sphincterotomy (ES) has been considered the best preoperative diagnostic tool for examination of the bile duct in patients with acute pancreatitis, but it is invasive, with complication rates of 5%-10% and mortality rates of up to 0.5%[1,2].
The management of patients with ABP is still a matter of debate, and in clinical practice it is often based on biochemical and radiological criteria[3]. However, studies have shown that commonly used biochemical and radiological predictors of the presence of CBD stones in patients with ABP may be unreliable[4].
Despite most guidelines and meta-analyses suggest that ERCP/ES is indicated in patients with ABP and coexisting cholangitis, a recent systematic review by van Geenen et al[5] revealed a lack of consensus on the role of routine early ERCP/ES in all patients with predicted severe ABP, regardless of cholestasis. The recently published ASGE guidelines on the role of endoscopy in the management of suspected choledocholithiasis indicate that clinicians should always perform a noninvasive test [endoscopic ultrasonography (EUS) or magnetic resonance cholangiopancreatography (MRCP)] in patients with ABP to select those who can benefit from ERCP/ES[6].
Accordingly, the present single-center prospective pilot study was designed to investigate the clinical usefulness of early EUS in the management of ABP.
MATERIALS AND METHODS
This prospective study was carried out at the Gastroenterology and Endoscopy Department of a secondary referral university hospital in northwestern Italy, with annual EUS and ERCP case loads of about 600 and 350, respectively. The study complied with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of our hospital. Written informed consent was obtained from all the patients enrolled. All consecutive patients entering the emergency department between January 2010 and December 2012 due to acute abdominal pain and showing biochemical and/or radiological findings consistent with possible ABP were prospectively enrolled. Patients were classified as having a low, moderate, or high probability of CBD stones, according to the established risk stratification[7,8]. Since our patient were all symptomatic for abdominal pain (as for inclusion criteria), serum AST and ALT level were indeed elevated in all the patients, therefore to better stratify the patients we have taken into account only the following markers: Bilirubin level and CBD dilation at transabdominal US, as previously described[9]. We therefore have considered patient at low risk if bilirubin level was < 2 mg/dL and CBD not dilated, high risk if bilirubin level was > 4 or > 2 with concomitant CBD dilation, intermediate risk any of the other combination.
Diagnosis of acute pancreatitis required two of the following three features: (1) abdominal pain characteristic of acute pancreatitis; (2) serum amylase and/or lipase ≥ 3 times the upper limit of normal; and (3) characteristic findings of acute pancreatitis on computed tomography (CT) scan, according to the guidelines[10]. The severity of acute pancreatitis was classified according to the Glasgow criteria[11].
A biliary etiology was defined as the presence of dilated CBD on ultrasonography (US) or CT or two of the following three laboratory abnormalities: (1) serum bilirubin concentration > 1.9 mg/dL; (2) alanine aminotransferase (ALT) activity > 100 U/L with an ALT activity higher than the aspartate aminotransferase (AST) activity; and (3) alkaline phosphatase activity > 195 U/L with a γ-glutamyltransferase (GGT) activity > 45 U/L. Patients with other causes of acute pancreatitis (e.g., alcohol abuse) and chronic pancreatitis (clinical history and CT) were excluded from the study[4].
Exclusion criteria were refusal to participate in the study, inability to give informed consent, unsuitability for endoscopy, previous history of gastrectomy, previous history of sphincterotomy, ongoing acute cholecystitis (defined as increased wall thickness upon US, altered morphology as double-track, vascular spot upon color Doppler, or the presence of pericholecystic fluid), cholangitis, or previous clear identification of a disorder responsible for biliary obstruction upon US or CT, severe pancreatitis. Prior cholecystectomy was not an exclusion criterion.
All enrolled patients underwent EUS within 48 h of their admission. CBD stones were diagnosed by the presence of a reproducible hyperechoic focus with an associated acoustic shadowing, whereas the lack of associated acoustic shadowing within the extrahepatic bile duct was considered consistent with biliary sludge[1]. The number and size of all the stones detected were recorded. ERCP was performed immediately after EUS only in those cases with proven CBD stones or sludge. Patients defined negative for EUS were followed for a 6-mo period with telephone calls at 1, 3, and 6 mo after EUS.
