Abstract
Background
Although guidelines for antiarrhythmic drug therapy in atrial fibrillation (AF) were published in 2006, it remains uncertain whether adherence to these guidelines affects patient outcomes.
Methods and Results
We retrospectively evaluated the records of 5976 consecutive AF patients who were prescribed at least 1 antiarrhythmic drug between 2006 and 2013. Patients with 1 or more prescribed antiarrhythmic drugs that did not comply with guideline recommendations comprised the non–guideline‐directed group (=2920); the remainder constituted the guideline‐directed group (=3056). Time to events was assessed using the survival analysis method and adjusted for covariates using Cox regression. Rates of adherence to the guidelines increased significantly with a higher degree of prescriber specialization in arrhythmias (49%, 55%, and 60% for primary care physicians, general cardiologists, and cardiac electrophysiologists, respectively, P=0.001) for the first prescribed antiarrhythmic drug. Compared to the non–guideline‐directed group, the guideline‐directed group had higher rates of heart failure, but lower baseline CHADS2‐VASc scores (P<0.001) and lower rates of coronary artery disease, valvular disease, hypertension, hyperlipidemia, pulmonary disease, and renal insufficiency (P<0.05 for all). During 45±26 months follow‐up, the guideline‐directed group had a lower risk of AF recurrence (hazard ratio=0.86, 95% CI=0.80 to 0.93), fewer hospital admissions for AF (hazard ratio=0.87, 95% CI=0.79 to 0.97), and fewer procedures for recurrent AF, including electrical cardioversion, pacemaker implantation, and atrioventricular nodal ablation (P<0.01 for all). The mortality and stroke risks were similar between the groups.
Conclusions
Adherence to published guidelines in the antiarrhythmic management of AF is associated with improved patient outcomes.
Keywords: antiarrhythmic drugs, atrial fibrillation, guidelines, outcome
Introduction
Atrial fibrillation (AF) affects a large number of patients and is associated with significant morbidity and mortality because of thromboembolic complications, diminished quality of life, and heart failure, leading to significant resource utilization in our healthcare system.1–6 Rhythm control is therefore a desired strategy in managing many patients with AF,7–10 and this strategy often requires the use of antiarrhythmic drugs (AAD) that alter the function of cardiac myocyte membrane ion channels.7,11–13 However, chronic AAD therapy is associated with potentially serious side effects, limiting their use and complicating drug selection. In 2006, guidelines published by the American College of Cardiology, American Heart Association, and the European Society of Cardiology made important recommendations on the use of AAD in AF patients.14 However, because of the complex nature of AAD therapy and a relative scarcity of evidence, it remains unclear how well these guideline recommendations have been adopted in real‐world clinical practice by various physician specialties, and whether adherence to the guidelines impacts clinical outcomes of AF patients.
This study was designed to examine the rates of adherence by physicians to the 2006 published guidelines in the AAD management of AF and the impact of this adherence on the long‐term outcomes of AF patients, including mortality, AF recurrence, stroke, cardiovascular hospitalizations, and AF‐related procedures.
Methods
Study Population
This retrospective, observational study cohort consists of 5976 consecutive patients diagnosed with paroxysmal (58%) or persistent (42%) AF who were prescribed 1 or more AAD at the hospitals and clinics of the University of Pittsburgh Medical Center from January 2006 to November 2013 with the goal of achieving rhythm control. The cohort was assembled via query of the University of Pittsburgh Medical Center electronic medical record for encounters in which International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis of AF (427.31) was assigned and by searching associated pharmacologic databases for a prescription of Vaughan Williams Class IA, IC, or Class III AAD.15–16 All patients with AF and a prescribed AAD were included in this analysis (6219). After chart review, patients were excluded if the use of AAD was for the purpose of controlling ventricular arrhythmias (n=243). The remaining 5976 patients constituted the study cohort. The cohort was followed starting from the date of first AAD prescription (after January 1, 2006) through May 30, 2014 with prospective review of outpatient and inpatient medical records. Patients who died or were lost to follow‐up during this period were censored at their date of death or last encounter. The University of Pittsburgh Institutional Review Board approved this study.
