Abstract
AIM: To assess the correlation between decreased Muc5AC expression and patients’ survival and clinicopathological characteristics by conducting a meta-analysis.
METHODS: Literature searches were performed in PubMed and EMBASE, and 11 studies met our criteria. Summary hazard ratios or odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to estimate the effect. For the pooled analysis of the correlation between decreased Muc5AC expression and clinicopathological characteristics (tumour invasion depth, lymph node metastasis, tumour-node-metastasis stage, tumour size, venous invasion and lymphatic invasion), ORs and their variance were combined to estimate the effect.
RESULTS: Eleven retrospective cohort studies comprising 2135 patients were included to assess the association between Muc5AC expression and overall survival and/or clinicopathological characteristics. Decreased Muc5AC expression was significantly correlated with poor overall survival of gastric cancer patients (pooled HR = 1.35, 95%CI: 1.08-1.7). Moreover, decreased Muc5AC expression was also significantly associated with tumour invasion depth (pooled OR = 2.12, 95%CI: 1.56-2.87) and lymph node metastasis (pooled OR = 1.56, 95%CI: 1.00-2.44) in gastric cancer.
CONCLUSION: Decreased Muc5AC expression might be a poor prognostic predictor for gastric cancer.
Keywords: Prognosis, Muc5AC, Gastric cancer
Core tip: The association of decreased Muc5AC expression in gastric cancer and its prognostic value have been investigated for years; however, the results are controversial and inconclusive. To the best of our knowledge, this is the first meta-analysis suggesting that decreased Muc5AC expression is an unfavourable prognostic biomarker for gastric cancer patients. Patients with decreased Muc5AC expression are more likely to have poor overall survival and aggressive histopathological features.
INTRODUCTION
The incidence rate of gastric cancer has decreased substantially today as a result of healthy diet, reduction of Helicobacter pylori (H. pylori) infection and introduction of screening using photofluorography. However, 989600 new gastric cancer cases are estimated to have occurred in 2008. Although the highest incidence rates were observed in East Asia, gastric cancer is still the second most frequent cause of cancer-related death worldwide, leading to 738000 deaths in 2008[1,2]. Despite the availability of new treatments[3], the 5-year survival rate for gastric cancer patients remains lower than 25%[2]. It is imperative to identify an established marker possessing predicative value for the survival of gastric cancer patients. The prognosis of gastric cancer depends mostly on the histopathological grade and the stage. However, these findings are not always sufficient to predict the probability of relapse, metastasis and overall survival[4-6]. Therefore, new prognostic factors are needed and have been detected in many studies. Furthermore, the discovery of new prognostic factors may aid in a more accurate prediction of clinical outcome and may also reveal new therapeutic targets[7]. Many studies have evaluated prognostic markers associated with clinical outcomes, typically overall survival, in gastric cancer. Of these, Muc5AC, considered a very important prognostic marker, has been widely investigated.
Mucins are a group of diverse, complex, high molecular-weight glycoproteins that are major components of the mucus gel covering the gastric mucosa. Normal functions of mucins include protection against mechanical and chemical aggression, lubrication and acid resistance. Two forms of mucins have been reported: secreted and membrane-bound[8,9]. Muc5AC is a secreted gel-forming mucin and is limited to the cytoplasm of the foveolar epithelium and mucous neck cells throughout the stomach. Besides being a protective layer and a diffusion barrier for hydrochloric acid, Muc5AC was also reported to suppress the release of tumour cells, resulting in a decrease in invasion and metastasis[10-12]. The process of neoplastic transformation in the stomach is associated with decreased expression of Muc5AC. Muc5AC expression was reported to be absent or low in some gastric cancer cases[13-15]. Controversies and conflicting results about the prognostic role of low Muc5AC expression in gastric cancer have been published. Kocer et al[16] reported that gastric cancer patients with decreased Muc5AC expression have better overall survival. However, Suh et al[17] reported that decreased Muc5AC expression possesses no prognostic significance in determining the overall survival of gastric cancer patients. Wang et al[18] reported that decreased Muc5AC expression was a poor prognostic marker in gastric cancer. Thus we conducted this meta-analysis to assess the prognostic significance of decreased Muc5AC expression for gastric cancer patients.
