Abstract
Background
The effects of right ventricular apical pacing (RVAP) and right ventricular outflow tract (RVOT) septal pacing on atrial and ventricular electrophysiology have not been thoroughly compared.
Methods and Results
To identify a more favorable pacing strategy with fewer adverse effects, 80 patients who had complete atrioventricular block with normal cardiac function and who were treated with either RVAP (n=42) or RVOT septal pacing (n=38) were recruited after an average of 2 years of follow‐up. The data from electrocardiography and echocardiography performed before pacemaker implantation and at the end of follow‐up were collected. The patients in the RVOT septal pacing and RVAP groups showed similar demographic and clinical characteristics before pacing treatments. After a mean follow‐up of 2 years, the final maximum P‐wave duration; P‐wave dispersion; Q‐, R‐, and S‐wave complex duration; left atrial volume index; left ventricular end‐systolic diameter; ratio of transmitral early diastolic filling velocity to mitral annular early diastolic velocity; and interventricular mechanical delay in the RVOT septal pacing group were significantly less than those in the RVAP group (P<0.05). The final left ventricular ejection fraction of the RVOT septal pacing group was significantly higher than that of the RVAP group (P<0.05).
Conclusions
Compared with RVAP, RVOT septal pacing has fewer adverse effects regarding atrial electrical activity and structure in patients with normal cardiac function.
Keywords: cardiovascular diseases, electrophysiology, pacemaker
Introduction
Artificial cardiac pacing refers to a type of treatment that uses electrical impulses to maintain a suitable heart rate and rhythm, primarily in patients with bradyarrhythmias arising from disease in the heart's electrical conduction system, such as the sinoatrial node, atrioventricular node, or His‐Purkinje system.1 Right ventricular apical pacing (RVAP) was established in the mid‐1950s and was widely adopted in bradyarrhythmia therapy around the world.2
For decades, a growing number of short‐ and long‐term studies have suggested that RVAP may induce or worsen dyssynchronous left ventricle (LV) contraction, LV longitudinal shortening and twist, atrial fibrillation, and heart failure.3–4 These pathophysiological consequences are now attributed to the violation of several electrophysiological properties of the myocardium.5 Electrical conduction in the myocardium is at least 4 times slower than that in the Purkinje system; conduction along muscle fibers is 2 times faster than that with perpendicular activation.6 In addition, the conduction characteristics between the endocardial and epicardial layers are different.7–8 In the setting of RVAP, the activation front becomes ellipsoidal, and slowed conduction results, particularly in the intermediate and epicardial layers.1,5 For these reasons, alternative pacing sites with a more synchronous ventricular activation pattern have been explored.1,5
The region and pacing alternatives to RVAP are numerous, including His‐bundle pacing and para‐His pacing, medial septum, lower septum, right ventricular outflow tract (RVOT) in the septal region, and pulmonary infundibulum.9–10 Although these strategies are not well defined and standardized because of the ease of catheter implantation and catheter stability in this region, the most preferred area of pacing is the RVOT in the septal region.11–13 The ideal pacing mode for improving cardiac function requires the participation of Purkinje fibers, an effect that is difficult to establish.1,5 The RVOT septal region nearest the Purkinje system has been selected as favoring the pacing site1,5; however, clinical evidence to support RVOT septal pacing is still rare and confusing.14–16 Favorable studies have shown that RVOT septal pacing produces interventricular synchronous contraction denoted by narrow Q‐, R‐, and S‐wave complexes, preventing the deterioration of LV structure and function.14–15In contrast, another study suggested that although RVOT pacing caused more synchronous LV contraction than RVAP, it had no benefit over RVAP regarding the prevention of cardiac remodeling and preserving LV systolic function.16 These conflicting studies appear to be associated with study designs that were not homogeneous regarding the methodology; judgment criteria; follow‐up; and, particularly, statistical power used.5 In addition, limited studies have focused on the effect of pacing sites on structure and function of the left atrium, findings that may be important for the development of atrial arrhythmias.15,17 P‐wave dispersion (PWD) is a novel prediction index of atrial arrhythmia.18 Short‐term ventricular demand pacing induces prolongation of maximum P‐wave duration with increased PWD19; however, whether right ventricular pacing sites affect PWD is unclear.
