Abstract
Background
Serum bicarbonate varies over time in chronic kidney disease (CKD) patients, and this variability may portend poor cardiovascular outcomes. The aim of this study was to conduct a time‐updated longitudinal analysis to evaluate the association of serum bicarbonate with long‐term clinical outcomes: heart failure, atherosclerotic events, renal events (halving of estimated glomerular filtration rate [eGFR] or end‐stage renal disease), and mortality.
Methods and Results
Serum bicarbonate was measured annually, in 3586 participants with CKD, enrolled in the Chronic Renal Insufficiency Cohort (CRIC) study. Marginal structural models were created to allow for integration of all available bicarbonate measurements and proper adjustment for time‐dependent confounding. During the 6 years follow‐up, 512 participants developed congestive heart failure (26/1000 person‐years) and 749 developed renal events (37/1000 person‐years). The risk of heart failure and death was significantly higher for participants who maintained serum bicarbonate >26 mmol/L for the entire duration of follow‐up (hazard ratio [HR] 1.66; 95% confidence interval [CI], 1.23 to 2.23, and HR 1.36, 95% CI 1.02 to 1.82, respectively) compared with participants who kept their bicarbonate 22 to 26 mmol/L, after adjusting for demographics, co‐morbidities, medications including diuretics, eGFR, and proteinuria. Participants who maintained serum bicarbonate <22 mmol/L had almost a 2‐fold increased risk of renal disease progression (HR 1.97; 95% CI, 1.50 to 2.57) compared with participants with bicarbonate 22 to 26 mmol/L.
Conclusion
In this large CKD cohort, persistent serum bicarbonate >26 mmol/L was associated with increased risk of heart failure events and mortality. Further studies are needed to determine the optimal range of serum bicarbonate in CKD to prevent adverse clinical outcomes.
Keywords: CKD, heart failure, serum bicarbonate
Introduction
It is estimated that over 20 million Americans have chronic kidney disease (CKD).1 In addition to progression to end‐stage disease, cardiovascular disease is the major cause of morbidity and mortality in patients with CKD.2 Therefore, the need to identify novel risk factors that increase risk for cardiovascular disease in the setting of CKD has been well recognized.3 Development of CKD is associated with retention of hydrogen ions, increase in kidney interstitial acidity, and increase endothelin and aldosterone, even in the absence of a low plasma bicarbonate.4 Metabolic acidosis is a predictor of kidney disease progression5 and a limited number of interventional trials have shown that base therapy in the form of sodium bicarbonate or simply a diet rich in fruits and vegetables improves the interstitial acid‐base milieu, reduces endothelin and aldosterone levels, and slows the CKD progression.6–10 However, whether changes in acid base milieu have an impact on cardiovascular outcomes is less well studied. Additionally, the optimal level of serum bicarbonate for the cardiovascular system has not been evaluated in any of these trials. Indeed, while most literature has focused on metabolic acidosis, the effect of high serum bicarbonate in CKD patients has been less studied.
Observational studies have shown that serum bicarbonate concentration is associated with renal outcomes and mortality, with a presumed optimal level in the 24 to 26 mmol/L range,11–16 resulting in the current recommendation to maintain serum bicarbonate above 22 mmol/L in CKD patients.17 Our initial work in the Chronic Renal Insufficiency Cohort (CRIC) Study showed a 14% higher risk of heart failure events (HR, 1.14; 95% CI, 1.03 to 1.26; P=0.02) per 1 mmol/L increase in serum bicarbonate level over 24 mmol/L, and a 3% lower risk for renal events per 1 mmol/L increase in serum bicarbonate (HR, 0.97; 95% CI, 0.94 to 0.99; P=0.01).5 All the above‐mentioned studies used a single point appraisal of the serum bicarbonate level at study baseline, without taking into consideration the bicarbonate variation overtime, which is a frequent occurrence in CKD population. Marginal structural models (MSMs) are modern statistical tools available to estimate the effect of time‐updated exposures, heavily influenced by time‐updated confounders in longitudinal studies.18
The goal of this analysis was to investigate the relationship between serum bicarbonate levels and heart failure events, CKD progression, and mortality utilizing time‐updated serum bicarbonate measurements, and appropriate adjustment for time‐dependent confounders. We also sought to explore the potential effect modification by demographic and clinical characteristics, including level of kidney function.
Methods
Study Design and Population
The Chronic Renal Insufficiency Cohort (CRIC) Study enrolled 3939 individuals aged 21 to 74 years with estimated glomerular filtration rate (eGFR) of 0.33 to 1.17 mL/s per m2 (20 to 70 mL/min per 1.73 m2), from June 2003 to December 2008 at 7 clinical centers across the United States (Ann Arbor, MI; Baltimore, MD; Chicago, IL; Cleveland, OH; New Orleans, LA; Philadelphia, PA; and Oakland, CA). Study design and baseline participant characteristics were previously published.19–21 Major exclusion criteria included prior dialysis treatment lasting more than 1 month, New York Heart Association Class III/IV heart failure, polycystic kidney disease, or other primary renal diseases requiring active immunosupression, human immunodeficiency virus (HIV) infection, pregnancy, inability to give informed consent or institutionalization. Participants underwent annual study visits and telephone follow‐up twice a year. The final study population for this analysis included 3586 participants after exclusion of 353 with missing serum bicarbonate and other covariates at baseline. There was no statistically significant difference between study participants and those excluded from the analyses. Study participants provided written informed consent and authorized release of medical records for study purposes, and are followed annually under protocols approved by institutional review boards at each of the CRIC Study clinical centers.
