Abstract
Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is a tumor suppressor gene that induces cell apoptosis by inhibiting the PI3K/Akt signaling pathway. Glioblastoma (GBM) is a brain tumor that is resistant to irradiation and chemotherapy and, thus, is difficult to cure. GBM stem-like cells (GSCs) have been implicated as a cause of this resistance. microRNA (miRNA/miR) inhibits the expression of proteins. The objective of the present study was to identify miRNAs that target PTEN, which induces apoptosis, in irradiation-resistant GSCs. When the expression of miRNAs was examined in GSCs irradiated at 60 Gy using the human GBM A172 cell line, the expression of PTEN-targeting miR-17-5p, -19a-3p, -19b-3p, -21-5p, -130b-3p, -221-3p and -222-3p was significantly higher in irradiated GSCs than in non-irradiated cells, and the PTEN expression levels, as revealed by immunostaining, were lower in the irradiated GSCs than in the non-irradiated cells. These results suggested that the expression of PTEN was suppressed through the overexpression of PTEN-targeting miRNAs in GSCs following irradiation.
Keywords: microRNA, phosphatase and tensin homolog deleted on chromosome 10, glioblastoma, glioblastoma stem-like cell
Introduction
Glioblastoma (GBM) is the most malignant central nervous system tumor. GBM is typically treated with a combination of surgery, radiotherapy and chemotherapy; however, the effect of this treatment is not satisfactory with regard to prognosis. GBM is characterized by a strong resistance to postoperative radiotherapy and chemotherapy. The cause of this resistance may be related to GBM stem-like cells (GSCs).
Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is a tumor suppressor that inhibits the PI3K/Akt pathway. PTEN dephosphorylates the inositol ring D3 position of the substrate, PIP3, and transfers it to the cell membrane, which inhibits the activation of cell proliferation-inducing Akt and induces cell apoptosis (1,2). The PI3K/Akt pathway was previously shown to be abnormally enhanced and inhibited apoptosis in malignant tumors in which PTEN was deleted and mutated, and this promoted cell proliferation and the process of cells becoming malignant and resistant to irradiation and chemotherapy (3–6).
Nuclear division and necrosis have frequently been detected in GBM tissue of patients predicted to have a survival time of shorter than one year, and tumors were found to rapidly infiltrate the surrounding tissue and disseminate to the meninx (7). A previous study reported that microRNAs (miRNAs/miR) were complementarily bound to a specific target nucleotide of the 3′-untranslated region (UTR) of mRNA and inhibited the expression of mRNA and protein (8). Secretory miRNAs (circulating miRNAs) may be applied clinically, as they are embedded in the granular vesicles of exosomes and are then secreted so that they are stable in body fluids, such as blood and cerebrospinal fluid, and analyzable in histopathological preparations.
The presence of GSCs scattered in GBM tissue has recently been identified as a factor involved in the development of resistance to GBM treatments (9–11). GSCs have been detected among cells that are positive for the surface antigen cluster of differentiation (CD)133, as well as side population cells. GSC were previously shown to be resistant to irradiation and chemotherapy, as they are in the G0 phase of the cell cycle, which leads to the recurrence of tumors (10,12). Molecular targeted therapies that target GCSs and PTEN are now being investigated (13–15); however, the radiation doses investigated in these studies are lower than those used for cancer therapy in a clinical setting.
The present study analyzed miRNAs in GSCs irradiated at a dose (total 60 Gy) applied in clinical practice to induce apoptosis in GSCs, which are resistant to irradiation and cause recurrence. The objective of this study was to identify miRNAs that targeted PTEN, which induces apoptosis in irradiation-resistant GSCs, using A172 cells.
Materials and methods
Cell line and culture conditions
The human glioblastoma A172 cell line, which was derived from the Japanese Cancer Resources Bank (Japanese Cancer Resources Bank, Osaka, Japan) was used in the present study. The selected culture medium contained 10% fetal bovine serum and 100 mg/ml streptomycin (Gibco penicillin-streptomycin liquid; Gibco Life Technologies, Carlsbad, CA, USA) in Dulbecco's modified Eagle's medium Ham/F12 (Sigma-Aldrich, Oberhaching, Germany). The cells were incubated at 37°C with 5% carbon dioxide.