Patient Characteristics and Medication Prescription
Demographic data were obtained from the clinical records. Information on comorbidities was generated from International Classification of Diseases, Ninth Revision, Clinical Modification codes in the clinical database with coding algorithms as described by Quan et al17 The CHA2DS2‐VASc score and Charlson comorbidity index were also calculated for each patient for risk stratification.18–20
For each AAD, we ascertained the initiation date and discontinuation date via review of the institutional pharmacologic database and clinical notes and orders in the electronic medical record. We first generated a list of AAD used by each patient from the pharmacologic database. Two reviewers then separately reviewed the information on each AAD in the electronic medical record for accuracy. The results from the 2 reviewers were then compared, and in cases of disagreement, a third reviewer adjudicated the findings. The initial agreement rate for AAD and dates between the 2 reviewers was 80.6%. Information on other medications (excluding the AAD) was obtained from the pharmacologic database search, which generated results for 4311 (72.1%) patients.
Group Analysis
We determined the adherence of each AAD to the recommendations of the 2006 published guidelines with a custom‐made STATA‐based algorithm, the accuracy of which was confirmed with manual verification of compliance in a random sample of 120 patients (100% accuracy). We then assigned patients in our cohort to 1 of 2 separate groups: the guideline‐directed (GD) group, in which all of the AADs prescribed complied with the 2006 guidelines, including the first prescribed AAD, which had to be consistent with the first‐line therapy recommendation, and the non–guideline‐directed (NGD) group, in which 1 or more of the AAD prescribed to the patient were not adherent to the guidelines or the first prescribed AAD was not considered first‐line therapy. Patients who were prescribed dronedarone, disopyramide, procainamide, and quinidine were automatically classified into the NGD group, as these medications were not included in the 2006 guideline recommendations.14 Because new AF management guidelines were published in 2014,21 we also determined the adherence of each AAD used in our patient cohort to the recommendations of the 2014 published guidelines, and calculated the concordance rate between the 2006 and 2014 guidelines.
Antiarrhythmic Drug Prescribers
The primary medical specialty of AAD prescribers could be ascertained in 4604 (77%) patients (primary care physician or family doctor = 249 [5%], general cardiologist = 3017 (66%), cardiac electrophysiologist [EP = 1338 [29%)] for the first prescribed AAD.
Clinical Outcomes
Clinical outcomes evaluated in this study included death, AF recurrence, stroke, admission for AF, admission for congestive heart failure, admission for other cardiovascular conditions, and need for AF‐related procedures, including direct‐current electrical cardioversion, AF ablation, pacemaker implantation, atrioventricular nodal ablation, and surgical Maze procedures. Dates of AF recurrence were ascertained from clinical notes documenting recurrence of AF by ECG, electrocardiographic monitors, or recurrence of AF symptoms. Causes for admission to the hospital were adjudicated by review of admission notes performed independently by 2 members of the research team who were blinded to the group assignment of patients. Cardiovascular admissions were subclassified into AF, congestive heart failure, and other cardiovascular reasons.
Statistical Analysis
Baseline characteristics are presented as means±SD for continuous variables and as occurrence rates for dichotomous variables and were compared using the Student t and χ2 tests, respectively. A P‐value < 0.05 was considered statistically significant. Kaplan–Meier curves were constructed for overall survival and Nelson‐Aalen cumulative hazard curves were constructed for other major clinical outcomes and were compared using the log‐rank test for univariate analysis. Cox proportional‐hazard models were constructed for each clinical outcome to adjust for any unbalanced (P<0.10) covariates affecting the outcome of interest. These included, after adjusting for possible interactions between covariates, age, CHA2DS2‐VASc score, congestive heart failure, coronary artery disease, hypertension, valvular heart disease, hyperlipidemia, chronic obstructive lung disease, and chronic kidney disease. Patient gender and Charlson comorbidity index were also included for their importance. Analyses were primarily conducted between the GD and the NGD groups using the 2006 guideline classification.14 The same analyses were conducted again using the 2014 guideline21 classification of patients between the GD and NGD groups in order to test the robustness of our results.
Results
Study Population
The study cohort comprised 5976 patients with AF, of whom 3056 patients (51.1%) were in the GD group and 2920 patients (48.9%) were in the NGD group. Table 1 includes reasons for classification in the NGD group and Table 2 compares the baseline characteristics of the study groups. GD patients were younger and had longer follow‐up, lower CHA2DS2‐VASc score, and lower rates of coronary artery disease, hypertension, hyperlipidemia, valvular heart disease, chronic obstructive pulmonary disease, and chronic kidney disease, but they had a higher rate of congestive heart failure. The 2 groups had similar Charlson comorbidity index scores and similar anticoagulation rates.
Table 1.