Data Collection
Main predictor
Serum bicarbonate was measured annually using an enzymatic procedure with phosphoenolpyruvate carboxylase on the Ortho Vitros platform at the University of Pennsylvania Core Laboratory. Time‐updated mean serum bicarbonate level was defined as the average serum bicarbonate at any given visit along with those from all prior visits. The current analysis examined time‐updated mean serum bicarbonate levels continuously per 1 mmol/L increase, and categorically using the following groups of serum bicarbonate: below 22 mmol/L, between 22 to 26 mmol/L (reference group) and above 26 mmol/L.
Outcomes
The study outcomes were adjudicated heart failure events, atherosclerotic events, renal disease progression, and mortality, ascertained from study entry through March 2012. Participants’ follow‐up was censored at the end of the follow‐up time period, loss to follow‐up, when they achieved the event of interest, or death, whichever occurred first.
Study personnel identified possible heart failure events by reviewing hospital billing codes. Two independent reviewers adjudicated heart failure events and classified them as probable or definite. Criteria for congestive heart failure events included a combination of symptoms (dyspnea on exertion, paroxysmal nocturnal dyspnea, and orthopnea), accompanied by radiographic changes (pulmonary edema, congestion) or pertinent findings on physical examination (evidence of 2 or more of the following: pulmonary rales, S3 gallop, jugular venous distension >5 cm, and peripheral edema).
Atherosclerotic events included adjudicated myocardial infarction, cerebrovascular accident, or peripheral vascular disease. Criteria for myocardial infarction classified as definite, probable, or possible, included a combination of chest pain, electrocardiographic abnormalities, and elevated cardiac biomarkers. Peripheral vascular disease procedures were ascertained using International Classification of Diseases, Ninth Revision codes. Two neurologists adjudicated cerebrovascular accidents.
Renal disease progression was defined by a composite of ESRD or halving of eGFR from baseline. ESRD was defined as receipt of chronic dialysis or kidney transplant and was ascertained primarily through self‐report. Estimated GFR was calculated from serum creatinine and cystatin C using a CRIC Study equation.22 Time to eGFR halving was imputed assuming a linear decline in kidney function between in‐person annual visit measures.
Mortality was ascertained from reports of next of kin, retrieval of death certificates, obituaries, reviews of hospital records, and linkage with the Social Security Death Master File.
Covariates
Demographic and clinical information were obtained at the baseline and follow‐up study visits by questionnaires, interviews, and physical examination. History of any cardiovascular disease included prior myocardial infarction, revascularization, heart failure, stroke, or peripheral arterial disease. Current smoking was defined as self‐report of current use of cigarettes and at least 100 cigarettes smoked. At each study visit, participants were queried about any medication usage in the prior 30 days. All anti‐hypertensive medications were categorized into drug classes, and the total number of anti‐hypertensive drug classes was calculated. Serum creatinine was measured at the CRIC Central Laboratory by an enzymatic method (www.orthoclinical.com) through October 2008 and by the Jaffe method (www.beckmancoulter.com) thereafter, and standardized to isotope dilution mass spectrometry‐traceable values.23–24 Twenty four‐hour urine total protein was measured using a turbidometric method (www.roche-diagnostics.us) through October 2008, and spectrophotometric quantitation (www.beckmancoulter.com) thereafter. Glucose was detected using a coupled enzymatic method (www.orthoclinical.com) through October 2008, and by an oxygen‐depletion method (www.beckmancoulter.com) thereafter. Diabetes mellitus was defined as a fasting glucose >7 mmol/L (126 mg/dL), a non‐fasting glucose >11.1 mmol/L (200 mg/dL), or use of insulin or other antidiabetic medication.25 Hypertension was defined as either systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, or use of antihypertensive medications.26
Statistical Analysis
Descriptive statistics and distributions of all available serum bicarbonate variables were created. Over each participant's entire follow‐up, serum bicarbonate was classified as the percentage of time spent with a serum bicarbonate level <22 mmol/L, between 22 to 26 mmol/L, and >26 mmol/L, each categorized as 0%, 1% to 20%, 20.1% to 40%, 40.1% to 99.9%, and 100% of follow‐up time. The proportion of participants in each of these categories of follow‐up time was depicted graphically. Crude event rates were calculated overall and within levels of baseline serum bicarbonate.
Marginal structural models were designed to estimate the cumulative effect of a treatment/exposure over some period when the confounders change over time.18 We used time‐updated information on serum bicarbonate by examining the mean bicarbonate over time at each study visit, to ascertain its average causal effect on clinical outcomes. Important covariates associated with heart failure and renal events, like proteinuria and eGFR vary over time and may also predict subsequent serum bicarbonate levels. Therefore, to account for this potential time‐dependent confounding while leaving any pathway effects intact, we fit marginal structural models (MSMs) that utilize a weighted discrete time logistic regression model. This model is comparable to the standard Cox proportional hazards model.18 The weight for each observation in the MSMs was inversely proportional to the probability that a subject's history of bicarbonate level is what was observed in the dataset. Hazards ratios and 95% confidence intervals (95% CI) were reported for all models. All models were adjusted for age, gender, race/ethnicity, clinical center, eGFR, proteinuria, diabetes, systolic blood pressure, cardiovascular disease at baseline, chronic obstructive pulmonary disease, tobacco use, diuretic and alkali medication used, low‐density lipoprotein, fibroblast growth factor 23, high‐sensitivity C‐reactive protein. Serum bicarbonate was modeled in terms of hazards ratios per 1 mmol/L increase and also across distinct categories (<22, 22 to 26, and >26 mmol/L).
MSMs compare different scenarios that would happen to subjects in the study. For example, what would happen if all subjects’ serum bicarbonate levels were above 26 mmol/L compared with what would happen if all subjects’ serum bicarbonate levels were between 22 to 26 mmol/L. For example, if the hazard ratio for heart failure is found to be 1.66 for the group of serum bicarbonate >26 mmol/L, the risk of heart failure would be 66% higher if all subjects’ bicarbonate level were >26 mmol/L during the whole follow‐up time, than if all subjects’ bicarbonate level were between 22 to 26 mmol/L. Similar interpretation applies to the other outcomes. More detailed explanation of MSM is included the Data S1.