Radiation instrument and radiation conditions
The cells were irradiated with 4-MV X-rays at a dose of 2.00 Gy/min over a 25×25-cm field at a radiation depth of 4.0 cm using a Mevatron KD2/50 (Toshiba, Tokyo, Japan) at room temperature. The cells were cultured for 7 h prior to roentgen irradiation. A total of 2×105 cells were irradiated in a 10-cm dish. The radiation conditions used were 2 Gy/day for 5 days over 1 week. The cells were irradiated for six weeks, up to a total dose of 60 Gy.
Isolation of CD133+/CD133− cells
Magnetic cell sorting (MiniMACS™ Separator and Starting Kit; cat. no. 130-090-312; Miltenyi Biotec GmbH, Begisch Gladbach, Germany) was used to separate CD133+ cells from CD133− cells. Cells were counted using a cell sorter (Z1™ Coulter Counter, Beckman Coulter, Brea, CA, USA). The culture became turbid in buffer at 350 µl/108 cells. The cells were labeled using the CD133 MicroBead Kit (MACS®; cat. no. 130-097-049; Miltenyi Biotec GmbH) containing CD133/1(AC133)-Biotin, and the CD133 MicroBead Kit (human; cat. no. 130-050-801; Miltenyi Biotec GmbH), containing an Fc receptor-blocking reagent, and anti-biotin MicroBeads in buffer. The solution was loaded onto the column and set in the magnetic field of the separator and the unlabeled (CD133−) cells were extracted. The CD133-labeled (CD133+) cells were eluted from the column with 1 ml autoMACS Rinsing Solution (cat. no. 130-091-222; Miltenyi Biotec GmbH). The separated CD133− and CD133+ cells were counted.
miRNA extraction and polymerase chain reaction (PCR)
Small RNA-enriched RNA (500 ng/µl) was isolated from the cells using the miRNeasy micro kit (Qiagen Inc., Valencia, CA, USA). Reverse transcription and quantitative PCR were performed using the RT2 miRNA PCR Array human brain cancer (catalogue no. MIHS-108ZA-12; Qiagen Inc.), and an ABI PRISM 7000 sequence detection system (Applied Biosystems, Tokyo, Japan) was used under the following cycling conditions: 1 cycle at 95°C for 15 min, followed by 40 cycles at 94°C for 15 sec, 55°C for 30 sec and 70°C for 30 sec. Comparative Ct analysis (2−ΔΔCT) was used to identify a set of 86 mRNAs that were differentially expressed between irradiated and non-irradiated CD133+ cells. Measurements were recorded in triplicate.
Fluorescent immunohistochemistry
CD133+ cells were fixed in 10% formaldehyde for 1 h. These cells were incubated with an anti-PTEN antibody (1:100; Abcam, Cambridge, MA, USA) for 1 h at room temperature, followed by a secondary, TRITC-conjugated anti-rabbit immunoglobulin G antibody (A21428; Life Technologies, Carlsbad, CA, USA) and bisbenzimide H33342 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) in a humid chamber at 37°C for 30 min. Images were captured on a microscope with MetaXpress Image Analysis software (Molecular Devices, Sunnyvale, CA, USA). Staining for PTEN expression by AlexaFluor 594 was scored using a 0–3 scale. Specimens with scores of 0 or 1 (no or negligible nuclear staining in <0–30% of GSC) were considered immunonegative. Specimens that showed an intermediate (borderline) score of 2 (weak to moderate nuclear staining in <30–60% of GSCs) were considered equivocal. Specimens with a score 3 (strong complete nuclear staining in >60% of GSCs) were considered immunopositive.