Patients with a definite diagnosis of cholecystolithiasis underwent laparoscopic or open cholecystectomy within 4 wk of their hospital discharge.
Procedures
All the EUS procedures were performed by two expert endosonographers with more than 5 years of experience in the procedure (A.A. and M.B.). The procedures were performed by means of a linear (GF-UCT140, Olympus Optical Co., Ltd., Tokyo, Japan) scanning echoendoscope using all frequencies (i.e., 5, 6, 7.5, and 10 MHz), but mainly 7.5 and 10 MHz to exclude CBD microlithiasis. The two endosonographers had extensive experience with linear probes (each about 100 examinations/year). After sedation by iv injection of midazolam (1-5 mg) and fentanyl (50-100 gamma), the EUS transducer was inserted up to the second duodenal portion and gradually withdrawn to visualize the main duodenal papilla, extrahepatic bile duct, cystic and hepatic ducts, and gallbladder. In case of CBD stone detection, therapeutic ERCP with ES and stone extraction was performed during the same endoscopic session under the same sedation by two independent investigators (A.A. and M.O.), with either 10 and 15 years of experience in the procedure. Cholangiography upon ERCP was obtained by injecting a 50%-diluted iodinated contrast agent (Ultravist iopromide injection; Bayer Healt Care), starting proximally in the duct in order to avoid pushing any small stones into the intrahepatic ducts.
Outcomes
Based on patient follow-up, we assessed the following two outcomes: (1) reliability of EUS, as an early approach in patients with ABP, to correctly identify the presence of CBD stones and consequent need of early ERCP with respect to the risk stratification based on clinical criteria; and (2) feasibility of the early sequential approach EUS/ERCP in diagnosis and treatment of CBD stones in the setting of ABP.
Statistical analysis
Clinical data were recorded in a computerized database by a single research assistant (M.G.). Continuous data are expressed as median with interquartile range, unless otherwise stated. Proportions are given as numbers and percentages. Differences in clinical parameters among the three risk groups were assessed by means of the Mann-Whitney U-test for continuous data or the chi-squared Fisher’s exact test, when appropriate, for proportions. All reported P-values are two-tailed. A P-value < 0.05 was considered statistically significant.
The following predictors were investigated: age, gender, bilirubin, AST, ALT, GGT, alkaline phosphatase, amylase, fever, and CBD dilation (defined as CBD diameter ≥ 6 mm or ≥ 8 mm in case of cholecystectomy, upon US). The maximum value for normal CBD diameter is still controversial, but generally accepted to be between 6 and 8 mm. Conventionally, the upper limit of normality for the CBD as measured by US is considered to be 6 mm. The possible association between EUS evidence of CBD stones and individual pre-procedure predictors was first assessed by univariate logistic regression analysis, and then by entering the variables significantly associated with CBD stones (P < 0.05) in a multivariate logistic regression model.
RESULTS
Patient recruitment
A total of 181 patients with pancreatitis were admitted to the emergency department between January 2010 and December 2012. Seventeen of them were excluded from the study because they were diagnosed as having severe pancreatitis and were recovered in others wards. Of the remaining 164 patients, 9 were excluded because of hypertriglyceridemic pancreatitis, 8 alcoholic pancreatitis, 3 chronic pancreatitis, and 1 autoimmune pancreatitis. Thus, 143 (88%) patients diagnosed as having ABP were considered eligible for the study, but 22 were excluded because of cholangitis, 10 because CT revealed choledocholithiasis, 32 because they were unsuitable for endoscopy, 5 because CT showed pancreatic neoplasia, 1 because CT found biliary prosthesis obstruction, and 2 did not give their consent. Accordingly, a total of 71 patients (38 females, 53.5%, mean age 58 ± 20.12 years, range 27-89 years; 33 males, 46.5%, mean age 65 ± 11.86 years, range 41-91 years) were included in the present study (Figure 1).
Figure 1.