We explored effect modification by an a priori selected set of baseline characteristics including race/ethnicity, diabetes status, and level of kidney function (eGFR <45 and ≥45 mL/min per 1.73 m²). Interaction terms were included in the marginal structural models and stratified analyses across these variables were reported. All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC). All statistical tests were 2 sided, and P<0.05 was considered significant.
Results
The study included 3586 participants with CKD from CRIC. Mean age at study entry was 58±11 (SD) years, the mean eGFR was 0.75 mL/s per m2 (44.9 mL/min per 1.73 m2), median 24‐hour urine total protein excretion was 0.2 g/day and median serum bicarbonate level was 24; interquartile range, 22 to 26) mmol/L. In the study cohort, 1631 (41.8%) were African‐Americans, 1763 (45.2%) were females and 1896 (48.6%) had diabetes. At baseline, participants who were African‐Americans, older, diabetics, on diuretics, and with higher body mass index were more likely to have serum bicarbonate above 26 mmol/L. At the other end of the spectrum, participants who were younger, male, Hispanic, and hypertensive were more likely to have a serum bicarbonate below 22 mmol/L. Likewise, participants with lower eGFR and hemoglobin, and higher creatinine, proteinuria, parathyroid hormone, phosphorus, and FGF23 levels had low serum bicarbonate (<22 mmol/L) at baseline. See Table 1 for additional baseline study cohort characteristics.
Table 1.
Characteristics of CRIC Study Participants by Baseline Serum Bicarbonate
| Characteristic | All Participants (n=3908) | Groups of Baseline Serum Bicarbonate (mmol/L) | P Value* | ||
|---|---|---|---|---|---|
| <22 (n=610) | [22 to 26] (n=2071) | >26 (n=905) | |||
| Serum bicarbonate, mmol/L | 24.4 (3.2) | 19.5 (1.7) | 24.1 (1.4) | 28.4 (1.5) | <0.0001 |
| Demographic data | |||||
| Age, y | 57.7 (11.0) | 56.1 (12.0) | 57.6 (11.1) | 59.1 (9.9) | <0.0001 |
| Women | 1763 (45.2) | 291 (43.0) | 1020 (45.2) | 452 (46.5) | 0.39 |
| Race | |||||
| Non‐hispanic white | 1625 (41.6) | 226 (33.4) | 987 (43.8) | 412 (42.3) | <0.0001 |
| Non‐hispanic black | 1631 (41.8) | 275 (40.7) | 892 (39.6) | 464 (47.7) | <0.0001 |
| Hispanic | 495 (12.7) | 156 (23.1) | 280 (12.4) | 59 (6.1) | <0.0001 |
| Other | 153 (3.9) | 19 (2.8) | 96 (4.3) | 38 (3.9) | <0.0001 |
| Hypertension | 3360 (86.1) | 601 (88.9) | 1937 (85.9) | 822 (84.5) | 0.04 |
| Diabetes | 1896 (48.6%) | 362 (53.6%) | 1079 (47.8%) | 455 (46.8%) | 0.01 |
| Any cardiovascular disease | 1303 (33.4) | 223 (33.0) | 745 (33.0) | 335 (34.4) | 0.72 |
| Current smoking | 508 (13.0) | 128 (18.9) | 289 (12.8) | 91 (9.4) | <0.0001 |
| Chronic obstructive pulmonary disease | 121 (3.1) | 20 (3.0) | 63 (2.8) | 38 (4.0) | 0.24 |
| Body mass index, kg/m2 | 32.1 (7.8) | 31.3 (7.2) | 32.2 (7.9) | 32.5 (8.0) | 0.01 |
| Systolic blood pressure, mm Hg | 128.5 (22.2) | 130.0 (22.9) | 128.1 (21.9) | 128.5 (22.5) | 0.15 |
| Diastolic blood pressure, mm Hg | 71.5 (12.8) | 71.5 (12.8) | 71.6 (12.7) | 71.4 (13.2) | 0.88 |
| LDL cholesterol, mmol/L | 2.7 (0.9) | 2.6 (1.0) | 2.7 (0.9) | 2.7 (0.9) | 0.03 |
| HDL cholesterol, mmol/L | 1.2 (0.4) | 1.2 (0.4) | 1.2 (0.4) | 1.3 (0.4) | <0.0001 |
| Medications | |||||
| Aspirin | 1662 (42.9) | 282 (41.8) | 943 (42.1) | 437 (45.3) | 0.20 |
| Beta‐blockers | 1913 (49.3) | 339 (50.3) | 1074 (48.0) | 500 (51.9) | 0.11 |