In the present study, to assess whether RVOT septal pacing could maintain more favorable atrial and ventricular electrical activity to reduce the incidence of atrial fibrillation compared with RVAP, cardiac electrophysiology and cardiac structure and function of RVOT septal pacing and RVAP were assessed with 2‐year follow‐up in 80 Chinese patients who had complete atrioventricular block with normal cardiac function.
Methods
Patients
From January 2008 to December 2012, 80 patients who had complete atrioventricular block with normal cardiac function and who received either RVAP or RVOT septal pacing at our cardiology department were enrolled in the study retrospectively. The following exclusion criteria were used: history of atrial fibrillation and/or heart failure; PWD >40 ms before implantation of the pacemaker; severe tricuspid valve disease or tricuspid valve surgery history; and documented coronary artery disease, pulmonary disease, and renal insufficiency. All of the patients underwent electrocardiography, echocardiography, and clinical assessment. The mean follow‐up duration was 2.02±0.4 years.
Ethical Issues
Written informed consent regarding the procedures and medical data to be used was obtained from all patients, according to the guidelines of the Chinese National Ethics Regulation Committee. The review board of the First Affiliated Hospital of Soochow University approved this protocol in accordance with the amended Declaration of Helsinki (reference number: FAHSU 2013–023).
Pacemaker and Lead Implantation
All implantations were performed in a sterile manner with the patient in a conscious state under local anesthesia and by an experienced operator. In the RVAP group, the passive fixation electrodes were positioned toward the right ventricular apex (Figure). In the RVOT septal pacing group, the active helix electrodes were positioned against the septum of the RVOT (Figure). The RVOT septum was confirmed according to the tip of the electrode directed to the spine 45° to the left anterior oblique view using fluoroscopic radiographs and producing a negative or isoelectric vector in lead I and positive Q, R, and S waves in leads II, III, and aVF in the pacemaker electrocardiogram (ECG) (Figure). In both groups, the passive fixation electrodes were used as a right atrial appendage. After pacemaker implantation, all patients were followed regularly as outpatients. Pacing parameters, including pacing threshold, sensitivity, electrode impedance, and percentage of ventricular pacing, were assessed.
Figure 1.
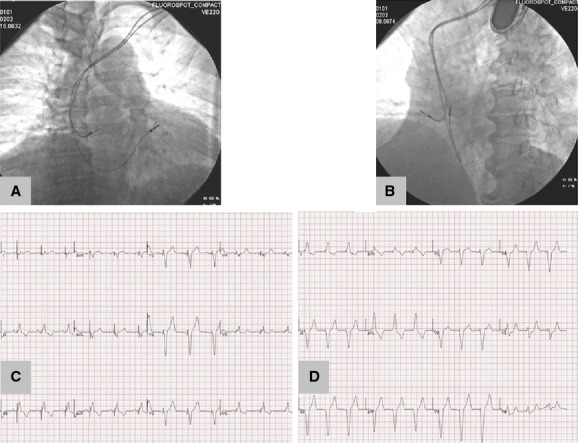
Exemplificative diagram for RVOT septal and RVAP pacing. Chest x‐ray showing the position of the right ventricular lead at the right ventricular outflow tract septum in right anterior oblique (30°) (A) and left anterior oblique (45°) (B) views. Twelve‐lead electrocardiograms indicating the site of RVOT septal pacing (C) and RVAP (D). RVAP indicates right ventricular apical pacing; RVOT, right ventricular outflow tract.
Twelve‐Lead Electrocardiography
A 12‐lead surface ECG was obtained after a 20‐minute resting period in the supine position at a paper speed of 50 mm/s and 2 mV/cm before pacemaker implantation, immediately after implantation, and at the end of the follow‐up. The P‐wave duration was measured manually in all of the simultaneously recorded 12 leads of the surface ECG. For greater accuracy, measurements were performed with calipers and a magnifying lens. The onset of the P wave was defined as the point of the first visible upward departure from the baseline for positive waveforms and as the point of the first downward departure from the baseline for negative waveforms. The return to the baseline was considered the end of the P wave. Three continuous sinus P‐wave durations in the same lead were measured, and their average value was defined as the same lead P‐wave duration. The maximum P‐wave duration (Pmax) measured in any of the 12 leads of the surface ECG was used as the longest atrial conduction time. The difference between the Pmax and minimum P‐wave duration was calculated and defined as PWD.19 The Q‐, R‐, and S‐wave complex duration (QRSd) was calculated using the first to last sharp vector crossing the isoelectric line in all of the leads. Three continuous QRSd values were detected in the lead with the widest QRS complex in the 12 leads, and their average value was defined as QRSd. Twelve‐lead electrocardiography was performed and analyzed by the same experienced technician. The final electrocardiographic result for each patient was reviewed by the chief technician. Any uncertainties regarding electrocardiographic results were resolved by discussion. Both technicians were blinded to clinical data and group division.