MATERIALS AND METHODS
Literature retrieval
We performed a computerized search in PubMed and EMBASE to identify studies by using the terms “Muc5AC,” “Mucin 5AC,” “Muc-5AC,” “gastric cancer,” “gastric carcinoma,” “gastric neoplasm,” “stomach cancer” and “prognosis”, “prognostic” and “survival” on November 8th, 2013. We did not consider conference abstracts because of limited data reported in them.
Eligibility criteria
Studies meeting the following criteria were included: (1) the correlation between MUC5AC expression and overall survival and/or clinicopathological characteristics of gastric cancer was evaluated; (2) MUC5AC expression was measured by immunohistochemistry (IHC) in the primary tumour tissues; (3) sufficient information was provided to allow for estimation of hazard ratios (HRs) and/or odds ratios (ORs) and their invariance; and (4) study was published as a full paper in the English language. Studies were excluded for the following reasons: (1) duplicate; (2) cell or animal experiment; and (3) letters to the editor or reviews.
Data extraction and management
Articles were reviewed independently by two investigators (He KC and Zhang CT) for data extraction. Disagreements were resolved by consensus. Data were extracted from eligible studies independently by the two investigators. If the results reported in the identified studies overlapped (e.g., same authors and institutions), only the most recent or the most complete study was involved in the analysis. The two investigators reviewed each eligible study and extracted data into a table that included the author’s name, year of publication, study location, number of patients, medium age, cut-off value and primary antibody.
Statistical analysis
For the quantitative aggregation of the survival outcome, the ability of decreased Muc5AC expression to predict survival was measured by HR, which was pooled from the HR estimates of included studies using the fixed effects model with the assumption of the heterogeneity of the HR estimates[19]. The between-study heterogeneity was tested by performing I2 and χ2 measures. A random effect model was performed when the P-value of heterogeneity test was < 0.1[20]. For each study, the log hazard ratio estimate and its standard error (SE) were calculated from the data offered in the manuscript or read from Kaplan-Meier survival curve using an approach reported in a previous publication[21]. Subgroup analyses by stratifying on study location, number of patients and cut-off value were conducted. For the pooled analysis of the correlations between decreased Muc5AC expression and clinicopathological characteristics (depth of tumour invasion, lymph node metastasis, tumour-node-metastasis (TNM) stage, tumour size, venous invasion and lymphatic invasion), ORs and their variance were combined to estimate the effect[22].
An observed HR or OR > 1 implied a worse prognosis for the group with decreased Muc5AC expression and would be considered to be statistically significant if the 95%CI did not cross 1. These results are presented in forest plot graphs and the funnel plot was examined to explore the possibility of publication bias. In addition, sensitivity analysis was also conducted by sequential omission of individual studies to evaluate stability of the results. All statistical analyses with a P-value < 0.05 were considered statistically significant. Statistical analyses were performed using Review Manager (RevMan) Version 5.1.
RESULTS
Eligible studies and characteristics
As shown in Figure 1, a total of 132 studies were identified using the search strategy as described above. After the first screening, 79 citations were excluded from analysis based on abstracts, leaving 53 studies for full-text review. Upon further review, 18 were excluded because there was no data about survival analysis or clinicopathological characteristics, 16 were excluded because of insufficient reported data to calculate HR or OR, five were excluded because their data overlapped with other studies and one was excluded because the authors assessed Muc5AC expression using a method other than IHC. After careful review, 11 retrospective studies were enrolled to assess the prognostic value of Muc5AC expression in gastric cancer[12,16-18,23-29], and no prospective study was found.
Figure 1.
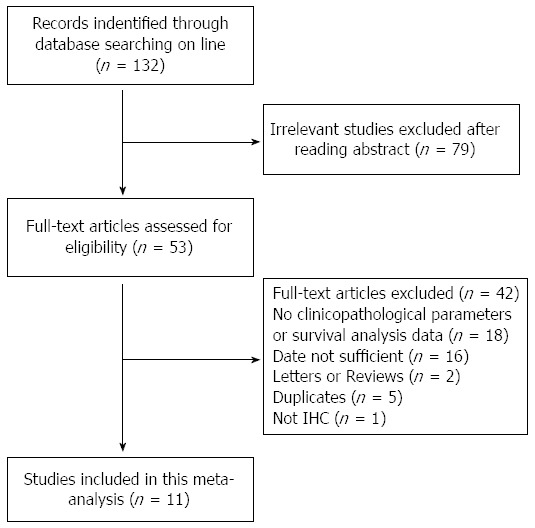
Flow chart of the study selection process.