| Statins | 2136 (55.1) | 396 (58.8) | 1215 (54.3) | 525 (54.5) | 0.11 |
| ACE inhibitor/ARB | 2668 (68.8) | 481 (71.4) | 1545 (69.0) | 642 (66.6) | 0.12 |
| Anti‐acidosis medications* | 91 (2.3) | 20 (3.0) | 46 (2.1) | 25 (2.6) | 0.33 |
| Any diuretic | 2309 (59.6) | 387 (57.4) | 1286 (57.4) | 636 (66.0) | <0.0001 |
| Laboratory data | |||||
| eGFR, mL/min per 1.73 m2* | 44.9 (16.9) | 35.2 (13.8) | 45.5 (16.7) | 50.1 (16.4) | <0.0001 |
| Creatinine, mg/dL | 1.84 (0.65) | 2.26 (0.79) | 1.80 (0.60) | 1.64 (0.51) | <0.0001 |
| Creatinine, μmol/L | 162.7 (57.4) | 200.2 (69.9) | 159.1 (53.0) | 145.2 (44.7) | |
| 24 hours urine protein, g/day* | 0.19 (0.07 to 0.92) | 0.48 (0.11 to 1.95) | 0.18 (0.07 to 0.93) | 0.11 (0.06 to 0.47) | <0.0001 |
| Urine albumin/creatinine, μg/mg* | 52.3 (8.7 to 461.4) | 218.4 (27.0 to 1105) | 51.2 (8.5 to 446.6) | 22.1 (6.1 to 191.6) | <0.0001 |
| Calcium, mmol/L | 2.3 (0.1) | 2.3 (0.2) | 2.3 (0.1) | 2.3 (0.1) | 0.002 |
| Phosphorus, mmol/L | 1.2 (0.2) | 1.3 (0.3) | 1.2 (0.2) | 1.2 (0.2) | <0.0001 |
| Parathyroid hormone, ng/L | 54.0 (35.0 to 90.0) | 76.0 (46.8 to 131.9) | 53.0 (34.0 to 85.7) | 45.2 (32.1 to 74.9) | <0.0001 |
| Albumin, g/L | 39.4 (4.7) | 38.6 (5.1) | 39.5 (4.7) | 39.5 (4.5) | <0.0001 |
| Hemoglobin, g/L | 126 (18) | 120 (18) | 126 (17) | 129 (17) | <0.0001 |
| Fibroblast growth factor 23, RU/mL* | 145.6 (95.7 to 239.3) | 193.0 (122.3 to 299.5) | 144.5 (95.3 to 235.9) | 121.3 (85.6 to 190.7) | <0.0001 |
| High sensitivity C reactive protein, nmol/L* | 24.8 (10.5 to 61.9) | 25.7 (10.5 to 63.8) | 23.8 (9.5 to 61.0) | 24.8 (9.5 to 61.0) | 0.33 |
| Dietary protein, g/kg per day | 0.8 (0.4) | 0.8 (0.5) | 0.8 (0.4) | 0.8 (0.4) | 0.21 |
| Urine urea Nitrogen, g/day* | 7.7 (5.7 to 10.5) | 7.5 (5.3 to 10.1) | 7.8 (5.7 to 10.7) | 7.6 (5.8 to 10.5) | 0.06 |
Unless otherwise noted, values are n (%) or means±SDs. ACE indicates angiotensin‐converting enzyme; ARB, angiotensin‐receptor blocker; CRIC, Chronic Renal Insufficiency Cohort; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
P value obtained from the Kruskal–Wallis ranking test.
Anti‐acidosis medications are represented by: calcium citrate, magnesium citrate, potassium citrate, sodium bicarbonate, sodium lactate, sodium citrate, sodium acetate, tromethamine, and lactated potassium saline.
Estimated GFR was calculated from serum creatinine and cystatin C using a CRIC Study equation.25
Median (interquartile range).
Each participant's serum bicarbonate values over their course of participation in the CRIC study was averaged; the within‐participant mean serum bicarbonate level over time ranged from 11 to 39 mmol/L (mean [SD]: 24.4 [2.7] mmol/L) (Figure 1).
Figure 1.
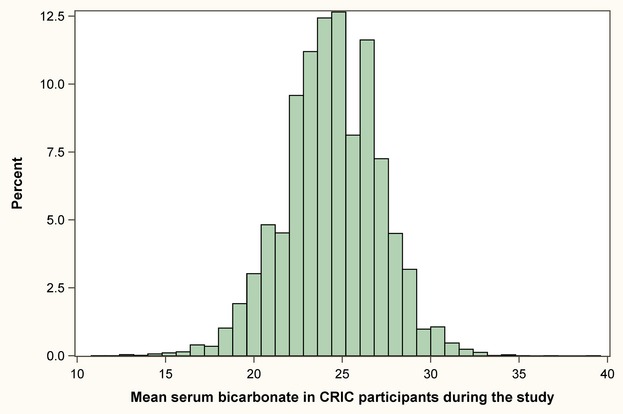
Distribution of mean serum bicarbonate in CRIC participants over their course of participation in the study. CRIC indicates Chronic Renal Insufficiency Cohort.
Figure 2 depicts the distribution of follow‐up time spent with serum bicarbonate above 26 and below 22 mmol/L among all participants. In almost 5% of CRIC participants, all measured serum bicarbonate levels were greater than 26 mmol/L. Conversely, in 55.1% of study participants, all measured serum bicarbonate levels were less than 22 mmol/L.
Figure 2.
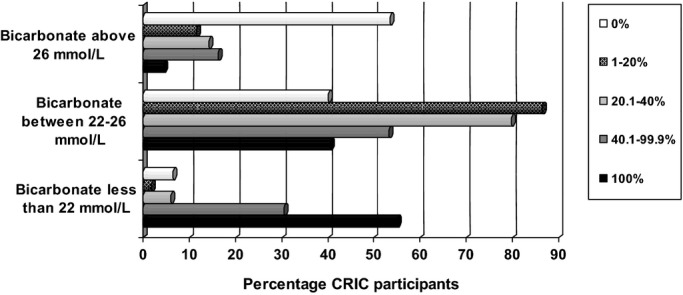
Percentage of follow‐up time spent with high, normal and low serum bicarbonate (N=3586 CRIC participants). CRIC indicates Chronic Renal Insufficiency Cohort.