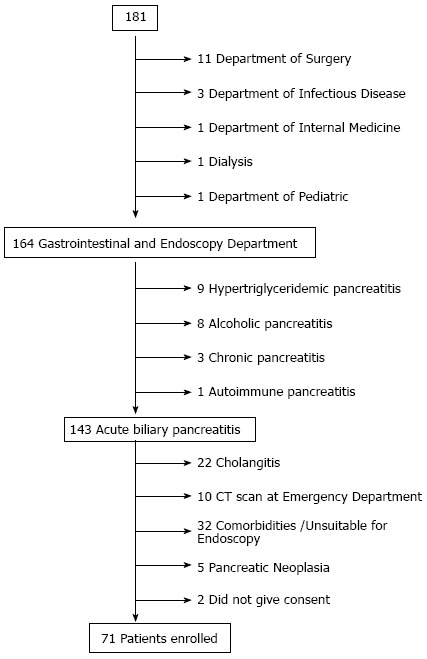
Patient recruitment flow chart.
The patient’s characteristics, with pertinent clinical and laboratory data, are summarized in Table 1. According to the Glasgow criteria[11], all patients were diagnosed as having mild pancreatitis. Based on the previously described criteria, the probability of CBD stones was considered low in 21 cases (29%), moderate in 26 (37%), and high in the remaining 24 (34%).
Table 1.
Characteristics of the 71 patients according to the level of risk for common bile duct stones
| Patient features |
Risk of common bile duct stones |
||
| Low (n = 21; 29%) | Moderate (n = 26; 37%) | High (n = 24; 34%) | |
| Age (yr, mean ± SD) | 59 ± 17 | 59 ± 17 | 62 ± 18 |
| Male, n (%) | 9 (43) | 12 (46) | 13 (54) |
| Bilirubin (mg/dL, mean ± SD) | 1.3 ± 0.5 | 2.1 ± 1.4 | 5.0 ± 1.7 |
| AST (IU/mL, mean ± SD) | 201 ± 186 | 232 ± 175 | 237 ± 237 |
| ALT (IU/mL, mean ± SD) | 169 ± 182 | 237 ± 146 | 302 ± 268 |
| Alkaline phosphatase (U/L, mean ± SD) | 341 ± 183 | 328 ± 192 | 495 ± 316 |
| GGT (IU/mL, mean ± SD) | 306 ± 253 | 634 ± 167 | 541 ± 390 |
| Fever | 1 | 1 | 2 |
| Previous cholecystectomy | 1 | 2 | 2 |
| Common bile duct dilation | 0 | 14 | 11 |
| Gallstones on US | 13 | 20 | 23 |
| Gallstones on EUS in patients negative on US | 2 | 0 | 0 |
AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; GGT: γ-glutamyltransferase; US: Ultrasonography; EUS: Endoscopic ultrasonography.
In detail, no statistically significant differences were found among the three groups concerning age (P = 0.20, Bonferroni test), sex (P = 0.38, Fisher exact test), comorbidities (P = 0.29, Fisher exact test), or cholecystolithiasis (P = 0.07, Fisher exact test). Dilatation of CBD was present in about half the patients included in the moderate and high risk groups. Five of those patients had already undergone cholecystectomy 3 mo to 43 years earlier, and 2 had been previously treated by sphincterotomy for CBD stones 3 mo to 15 years earlier. Gallstones were present in more than 50% of patients belonging to each group.
The 71 patients included in the study underwent EUS, which allowed for a complete evaluation of the target sites in all the cases. The procedure was completed in a mean time of 14.7 min (range 9-34 min), without any notable complications.
The overall CBD stone frequency was 44% (31 of 71), with a significant increase from the group at low pretest probability to that at moderate (OR = 5.79, P = 0.01) and high (OR = 4.25, P = 0.03) pretest probability. The pretest probability of CBD stones in the low-risk group was 19%, in the moderate-risk group 58%, and in the high-risk group 50% (Table 2). EUS detected CBD stones in 4 low-risk patients and found none in 12 high-risk patients.
Table 2.
Common bile duct stones detected on endoscopic ultrasonography, according to the predetermined level of risk n (%)
| EUS |
Results |
||
| Low risk | Moderate risk | High risk | |
| Neg. | 17 (81) | 11 (42) | 12 (50) |
| Pos. | 4 (19) | 15 (58) | 12 (50) |
| Total | 21 | 26 | 24 |
Univariate logistic regression indicated that CBD diameter, cholecystolithiasis, and risk (moderate and high) were significantly associated with CBD stones. In the subsequent multivariate analysis, the CBD diameter was confirmed as an independent predictor of CBD stones (Z = 3.91; P < 0.001; 95%CI: 3.12-30.67), with an odds ratio of 9.78 (stepwise forward selection).