Reason for Noncompliance With Guidelines
| AAD | Reason for Noncompliance With Guidelines | Number |
|---|---|---|
| Amiodarone | Prescribed to patients without CHF and/or LVH as first‐line therapy | 1455 |
| Dronedarone | Prescribed without guideline recommendation | 615 |
| Dofetilide | Prescribed to patients without CAD and/or CHF as first‐line therapy | 280 |
| Flecainide | Used in patients with CHF and/or CAD | 77 |
| Used in patients with LVH | 4 | |
| Propafenone | Used in patients with CHF and/or CAD | 94 |
| Used in patients with LVH | 1 | |
| Sotalol | Used in patients with decompensated CHF | 342 |
| Used in patients with LVH | 22 | |
| Disopyramide | Prescribed without guideline recommendation | 19 |
| Procainamide | Prescribed without guideline recommendation | 5 |
| Quinidine | Prescribed without guideline recommendation | 6 |
| Total | 2920 |
AAD indicates antiarrhythmic drug; CAD, coronary artery disease; CHF, congestive heart failure; LVH, left ventricular hypertrophy.
Table 2.
Baseline Characteristics
| GD Group (n=3056) | NGD Group (n=2920) | P Value | |
|---|---|---|---|
| Age, y | 69±13 | 71±12 | <0.001 |
| Male gender | 1820 (59.6%) | 1708 (58.5%) | 0.404 |
| Follow‐up (months) | 47±27 | 43±25 | <0.001 |
| CHA2DS2‐VASc score | 2.84±1.76 | 3.00±1.74 | <0.001 |
| 0 to 2 | 1387 (45.4%) | 1203 (41.2%) | 0.011 |
| 3 to 5 | 1438 (47.1%) | 1462 (50.1%) | |
| 6 to 9 | 231 (7.5%) | 255 (8.7%) | |
| Charlson comorbidity index | 1.54±1.73 | 1.61±1.72 | 0.127 |
| Congestive heart failure | 870 (28.5%) | 535 (18.3%) | <0.001 |
| Coronary artery disease | 986 (32.3%) | 1153 (39.5%) | <0.001 |
| Hypertension | 1910 (62.5%) | 1927 (66.0%) | 0.005 |
| Left ventricular hypertrophy | 45 (1.5%) | 37 (1.3%) | 0.495 |
| Valvular heart disease | 539 (17.6%) | 708 (24.3%) | <0.001 |
| Ventricular tachycardia | 221 (7.2%) | 179 (6.1%) | 0.089 |
| Atrial flutter | 203 (6.6%) | 199 (6.8%) | 0.790 |
| Diabetes mellitus | 631 (20.7%) | 638 (21.9%) | 0.256 |
| Hyperlipidemia | 1645 (53.8%) | 1697 (58.1%) | <0.001 |
| Chronic obstructive pulmonary disease | 286 (9.4%) | 338 (11.6%) | 0.005 |
| Chronic kidney disease | 233 (7.6%) | 267 (9.1%) | 0.034 |
| Cancer | 305 (10.0%) | 341 (11.7%) | 0.035 |
| Medications | N=2210 | N=2101 | |
| Anticoagulation | 1748 (79.1%) | 1706 (81.2%) | 0.084 |
| Aspirin | 1580 (71.5%) | 1571 (74.8%) | 0.015 |
| Clopidogrel | 279 (12.6%) | 334 (15.9%) | 0.002 |
| ACE inhibitor/ARB | 1378 (62.4%) | 1402 (66.7%) | 0.003 |
| β‐Blocker | 1527 (69.1%) | 1654 (78.7%) | <0.001 |
| Calcium channel blocker | 901 (40.8%) | 1003 (47.7%) | <0.001 |
| Digoxin | 573 (25.9%) | 559 (26.6%) | 0.613 |
| Statins | 1308 (59.2%) | 1338 (63.7%) | 0.002 |
ACE inhibitor indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; GD, guideline‐directed group; NGD, non–guideline‐directed group.
Prescriber Adherence to Guidelines by Specialty
Rates of guideline adherence increased significantly with a higher degree of prescriber specialization in treatment of cardiac arrhythmias; this included both the first prescribed AAD (49%, 55%, and 60% for the primary care physician, general cardiologist, and EP groups, respectively, P=0.001) and the second prescribed AAD (74% and 84% for the general cardiologist, and EP groups [only 8 patients had a second AAD prescribed by a primary care physician], respectively, P<0.001). The choice of AAD also differed significantly according to physician specialty; there was greater use of dofetilide and Class IC AAD and lower use of amiodarone and sotalol (P<0.001 for all comparisons) in the EP group compared to other prescriber groups.