Echocardiography
A7 transthoracic echocardiographic examination was performed separately at baseline and at the end of follow‐up using a Sonos 5500 ultrasound machine (Philips) with a 2.5‐Hz transducer. All echocardiographic examinations for each patient were performed and analyzed by the same experienced echocardiographer who was blinded to clinical data and group division. Any uncertainties regarding echocardiographic examination results were resolved by discussion among senior technicians of the department of echocardiography. The measured parameters with the M‐mode technique included interventricular septum thickness (normal range: 6 to 10 mm), LV posterior wall thickness (normal range: 6 to 10 mm), LV end‐diastolic diameter (normal range 35 to 56 mm), LV end‐systolic diameter (normal range: 20 to 40 mm), transmitral early diastolic filling velocity, and mitral annular early diastolic velocity. The measurement of the LV ejection fraction (LVEF; normal value: >0.50) was performed using the Simpson's biplane method. The ratio of transmitral early diastolic filling velocity to mitral annular early diastolic velocity, designated as the E/Ea ratio (normal value: <8), represents LV filling pressure and indirect left atrial (LA) pressure. The LA volume was measured at end‐ventricular systole from the apical 2‐ and 4‐chamber views immediately before mitral valve opening. The LA volume was indexed to the body surface area to provide the LA volume index (normal value: <24 mL/m2).20
The intervals between the onset of QRS to the beginning of ejection at the aortic and pulmonary valve levels using pulse‐wave Doppler were defined as the aortic and pulmonary pre‐ejection intervals, respectively. The interventricular mechanical delay (IVMD) was defined as the difference between aortic and pulmonary pre‐ejection intervals.21 An IVMD value ≥40 ms was considered an indicator of interventricular desynchrony.
Statistical Analysis
Continuous data were expressed as mean±SD. Categorical data were summarized as frequencies and percentages, which were analyzed using the chi‐square test. Paired Student t test analysis was used for comparison within groups, and nonpaired Student t test analysis was used for comparison between groups. All tests were 2‐sided. A P value <0.05 was deemed statistically significant. Statistical analyses were performed using SPSS version 17.0 (SPSS Inc.).
Results
Clinical Characteristics of the RVAP and RVOT Septal Pacing Groups
Eighty patients (42 RVAP patients and 38 RVOT septal pacing patients) were included retrospectively in this comparative analysis. As shown in Table 1, there was no statistical difference between the 2 groups regarding age, sex, and rates of patients with hypertension, diabetes mellitus, and/or coronary artery disease. In the RVAP and RVOT septal pacing groups, respectively, 19.1% and 18.4% of patients took β‐blocker medication, and 38.1% and 34.1% of patients took angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers. No significant difference was observed regarding this medical history (Table 1).
Table 1.
