Abstract
Background
Recent evidence suggests that left atrial (LA) dysfunction may be mechanistically contributing to cerebrovascular events in patients with atrial fibrillation (AF). We investigated the association between regional LA function and a prior history of stroke during sinus rhythm in patients referred for catheter ablation of AF.
Methods and Results
A total of 169 patients (59±10 years, 74% male, 29% persistent AF) with a history of AF in sinus rhythm at the time of pre‐ablation cardiac magnetic resonance (CMR) were analyzed. The LA volume, emptying fraction, strain (S), and strain rate (SR) were assessed by tissue‐tracking cardiac magnetic resonance. The patients with a history of stroke or transient ischemic attack (n=18) had greater LA volumes (Vmax and Vmin; P=0.02 and P<0.001, respectively), lower LA total emptying fraction (P<0.001), lower LA maximum and pre‐atrial contraction strains (Smax and SpreA; P<0.001 and P=0.01, respectively), and lower absolute values of LA SR during left ventricular (LV) systole and early diastole (SRs and SRe; P=0.005 and 0.03, respectively) than those without stroke/transient ischemic attack (n=151). Multivariable analysis demonstrated that the LA reservoir function, including total emptying fraction, Smax, and SRs, was associated with stroke/transient ischemic attack (odds ratio 0.94, 0.91, and 0.17; P=0.03, 0.02, and 0.04, respectively) after adjusting for the CHA2DS2‐VASc score and LA Vmin.
Conclusions
Depressed LA reservoir function assessed by tissue‐tracking cardiac magnetic resonance is significantly associated with a prior history of stroke/transient ischemic attack in patients with AF. Our findings suggest that assessment of LA reservoir function can improve the risk stratification of cerebrovascular events in AF patients.
Keywords: atrial fibrillation, atrial strain, magnetic resonance imaging, stroke, tracking
Introduction
Atrial fibrillation (AF)—the most common arrhythmia—affects 6 million individuals in the United States. AF is associated with an increased risk of stroke1–2 that can be fatal, and survivors are often left permanently disabled. Mechanistically, cerebrovascular events in AF patients are thought to result from ineffective contraction during AF and subsequent intracardiac thrombosis. However, recent evidence suggests that underlying atrial fibrosis and subsequent atrial dysfunction may also be mechanistically contributing to cerebrovascular events in AF patients. For example, an increased left atrial (LA) volume and global LA dysfunction in individuals without clinically recognized AF are an independent predictor of clinical stroke/transient ischemic attack (TIA)3–4 as well as subclinical cerebrovascular events detected by brain magnetic resonance imaging (MRI).5 Our previous study demonstrated that the degree of regional LA dysfunction during sinus rhythm is proportional to the extent of underlying fibrosis quantified by late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR) in AF patients.6 In addition, regional LA function during AF is significantly depressed in patients with a prior history of stroke compared with those without, independent of the CHA2DS2‐VASc score,7–8 the standard system of risk stratification for stroke based on age, sex, and comorbidities.9
To further support the concept that the underlying atrial fibrosis and subsequent LA dysfunction may be mechanistically contributing to cerebrovascular events in AF patients, we investigated the association between regional LA function and a prior history of stroke during sinus rhythm in patients referred for catheter ablation of AF. We used tissue‐tracking CMR10–11 to quantify the LA volume and regional LA function.
Methods
Study Design
To examine the association of LA structure and function as determined by tissue‐tracking CMR with a prior history of stroke or TIA, a single‐center, retrospective, cross‐sectional study was performed within a longitudinal, prospectively enrolled database for all the patients referred to the Johns Hopkins Hospital for catheter ablation of AF. Between June 2010 and August 2013, 525 consecutive patients were referred for AF ablation (Figure 1). Among these, 300 patients underwent a routine pre‐ablation CMR. We excluded patients who were in AF at the time of CMR (n=92, 31%) because the CMR image quality is often poor and the measured LA strains are depressed during AF compared with those in sinus rhythm.12 We also excluded patients who had a prior AF ablation procedure (n=33), severe valvular disease in echocardiography (n=3), or poor‐quality CMR images (n=3). Thus, 169 patients were included in the final analysis. The stroke group (n=18, 11.8%) was identified as those with a prior history of stroke or TIA at the time of CMR; the remaining patients were designated as the control group (n=151). The patients were classified as having either paroxysmal or persistent AF based on the guidelines,9 and the thromboembolic risk was assessed using the CHADS2 and the CHA2DS2‐VASc scores.13–14 In general, the patients with persistent AF were placed on antiarrhythmic medications and referred for external cardioversion 3 to 4 weeks prior to CMR.15 All patients gave an informed consent to be included in the prospective patient database prior to the pre‐ablation CMR, and the protocol was approved by the Institutional Review Board of the Johns Hopkins Medicine.