Antiarrhythmic Medication Use
Table 3 details the use of the various AAD in the 2 study groups. The most commonly prescribed AAD was amiodarone (52%) for patients in the NGD group and sotalol (43%) for patients in the GD group. Class IC agents were more commonly used in the GD group.
Table 3.
Antiarrhythmic Medication Use
| Frequency | Mean Duration (Months) | |||
|---|---|---|---|---|
| GD Group | NGD Group | GD Group | NGD Group | |
| First prescribed antiarrhythmic medication | ||||
| N | 3056 | 2920 | 30.3 | 21.8 |
| Flecainide | 454 (14.9%) | 91 (3.1%) | 32.0 | 24.6 |
| Propafenone | 297 (9.7%) | 99 (3.4%) | 32.0 | 26.8 |
| Amiodarone | 749 (24.5%) | 1517 (52.0%) | 22.3 | 20.4 |
| Dofetilide | 245 (8.0%) | 294 (10.1%) | 34.3 | 31.6 |
| Sotalol | 1311 (42.9%) | 407 (13.9%) | 33.1 | 26.1 |
| Dronedarone | 0 | 488 (16.7%) | 15.1 | |
| Disopyramide | 0 | 15 (0.5%) | 21.3 | |
| Procainamide | 0 | 4 (0.1%) | 16.7 | |
| Quinidine | 0 | 5 (0.2%) | 24.7 | |
| P value | <0.001 | <0.001 | ||
| Second prescribed antiarrhythmic medication | ||||
| N | 562 (18.4%) | 794 (27.2%) | 19.5 | 15.8 |
| Flecainide | 82 (14.6%) | 77 (9.7%) | 23.5 | 16.8 |
| Propafenone | 30 (5.3%) | 36 (4.5%) | 21.8 | 17.8 |
| Amiodarone | 226 (40.2%) | 213 (26.8%) | 14.8 | 14.8 |
| Dofetilide | 169 (30.1%) | 114 (14.4%) | 23.8 | 15.9 |
| Sotalol | 55 (9.8%) | 173 (21.8%) | 18.2 | 18.1 |
| Dronedarone | 0 | 172 (21.7%) | 14.2 | |
| Disopyramide | 0 | 6 (0.8%) | 12.0 | |
| Procainamide | 0 | 2 (0.3%) | 14.6 | |
| Quinidine | 0 | 1 (0.1%) | 5.7 | |
| P value | <0.001 | <0.001 | ||
GD indicates guideline‐directed group; NGD, non–guideline directed group.
Clinical Outcomes
Table 4 details clinical event rates in the GD and NGD groups. During a mean follow‐up of 45±26 months, 785 (13%) patients died and 2877 (48%) patients had AF recurrence. GD patients had lower 1‐year rates of AF recurrence and pacemaker implantation. The 1‐year rates of death, stroke, admissions for heart failure, AF, and congestive heart failure and need for AF‐related procedures except pacemaker implantation otherwise were similar between the 2 groups.
Table 4.
Event Rates for Major Clinical Outcomes
| GD Group (N=3056) | NGD Group (N=2920) | Hazard Ratio | P Value | |
|---|---|---|---|---|
| Death | 411 (13.5%) | 374 (12.7%) | 1.05 | 0.417 |
| 1‐year death rate | 2.3% | 2.9% | 0.79 | 0.376 |
| First AF recurrence | 1463 (47.9%) | 1414 (48.4%) | 0.99 | 0.670 |
| 1‐year AF recur rate | 23.6% | 25.1% | 0.94 | 0.005 |
| Stroke | 120 (3.9%) | 108 (3.7%) | 1.05 | 0.645 |
| 1‐year stroke rate | 0.9% | 1.0% | 0.90 | 0.759 |
| 1st cardiac admission | 1224 (40.5%) | 1115 (38.2%) | 1.05 | 0.139 |
| 1‐year cardiac admission rate | 17.4% | 17.5% | 0.99 | 0.351 |
| 1st AF admission | 756 (24.7%) | 726 (24.9%) | 0.99 | 0.911 |
| 1‐year AF admission rate | 10.9% | 11.3% | 0.96 | 0.083 |
| 1st CHF admission | 302 (9.9%) | 249 (8.5%) | 1.16 | 0.070 |
| 1‐year CHF admission rate | 4.00% | 3.00% | 1.33 | 0.428 |
| AF‐related procedures | ||||
| Electrical cardioversion | 427 (14.0%) | 436 (14.9%) | 0.94 | 0.292 |
| AF ablation | 315 (10.3%) | 273 (9.4%) | 1.10 | 0.214 |
| Pacemaker implantation | 233 (7.6%) | 280 (9.6%) | 0.79 | 0.007 |
| AV nodal ablation | 153 (5.0%) | 176 (6.0%) | 0.83 | 0.084 |
| Maze surgery | 60 (2.0%) | 66 (2.3%) | 0.87 | 0.424 |
AF indicates atrial fibrillation; AV, atrioventricular; CHF, congestive heart failure; GD, guideline‐directed group; NGD, non–guideline directed group.