Summary of Patient Characteristics
| Indexes | RVAP Group (n=42) | RVOT Septal Pacing Group (n=38) | P Value |
|---|---|---|---|
| Demographic data and medical history | |||
| Age, y±SD | 61±8.5 | 63±9.7 | 0.886 |
| Sex, male/female | 23/19 | 20/18 | 1.000 |
| Hypertension, n (%) | 16 (38.1) | 14 (36.8) | 0.920 |
| Diabetes mellitus, n (%) | 4 (9.5) | 5 (13.2) | 0.729 |
| Coronary artery disease, n (%) | 1 (2.4) | 2 (5.3) | 0.602 |
| Mitral regurgitation | |||
| No or trivial | 28 (66.7) | 23 (60.5) | 0.740 |
| Mild | 13 (31.0) | 14 (36.8) | 0.752 |
| Moderate | 1 (2.4) | 1 (2.6) | 1.000 |
| Severe | 0 (0.0) | 0 (0.0) | — |
| Tricuspid regurgitation | |||
| No or trivial | 34 (80.9) | 29 (76.3) | 0.823 |
| Mild | 5 (11.9) | 6 (15.8) | 0.862 |
| Moderate | 3 (7.1) | 3 (7.9) | 1.000 |
| Severe | 0 (0.0) | 0 (0.0) | — |
| Medication use | |||
| β‐blockers, n (%) | 8 (19.1) | 7 (18.4) | 0.823 |
| ACEI/ARB, n (%) | 16 (38.1) | 13 (34.1) | 0.823 |
| Pacemaker type | |||
| DDD, n (%) | 31 (73.8) | 29 (76.3) | 1.000 |
| VVI, n (%) | 11 (26.2) | 9 (23.7) | 1.000 |
| Sinus rhythm, n (%) | 26 (61.9) | 24 (63.2) | 0.920 |
| Atrial pacing, n (%) | 16 (38.1) | 14 (36.8) | 0.920 |
| Atrial pacing, mean±SD | 20.5±4.3 | 18.9±5.8 | 0.719 |
Continuous variables are presented as mean±SD, and categorical data are presented as number (percentage). Differences between the 2 groups were examined using the Student t test and chi‐square or Fisher exact probability tests according to the characteristics of the data distribution. ACEI/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; DDD, dual‐chamber pacemaker; RVAP, right ventricular apical pacing; RVOT, right ventricular outflow tract; VVI, ventricular demand pacing.
Most patients had no or trivial mitral regurgitation and/or tricuspid regurgitation. No statistical difference was observed between the 2 groups regarding the rates of patients with moderate and severe mitral regurgitation and/or tricuspid regurgitation (Table 1). The leads were successfully implanted in all patients. No serious complications related to the surgery were detected during implantation or follow‐up. Detailed pacing parameters, including the atrial and ventricular pacing threshold, sensitivity, and electrode impedance were recorded, and no intergroup or intragroup difference was significant.
In the RVAP group, 31 patients (73.8%) were implanted with the dual‐chamber pacemaker, and 11 patients (26.2%) were implanted with ventricular demand pacing; among these patients, 26 (61.9%) underwent sinus rhythm mapping, and others had atrial pacing (20.5±4.32%). In the RVOT septal pacing group, 29 patients (76.3%) were implanted with a dual‐chamber pacemaker, and 9 patients (23.7%) were implanted with a ventricular demand pacemaker; among these patients, 24 (63.2%) underwent sinus rhythm mapping, and others had atrial pacing (18.9±5.8%). There were no significant differences in atrial pacing and sinus rhythm mapping between the 2 groups (Table 1). The mean follow‐up duration was 2.02±0.4 years. There was no significant difference in follow‐up duration between the RVAP group and the ROVT septal pacing group. During the 2 years of follow‐up after implantation, no patients were lost, and 6 patients had new onset of atrial fibrillation in the RVAP group; however, only 1 patient with new‐onset atrial fibrillation was observed in the RVOT septal pacing group.
Cardiac Electrophysiology Evaluation
To compare the difference in the 2 pacing strategies in cardiac electrophysiology, the P‐wave and QRS complex values were checked by ECG. As shown in Table 2, initial Pmax, PWD, and QRSd were similar between the RVAP and RVOT septal pacing groups. After a mean follow‐up of 2 years, the final PWD and QRSd increased significantly in the 2 groups compared with each baseline level (Table 2). In the RVOT septal pacing group, however, Pmax, PWD, and QRSd were significantly shorter than those in the RVAP group (Pmax: 107±8 ms versus 135±5 ms, P=0.043; PWD: 35±8 ms versus 46±10 ms, P=0.040; QRSd: 130±12 ms versus 154±13 ms, P=0.048) (Table 2). These data suggest that RVOT septal pacing displays lower Pmax, PWD, and QRSd compared with RVAP.
Table 2.
Electrocardiogram Changes in Both Groups During Follow‐up
| Electrocardiographic Parameters | RVAP Group (n=42) | RVOT Septal Pacing Group (n=38) | P Value | ||||
|---|---|---|---|---|---|---|---|
| Initial | Final | P Value | Initial | Final | P Value | ||
| Pmax, ms | 102±11 | 135±5 | 0.029 | 101±10 | 107±8 | 0.664 | 0.043 |
| Pmin, ms | 75±9 | 79±10 | 0.680 | 75±9 | 77±10 | 0.843 | 0.843 |
| PWD, ms | 27±7 | 46±10 | <0.001 | 26±9 | 35±8 | 0.029 | 0.040 |
| QRSd, ms | 102±11 | 154±13 | 0.002 | 101±10 | 130±12 | 0.043 | 0.048 |
Data are presented as mean±SD. Pmax indicates maximum P‐wave duration; Pmin, minimum P‐wave duration; PWD, P‐wave dispersion; QRSd, QRS complex duration; RVAP, right ventricular apical pacing; RVOT, right ventricular outflow tract.