All patients who had positive EUS results for CBD stones underwent ERCP and ES (Figure 2). EUS findings were confirmed by ERCP in 28 of 31 patients with CBD stones (90%), and a total of 47 stones (mean ± SD = 4.6 ± 2.2 mm in diameter) were extracted (Table 3). The number of the stones was similar among the three risk groups, and the size of stones did not increase significantly between the risk groups (one-way ANOVA, F2,28 = 1.80, P = 0.18).
Figure 2.
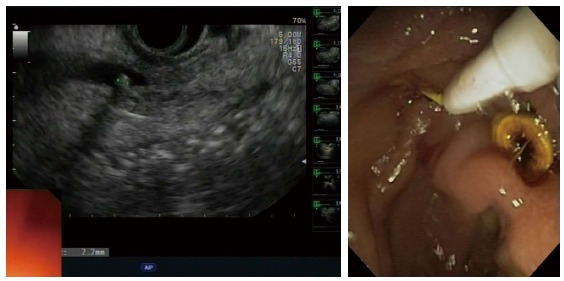
Endoscopic ultrasonography findings of common bile duct stone of 2.2 mm in a nondilated common bile duct (left); common bile duct stone extraction during endoscopic retrograde cholangiopancreatography (right).
Table 3.
Mean number and size (mm) of stone(s) in the 31 patients with common bile duct stones according to the predetermined level of risk
| Risk of common bile duct stones | Number, mean ± SD | Size, mean ± SD |
| Low (n = 4; 19%) | 1.5 ± 0.6 | 3.5 ± 3.9 |
| Moderate (n = 15; 58%) | 1.5 ± 0.5 | 4.2 ± 1.8 |
| High (n = 12; 50%) | 1.5 ± 0.5 | 5.6 ± 2.6 |
| Overall (n = 31; 44%) | 1.5 ± 0.5 | 4.6 ± 2.2 |
In 3 patients (2 at moderate, 1 at high risk for CBD stones), CBD stones were not found after both cholangiography and ES, despite repeated passages with a Dormia basket or a balloon catheter. Another 2 patients who had been negative at cholangiography (1 with high probability and 1 with moderate probability of CBD stones) were positive for CBD stones only after ES. Five patients with prior cholecystectomy (Table 1) had CBD stones at EUS and ERCP.
Only 3 of 31 patients (9%) who underwent ERCP had procedure-related uneventful complications (post-sphincterotomy self-limiting bleeding in 1 patient, transient hepatic enzyme increase in 1 patient, and transient oxygen desaturation in 1 patient).
The 40 patients who tested negative for CBD stones at EUS were closely monitored for 1 wk after the EUS procedure. Once discharged, these patients were followed for a 6-mo period with telephone calls at 1, 3, and 6 mo after EUS. All the patients were followed regularly for 6 mo or until they either underwent cholecystectomy or a second episode of biliary colic or ABP. During this follow-up none of the patients with high risk of CBD stones at pre-EUS stratification and negative EUS had new episodes of biliary or cholic pancreatitis in the follow-up.
DISCUSSION
Acute pancreatitis is a potentially life-threatening condition, with a reported mortality as high as 20%-30% in severe cases[12-14]. Gallstone disease represents the most common cause of acute pancreatitis, and the duration of bile duct obstruction, which is responsible for the increased pressure in the pancreatic duct, seems to be the main factor contributing to the severity of pancreatitis.
In guidelines published in the New England Journal of Medicine[15], the Indianapolis group proposed an early ERCP (within 24-48 h from onset of symptoms) in patients supposed to have cholangitis (fever, jaundice, and sepsis) or persistent biliary obstruction (conjugated bilirubin level > 5 mg/dL) and to consider it in patients with worsening clinical symptoms (e.g., worsening pain, leukocytosis, and change in vital signs). However, fever, tachycardia, tachypnea, and leucocytosis associated with systemic inflammatory response syndrome may occur early in the course of acute pancreatitis and may be indistinguishable from sepsis syndrome. According to the recent guidelines, ERCP is indicated in biliary pancreatitis with CBD obstruction and/or in the presence of cholangitis[16].