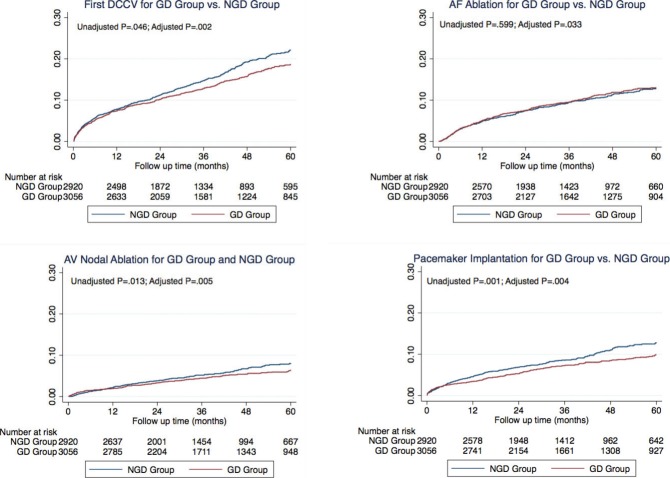
After adjusting for baseline characteristics, overall survival was similar in the 2 groups (Figure 1, hazard ratio [HR]=0.97, P=0.698). However, time to first AF recurrence and the time to first AF hospitalization were longer in GD patients than in the NGD patients (Table 5, Figure 1, HR=0.86, P<0.001 for AF recurrence; HR=0.87, P=0.007 for AF hospitalization). In addition, time to first electrical cardioversion, AF ablation, pacemaker implantation, and atrioventricular nodal ablation, but not surgical Maze procedure were all significantly longer in the GD group compared to the NGD group (Table 5, Figure 2, HR=0.81, P=0.002 for electrical cardioversion; HR=0.84, P=0.033 for AF ablation; HR=0.77, P=0.004 for pacemaker implantation; HR=0.73, P=0.005 for atrioventricular nodal ablation; HR=0.78, P=0.176 for surgical Maze procedure). There was no significant difference, however, in time to first stroke (HR=1.08, P=0.581), first cardiovascular hospitalization (HR=0.93, P=0.090), or first congestive heart failure hospitalization (HR=1.04, P=0.719) between the GD and NGD groups.
Figure 1.
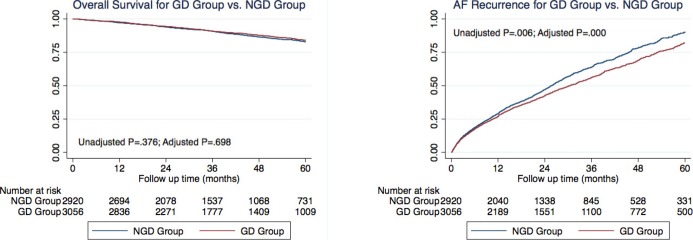
Kaplan–Meier curve for overall survival (left panel) and Nelson‐Aalen curve for atrial fibrillation recurrence (right panel). AF indicates atrial fibrillation; GD, guideline‐directed group; NGD, non–guideline‐directed group.
Table 5.
Cox Proportional‐Hazard Model for Major Clinical Outcomes
| Baseline Variables Included in the Model (P<0.10) | GD vs NGD Group | |||
|---|---|---|---|---|
| Adjusted Hazard Ratio | Un‐Adjusted P Value | Adjusted P Value | ||
| Death | Age, sex, Charlson index, CHF, CAD, HTN, HL | 0.97 | 0.376 | 0.698 |
| First AF recurrence | Age, Charlson index, CAD | 0.86 | 0.005 | <0.001 |
| Stroke | Age, sex, CHA2DS2‐VASc score, CHF, HTN, COPD | 1.08 | 0.759 | 0.581 |
| First cardiac admission | Age, sex, CHF, CAD | 0.93 | 0.351 | 0.090 |
| First AF admission | Age, sex, CHA2DS2‐VASc score, HTN, HL, CKD | 0.87 | 0.083 | 0.007 |
| First CHF admission | Age, CHA2DS2‐VASc score, CAD, CHF, HTN, COPD, CKD | 1.04 | 0.428 | 0.719 |
| Electrical cardioversion | Age, sex, CHA2DS2‐VASc score, Charlson index, CHF, CAD, valvular disease, COPD | 0.81 | 0.046 | 0.002 |
| AF ablation | Age, CHA2DS2‐VASc score, Charlson index, CAD, HTN, valvular disease, HL, CKD | 0.84 | 0.599 | 0.033 |
| Pacemaker implantation | Age, sex, valvular disease, HL | 0.77 | 0.001 | 0.004 |
| AV nodal ablation | Sex, CHF, CKD | 0.73 | 0.013 | 0.005 |
| Maze surgery | Age, CHA2DS2‐VASc score, valvular disease, HL, COPD | 0.78 | 0.162 | 0.176 |
AF indicates atrial fibrillation; AV, atrioventricular; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; GD, guideline‐directed group; HL, hyperlipidemia; HTN, hypertension; NGD, non–guideline‐directed group.