Cardiac Structure and Function Assessment
To monitor cardiac structure and function, echocardiography was performed for all patients before and after pacing. As shown in Table 3, of the patients treated with RVAP, LA volume index, LV end‐diastolic diameter, LV end‐systolic diameter, E/Ea ratio, and IVMD increased significantly compared with initial levels. Compared with the baseline value, the final LVEF decreased in the RVAP group (from 0.66±0.12 to 0.51±0.10; P=0.048). Of the patients treated with RVOT septal pacing, LA volume index, LV end‐systolic diameter, and IVMD also increased significantly compared with initial levels (Table 3). Of both groups, interventricular septum thickness displayed no significant change after pacing, and RVOT septal pacing did not alter the LV end‐diastolic diameter level (Table 3). Although both RVAP and RVOT septal pacing increased the LVAI and IVMD levels, the increasing rates of the RVOT septal pacing group were significantly lower than those of the RVAP group (Table 3). The final LVEF of the RVOT septal pacing group was 0.62±0.14, a value that was significantly higher than that of the RVAP group (0.51±0.10).
Table 3.
Echocardiographic Parameters in the 2 Groups During the Follow‐up
| Echocardiographic Parameters | RVAP Group (n=42) | RVOT Septal Pacing Group (n=38) | P Value | ||||
|---|---|---|---|---|---|---|---|
| Initial | Final | P Value | Initial | Final | P Value | ||
| LAVI, mL/m2 | 24.2±6.3 | 37.4±11.4 | 0.001 | 23.6±7.8 | 29.8±9.7 | 0.048 | 0.047 |
| IVST, mm | 10.1±1.6 | 10.7±1.8 | 0.648 | 9.6±2.1 | 10.4±1.7 | 0.547 | 0.826 |
| LVEDd, mm | 48.3±6.9 | 53.8±11.2 | 0.049 | 50.3±5.1 | 52.8±7.2 | 0.715 | 0.885 |
| LVESd, mm | 29.6±8.1 | 40.7±10.2 | 0.014 | 30.1±6.3 | 35.2±6.1 | 0.047 | 0.049 |
| LVEF | 0.66±0.12 | 0.51±0.10 | 0.048 | 0.65±0.09 | 0.62±0.14 | 0.722 | 0.047 |
| E/Ea ratio | 7.1±1.4 | 11.3±2.8 | <0.001 | 6.7±1.2 | 8.3±3.2 | 0.111 | 0.021 |
| IVMD, ms | 16.6±5.0 | 45.2±18.7 | <0.001 | 18.3±4.3 | 30.4±16.8 | <0.001 | 0.004 |
Data are presented as mean±SD. E/Ea ratio indicates ratio of transmitral early diastolic filling velocity to mitral annular early diastolic velocity; IVMD, difference between the aortic and pulmonary pre‐ejection intervals; IVST, interventricular septum thickness; LAVI, left atrial volume index; LVEDd, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESd, left ventricular end‐systolic diameter; RVAP, right ventricular apical pacing; RVOT, right ventricular outflow tract.
Discussion
In the present study, we confirmed that RVOT septal pacing induced shorter QRSd and less interventricular mechanical delay than RVAP, features that were associated with fewer adverse effects on LVEF and E/Ea ratio. In addition, our study demonstrated that RVOT septal pacing protected the atrial structure and electroactivity with less increase in LA volume index, Pmax, and PWD than in those with RVAP. These results indicate that RVOT septal pacing maintains a more satisfactory atrial and ventricular electroactivity sequence than RVAP.