The characteristics of included studies eligible for the meta-analysis are presented in Table 1. Four studies evaluated the patients from Korea, two from Turkey, two from Japan, two from China and one from Germany. The 11 studies contained 2135 patients with sample sizes ranging from 44 to 450 patients. Two studies had data only for overall survival, while five studies had data only for the clinicopathological characteristics. Four studies with data on overall survival also had data on clinicopathological characteristics. In particular, Dae and Sung used the same patients sample and we extracted the HR from the Dae’s study and clinicopathological parameters from Sung’s.
Table 1.
Characteristics of the included studies
| No. | Ref. | Year | Area | No. of patients | Gender M/F | Medium age | Therapy before surgical resection | Antibody used | Cut-off value | Purpose |
| 1 | Baldus et al[23] | 2002 | Germany | 200 | 107/93 | 60.8 | No | CLH2 | 35% | C/HR |
| 2 | Dae et al[25] | 2013 | South Korea | 412 | 286/126 | 58.5 | No | CLH2 | 10% | HR |
| 3 | Ilhan et al[24] | 2010 | Turkey | 257 | 201/56 | NR | No | 45M1 | 5% | C |
| 4 | Kocer et al[16] | 2004 | Turkey | 44 | 31/13 | 59.75 | No | 45M1 | 10% | C/HR |
| 5 | Lee et al[26] | 2001 | South Korea | 300 | 203/97 | NR | NR | CLH2 | 20% | C |
| 6 | Shiratsu et al[27] | 2013 | Japan | 214 | 150/64 | NR | No | CLH2 | 5% | C/HR |
| 7 | Suh et al[17] | 2012 | South Korea | 450 | 328/122 | 57.5 | No | NR | 10% | HR |
| 8 | Sung et al[12] | 2013 | South Korea | 412 | 286/126 | 58.5 | No | CLH2 | 5% | C |
| 9 | Tajima et al[28] | 2001 | Japan | 136 | 82/54 | 61.7 | No | 45M1 | 10% | C |
| 10 | Wang et al[18] | 2003 | China | 76 | 52/24 | 65.0 | NR | 45M1 | 5% | C/HR |
| 11 | Wang et al[29] | 2003 | China | 46 | 34/12 | 54.6 | NR | 45M1 | 0% | C |
NR: Not reported; M: Male; F: Female; C: Clinicopathological characteristics; HR: Hazard ratio.
Correlation between Muc5AC expression and overall survival
A forest plot of the HR estimates and results from the meta-analysis is presented in Figure 2. Six studies (n = 1396) of correlation between decreased Muc5AC expression and overall survival were included to conduct a quantitative aggregation of the survival results. The gastric cancer patients with decreased Muc5AC expression demonstrated a significantly poorer overall survival than those with preserved Muc5AC expression (pooled HR = 1.35, 95%CI: 1.08-1.7, fixed effects). The between-heterogeneity was nonsignificant (I2 = 31%, P = 0.21). We then performed subgroup analysis by study location, cut-off value and number of patients (Table 2). Subgroup analysis indicated that there still was a significant relation between decreased Muc5AC expression and overall survival of patients from east Asian countries (pooled HR = 1.39, 95%CI: 1.03-1.88, fixed effects), from the studies with more than 200 patients (pooled HR = 1.34, 95%CI: 1.05-1.71, fixed effects) and from the studies with cut-off values > 10% (pooled HR = 1.32, 95%CI: 1.03-1.7, fixed effects).
Figure 2.
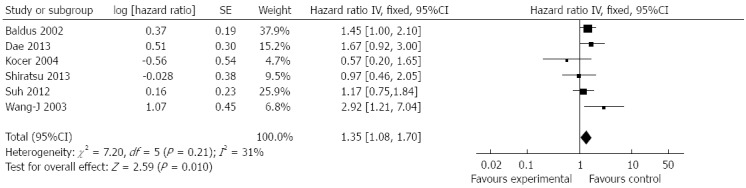
Forest plot of hazard ratio for association between decreased Muc5AC expression and overall survival of patients with gastric cancer.