Statistical analysis
Data were tested for significance using analysis of variance, performed using miScript miRNA PCR Array Data Analysis software (Qiagen Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Analysis of miRNA expression in 60-Gy-irradiated CD133+ A172 cells
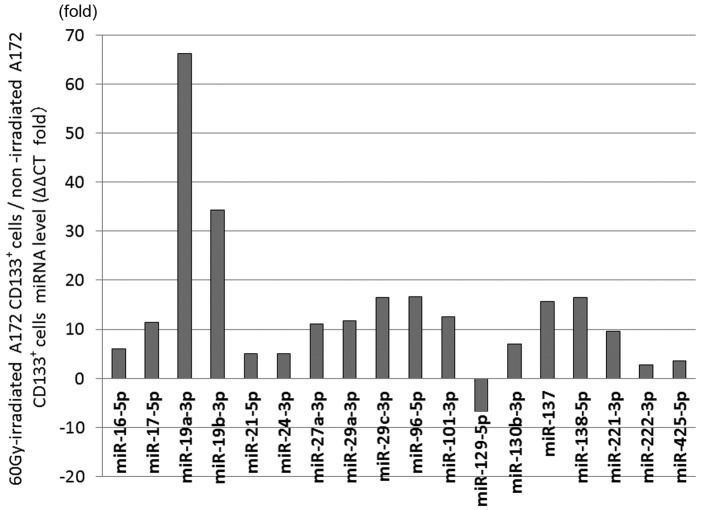
The 60-Gy-irradiated CD133+ A172 cells showed significant differential expression in 18 miRNAs compared with the non-irradiated cells. The expression was increased in the 60-Gy-irradiated CD133+ A172 cells in miR-16-5p by 5.97 times (P=0.0095), in miR-17-5p by 11.48 times (P=0.0054), in miR-19a-3p by 66.18 times (P=0.000023), in miR-19b-3p by 34.25 times (P=0.0023), in miR-21-5p by 4.99 times (P=0.045), in miR-24-3p by 4.97 times (P=0.024), in miR-27a-3p by 11.04 times (P=0.002), in miR-29a-3p by 11.75 times (P=0.0012), in miR-29c-3p by 16.46 times (P=0.014), in miR-96-5p by 16.68 times (P=0.028), in miR-101-3p by 12.53 times (P=0.005), in miR-130b-3p by 6.92 times (P=0.037), in miR-137 by 15.65 times (P=0.044), in miR-138-5p by 16.47 times (P=0.012), in miR-221-3p by 9.59 times (P=0.017), in miR-222-3p by 2.72 times (P=0.045) and in miR-425-5p by 3.6 times (P=0.039). The expression was decreased in the 60-Gy-irradiated CD133+ A172 cells in miR-129-5p by 6.71 times (P=0.048) (Fig. 1).
Figure 1.
Analysis of microRNA (miRNA/miR) expression in 60 Gy-irradiated cluster of differentiation (CD)133+ A172 cells. The expression levels of 17 miRNAs were significantly higher, while that of miR-129-5p was significantly lower in the 60-Gy-irradiated glioblastoma stem-like cells than in the non-irradiated cells.
Immunohistochemistry
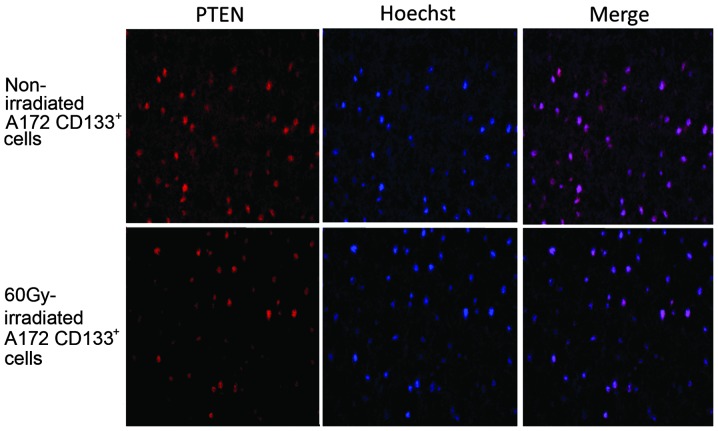
Immunostaining for PTEN was positive in the non-irradiated A172 CD133+ cells (positive rate, 80%; score, 3). The expression of PTEN was markedly decreased in the A172 CD133+ cells following irradiation with 60 Gy (positive rate, 10%; score, 1) (Fig. 2).