Several recent studies have identified clinical, biochemical, and radiological tests to predict the presence of CBD stones in patients presenting with biliary pain[7,17]. The recommended approach is to perform therapeutic ERCP in high-risk patients, to carry out MRCP or EUS prior to ERCP in moderate-risk patients, and to clinically observe low-risk patients[18].
Both MRCP and EUS are now indicated as the best imaging methods for CBD stone detection[10,19], and they have been proposed as alternative noninvasive tests to assess the presence of CBD stones[20-22]. MRCP and EUS were demonstrated to have high diagnostic accuracy[20,23-25], with similar sensitivity, specificity, accuracy, negative predictive value, and positive predictive value for detection of CBD stones[26]. However, EUS is more accurate than MRCP in the detection of small stones (< 5 mm), which are responsible for at least half of all cases of ABP. Other imaging methods to detect CBD stones in patients with suspected ABP are CT scan and helical CT scan. Although helical CT scan was shown to be more accurate than US and conventional CT scan by Tse et al[8], it has never been compared to MRCP or EUS, which do not expose the patient to radiation or contrast medium.
Therefore, EUS has recently been proposed as the new gold standard in the diagnosis of choledocholithiasis[27]. Furthermore, some reports showed a superiority of EUS for small stones and biliary sludge, especially if the bile duct is not dilated[26]. In particular, EUS has emerged as an accurate diagnostic tool, as demonstrated by the results of a recent meta-analysis[28]. Moreover the use of EUS significantly reduces the risk of overall complications of interventional ERCP; by performing EUS first, ERCP may be safely avoided in two-thirds of patients with suspected CBD stones[21].
Our study confirms the value of early EUS to correctly stratify patients with mild ABP for the presence of CBD stones. In particular, it is worth noting that 20% of patients stratified in the low-risk group according to clinical parameters were found to have CBD stones by EUS, thus undergoing ERCP and avoiding the risk of further pancreatic damage. By contrast, in 50% of patients allocated in the high-risk group based on clinical parameters, CBD stones were not found by EUS, thus avoiding unnecessary ERCP.
Our data confirm that the commonly used clinical, biochemical and radiological predictors of the presence of choledocholithiasis are unreliable for predicting the presence of CBD stones, with the exception of CBD dilatation at transabdominal US. All the other predictors, including bilirubin, AST, ALT, GGT, alkaline phosphatase, and cholecystolithiasis at transabdominal US were not significantly associated with CBD stones.
Our study showed that the pretest probability of CBD stones in the low-risk group was about 20%, in the moderate-risk group about 58%, and in the high-risk group 50%; these data confirm only in part the probability rates in the three risk levels published by the ASGE (10%, 50%, and > 50%, respectively)[6]. As expected, the relative risk of having choledocholithiasis was 3.03 between the low- and the moderate-risk group and 2.63 between the low- and the high-risk group. The relative risk between the moderate- and the high-risk group was 0.86. These findings highlight that the risk increases from the low-risk to other risk groups, but it does not occur between the moderate- and high-risk groups.
In the group of patients with normal gallbladder (no cholecystolithiasis) at US, EUS revealed microlithiasis in 20% of patients (2/10), thus allowing a better evaluation and diagnosis of such cases[29]. No association was found between the size of stones and the risk groups.
In our study, 3 patients (2 in the moderate-risk group, 1 in the high-risk group) who had positive EUS results for choledocholithiasis did not have CBD stones at cholangiography and after sphincterotomy. These 3 patients can be considered as false-positive on EUS, although for technical reasons ERCP can miss small stones (< 5 mm) either at cholangiography or after sphincterotomy. Moreover, cholangiography did not detect the presence of stones in 2 patients who were positive after sphincterotomy. This is due to the lower sensitivity of cholangiography compared to EUS[18,30,31].
Our study has also shown that all 40 patients negative for choledocholithiasis on EUS improved and showed the normalization of biochemical values within 7 d of hospitalization. Furthermore, all patients negative for CBD stones on EUS who underwent cholecystectomy did not present a second biliary episode for 6 mo. In particular, patients with high risk for CBD stones according to clinical parameters but negative EUS results were strictly followed for 6 mo after index examination: none of them experienced a second episode of ABP or biliary cholic, further validating the role of EUS as a good predictor of choledocholithiasis in the setting of ABP.