Heart Failure Events
Over an average of 6 years of follow‐up, 512 participants developed adjudicated congestive heart failure events that were classified as probable or definite (26 events per 1000 person‐years). Heart failure increased with higher levels of serum bicarbonate. In marginal structural models that incorporated time‐updated serum bicarbonate levels and adjustment for other time‐updated covariates, the risk for heart failure was 8% higher with each 1 mmol/L increase in mean serum bicarbonate over time (hazard ratio [HR] 1.08, 95% confidence interval [CI] 1.04 to 1.13, P<0.001). The rates of heart failure were nearly 2‐fold higher if participants’ mean serum bicarbonate level were above 26 mmol/L over time compared with between 22 to 26 mmol/L (HR 1.66, 95% CI, 1.23 to 2.23, P<0.001). There were no detectably different rates of heart failure events for those whose mean serum bicarbonate remained <22 mmol/L over time, compared with those with mean levels 22 to 26 mmol/L (HR 1.00; 95% CI, 0.70 to 1.44; P=0.99) (Table 2). Similar findings were observed when the risk for composite outcome of heart failure or death was evaluated in marginal structural models (Table S1).
Table 2.
Multivariable‐Adjusted Hazard Ratios for Time‐Updated Serum Bicarbonate on Heart Failure and Renal Events in the CRIC Using Marginal Structural Models
| Marginal Structural Model Hazard Ratio (95% Confidence Interval) | |
|---|---|
| Heart failure | |
| Continuous serum bicarbonate | Per 1 mmol/L increase in mean bicarbonate over time* |
| 1.08 (1.04 to 1.13) | |
| Categorical serum bicarbonate* (reference 22 to 26 mmol/L) | |
| Serum bicarbonate <22 mmol/L | 1.00 (0.70 to 1.44) |
| Serum bicarbonate >26 mmol/L | 1.66 (1.23 to 2.23) |
| Renal event* | |
| Continuous serum bicarbonate | Per 1 mmol/L increase in mean bicarbonate over time* |
| 0.93 (0.89 to 0.96) | |
| Categorical serum bicarbonate (reference 22 to 26 mmol/L) | |
| Serum bicarbonate <22 mmol/L | 1.97 (1.50 to 2.57) |
| Serum bicarbonate >26 mmol/L | 1.07 (0.75 to 1.53) |
Total of 3586 participants were included in each model. There were 512 participants with a heart failure event and 749 with a renal event. CRIC indicates Chronic Renal Insufficiency Cohort; eGFR, estimated glomerular filtration rate.
Results generated from a Marginal Structural Model with updated mean serum bicarbonate over time. All models are adjusted for age, gender, race/ethnicity, clinical center, eGFR, proteinuria, diabetes, hypertension, cardiovascular disease at baseline, chronic obstructive pulmonary disease, tobacco use, diuretic and alkali medication used, low density lipoprotein, fibroblast growth factor 23, high‐sensitivity C‐reactive protein. In order to evaluate the risk of events for participants with bicarbonate belonging to any category only a certain percent of time, the risk should be multiplied by that percent (ie, if a participant spends 50% of the time with bicarbonate >26 mmol/L, the risk of heart failure is 66×0.5=33%).
The 3 categories of serum bicarbonate, 22 to 26, <22 and >26 mmol/L, had the following distribution of CRIC participants: 2071, 610, and 905, with corresponding 259, 99 and 154 heart failure events and 401, 231 and 117 renal events in each group, respectively.
Atherosclerotic Cardiovascular Events
There was no association between time updated serum bicarbonate levels and atherosclerotic cardiovascular events examined as a continuous or categorical variable in marginal structural models adjusted for time updated covariates (Table S2).
Renal Events
Renal events were observed in 749 participants (37 events per 1000 person‐years) during follow‐up time. Renal disease progression was more likely in participants with lower levels of serum bicarbonate. In marginal structural models that adjusted for time updated covariates the risk for renal events that was 7% lower with each 1 mmol/L increase in mean serum bicarbonate over time (HR 0.93, 95% CI 0.89 to 0.96, P<0.001). The rates of renal events were nearly 2‐fold higher if participants’ mean serum bicarbonate were less than 22 mmol/L over time compared to mean levels of 22 to 26 mmol/L (HR 1.97, 95% CI 1.50 to 2.57, P<0.001). There were no detectably different rates of renal events for those whose mean serum bicarbonate remained above 26 mmol/L over time, compared with those with mean levels 22 to 26 mmol/L (HR 1.07, 95% CI 0.75 to 1.53, P=0.69) (Table 2).
Mortality
There were 639 deaths during follow‐up in the study cohort (28.7 events per 1000 person‐years). The risk of death associated with mean serum bicarbonate above 26 mmol/L was higher than the risk associated with mean levels 22 to 26 mmol/L (HR 1.36, 95% CI, 1.02 to 1.82, P=0.03). There was no association between time updated serum bicarbonate levels and mortality examined as a continuous variable in marginal structural models adjusted for time updated covariates (Table 3).
Table 3.
Multivariable‐Adjusted Hazard Ratios for Time‐Updated Serum Bicarbonate on Mortality in the CRIC Study Using Marginal Structural Models
| Marginal Structural Model Hazard Ratio (95% Confidence Interval) | |
|---|---|
| Death* | |
| Continuous serum bicarbonate | Per 1 mmol/L increase in mean bicarbonate over time* |
| 1.02 (0.98 to 1.06) | |
| Categorical serum bicarbonate (reference 22 to 26 mmol/L) | |
| Serum bicarbonate <22 mmol/L | 1.26 (0.92 to 1.74) |
| Serum bicarbonate >26 mmol/L | 1.36 (1.02 to 1.82) |
Total of 3586 participants were included in the model. There were a total of 639 deaths. CRIC indicates Chronic Renal Insufficiency Cohort; eGFR, estimated glomerular filtration rate.
The 3 categories of serum bicarbonate, 22 to 26, <22 and >26 mmol/L, had the following distribution of CRIC participants: 2071, 610, and 905, with corresponding 358, 130, and 151 deaths in each group, respectively.