Figure 2.
Immunostaining for phosphatase and tensin homolog deleted on chromosome 10 (PTEN) in non-irradiated and 60-Gy-irradiated A172 cluster of differentiation (CD)133+ cells. The nuceli were stained by Hoechst 33342 (blue) stain and PTEN by AlexaFluor 594 (Texas red). Images were captured at x200 magnification.
Discussion
Significant differences were noted in the expression levels of 18 miRNAs between the 60-Gy-irradiated GSCs and the non-irradiated cells in the present study. The expression levels of 17 miRNAs (miR-16-5p, -17-5p, -19a-3p, -19b-3p, -21-5p, -24-3p, -27a-3p, -29a-3p, -29c-3p, -96-5p, -101-3p, -130b-3p, -137, -138-5p, -221-3p, -222-3p and -425-5p) were significantly higher, while that of miR-129-5p was significantly lower in the 60-Gy-irradiated GSCs compared with the non-irradiated cells. Of these, miR-17-5p, -19a-3p, -19b-3p, -21-5p, -130b-3p, -221-3p and -222-3p were previously shown to target PTEN, indicating that the regulation of PTEN expression through these miRNAs induces apoptosis in cultured brain tumor cells (16–18)
miRNA complementarily binds to a specific nucleotide in the 3′-UTR of mRNA, and inhibits target mRNA translation and protein expression. The inhibition of PTEN expression by the upregulated expression of miR-17-5p, -19a-3p and -19b-3p in malignant lymphomas, oligodendrocytes and oncogenic cells has been reported previously (19,20), and an inverse correlation was found between the expression of miR-130 and PTEN in osteosarcoma cell lines (21). A previous study showed that miR-21 acted together with miR-155 in a downstream signaling pathway in natural killer cell tumors and cell lines, and inhibited the expression of the target PTEN and PDCD4 (22). Furthermore, the increased expression of miR-221-3p and -222-3p inhibited that of PTEN in glioma cells (16,17). These findings suggest that the expression of miR-17-5p, -19a-3p, -19b-3p, -21-5p, -130b-3p, -221-3p and -222-3p enhanced and inhibited the PTEN expression in the 60-Gy-irradiated GSCs in the present study. Immunostaining confirmed the localization of PTEN expression in the nucleus, and revealed that its expression was weaker in the 60-Gy-irradiated GSCs than in the non-irradiated cells. The presence of PTEN in the cytoplasm has been shown to inhibit the activation of Akt by dephosphorylating PIP3, which induces cell apoptosis (23,24). However, PTEN has also been found to be strongly localized in the nuclei of undifferentiated cells in the resting G0 phase, and to inhibit cell proliferation through an Akt-independent molecular mechanism (18,25,26). PTEN was expressed in the nuclei of the non-irradiated and 60-Gy-irradiated GSCs in the present study, suggesting that the proliferation of the GSCs was inhibited through an Akt-independent molecular mechanism, as were their self-differentiation and replication abilities, which are characteristic of GSCs. In addition, the expression of PTEN was weaker in the 60-Gy-irradiated GSCs than in the non-irradiated cells, which indicated that the inhibition of cell proliferation, self-differentiation and self-replication was weaker in the 60-Gy-irradiated GSCs.
PTEN was previously shown to suppress tumors through the Rb/E2F signaling pathway, and to promote Rb- and E2F-related apoptotic reactions (27), suggesting that the suppressed expression of PTEN in 60-Gy-irradiated GSCs leads to the resistance to apoptosis.
Although PTEN has been identified as a protein that induces apoptosis in cells, only a few treatments currently target the PTEN protein. The PTEN protein may be applied to treatments in order to induce apoptosis in irradiation-resistant GSCs.