Figure 2.
Nelson‐Aalen cumulative hazard curve for major outcomes including electrical cardioversion of atrial fibrillation (left upper panel), pacemaker implantation (right lower panel), atrioventricular nodal ablation (left lower panel), and atrial fibrillation ablation (right upper panel). AF indicates atrial fibrillation; DCCV, direct‐current cardioversion; GD, guideline‐directed group; NGD, non–guideline‐directed group.
Analysis According to the 2014 Guideline Classification
The study cohort was reclassified according to the 2014 AF management published guidelines21 into the GD group with 3558 patients (59.5%) and the NGD group with 2418 patients (40.5%). A total of 556 patients were reclassified from the NGD to the GD group (n=529) or from the GD to the NGD group (n=27), yielding a concordance rate of 91% between the 2006 and 2014 guideline classifications. As was the case with the 2006 guidelines, physician compliance with the 2014 guideline recommendations increased significantly with increasing physician specialization in arrhythmia management, with adherence rates for the first prescribed AAD of 54%, 60%, and 78% for the primary care physician, general cardiologist, and EP groups, respectively (P<0.001). In addition, the impact of adherence to guidelines on patients' outcomes was similar when patients were classified according to the 2006 or 2014 guidelines. Using the 2014 guideline classification, patients in the GD group had lower risk of AF recurrence (HR=0.83, P<0.001), AF hospitalizations (HR=0.71, P<0.001), and AF‐related procedures including pacemaker implantation (HR=0.77, P=0.004), atrioventricular nodal ablation (HR=0.78, P=0.024), and electrical cardioversion (HR=0.70, P<0.001). Mortality and stroke risks were similar between the 2 groups.
The outcomes associated with compliance to the 2006 and 2014 published guidelines were clinically significant. AF patients who had antiarrhythmic medications prescribed in accordance with guideline recommendations achieved better rhythm control, with less AF recurrence (relative risk reduction 14% for 2006 guideline and 17% for 2014 guideline), fewer AF hospitalizations (relative risk reduction 13% for 2006 guideline and 29% for 2014 guideline), and less requirement for AF‐related procedures (relative risk reduction 16% to 30% for the different procedures).
Discussion
This study, which included nearly 6000 AF patients who were prescribed AAD after 2006, demonstrates that overall adherence to the 2006 American College of Cardiology/American Heart Association/European Society of Cardiology published guidelines is approximately 50% and is associated with improved clinical outcomes relating to AF rhythm control. It also demonstrates that a higher level of specialization in arrhythmia management among prescribing physicians is associated with higher adherence rates. These results, which remained significant after multivariate adjustment for differences in baseline characteristics between the 2 study groups and after reclassifying patients based on the recently published 2014 guidelines, may have important clinical implications in the management of AF patients.
To date, few controlled clinical trials11,22 have demonstrated benefit of AAD on outcomes beyond improving quality of life in symptomatic patients. Moreover, concerns over deleterious side effects and even toxicities have limited the clinical use of AAD and makes their prescribing difficult for many clinicians. The recommendations of the 2006 published guidelines provide an important and practical roadmap for prescribing AAD in clinical practice. These guidelines, however, are primarily supported by randomized, controlled trials that have included highly selected patients followed for a relatively short period of time.11,14 Also, these trials focused primarily on maintenance of sinus rhythm while providing inadequate information on other important outcome measures, such as AF‐related hospitalizations or procedures.11,13 Our study examined the use of AAD in a real‐world clinical setting, without any patient selection or exclusion, focusing on an array of important outcomes over a mean follow‐up duration of more than 3.5 years. Moreover, rather than examining the effect of individual medications, our study evaluated the AAD selection strategy (adherent or nonadherent to published guidelines), which has broader implications for clinical practice. We demonstrated similar mortality, stroke, and cardiovascular hospitalization rates between the 2 study groups in our study, which is consistent with findings from most large randomized trials except the ATHENA trial,11,13,22 but lower AF recurrence rates leading to fewer AF‐related hospitalizations and procedures.