Results generated from a Marginal Structural Model with updated mean serum bicarbonate over time. All models are adjusted for age, gender, race/ethnicity, clinical center, eGFR, proteinuria, diabetes, hypertension, cardiovascular disease at baseline, chronic obstructive pulmonary disease, tobacco use, diuretic and alkali medication used, Low Density Lipoprotein, Fibroblast Growth Factor 23, High‐sensitivity C‐reactive protein. In order to evaluate the risk of events for participants with bicarbonate belonging to any category only a certain percent of time, the risk should be multiplied by that percent (ie, if a participant spends 50% of the time with bicarbonate >26 mmol/L, the risk of mortality is 36×0.5=18%).
Subgroup Analyses
We performed a series of a priori subgroup analyses to verify the associations between serum bicarbonate levels and heart failure and renal events, by race/ethnicity, diabetes status, proteinuria, and eGFR levels. The relationship between serum bicarbonate and heart failure and renal events was robust and consistent across categories of race/ethnicity, diabetes, or baseline kidney function (Figure 3).
Figure 3.
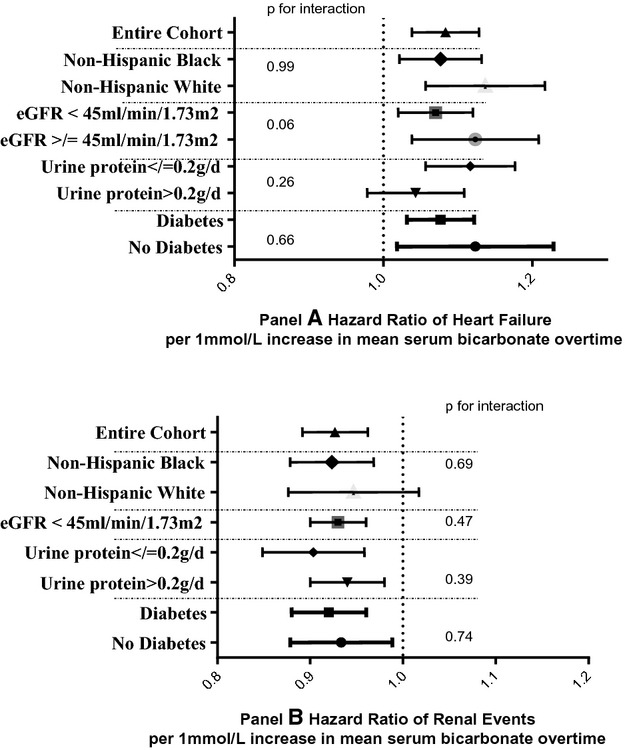
Multivariable‐adjusted hazard ratios for time‐updated serum bicarbonate on heart failure (A) and renal events (B)* by subgroups in the CRIC Study using Marginal Structural Models. CRIC indicates Chronic Renal Insufficiency Cohort; eGFR, estimated glomerular filtration rate.
The strength of association between serum bicarbonate >26 mmol/L and heart failure events persisted after excluding participants taking anti‐acidosis medications (calcium citrate, magnesium citrate, potassium citrate, sodium bicarbonate, sodium lactate, sodium citrate, sodium acetate, tromethamine, and lactated potassium saline) (HR 1.66, 95% CI 1.23 to 2.24), had chronic obstructive pulmonary disease (HR 1.54, 95% CI 1.13 to 2.09) or cardiovascular disease at baseline (HR 1.72, 95% CI 1.04 to 2.85). Similarly, the association between serum bicarbonate <22 mmol/L and renal events remained strong for the above groups. Analyses excluding participants on diuretic were not feasible due to the lack of power – almost 60% of the study cohort were taking diuretics.
Discussion
In this large cohort of individuals with mild to moderate CKD, the relationship between high‐serum bicarbonate and increased risk of heart failure and death was robust after accounting for time‐updated measurements of kidney function, markers of inflammation, and medication use. Persistently elevated serum bicarbonate above 26 mmol/L was associated with higher risk of heart failure events and all‐cause mortality. At the opposite end of the spectrum, CKD patients with serum bicarbonate persistently below 22 mmol/L had an increased risk of eGFR halving or progression of renal disease to ESRD. There was no statistically significant association between serum bicarbonate levels and atherosclerotic cardiovascular events.
In addition to the traditional risk factors, including the high prevalence of atherosclerotic heart disease, anemia, hypertension, volume overload due to impaired sodium excretion, novel risk factors for heart failure in CKD have emerged, including elevated FGF23 and serum bicarbonate levels.27 Though it is known that patients with CKD are predisposed to volume overload due to reduced eGFR, the independent association between serum bicarbonate and heart failure events was robust in this study, regardless of level of eGFR or proteinuria. Furthermore, changes in diuretic use overtime, that could potentially affect serum bicarbonate levels, did not influence the strength of the association.
Heart failure and CKD share several pathophysiologic mechanisms such as activation of the renin‐angiotensin‐aldosterone system, sympathetic nervous system, inflammation, and oxidative stress.28 CKD is the most prevalent co‐morbidity in heart failure and confers the highest risk of mortality and hospitalizations for heart failure.29 There is a bidirectional relationship between CKD and heart failure, each being capable of causing or worsening the other. However, it is important to note that subjects with New York Heart Association class III and IV heart failure were excluded from the CRIC study.