References
- 1.BlancoAparicio C, Renner O, Leal JF, Carnero A. PTEN, more than the AKT pathway. Carcinogenesis. 2007;28:1379–1386. doi: 10.1093/carcin/bgm052. [DOI] [PubMed] [Google Scholar]
- 2.Carnero A, BlancoAparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8:187–198. doi: 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- 3.Hou W, Liu J, Chen P, Wang H, Ye BC, Qiang F. Mutation analysis of key genes in RAS/RAF and PI3K/PTEN pathways in Chinese patients with hepatocellular carcinoma. Oncol Lett. 2014;8:1249–1254. doi: 10.3892/ol.2014.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kechagioglou P, Papi RM, Provatopoulou X, Kalogera E, Papadimitriou E, Grigoropoulos P, Nonni A, Zografos G, Kyriakidis DA, Gounaris A. Tumor suppressor PTEN in breast cancer: Heterozygosity, mutations and protein expression. Anticancer Res. 2014;34:1387–1400. [PubMed] [Google Scholar]
- 5.Appin CL, Gao J, Chisolm C, Torian M, Alexis D, Vincentelli C, Schniederjan MJ, Hadjipanayis C, Olson JJ, Hunter S, et al. Glioblastoma with oligodendroglioma component (GBM-O): Molecular genetic and clinical characteristics. Brain Pathol. 2013;23:454–461. doi: 10.1111/bpa.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reifenberger G, Collins VP. Pathology and molecular genetics of astrocytic gliomas. J Mol Med Berl. 2004;82:656–670. doi: 10.1007/s00109-004-0564-x. [DOI] [PubMed] [Google Scholar]
- 7.Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, BarnholtzSloan JS, Villano JL. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014;23:1985–1996. doi: 10.1158/1055-9965.EPI-14-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin J, Xu Q. Functions and application of exosomes. Acta Pol Pharm. 2014;71:537–543. [PubMed] [Google Scholar]
- 9.Yan K, Yang K, Rich JN. The evolving landscape of glioblastoma stem cells. Curr Opin Neurol. 2013;26:701–707. doi: 10.1097/WCO.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahlrot RH, Hansen S, Jensen SS, Schrøder HD, Hjelmborg J, Kristensen BW. Clinical value of CD133 and nestin in patients with glioma: A population-based study. Int J Clin Exp Pathol. 2014;7:3739–3751. [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SH, Kwon CH, Nakano I. Detoxification of oxidative stress in glioma stem cells: Mechanism, clinical relevance, and therapeutic development. J Neurosci Res. 2014;92:1419–1424. doi: 10.1002/jnr.23431. [DOI] [PubMed] [Google Scholar]
- 12.Hide T, Takezaki T, Nakamura H, Kuratsu J, Kondo T. Brain tumor stem cells as research and treatment targets. Brain Tumor Pathol. 2008;25:67–72. doi: 10.1007/s10014-008-0237-5. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki A, Nakajo T, Tsunoda Y, Yamamoti G, Kobayashi Y, Tsuji M, Udaka Y, Mizutani T, Oguchi K. Gene analysis and dynamics of tumor stem cells in human glioblastoma cells after radiation. Hum Cell. 2013;26:73–79. doi: 10.1007/s13577-013-0060-0. [DOI] [PubMed] [Google Scholar]
- 14.Lv S, Teugels E, Sadones J, De Brakeleer S, Duerinck J, Du Four S, Michotte A, De Grève J, Neyns B. Correlation of EGFR, IDH1 and PTEN status with the outcome of patients with recurrent glioblastoma treated in a phase II clinical trial with the EGFR-blocking monoclonal antibody cetuximab. Int J Oncol. 2012;41:1029–1035. doi: 10.3892/ijo.2012.1539. [DOI] [PubMed] [Google Scholar]