Figure 1.
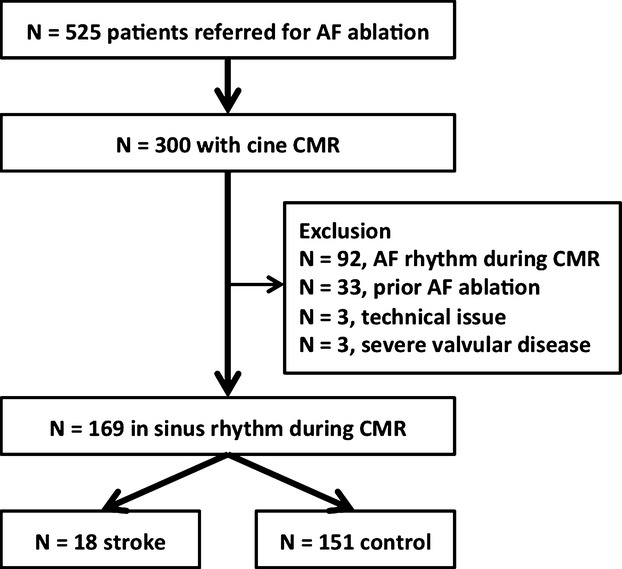
Patient enrollment. AF indicates atrial fibrillation; CMR, cardiac magnetic resonance.
CMR Protocol
CMR was performed with a 1.5‐T scanner (Avanto; Siemens Medical Systems, Erlangen, Germany) and a 6‐channel phased array body coil in combination with a 6‐channel spine matrix coil. All images were ECG gated and acquired with breath‐holding, with the patient in a supine position. Cine CMR images were scanned in the radial long axis by True Fast Imaging with Steady‐State Precession (TrueFISP) sequence with a TE/TR and flip angle of 1.2/2.4 ms and 80°, an in‐plane resolution of 1.4×1.4 mm, a slice thickness of 8 mm, and a spacing of 2 mm. The images were acquired with 30 frames during the time interval between the R‐peak of the ECG (temporal resolution, 20 to 40 ms). Among the 169 patients included in the final analysis, 85 (n=5 in the stroke group and n=80 in control group) also underwent late gadolinium enhancement (LGE) to quantify LA fibrosis. LGE‐MRI scans were acquired within a range of 15 to 25 minutes after the injection of gadopentetate dimeglumine (0.2 mmol/kg, Bayer Healthcare Pharmaceuticals, Montville, NJ) using a fat‐saturated 3‐dimensional (3‐D) inversion recovery‐prepared fast spoiled gradient‐recalled echo sequence with the following: respiratory navigation and ECG gating with TE/TR and flip angle of 1.52/3.8 ms and 10°, an in‐plane resolution of 1.3×1.3 mm, and a slice thickness of 2.0 mm. Trigger time for 3‐DLGE‐MRI images was optimized to acquire imaging data during diastole of LA as observed from the cine images. The optimal inversion time was identified with an inversion time scout scan (median 270 ms; range 240 to 290 ms) to maximize nulling of the LA myocardium. A parallel imaging technique—generalized autocalibrating partially parallel acquisition (reduction factor 2)—was used. Image processing was conducted in QMass MR (version 7.2; Leiden University Medical Center, Leiden, The Netherlands) on multiplanar reformatted axial images from 3‐D axial image data. To define LA fibrosis, we used the cutoff value of >0.97 for image intensity ratio, which has been shown to correspond to the bipolar voltage <0.5 mV.16
Tissue‐Tracking CMR
We used off‐line semiautomated multimodality tissue‐tracking software version 6.0 (Toshiba, Tokyo, Japan) to analyze the LA and left ventricular (LV) structure and function in long‐axis 2‐ and 4‐chamber cine images (Figure 2A and 2B).1 The endo‐ and epicardial borders, excluding pulmonary veins and LA appendage, were manually traced. The number of pixels that lay across the LA and LV wall was 2.2±1.2 and 8.8±2.7, respectively. Multimodality tissue‐tracking is similar in concept to 2‐dimensional (2‐D) speckle‐tracking imaging. Briefly, multimodality tissue‐tracking reads characteristic pixel patterns in each 10×10 mm