However, our study had several limitations. First, these findings represent the experience of a single center with the potential limitation of a small number of patients enrolled. Another limitation could be that a second diagnostic test to confirm the absence of CBD stones in negative EUS patients was not scheduled. However, the only reliable test in this setting, notably MRI, is not a true gold standard mainly due to its low sensitivity for microlithiasis[26].
In conclusion, the present results confirm that EUS-guided ERCP is an accurate, safe, and quick strategy as a first step in the management of patients with ABP. This approach allows clinicians not only to identify patients who will benefit from therapeutic ERCP, thus reducing the risk of further pancreatic damage, but also to select patients who do not need ERCP, thus avoiding unnecessary operative procedures and possible related complications. Therefore, as the current guidelines suggest, we confirm that EUS should be considered as a routine procedure for all patients with ABP. Ideally the gastroenterologists responsible for ERCP should be trained in EUS and vice versa. In cases of ABP, an early EUS approach could also be relevant terms of cost-effectiveness. Although a cost analysis has not been performed in this study, EUS offers the advantage of sharing the same sedation procedures as ERCP, so that patients can be submitted to both exams in the same session.
COMMENTS
Background
The role and timing of endoscopy in the setting of acute biliary pancreatitis (ABP) is still being debated. In clinical practice the decision to perform an endoscopic retrograde cholangiopancreatography (ERCP) is often based on biochemical and radiological criteria despite they already have been shown to be unreliable predictors of common bile duct (CBD) stone presence. Endoscopic ultrasonography (EUS) has recently been proposed as the new gold standard in the diagnosis of choledocholithiasis. Accordingly, the present single-center prospective pilot study was designed to investigate the clinical usefulness of early EUS in the management of ABP.
Research frontiers
Accurate prediction of CBD stones is warranted to select patients for early therapeutic ERCP. Other noninvasive (or minimally invasive) imaging techniques such as EUS and MRCP have been used to select patients for therapeutic ERCP to minimize the risk of complications associated with unnecessary diagnostic ERCPs. However, EUS is more accurate than MRCP in the detection of small stones (< 5 mm), which are responsible for at least half of all cases of ABP.
Innovations and breakthroughs
Early EUS in ABP allows, if appropriate, immediate endoscopic treatment and significant spare of unnecessary operative procedures thus reducing possible related complications.
Applications
A preliminary EUS may help in decision-making: if a stone is present, ERCP with extraction can be performed in the same endoscopic session, whereas if no stone is found, the patient can be spared the added risk, which is important in terms of cost effectiveness.
Peer-review
The study tried to clarify a very controversial problem such as the best diagnostic approach to CDS and pancreatitis. The idea is interesting and there is a lack of consensus in the literature considering this issue The study is well organized and present very interesting data. The article is well written, conclusions are practical and clear.
Footnotes
Institutional review board statement: The study complied with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of our hospital.
Clinical trial registration statement: NCT02430285.
Informed consent statement: Written informed consent was obtained from all the patients enrolled.
Conflict-of-interest statement: None declared.
Data sharing statement: Consent for data sharing was not obtained but this was a single centre pilot study and the presented data are anonymized and the risk of identification is low.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 10, 2015
First decision: April 1, 2015
Article in press: June 10, 2015