Table 2.
Subgroup analysis of pooled hazard ratio in gastric cancer patients with decreased Muc5AC expression
| No. of studies | No. of patients | Pooled HR (95%CI) |
Heterogeneity |
||
| I2 | P-value | ||||
| Study location | |||||
| East Asia | 4 | 1152 | 1.39 (1.03-1.88) | 33% | 0.21 |
| Non-East Asia | 2 | 244 | 1.31 (0.92-1.86) | 62% | 0.10 |
| Sample size | |||||
| ≥ 200 | 4 | 1276 | 1.34 (1.05-1.71) | 0% | 0.63 |
| < 200 | 2 | 120 | 1.49 (0.76-2.94) | 81% | 0.02 |
| Cut-off value | |||||
| ≥ 10% | 4 | 1106 | 1.32 (1.03-1.7) | 14% | 0.32 |
| < 10% | 2 | 290 | 1.54 (0.87-2.71) | 71% | 0.06 |
The funnel plots for publication bias did not exhibit asymmetry (Figure 3). Thus, no evidence of publication bias was detected.
Figure 3.
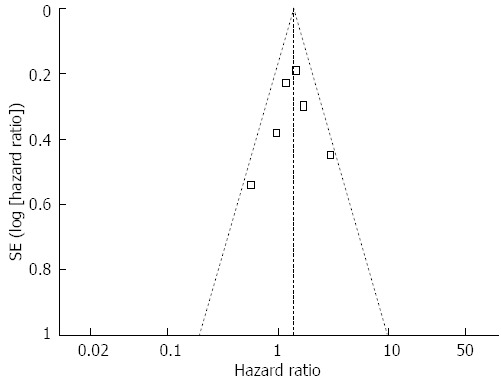
Funnel plot of decreased Muc5AC expression and overall survival of patients with gastric cancer.
Association of Muc5AC expression with depth of tumour invasion and lymph node metastasis
We evaluated the correlation between Muc5AC expression and depth of tumour invasion and lymph node metastasis of gastric cancer. As shown in Figure 4, the data combined from five studies including 990 patients indicated that low expression of Muc5AC was significantly associated with depth of tumour invasion (pooled OR = 2.12, 95%CI: 1.56-2.87, fixed effects) and there was no significant between-study heterogeneity (I2 = 2%, P = 0.4). Moreover, decreased Muc5AC expression was also associated with lymph node metastasis (pooled OR = 1.56, 95%CI: 1.00-2.44, random effects). Eight studies including 1384 patients were involved in this analysis, and the between-study heterogeneity was significant (I2 = 66%, P = 0.005). Formal statistical significance with no significant between-study heterogeneity was claimed when analyses were limited to studies with over 200 patients (pooled OR = 1.61, 95%CI: 1.13-2.3, random-effect) (Figure 5). Sensitivity analysis indicated that the results were not significantly influenced by omitting any single study.
Figure 4.
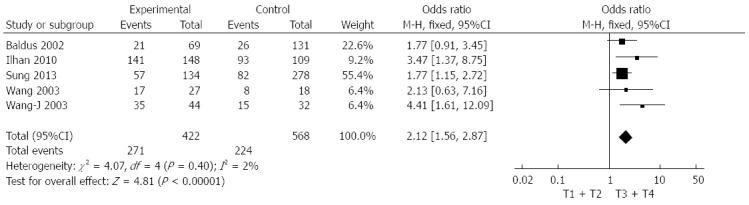
Forest plot of odds ratio for association between decreased Muc5AC expression and depth of tumour invasion in patients with gastric cancer.
Figure 5.
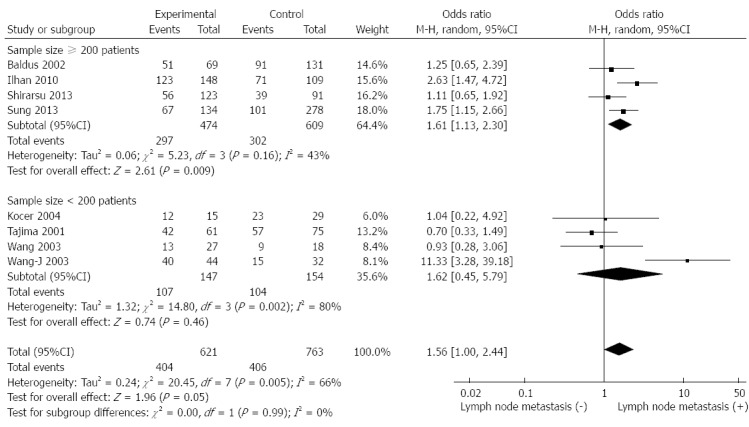
Forest plot of odds ratio for association between decreased Muc5AC expression and lymph node metastasis in patients with gastric cancer.