The role of alkalosis on cardiac muscle is not completely understood and relatively limited literature exists in the field. It is known from animal models that mild renal insufficiency in rats results in early cardiac fibrosis and impaired diastolic function, which progresses to more global left ventricular remodeling and dysfunction.30 Our previous work was remarkable in showing a 14% increased risk of heart failure events with each 1 mmol/L increased in serum bicarbonate above 24 mmol/L.5 This current study takes a step forward and describes a powerful relationship between time‐updated serum bicarbonate and heart failure events. Though our study is observational and does not imply causality, it does complement the evidence from animal studies that link an alkalotic milieu with restructuring of the cardiac muscle with consequent heart failure.30
Vascular precipitation of calcium and phosphorus is probably the most extensively studied mechanism of alkalosis leading to adverse cardiovascular events and death.31 The pathophysiology of vascular calcification in CKD is not clearly understood.31 Experimental studies looking at the effects of alkalosis on the regulation of cardiac myocytes showed that metabolic alkalosis promotes specific changes in regulatory proteins responsible for gene transcription and cell survival.32 It is also known that alkalinization increases vascular calcification in cultured cells and uremic rats.33–34 In vivo experiments on uremic rats showed that aortic calcification was prevented by metabolic acidosis that was induced by ingestion of ammonium chloride.35
Prior observational studies and clinical trials provide evidence of the link between serum bicarbonate and kidney disease progression. Observational findings are widely consistent in demonstrating a graded increase in renal events associated with decline in serum bicarbonate.5,12–13,15 Furthermore, reports from small clinical trials, have provided evidence of improvement in renal outcomes with treatment of chronic metabolic acidosis with either sodium bicarbonate supplementation or a diet rich in fruits and vegetables.6–10
Although the observational nature of CRIC Study data limits causal inference given the mutual relationship between serum bicarbonate level and severity of kidney dysfunction, novel approaches to analyzing observational data substantially improve our ability to employ such inference. The current analysis describes the time‐updated effect of serum bicarbonate on CKD progression while simultaneously adjusting for the confounding effects of time‐updated level of kidney function, proteinuria, diuretic and anti‐acidosis medication use, while leaving intact these factors’ potential intermediary effects. The greater magnitude of the serum bicarbonate effect in the Marginal Structural Models compared with our previous analysis using the baseline data highlights the benefit of updating this dynamic exposure over time to better estimate its impact on clinical outcomes. To our knowledge, this analysis is the first to assess this question using appropriate techniques for dealing with time‐dependent confounding.
However, some important limitations need to be noted. First, a blood gas analysis would truly assess the acid base status, compared with serum bicarbonate alone. Though we did adjust for the presence of lung disease, some participants might have high bicarbonate as a compensatory mechanism for respiratory acidosis. Second, serum bicarbonate measurements occurred in most instances a couple of days after the original blood draw, and one has to account for a change up to 4 mmol/L in true bicarbonate concentration for all determinations done after 24 hours from collection.36 Third, the diagnosis of heart failure was made using standard algorithms based on clinical, imaging, and laboratory data that are validated in the general population, but not for heart failure in the setting of CKD.37 Fourth, the marginal structural models used annual measurements of serum bicarbonate, which assumes the values remain constant in between 2 assessments.
The current study of a large cohort of participants with CKD stages 2 to 4 demonstrates a potent relationship between time‐updated serum bicarbonate and heart failure and renal events. The deviation of serum bicarbonate from normal in either direction, one portending adverse renal outcomes and the other heart failure events reinforces the need to keep a balanced acid base status in CKD patients and find a “sweet spot” for the serum bicarbonate that correlates with the best clinical outcomes. The use of time‐updated serum bicarbonate and appropriate adjustment for time‐dependent confounders in this analysis provides improved evidence of this important predictor of heart failure events and CKD progression compared with baseline models. These findings suggest that changes in acid base status could represent a novel mechanism of congestive heart failure in CKD. Further studies are needed to determine the optimal range for serum bicarbonate to prevent cardiovascular and renal events in patients with CKD.
Appendix
CRIC Study Investigators: Alan S. Go, MD, Jiang He, MD, John W. Kusek, PhD, Akinlolu Ojo, MD, James P. Lash, MD, Raymond R. Townsend, MD.
Supplementary Material
Appendix Analyses of time-updated serum bicarbonate.
Sources of Funding
Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR‐000424, University of Maryland GCRC M01 RR‐16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente NIH/NCRR UCSF‐CTSI UL1 RR‐024131. Dobre was supported by 13FTF15920005. Scialla was supported by K23DK095949. Wolf was supported by R01DK081374. Fischer was supported by Department of Veterans Affairs Health Services Research and Development Service.
Disclosures
None.