In our cohort, the implementation of guideline recommendations was far from ideal. Nearly half of all patients were prescribed at least 1 antiarrhythmic medication that was not compliant with the 2006 guidelines. Among these, 1455 patients were prescribed amiodarone as a first‐line AAD in the absence of a history of congestive heart failure or left ventricular dysfunction, which contradicts the guideline recommendations.14 Similarly, about 160 patients with coronary artery disease and/or congestive heart failure in our cohort were prescribed a class IC AAD (flecainide or propafenone) despite well‐publicized data implicating these agents for increased risk for proarrhythmia and death in this patient population.14,23 Moreover, about 700 patients who were prescribed dronedarone were also noncompliant as this AAD was not included in the 2006 published guidelines nor in the 2011 focused updates.14,24 However, when dronedarone was included in the 2014 AF management guidelines,21 most of those patients became compliant with the recommendations.
Implications for Clinical Practice
Our data have important implications on the AAD management of AF patients. They support adherence to the guideline recommendations for the prescription of AAD for AF, as compliance with these guidelines is associated with improved clinical outcomes, largely because of a greater likelihood of successful rhythm control and therefore fewer additional procedures being required. Our findings also demonstrate a need for disseminating knowledge to healthcare professionals of all specialties, particularly those less specialized in arrhythmia management, regarding the contents of published guidelines and the importance of adhering to them when possible, particularly that nonadherence can potentially be dangerous, such as when a class IC agent is used in the context of coronary disease and prior myocardial infarction,23 for example. Our findings also suggest the need for early referral to an arrhythmia specialist when rhythm control of AF is being entertained, particularly that invasive options, such as AF ablation, have to be considered in these situations and patient counseling regarding these options is best provided by physicians who perform them.
Study Limitations
Our study is a cohort study, therefore carrying the inherent limitations of selection bias and information bias. The effect of selection bias on the final outcome is likely limited because, although statistically significant, baseline differences were of small magnitude and were controlled for in multivariate analyses, which reached similar conclusions as the univariate analyses. For information bias, underreporting of clinical events cannot be excluded but is likely to be of small magnitude, if present, because the clinical event rates in our cohort are similar to those reported in randomized controlled AF trials. In addition, the quality of event reporting and data collection is similar between the 2 study groups, as these data were collected simultaneously, using the same institutional electronic medical record system and since study group assignment was automated and performed after data collection was completed. Nevertheless, the lack of formal cardiac monitoring to determine recurrence of AF is a limitation of this analysis. Our study was also performed at a single center and therefore our results may not be reproducible at other institutions with patient populations and different clinical settings. It is worth noting, however, that University of Pittsburgh Medical Center comprises a network of more than 25 hospitals, ranging from rural and suburban community hospitals to tertiary care urban centers, as well as many outpatient clinics encompassing a large geographical area in Western Pennsylvania. With a cohort of nearly 6000 AF patients derived from these sites, this study has a wide representation of varying practice settings and patient demographics.
Conclusions
AF patients who had antiarrhythmic medications prescribed in accordance with the 2006 American College of Cardiology/American Heart Association/European Society of Cardiology AF treatment guideline recommendations had lower rates of AF recurrence, resulting in fewer AF hospitalizations and AF treatment‐related procedures. Although physicians' adherence to the recommendations of the published guidelines was modest, physicians with greater training in treating arrhythmias achieved higher rates of adherence to these guidelines.
Disclosures
Dingxin Qin, George Leef, Mian Bilal Alam, Rohit Rattan, Mohamad Bilal Munir, Divyang Patel, Furqan Khattak, and Nishit Vaghasia: none. Evan Adelstein: Research Support from St Jude Medical. Sandeep K. Jain: Research Support from St Jude Medical and Medtronic. Samir Saba: Research Support and consultation from St Jude Medical, Boston Scientific, and Medtronic.