- 15.Stechishin OD, Luchman HA, Ruan Y, Blough MD, Nguyen SA, Kelly JJ, Caimcross JG, Weiss S. On-target JAK2/STAT3 inhibition slows disease progression in orthotopic xenografts of human glioblastoma brain tumor stem cells. Neuro Oncol. 2013;15:198–207. doi: 10.1093/neuonc/nos302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Q, Yan Y, Huang Z, Zhong X, Huang L. MicroRNA-221 targeting PI3-K/Akt signaling axis induces cell proliferation and BCNU resistance in human glioblastoma. Neuropathology. 2014;34:455–464. doi: 10.1111/neup.12129. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Guo F, Wang P, Hong S, Zhang C. miR-221/222 confers radioresistance in glioblastoma cells through activating Akt independent of PTEN status. Curr Mol Med. 2014;14:185–195. doi: 10.2174/1566524013666131203103147. [DOI] [PubMed] [Google Scholar]
- 18.Chung JH, Eng C. Nuclear-cytoplasmic partitioning of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) differentially regulates the cell cycle and apoptosis. Cancer Res. 2005;65:8096–8100. doi: 10.1158/0008-5472.CAN-05-1888. [DOI] [PubMed] [Google Scholar]
- 19.Tagawa H, Ikeda S, Sawada K. Role of microRNA in the pathogenesis of malignant lymphoma. Cancer Sci. 2013;104:801–809. doi: 10.1111/cas.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao E, Jiang C, Ji M, Huang X, Iqbal J, Lenz G, Wright G, Staudt LM, Zhao Y, McKeithan TW, et al. The miRNA-17-92 cluster mediates chemoresistance and enhances tumor growth in mantle cell lymphoma via PI3K/AKT pathway activation. Leukemia. 2012;26:1064–1072. doi: 10.1038/leu.2011.305. [DOI] [PubMed] [Google Scholar]
- 21.Namløs HM, Meza-Zepeda LA, Barøy T, Østensen IH, Kresse SH, Kuijjer ML, Serra M, Bürger H, Cleton-Jansen AM, Myklebost O. Modulation of the osteosarcoma expression phenotype by microRNAs. PLoS One. 2012;7:e48086. doi: 10.1371/journal.pone.0048086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamanaka Y, Tagawa H, Takahashi N, Watanabe A, Guo YM, Iwamoto K, Yamashita J, Saitoh H, Kameoka Y, Shimizu N, et al. Aberrant overexpression of microRNAs activate AKT signaling via down-regulation of tumor suppressors in natural killer-cell lymphoma/leukemia. Blood. 2009;114:3265–3275. doi: 10.1182/blood-2009-06-222794. [DOI] [PubMed] [Google Scholar]
- 23.Ristorcelli E, Beraud E, Verrando P, Villard C, Lafitte D, Sbarra V, Lombardo D, Verine A. Human tumor nanoparticles induce apoptosis of pancreatic cancer cells. FASEB J. 2008;22:3358–3369. doi: 10.1096/fj.07-102855. [DOI] [PubMed] [Google Scholar]
- 24.Asano T, Fujishiro M, Kushiyama A, Nakatsu Y, Yoneda M, Kamata H, Sakoda H. Role of phosphatidylinositol 3-kinase activation on insulin action and its alteration in diabetic conditions. Biol Pharm Bull. 2007;30:1610–1616. doi: 10.1248/bpb.30.1610. [DOI] [PubMed] [Google Scholar]
- 25.Chung JH, GinnPease ME, Eng C. Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) has nuclear localization signal-like sequences for nuclear import mediated by major vault protein. Cancer Res. 2005;65:4108–4116. doi: 10.1158/0008-5472.CAN-05-0124. [DOI] [PubMed] [Google Scholar]
- 26.Liu JL, Sheng X, Hortobagyi ZK, Mao Z, Gallick GE, Yung WK. Nuclear PTEN-mediated growth suppression is independent of Akt down-regulation. Mol Cell Biol. 2005;25:6211–6224. doi: 10.1128/MCB.25.14.6211-6224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morales LD, Casillas Pavón EA, Shin JW, Garcia A, Capetillo M, Kim DJ, Lieman JH. Protein tyrosine phosphatases PTP-1B, SHP-2, and PTEN facilitate Rb/E2F-associated apoptotic signaling. PLoS One. 2014;9:e97104. doi: 10.1371/journal.pone.0097104. [DOI] [PMC free article] [PubMed] [Google Scholar]