References
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. J Am Med Assoc. 2007; 298:2038-2047. [DOI] [PubMed] [Google Scholar]
- Yuyun MF, Khaw KT, Luben R, Welch A, Bingham S, Day NE, Wareham NJ. Microalbuminuria independently predicts all‐cause and cardiovascular mortality in a British population: the European Prospective Investigation into Cancer in Norfolk (EPIC‐Norfolk) population study. Int J Epidemiol. 2004; 33:189-198. [DOI] [PubMed] [Google Scholar]
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PWAmerican Heart Association Councils on Kidney in Cardiovascular Disease HBPRCC, Epidemiology, Prevention. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003; 108:2154-2169. [DOI] [PubMed] [Google Scholar]
- Wesson DE, Simoni J. Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int. 2010; 78:1128-1135. [DOI] [PubMed] [Google Scholar]
- Dobre M, Yang W, Chen J, Drawz P, Hamm LL, Horwitz E, Hostetter T, Jaar B, Lora CM, Nessel L, Ojo A, Scialla J, Steigerwalt S, Teal V, Wolf M, Rahman M. Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: a report from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2013; 62:670-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito‐Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009; 20:2075-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goraya N, Simoni J, Jo CH, Wesson DE. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol. 2013; 8:371-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goraya N, Simoni J, Jo C, Wesson DE. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int. 2012; 81:86-93. [DOI] [PubMed] [Google Scholar]
- Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010; 78:303-309. [DOI] [PubMed] [Google Scholar]
- Phisitkul S, Khanna A, Simoni J, Broglio K, Sheather S, Rajab MH, Wesson DE. Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int. 2010; 77:617-623. [DOI] [PubMed] [Google Scholar]
- Kovesdy CP, Anderson JE, Kalantar‐Zadeh K. Association of serum bicarbonate levels with mortality in patients with non‐dialysis‐dependent CKD. Nephrol Dial Transplant. 2009; 24:1232-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SN, Abramowitz M, Hostetter TH, Melamed ML. Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am J Kidney Dis. 2009; 54:270-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Tighiouart H, Vaughn NS, Beck GJ, Kusek JW, Collins AJ, Greene T, Sarnak MJ. Serum bicarbonate and long‐term outcomes in CKD. Am J Kidney Dis. 2010; 56:907-914. [DOI] [PubMed] [Google Scholar]
- Navaneethan SD, Schold JD, Arrigain S, Jolly SE, Wehbe E, Raina R, Simon JF, Srinivas TR, Jain A, Schreiber MJ, Jr, Nally JV., Jr Serum bicarbonate and mortality in stage 3 and stage 4 chronic kidney disease. Clin J Am Soc Nephrol. 2011; 6:2395-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael KL, Wei G, Baird BC, Greene T, Beddhu S. Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int. 2011; 79:356-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael KL, Zhang Y, Wei G, Greene T, Cheung AK, Beddhu S. Serum bicarbonate and mortality in adults in NHANES III. Nephrol Dial Transplant. 2013; 28:1207-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003; 42:S1-S201. [PubMed] [Google Scholar]
- Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000; 11:550-560. [DOI] [PubMed] [Google Scholar]
- Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin‐Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, III, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JTChronic Renal Insufficiency Cohort Study I. The Chronic Renal Insufficiency Cohort (CRIC) study: design and methods. J Am Soc Nephrol. 2003; 14:S148-S153. [DOI] [PubMed] [Google Scholar]
- Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI. Chronic Renal Insufficiency Cohort (CRIC) study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009; 4:1302-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer MJ, Go AS, Lora CM, Ackerson L, Cohan J, Kusek JW, Mercado A, Ojo A, Ricardo AC, Rosen LK, Tao K, Xie D, Feldman HI, Lash JPCric, Groups HCS. CKD in Hispanics: baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic‐CRIC Studies. Am J Kidney Dis. 2011; 58:214-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2012; 60:250-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe M, Hsu CY, Feldman HI, Weir M, Landis JR, Hamm LLChronic Renal Insufficiency Cohort Study. Variability of creatinine measurements in clinical laboratories: results from the CRIC study. Am J Nephrol. 2010; 31:426-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente FChronic Kidney Disease Epidemiology C. Expressing the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007; 53:766-772. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006; 29suppl 1:S43-S48. [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. J Am Med Assoc. 2003; 289:2560-2572. [DOI] [PubMed] [Google Scholar]
- Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, Zhang X, Nessel L, Hamano T, Grunwald JE, Raj DS, Yang W, He J, Lash JP, Go AS, Kusek JW, Feldman H, Wolf M. Fibroblast growth factor‐23 and cardiovascular events in CKD. J Am Soc Nephrol. 2014; 25:349-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000; 102:203-210. [DOI] [PubMed] [Google Scholar]
- van Deursen VM, Urso R, Laroche C, Damman K, Dahlstrom U, Tavazzi L, Maggioni AP, Voors AA. Co‐morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail. 2014; 16:103-111. [DOI] [PubMed] [Google Scholar]
- Martin FL, McKie PM, Cataliotti A, Sangaralingham SJ, Korinek J, Huntley BK, Oehler EA, Harders GE, Ichiki T, Mangiafico S, Nath KA, Redfield MM, Chen HH, Burnett JC., Jr Experimental mild renal insufficiency mediates early cardiac apoptosis, fibrosis, and diastolic dysfunction: a kidney‐heart connection. Am J Physiol Regul Integr Comp Physiol. 2012; 302:R292-R299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill WC, Lomashvili KA. Recent progress in the treatment of vascular calcification. Kidney Int. 2010; 78:1232-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulou K, Beis I, Gaitanaki C. MAPK signaling pathways are needed for survival of H9c2 cardiac myoblasts under extracellular alkalosis. Am J Physiol Heart Circ Physiol. 2008; 295:H1319-H1329. [DOI] [PubMed] [Google Scholar]
- de Solis AJ, Gonzalez‐Pacheco FR, Deudero JJ, Neria F, Albalate M, Petkov V, Susanibar L, Fernandez‐Sanchez R, Calabia O, Ortiz A, Caramelo C. Alkalinization potentiates vascular calcium deposition in an uremic milieu. J Nephrol. 2009; 22:647-653. [PubMed] [Google Scholar]
- Lomashvili K, Garg P, O'Neill WC. Chemical and hormonal determinants of vascular calcification in vitro. Kidney Int. 2006; 69:1464-1470. [DOI] [PubMed] [Google Scholar]
- Mendoza FJ, Lopez I, Montes de Oca A, Perez J, Rodriguez M, Aguilera‐Tejero E. Metabolic acidosis inhibits soft tissue calcification in uremic rats. Kidney Int. 2008; 73:407-414. [DOI] [PubMed] [Google Scholar]
- Boyanton BL, Jr, Blick KE. Stability studies of twenty‐four analytes in human plasma and serum. Clin Chem. 2002; 48:2242-2247. [PubMed] [Google Scholar]
- Piller LB, Davis BR, Cutler JA, Cushman WC, Wright JT, Jr, Williamson JD, Leenen FH, Einhorn PT, Randall OS, Golden JS, Haywood LJ, The ACRG. Validation of heart failure events in the antihypertensive and lipid lowering treatment to prevent heart attack trial (ALLHAT) participants assigned to doxazosin and chlorthalidone. Curr Control Trials Cardiovasc Med. 2002; 3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Analyses of time-updated serum bicarbonate.