Association of Muc5AC expression with TNM stage, tumour size, venous and lymphatic invasion
We observed a trend toward a correlation of decreased Muc5AC expression with advanced TNM stage (pooled OR = 1.25, 95%CI: 0.92-1.69, larger tumour size (≥ 5 cm vs < 5 cm: pooled OR = 1.38, 95%CI: 0.84-2.28), venous invasion (positive vs negative: pooled OR = 1.26, 95%CI: 0.82-1.94) and lymphatic invasion (positive vs negative: OR = 1.26, 95%CI: 0.89-1.8). The meta-analysis of the association between Muc5AC and TNM stage had between-study heterogeneity (I2 = 78%, P = 0.003), whereas analysis of other histological features presented no significant heterogeneity (I2 = 0%-35%) (Table 3).
Table 3.
Meta-analysis of decreased Muc5AC expression and clinicopathological characteristics of gastric cancer
| Clinicopathological characteristic | No. of studies | No. of patients | Pooled OR (95%CI) |
Heterogeneity |
|
| I2 | P-value | ||||
| TNM stage | 4 | 801 | 1.15 (0.56-2.34), Random | 78% | 0.003 |
| Tumor size | 3 | 257 | 1.38 (0.84-2.28), Fixed | 0% | 0.83 |
| Venous invasion | 3 | 394 | 1.26 (0.82-1.94), Fixed | 35% | 0.21 |
| Lymphatic invasion | 3 | 650 | 1.27 (0.89-1.80), Fixed | 25% | 0.26 |
DISCUSSION
Muc5AC is a secreted gel-forming mucin and is limited to the cytoplasm of the foveolar epithelium and mucous neck cells throughout the stomach, playing a very important role in protecting the stomach. Muc5AC was reported to have been decreased in some non-neoplastic and preneoplastic conditions. In H. pylori associated gastritis, H. pylori disrupts the assembly of the mucin molecule via inhibition of galactosyltransferase and reduces gastric mucus viscosity by elevating pH through urease secretion[30,31]. In intestinal metaplasia, the expression pattern of mucin peptides is altered, leading to reduced Muc5AC immunoreactivity, especially in type I intestinal metaplasia[32]. Muc5AC expression was reported to be absent or low in some gastric cancer cases. The molecular mechanisms that result in decreased Muc5AC expression remain poorly understood[13-15,33]. The prognostic role of decreased Muc5AC expression in gastric cancer has been widely investigated.
The aim of this meta-analysis was to assess the association between decreased Muc5AC expression and overall survival and clinicopathological features of gastric cancer. Our analysis pooled the HRs and ORs based on 11 studies comprising 2135 patients with gastric cancer, covering the period from 2001 to 2013, indicating that decreased Muc5AC expression significantly predicted poor overall survival of gastric cancer patients. Moreover, correlations between decreased Muc5AC expression and depth of tumour invasion and lymph node metastasis were also found to be significant. Although there were no significant correlations between decreased Muc5AC expression and TNM stage, tumour size, venous invasion and lymphatic invasion, we detected trends for advanced TNM stage (stages III and IV), larger tumour size (> 5 cm), venous invasion and lymphatic invasion to be associated with decreased Muc5AC expression. These results might be due to the small number of patients included in the meta-analysis. More patients and studies will be needed to confirm our findings in the future.