References
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001; 285:2370-2375. [DOI] [PubMed] [Google Scholar]
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. Arch Intern Med. 1987; 147:1561-1564. [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003; 107:2920-2925. [DOI] [PubMed] [Google Scholar]
- Stewart S, Hart CL, Hole DJ, McMurray JJ. A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study. Am J Med. 2002; 113:359-364. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014; 129:e28-e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011; 4:313-320. [DOI] [PubMed] [Google Scholar]
- Zimetbaum P. Antiarrhythmic drug therapy for atrial fibrillation. Circulation. 2012; 125:381-389. [DOI] [PubMed] [Google Scholar]
- Hagens VE, Ranchor AV, Van SE, Bosker HA, Kamp O, Tijssen JG, Kingma JH, Crijns HJ, Van Gelder ICRACE Study Group. Effect of rate or rhythm control on quality of life in persistent atrial fibrillation. Results from the Rate Control Versus Electrical Cardioversion (RACE) Study. J Am Coll Cardiol. 2004; 43:241-247. [DOI] [PubMed] [Google Scholar]
- Van Gelder IC, Haegeli LM, Brandes A, Heidbuchel H, Aliot E, Kautzner J, Szumowski L, Mont L, Morgan J, Willems S, Themistoclakis S, Gulizia M, Elvan A, Smit MD, Kirchhof P. Rationale and current perspective for early rhythm control therapy in atrial fibrillation. Europace. 2011; 13:1517-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhof P, Bax J, Blomstrom‐Lundquist C, Calkins H, Camm AJ, Cappato R, Cosio F, Crijns H, Diener HC, Goette A, Israel CW, Kuck KH, Lip GY, Nattel S, Page RL, Ravens U, Schotten U, Steinbeck G, Vardas P, Waldo A, Wegscheider K, Willems S, Breithardt G. Early and comprehensive management of atrial fibrillation: executive summary of the proceedings from the 2nd AFNET‐EHRA consensus conference ‘research perspectives in AF’. Eur Heart J. 2009; 30:2969c-2977c. [DOI] [PubMed] [Google Scholar]
- Lafuente‐Lafuente C, Longas‐Tejero MA, Bergmann JF, Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2012; 5:CD005049. [DOI] [PubMed] [Google Scholar]
- Freemantle N, Lafuente‐Lafuente C, Mitchell S, Eckert L, Reynolds M. Mixed treatment comparison of dronedarone, amiodarone, sotalol, flecainide, and propafenone, for the management of atrial fibrillation. Europace. 2011; 13:329-345. [DOI] [PubMed] [Google Scholar]
- Calkins H, Reynolds MR, Spector P, Sondhi M, Xu Y, Martin A, Williams CJ, Sledge I. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta‐analyses. Circ Arrhythm Electrophysiol. 2009; 2:349-361. [DOI] [PubMed] [Google Scholar]
- Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JLAmerican College of Cardiology/American Heart Association Task Force on Practice Guidelines; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006; 114:e257-e354. [DOI] [PubMed] [Google Scholar]
- ICD‐9‐CM Guidelines, Conversion Table, and Addenda. Classification of Diseases, Functioning, and Disability. National Center for Health Statistics, CDC; Available at: http://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed December 15, 2012. [Google Scholar]
- Vaughan Williams EM. In: Sandfte E, Flensted‐Jensen E, Olesen KH. (eds.). Classification of anti‐arrhythmic drugs. Symposium on Cardiac Arrhythmias. 1970Sodertalje, Sweden: AB ASTRA; 1970. 449-472. [Google Scholar]
- Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005; 43:1130-1139. [DOI] [PubMed] [Google Scholar]
- Lane DA, Lip GY. Use of the CHA(2)DS(2)‐VASc and HAS‐BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012; 126:860-865. [DOI] [PubMed] [Google Scholar]
- Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof PESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012; 33:2719-2747. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40:373-383. [DOI] [PubMed] [Google Scholar]
- January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Jr, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: a Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014; 64:e1-e76. [DOI] [PubMed] [Google Scholar]
- Hohnloser SH, Crijns HJ, Eickels M, Gaudin C, Page RL, Torp‐Pedersen C, Connolly SJATHENA Investigators. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009; 360:668-678. [DOI] [PubMed] [Google Scholar]
- Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias‐Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991; 324:781-788. [DOI] [PubMed] [Google Scholar]
- Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS, Smith SC, Jr, Priori SG, Estes NA, III, Ezekowitz MD, Jackman WM, January CT, Lowe JE, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Jacobs AK, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Kushner FG, Ohman EM, Stevenson WG, Tarkington LG, Yancy CWAmerican College of Cardiology Foundation/American Heart Association Task Force. ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011; 123:e269-e367. [DOI] [PubMed] [Google Scholar]