P- Reviewer: Basoli A, Seicean A, van Erpecum K S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.Fabbri C, Polifemo AM, Luigiano C, Cennamo V, Fuccio L, Billi P, Maimone A, Ghersi S, Macchia S, Mwangemi C, et al. Single session versus separate session endoscopic ultrasonography plus endoscopic retrograde cholangiography in patients with low to moderate risk for choledocholithiasis. J Gastroenterol Hepatol. 2009;24:1107–1112. doi: 10.1111/j.1440-1746.2009.05828.x. [DOI] [PubMed] [Google Scholar]
- 2.Kwon CI, Song SH, Hahm KB, Ko KH. Unusual complications related to endoscopic retrograde cholangiopancreatography and its endoscopic treatment. Clin Endosc. 2013;46:251–259. doi: 10.5946/ce.2013.46.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang MH, Chen TH, Wang SE, Tsai YF, Su CH, Wu CW, Lui WY, Shyr YM. Biochemical predictors for absence of common bile duct stones in patients undergoing laparoscopic cholecystectomy. Surg Endosc. 2008;22:1620–1624. doi: 10.1007/s00464-007-9665-2. [DOI] [PubMed] [Google Scholar]
- 4.van Santvoort HC, Bakker OJ, Besselink MG, Bollen TL, Fischer K, Nieuwenhuijs VB, Gooszen HG, Erpecum KJ. Prediction of common bile duct stones in the earliest stages of acute biliary pancreatitis. Endoscopy. 2011;43:8–13. doi: 10.1055/s-0030-1255866. [DOI] [PubMed] [Google Scholar]
- 5.van Geenen EJ, van Santvoort HC, Besselink MG, van der Peet DL, van Erpecum KJ, Fockens P, Mulder CJ, Bruno MJ. Lack of consensus on the role of endoscopic retrograde cholangiography in acute biliary pancreatitis in published meta-analyses and guidelines: a systematic review. Pancreas. 2013;42:774–780. doi: 10.1097/MPA.0b013e318287d208. [DOI] [PubMed] [Google Scholar]
- 6.Maple JT, Ben-Menachem T, Anderson MA, Appalaneni V, Banerjee S, Cash BD, Fisher L, Harrison ME, Fanelli RD, Fukami N, et al. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc. 2010;71:1–9. doi: 10.1016/j.gie.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 7.Barkun AN, Barkun JS, Fried GM, Ghitulescu G, Steinmetz O, Pham C, Meakins JL, Goresky CA. Useful predictors of bile duct stones in patients undergoing laparoscopic cholecystectomy. McGill Gallstone Treatment Group. Ann Surg. 1994;220:32–39. doi: 10.1097/00000658-199407000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tse F, Barkun JS, Barkun AN. The elective evaluation of patients with suspected choledocholithiasis undergoing laparoscopic cholecystectomy. Gastrointest Endosc. 2004;60:437–448. doi: 10.1016/s0016-5107(04)01457-9. [DOI] [PubMed] [Google Scholar]
- 9.Anderloni A, Ballarè M, Pagliarulo M, Conte D, Galeazzi M, Orsello M, Andorno S, Del Piano M. Prospective evaluation of early endoscopic ultrasonography for triage in suspected choledocholithiasis: results from a large single centre series. Dig Liver Dis. 2014;46:335–339. doi: 10.1016/j.dld.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 11.Corfield AP, Cooper MJ, Williamson RC, Mayer AD, McMahon MJ, Dickson AP, Shearer MG, Imrie CW. Prediction of severity in acute pancreatitis: prospective comparison of three prognostic indices. Lancet. 1985;2:403–407. doi: 10.1016/s0140-6736(85)92733-3. [DOI] [PubMed] [Google Scholar]
- 12.Hirano T, Manabe T. A possible mechanism for gallstone pancreatitis: repeated short-term pancreaticobiliary duct obstruction with exocrine stimulation in rats. Proc Soc Exp Biol Med. 1993;202:246–252. doi: 10.3181/00379727-202-43534. [DOI] [PubMed] [Google Scholar]
- 13.Runzi M, Niebel W, Goebell H, Gerken G, Layer P. Severe acute pancreatitis: nonsurgical treatment of infected necroses. Pancreas. 2005;30:195–199. doi: 10.1097/01.mpa.0000153613.17643.b3. [DOI] [PubMed] [Google Scholar]
- 14.Senninger N, Moody FG, Coelho JC, Van Buren DH. The role of biliary obstruction in the pathogenesis of acute pancreatitis in the opossum. Surgery. 1986;99:688–693. [PubMed] [Google Scholar]
- 15.Yaghoobi M. ERCP for gallstone pancreatitis. N Engl J Med. 2014;370:1955–1956. doi: 10.1056/NEJMc1403445. [DOI] [PubMed] [Google Scholar]