In this research, there was no significant between-study heterogeneity for most tests of the included studies, except Muc5AC expression with lymph node metastasis and TNM stage. Small sample size might contribute to the between-study heterogeneity of association of Muc5AC expression with lymph node metastasis, because the between-study heterogeneity was nonsignificant when the number of patients was expanded to 200. To identify the source of heterogeneity of correlation between Muc5AC expression and TNM stage, we found that TNM system used by Ilhan was the 6th edition whereas other authors used the 5th edition[34,35]. There was no significant heterogeneity of correlation between Muc5AC and TNM stage when we excluded the Ilhan’s study (I2 = 46%, P = 0.16). Furthermore, because an optimal threshold has not been defined, the cut-off value defining a gastric cancer with decreased Muc5AC expression is arbitrary, which might produce heterogeneity. However, there was no significant heterogeneity of correlation between decreased Muc5AC expression and overall survival of gastric cancer patients. The association of decreased Muc5AC expression and overall survival of patients from the studies with a cut-off value greater than 10% is still significant. The results indicated that cut-off value had no significant association with heterogeneity.
We enlarged the sample size by conducting this meta-analysis to obtain more accurate results. Certain unsolvable limitations in this meta-analysis need to be pointed out. First, the technique of detecting Muc5AC was IHC staining, which contains a variety of methodological factors, such as fixation method of paraffin-embedded tissues, storage time, different primary antibodies, protocols and different definitions of positivity. Second, unpublished studies and conference abstracts were not included in our analysis because of limited data for methodology assessment and data synthesis. This research was also restricted to studies published in English language because other languages were not available for most authors and readers. Therefore, publication bias and language bias might have occurred[36,37]. However, we did not find a publication bias of correlation between Muc5AC expression and overall survival of patients with gastric cancer in this study. We tried to get relevant data that were not available from the published reports, but it was unavoidable that some data that might reduce the significance of decreased Muc5AC expression as a poor prognostic maker in gastric cancer might be missed. Third, although decreased Muc5AC expression was associated with stage of disease, it was impossible to make a subgroup analysis of stage because there was no sufficient data in this meta-analysis[13]. Luckily, we found trends for correlations of Muc5AC with overall survival and histopathological features in whatever clinical stage. Therefore, although we minimized the bias by confirming a detailed protocol before carrying out this study, by performing a careful search for published studies and by using standard methods for study recruiting, data extraction and data analysis, conclusions about associations between decreased Muc5AC expression and overall survival and clinicopathological features of gastric cancer still need to be drawn discreetly.
We speculate that Muc5AC could be a candidate biomarker for the prediction of prognosis and aggressiveness in gastric cancer because Muc5AC is frequently used as a marker in the practice of surgical pathology and is easily detected by routine immunohistochemistry of biopsy samples. To our knowledge, this is the first meta-analysis that suggests decreased Muc5AC expression is an unfavourable prognostic biomarker for gastric cancer patients. Patients with decreased Muc5AC expression are more likely to have poor overall survival and aggressive histopathological features. However, larger studies using standardized unbiased methods are still required before measuring immunohistochemical Muc5AC as a prognostic tool.
COMMENTS
Background
Muc5AC is a secreted gel-forming mucin, playing a very important role in protecting the stomach. Muc5AC expression was reported to be absent or low in some gastric cancer cases. The prognostic role of decreased Muc5AC expression in gastric cancer has been widely investigated.
Research frontiers
The association of decreased Muc5AC expression in gastric cancer and prognosis value has been investigated for years, but the results are controversial and inconclusive. The aim of this meta-analysis is to assess the association between decreased Muc5AC expression and overall survival and clinicopathological features of gastric cancer.
Innovations and breakthroughs
This is the first meta-analysis that suggests decreased Muc5AC expression is an unfavourable prognostic biomarker for gastric cancer patients. A more convincing result was obtained by enlarging the sample size.
Applications
Patients with decreased Muc5AC expression are more likely to have poor overall survival and aggressive histopathological features. We speculate that Muc5AC could be a candidate biomarker for the prediction of prognosis and aggressiveness in gastric cancer.
Peer-review
This is nicely written manuscript providing important information about decreased Muc5AC as a poor prognostic maker for patients with gastric cancer.