Regarding the effect of RVOT pacing on ventricular electroactivity, Leong et al15 demonstrated that the pacing site directly affects ventricular synchrony. The electrical impulse of RVAP occurs from the right ventricular apex and then propagates through the myocardium instead of the His‐Purkinje conduction system, resulting in a wide QRS complex similar to the left bundle branch block.22–23 The RVOT septal pacing area, however, is close to a physiological conduction system; it would promote depolarization more physiologically through rapid conduction and produces a narrow QRS width on ECG.24–25 Restoring a narrower QRS duration may, in part, explain why RVOT septal pacing is associated with protective effects on interventricular synchrony compared with RVAP. In our study, RVOT septal pacing produced a shorter QRSd than RVAP, whereas the final QRSd of the RVOT septal pacing group was also increased compared with the initial values, and the median average value was >120 ms. This result may be associated with difficulty in obtaining consistent, accurate, and reliable placement of leads in the same position and near the His‐Purkinje conduction system. Consequently, the final IVMD in the RVOT septal pacing group was increased compared with the baseline value but was less than that of the RVAP group at the end of follow‐up.
Zhang et al compared RVOT pacing with RVAP in elderly patients with normal LVEF.26 Their results showed deterioration in the LVEF in patients with RVAP but not in those with RVOT pacing, suggesting that RVOT pacing is better than RVAP in terms of preventing cardiac dysfunction. In contrast, in a study by Gong et al,16 although RVOT pacing caused more synchronous LV contraction than RVAP, no benefit over RVAP was shown regarding the prevention of cardiac remodeling and preservation of LV systolic function after 12 months of pacing in patients with normal cardiac function. In their study, RVOT pacing also produced a wide QRS complex with a median QRSd of 161 ms, a value that was different from that in other studies concerning QRSd produced by RVOT septal pacing. Thus, the precise pacing site might be different from others. In addition, such prolonged QRS duration induced by RVOT septal pacing may increase heart failure risk similar to long‐term RVAP.27 In our study, RVOT septal pacing produced shorter QRSd (median value was 130 ms) and had fewer adverse effects on IVMD, LVEF, and E/Ea ratio than RVAP. Consequently, compared with RVAP, RVOT septal pacing is the better choice to maintain ventricular synchronism.28
PWD reflects the heterogeneity of atrial electroactivity.18 Clinical studies have shown that PWD is a new predictive index of atrial arrhythmia, particularly atrial fibrillation.29 In the present study, the final PWDs in the RVAP and RVOT septal pacing groups were significantly increased compared with their initial values, but these changes were fewer in the RVOT septal pacing group. In addition, the incidence of new onset of atrial fibrillation was 14.3% (6 of 42) in the RVAP group but only 2.6% (1 of 38) in the RVOT septal pacing group at 2‐year follow‐up. In the present study, there were no significant differences in the atrial pacing and ventricular pacing percentages between the 2 groups. Consequently, we considered that RVOT septal pacing might have fewer adverse effects on atrial electroactivity compared with RVAP.
P‐wave abnormalities include Pmax and PWD, which are detected on ECGs and have been thought to reflect LA enlargement, LA hypertension, and altered conduction.19 The abnormal ventricular systolic and diastolic dysfunction induced by RVAP results in increased left atrium after‐load; therefore, the left atrium pressure will increase, and the atrial myocardium will be stretched, possibly leading to left atrium remodeling and, later, the development of atrial arrhythmias. A previous study demonstrated that acute RVAP could induce LA enlargement and impaired atrial contractility.17 Another study documented that long‐term RVOT pacing was associated with less adverse left atrium remodeling.15 These 2 studies, however, did not discuss the effect of different pacing sites on P waves, which represent atrial electroactivity. In our study, we observed the effect of chronic RVAP and RVOT septal pacing on both the left atrium structure and electroactivity. Compared with RVAP, RVOT septal pacing maintained the ventricular synchronism and prevented LV remodeling, resulting in less increase in the LV filling pressure and E/Ea ratio and in left atrium pressure; therefore, the LA volume index, Pmax, and PWD of patients in the RVOT septal pacing group were less than those of the RVAP group.
In the current study, only patients with normal LV function were included. Consequently, whether the findings of this study are applicable to patients with underlying heart disease or LV dysfunction, or both, remains unclear. To control the systematic and accidental errors that can occur during electrocardiographic and echocardiographic data acquisition, all electrocardiographic and echocardiographic examinations were performed by the same experienced technicians of the departments of electrocardiography and echocardiography, respectively. The final result for each patient was reviewed by the chief technicians. Any uncertainties regarding results were resolved by discussion. All technicians were blinded to clinical data and group division. The number of patients in this study was insufficient to determine whether prolonged PWD increases the incidence of atrial fibrillation.