- 16.Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–115; 1416. doi: 10.1038/ajg.2013.218. [DOI] [PubMed] [Google Scholar]
- 17.Canto MI, Chak A, Stellato T, Sivak MV. Endoscopic ultrasonography versus cholangiography for the diagnosis of choledocholithiasis. Gastrointest Endosc. 1998;47:439–448. doi: 10.1016/s0016-5107(98)70242-1. [DOI] [PubMed] [Google Scholar]
- 18.De Lisi S, Leandro G, Buscarini E. Endoscopic ultrasonography versus endoscopic retrograde cholangiopancreatography in acute biliary pancreatitis: a systematic review. Eur J Gastroenterol Hepatol. 2011;23:367–374. doi: 10.1097/MEG.0b013e3283460129. [DOI] [PubMed] [Google Scholar]
- 19.Fusaroli P, Kypraios D, Caletti G, Eloubeidi MA. Pancreatico-biliary endoscopic ultrasound: a systematic review of the levels of evidence, performance and outcomes. World J Gastroenterol. 2012;18:4243–4256. doi: 10.3748/wjg.v18.i32.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pezzilli R, Billi P, Barakat B, Baroncini D, D’Imperio N, Miglio F. Effects of early ductal decompression in human biliary acute pancreatitis. Pancreas. 1998;16:165–168. doi: 10.1097/00006676-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Liu CL, Fan ST, Lo CM, Tso WK, Wong Y, Poon RT, Lam CM, Wong BC, Wong J. Comparison of early endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in the management of acute biliary pancreatitis: a prospective randomized study. Clin Gastroenterol Hepatol. 2005;3:1238–1244. doi: 10.1016/s1542-3565(05)00619-1. [DOI] [PubMed] [Google Scholar]
- 22.Güngör B, Cağlayan K, Polat C, Seren D, Erzurumlu K, Malazgirt Z. The predictivity of serum biochemical markers in acute biliary pancreatitis. ISRN Gastroenterol. 2011;2011:279607. doi: 10.5402/2011/279607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol. 2010;16:4888–4891. doi: 10.3748/wjg.v16.i39.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu CL, Lo CM, Chan JK, Poon RT, Lam CM, Fan ST, Wong J. Detection of choledocholithiasis by EUS in acute pancreatitis: a prospective evaluation in 100 consecutive patients. Gastrointest Endosc. 2001;54:325–330. doi: 10.1067/mge.2001.117513. [DOI] [PubMed] [Google Scholar]
- 25.Vázquez-Sequeiros E, González-Panizo Tamargo F, Boixeda-Miquel D, Milicua JM. Diagnostic accuracy and therapeutic impact of endoscopic ultrasonography in patients with intermediate suspicion of choledocholithiasis and absence of findings in magnetic resonance cholangiography. Rev Esp Enferm Dig. 2011;103:464–471. doi: 10.4321/s1130-01082011000900005. [DOI] [PubMed] [Google Scholar]
- 26.Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc. 2006;64:248–254. doi: 10.1016/j.gie.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 27.Gabbrielli A, Pezzilli R, Uomo G, Zerbi A, Frulloni L, Rai PD, Castoldi L, Costamagna G, Bassi C, Carlo VD. ERCP in acute pancreatitis: What takes place in routine clinical practice? World J Gastrointest Endosc. 2010;2:308–313. doi: 10.4253/wjge.v2.i9.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moon JH, Cho YD, Cha SW, Cheon YK, Ahn HC, Kim YS, Kim YS, Lee JS, Lee MS, Lee HK, et al. The detection of bile duct stones in suspected biliary pancreatitis: comparison of MRCP, ERCP, and intraductal US. Am J Gastroenterol. 2005;100:1051–1057. doi: 10.1111/j.1572-0241.2005.41057.x. [DOI] [PubMed] [Google Scholar]
- 29.del Pozo D, Tabernero S, Poves E, Sanz C, Beceiro I, Costero B, Villafruela M, Borrego G. Usefulness of endoscopic ultrasonography in the clinical suspicion of biliary disease. Rev Esp Enferm Dig. 2011;103:345–348. doi: 10.4321/s1130-01082011000700002. [DOI] [PubMed] [Google Scholar]
- 30.Şurlin V, Săftoiu A, Dumitrescu D. Imaging tests for accurate diagnosis of acute biliary pancreatitis. World J Gastroenterol. 2014;20:16544–16549. doi: 10.3748/wjg.v20.i44.16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimura Y, Arata S, Takada T, Hirata K, Yoshida M, Mayumi T, Hirota M, Takeda K, Gabata T, Amano H, et al. Gallstone-induced acute pancreatitis. J Hepatobiliary Pancreat Sci. 2010;17:60–69. doi: 10.1007/s00534-009-0217-0. [DOI] [PubMed] [Google Scholar]