Footnotes
Supported by Programs Foundation of Ministry of Education of China, No. 20115132120004; National Natural Science Foundation of China, No. 81202624, No. 81102720 and No. 81072902.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 24, 2014
First decision: July 15, 2014
Article in press: February 13, 2015
P- Reviewer: de Gramont A, Mayer RJ, Meropol NJ S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang DN
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Amedei A, Benagiano M, della Bella C, Niccolai E, D’Elios MM. Novel immunotherapeutic strategies of gastric cancer treatment. J Biomed Biotechnol. 2011;2011:437348. doi: 10.1155/2011/437348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allgayer H, Heiss MM, Schildberg FW. Prognostic factors in gastric cancer. Br J Surg. 1997;84:1651–1664. [PubMed] [Google Scholar]
- 5.Lazăr D, Tăban S, Dema A, Cornianu M, Goldiş A, Raţiu I, Sporea I. Gastric cancer: the correlation between the clinicopathological factors and patients' survival (I) Rom J Morphol Embryol. 2009;50:41–50. [PubMed] [Google Scholar]
- 6.Chiaravalli AM, Klersy C, Vanoli A, Ferretti A, Capella C, Solcia E. Histotype-based prognostic classification of gastric cancer. World J Gastroenterol. 2012;18:896–904. doi: 10.3748/wjg.v18.i9.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oldenhuis CN, Oosting SF, Gietema JA, de Vries EG. Prognostic versus predictive value of biomarkers in oncology. Eur J Cancer. 2008;44:946–953. doi: 10.1016/j.ejca.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Verma M, Davidson EA. Mucin genes: structure, expression and regulation. Glycoconj J. 1994;11:172–179. doi: 10.1007/BF00731215. [DOI] [PubMed] [Google Scholar]
- 9.Kim YS, Gum JR. Diversity of mucin genes, structure, function, and expression. Gastroenterology. 1995;109:999–1001. doi: 10.1016/0016-5085(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 10.Nordman H, Davies JR, Lindell G, Carlstedt I. Human gastric mucins--a major population identified as MUC5. Biochem Soc Trans. 1995;23:533S. doi: 10.1042/bst023533s. [DOI] [PubMed] [Google Scholar]
- 11.Corfield AP, Myerscough N, Longman R, Sylvester P, Arul S, Pignatelli M. Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut. 2000;47:589–594. doi: 10.1136/gut.47.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SM, Kwon CH, Shin N, Park do Y, Moon HJ, Kim GH, Jeon TY. Decreased Muc5AC expression is associated with poor prognosis in gastric cancer. Int J Cancer. 2014;134:114–124. doi: 10.1002/ijc.28345. [DOI] [PubMed] [Google Scholar]
- 13.Reis CA, David L, Nielsen PA, Clausen H, Mirgorodskaya K, Roepstorff P, Sobrinho-Simões M. Immunohistochemical study of MUC5AC expression in human gastric carcinomas using a novel monoclonal antibody. Int J Cancer. 1997;74:112–121. doi: 10.1002/(sici)1097-0215(19970220)74:1<112::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 14.Carrato C, Balague C, de Bolos C, Gonzalez E, Gambus G, Planas J, Perini JM, Andreu D, Real FX. Differential apomucin expression in normal and neoplastic human gastrointestinal tissues. Gastroenterology. 1994;107:160–172. doi: 10.1016/0016-5085(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 15.Ho SB, Shekels LL, Toribara NW, Kim YS, Lyftogt C, Cherwitz DL, Niehans GA. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res. 1995;55:2681–2690. [PubMed] [Google Scholar]
- 16.Kocer B, Soran A, Kiyak G, Erdogan S, Eroglu A, Bozkurt B, Solak C, Cengiz O. Prognostic significance of mucin expression in gastric carcinoma. Dig Dis Sci. 2004;49:954–964. doi: 10.1023/b:ddas.0000034554.96191.66. [DOI] [PubMed] [Google Scholar]