In conclusion, compared with RVAP, RVOT septal pacing showed fewer adverse effects on Pmax, PWD, and QRSd in patients with normal cardiac function, indicating that RVOT septal pacing maintains more satisfying atrial and ventricular electrophysiology.
Sources of Funding
This study was supported by grant for the training plan of “six talent peaks” from Jiangsu Province, China (no. 2013‐WSN‐067).
Disclosures
None.
References
- Hillock RJ, Mond HG. Pacing the right ventricular outflow tract septum: time to embrace the future. Europace. 2012; 14:28-35. [DOI] [PubMed] [Google Scholar]
- Furman S, Schwedel J. An intracardiac pacemaker for Stokes‐Adams seizures. N Engl J Med. 1959; 261:943-948. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang Z, Zhang Y, Gao M, Wang J, Zhang Y, Xie X, Hou Y. Effects of right ventricular nonapical pacing on cardiac function: a meta‐analysis of randomized controlled trials. Pacing Clin Electrophysiol. 2013; 36:1032-1051. [DOI] [PubMed] [Google Scholar]
- Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, Lamas GAMOde Selection Trial Investigators. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003; 107:2932-2937. [DOI] [PubMed] [Google Scholar]
- Da Costa A, Gabriel L, Romeyer‐Bouchard C, Géraldine B, Gate‐Martinet A, Laurence B, Levallois M, Isaaz K. Focus on right ventricular outflow tract septal pacing. Arch Cardiovasc Dis. 2013; 106:394-403. [DOI] [PubMed] [Google Scholar]
- Spach MS, Miller WT, III, Geselowitz DB, Barr RC, Kootsey JM, Johnson EA. The discontinuous nature of propagation in normal canine cardiac muscle. Evidence for recurrent discontinuities of intracellular resistance that affect the membrane currents. Circ Res. 1981; 48:39-54. [DOI] [PubMed] [Google Scholar]
- Myerburg RJ, Gelband H, Nilsson K, Castellanos A, Morales AR, Bassett AL. The role of canine superficial ventricular muscle fibers in endocardial impulse distribution. Circ Res. 1978; 42:27-35. [DOI] [PubMed] [Google Scholar]
- Frazier DW, Krassowska W, Chen PS, Wolf PD, Danieley ND, Smith WM, Ideker RE. Transmural activations and stimulus potentials in three‐dimensional anisotropic canine myocardium. Circ Res. 1988; 63:135-146. [DOI] [PubMed] [Google Scholar]
- Kaye G, Stambler BS, Yee R. Search for the optimal right ventricular pacing site: design and implementation of three randomized multicenter clinical trials. Pacing Clin Electrophysiol. 2009; 32:426-433. [DOI] [PubMed] [Google Scholar]
- Mond HG, Hillock RJ, Stevenson IH, McGavigan AD. The right ventricular outflow tract: the road to septal pacing. Pacing Clin Electrophysiol. 2007; 30:482-491. [DOI] [PubMed] [Google Scholar]
- Rosso R, Medi C, Teh AW, Hung TT, Feldman A, Lee G, Mond HG. Right ventricular septal pacing: a comparative study of outflow tract and mid ventricular sites. Pacing Clin Electrophysiol. 2010; 33:1169-1173. [DOI] [PubMed] [Google Scholar]
- Stambler BS, Ellenbogen K, Zhang X, Porter TR, Xie F, Malik R, Small R, Burke M, Kaplan A, Nair L, Belz M, Fuenzalida C, Gold M, Love C, Sharma A, Silverman R, Sogade F, Van Natta B, Wilkoff BLROVA Investigators. Right ventricular outflow versus apical pacing in pacemaker patients with congestive heart failure and atrial fibrillation. J Cardiovasc Electrophysiol. 2003; 14:1180-1186. [DOI] [PubMed] [Google Scholar]
- Vlay SC. Right ventricular outflow tract pacing: practical and beneficial. A 9‐year experience of 460 consecutive implants. Pacing Clin Electrophysiol. 2006; 29:1055-1062. [DOI] [PubMed] [Google Scholar]
- Tse HF, Yu C, Wong KK, Tsang V, Leung YL, Ho WY, Lau CP. Functional abnormalities in patients with permanent right ventricular pacing: the effect of sites of electrical stimulation. J Am Coll Cardiol. 2002; 40:1451-1458. [DOI] [PubMed] [Google Scholar]