- 17.Suh YS, Lee HJ, Jung EJ, Kim MA, Nam KT, Goldenring JR, Yang HK, Kim WH. The combined expression of metaplasia biomarkers predicts the prognosis of gastric cancer. Ann Surg Oncol. 2012;19:1240–1249. doi: 10.1245/s10434-011-2125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JY, Chang CT, Hsieh JS, Lee LW, Huang TJ, Chai CY, Lin SR. Role of MUC1 and MUC5AC expressions as prognostic indicators in gastric carcinomas. J Surg Oncol. 2003;83:253–260. doi: 10.1002/jso.10222. [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27:335–371. doi: 10.1016/s0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- 20.Ospina PA, Nydam DV, DiCiccio TJ. Technical note: The risk ratio, an alternative to the odds ratio for estimating the association between multiple risk factors and a dichotomous outcome. J Dairy Sci. 2012;95:2576–2584. doi: 10.3168/jds.2011-4515. [DOI] [PubMed] [Google Scholar]
- 21.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 23.Baldus SE, Mönig SP, Arkenau V, Hanisch FG, Schneider PM, Thiele J, Hölscher AH, Dienes HP. Correlation of MUC5AC immunoreactivity with histopathological subtypes and prognosis of gastric carcinoma. Ann Surg Oncol. 2002;9:887–893. doi: 10.1007/BF02557526. [DOI] [PubMed] [Google Scholar]
- 24.İlhan Ö, Han Ü, Önal B, Çelık SY. Prognostic significance of MUC1, MUC2 and MUC5AC expressions in gastric carcinoma. Turk J Gastroenterol. 2010;21:345–352. doi: 10.4318/tjg.2010.0119. [DOI] [PubMed] [Google Scholar]
- 25.Kim DH, Shin N, Kim GH, Song GA, Jeon TY, Kim DH, Lauwers GY, Park do Y. Mucin expression in gastric cancer: reappraisal of its clinicopathologic and prognostic significance. Arch Pathol Lab Med. 2013;137:1047–1053. doi: 10.5858/arpa.2012-0193-OA. [DOI] [PubMed] [Google Scholar]
- 26.Lee HS, Lee HK, Kim HS, Yang HK, Kim YI, Kim WH. MUC1, MUC2, MUC5AC, and MUC6 expressions in gastric carcinomas: their roles as prognostic indicators. Cancer. 2001;92:1427–1434. doi: 10.1002/1097-0142(20010915)92:6<1427::aid-cncr1466>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 27.Shiratsu K, Higuchi K, Nakayama J. Loss of gastric gland mucin-specific O-glycan is associated with progression of differentiated-type adenocarcinoma of the stomach. Cancer Sci. 2014;105:126–133. doi: 10.1111/cas.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tajima Y, Shimoda T, Nakanishi Y, Yokoyama N, Tanaka T, Shimizu K, Saito T, Kawamura M, Kusano M, Kumagai K. Gastric and intestinal phenotypic marker expression in gastric carcinomas and its prognostic significance: immunohistochemical analysis of 136 lesions. Oncology. 2001;61:212–220. doi: 10.1159/000055377. [DOI] [PubMed] [Google Scholar]
- 29.Wang RQ, Fang DC. Alterations of MUC1 and MUC3 expression in gastric carcinoma: relevance to patient clinicopathological features. J Clin Pathol. 2003;56:378–384. doi: 10.1136/jcp.56.5.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka S, Mizuno M, Maga T, Yoshinaga F, Tomoda J, Nasu J, Okada H, Yokota K, Oguma K, Shiratori Y, et al. H. pylori decreases gastric mucin synthesis via inhibition of galactosyltransferase. Hepatogastroenterology. 2003;50:1739–1742. [PubMed] [Google Scholar]
- 31.Celli JP, Turner BS, Afdhal NH, Keates S, Ghiran I, Kelly CP, Ewoldt RH, McKinley GH, So P, Erramilli S, et al. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. Proc Natl Acad Sci USA. 2009;106:14321–14326. doi: 10.1073/pnas.0903438106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reis CA, David L, Correa P, Carneiro F, de Bolós C, Garcia E, Mandel U, Clausen H, Sobrinho-Simões M. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res. 1999;59:1003–1007. [PubMed] [Google Scholar]
- 33.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sobin LH, Wittekind C. TNM Classification of Malignant Tumors. 5th ed. New York: Wiley; 1997. [Google Scholar]
- 35.Greene FL. Stomach. In: American Joint Committee on Cancer-AJCC Cancer Staging Manual., editor. 6th ed. New York: Springer; 2002. [Google Scholar]
- 36.Egger M, Zellweger-Zähner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet. 1997;350:326–329. doi: 10.1016/S0140-6736(97)02419-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Y, Li N, Zhuang W, Liu GJ, Wu TX, Yao X, Du L, Wei ML, Wu XT. P53 codon 72 polymorphism and gastric cancer: a meta-analysis of the literature. Int J Cancer. 2007;121:1481–1486. doi: 10.1002/ijc.22833. [DOI] [PubMed] [Google Scholar]