- Leong DP, Mitchell A, Salna I, Brooks AG, Sharma G, Lim HS, Alasady M, Barlow M, Leitch J, Sanders P, Young GD. Long‐term mechanical consequences of permanent right ventricular pacing: effect of pacing site. J Cardiovasc Electrophysiol. 2010; 21:1120-1126. [DOI] [PubMed] [Google Scholar]
- Gong X, Su Y, Pan W, Cui J, Liu S, Shu X. Is right ventricular outflow tract pacing superior to right ventricular apex pacing of cardiovascular disease in patients with normal cardiac function? Clin Cardiol. 2009; 32:695-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JM, Fang F, Zhang Q, Sanderson JE, Chan JY, Lam YY, Yu CM. Acute effects of right ventricular apical pacing on left atrial remodeling and function. Pacing Clin Electrophysiol. 2012; 35:856-862. [DOI] [PubMed] [Google Scholar]
- Dilaveris PE, Gialafos JE. P‐wave dispersion: a novel predictor of paroxysmal atrial fibrillation. Ann Noninvasive Electrocardiol. 2001; 6:159-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amasyali B, Köse S, Aytemir K, Can I, Kabakci G, Tokgozoglu L, Ozkutlu H, Nazli N, Isik E, Oto A. The effect of VVI pacing on P‐wave dispersion in patients with dual‐chamber pacemakers. Heart Vessels. 2006; 21:8-12. [DOI] [PubMed] [Google Scholar]
- Leung DY, Chi C, Allman C, Boyd A, Ng AC, Kadappu KK, Leung M, Thomas L. Prognostic implications of left atrial volume index in patients in sinus rhythm. Am J Cardiol. 2010; 105:1635-1639. [DOI] [PubMed] [Google Scholar]
- Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J, III, St John Sutton M, De Sutter J, Murillo J. Results of the predictors of response to CRT (PROSPECT) trial. Circulation. 2008; 117:2608-2616. [DOI] [PubMed] [Google Scholar]
- Sutton R. Cardiac pacing. Curr Opin Cardiol. 1993; 8:22-26. [DOI] [PubMed] [Google Scholar]
- Atlee JL, Bernstein AD. Cardiac rhythm management devices (part I): indications, device selection, and function. Anesthesiology. 2001; 95:1265-1280. [DOI] [PubMed] [Google Scholar]
- Wang F, Shi H, Sun Y, Wang J, Yan Q, Jin W, Zhang J, Meng W, Zhang F, Chen G, Sun B. Right ventricular outflow pacing induces less regional wall motion abnormalities in the left ventricle compared with apical pacing. Europace. 2012; 14:351-357. [DOI] [PubMed] [Google Scholar]
- Deshmukh P, Casavant DA, Romanyshyn M, Anderson K. Permanent, direct His‐bundle pacing: a novel approach to cardiac pacing in patients with normal His‐Purkinje activation. Circulation. 2000; 101:869-877. [DOI] [PubMed] [Google Scholar]
- Zhang H, Qian J, Hou F, Liu Y, Mao J. Comparison of right ventricular apex and right ventricular outflow tract septum pacing in the elderly with normal left ventricular ejection: long‐term follow‐up. Kardiol Pol. 2012; 70:1130-1139. [PubMed] [Google Scholar]
- Chen S, Yin Y, Lan X, Liu Z, Ling Z, Su L, Kiuchi MG, Li X, Zhong B, Krucoff MWPREDICT‐Heart Failure study international group. Paced QRS duration as a predictor for clinical heart failure events during right ventricular apical pacing in patients with idiopathic complete atrioventricular block: results from an observational cohort study (PREDICT‐HF). Eur J Heart Fail. 2013; 15:352-359. [DOI] [PubMed] [Google Scholar]
- Molina L, Sutton R, Gandoy W, Reyes N, Lara S, Limón F, Gómez S, Orihuela C, Salame L, Moreno G. Medium‐term effects of septal and apical pacing in pacemaker‐dependent patients a double‐blind prospective randomized study. Pacing Clin Electrophysiol. 2014; 37:207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani JW, Williamson MA, Ellinor PT, Monahan KM, Benjamin EJ. P wave indices: current status and future directions in epidemiology, clinical, and research applications. Circ Arrhythm Electrophysiol. 2009; 2:72-79. [DOI] [PMC free article] [PubMed] [Google Scholar]