area as template pieces from the reference image. The identified area as a template was searched in the next frame to find the best match according to the mean squared error of the image pixel intensity. This was used to accurately track pixel locations between subsequent image frames. Repeating the algorithm, the LA wall was automatically tracked through the cardiac cycle (Videos S1 and S2). With this 2‐D displacement field over time from the reference configuration to the deformed configuration, the differentiation with respect to the reference configuration gives the deformation gradient tensor (F), which depends on position. The Lagrangian Green's strain tensor (E) was then calculated as:
where FT is the transpose of F and I is the identity matrix. The strain (S) was defined as a stretch ratio along the longitudinal axis that represents the length normalized to its length at the reference configuration:
where Ell is the strain with respect to the local longitudinal axis calculated from the E.
Figure 2.
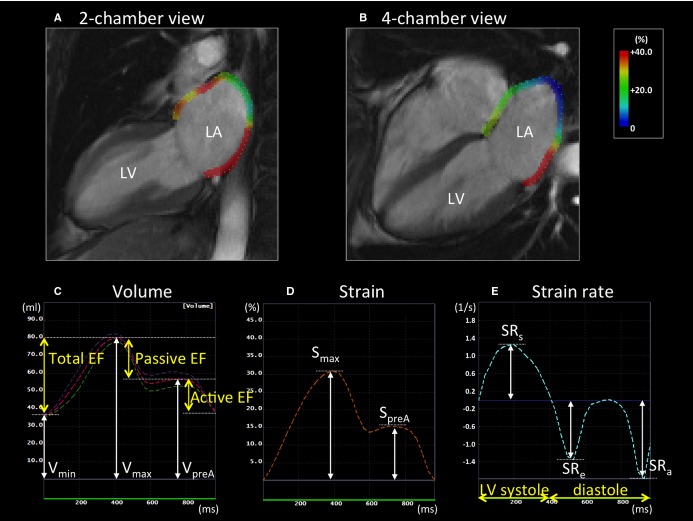
LA measurements by tissue‐tracking CMR in a patient without stroke. A and B, Left atrium (LA) longitudinal strain in the 2‐ and 4‐chamber views at the end of left ventricular (LV) systole. C, LA volume curve. The pink dotted line is the average of the values of volume in the 2‐ and 4‐chamber views. The LA maximum volume (Vmax), the pre‐atrial contraction volume (VpreA), and the minimum volume (Vmin) were identified. The LA emptying fractions (EFs) were calculated using Vmax, VpreA, and Vmin. D and E, The LA strain and strain rate curve. The LA maximum strain (Smax) and pre‐atrial contraction strain (SpreA) were identified from the strain curve. The strain rates during LV systole (SRs), LV early diastole (SRe), and atrial contraction (SRa) were also analyzed from the strain rate curve. CMR indicates cardiac magnetic resonance.
The global longitudinal strain and strain rate were calculated by averaging all of the strain values obtained in long‐axis 2‐ and 4‐chamber views. A positive and negative strain value indicates stretch and shortening, respectively, with respect to the reference configuration at the ventricular end‐diastole defined as the peak of R wave on surface ECG. LA maximum strain (Smax) and pre‐atrial contraction strain (SpreA) were identified from the strain curve (Figure 2D); the strain rates in LV systole (SRs), LV early diastole (SRe), and LA contraction (SRa) were obtained from the strain rate curve (Figure 2E).
The LA volume curve was generated by the biplane modified Simpson's method, which was validated using the area‐length method,17,10,18 and the maximum LA volume (Vmax), pre‐atrial contraction LA volume (VpreA), and minimum LA volume (Vmin) were extracted (Figure 2C). All the LA volumes were indexed by the body surface area (BSA) according to DuBois’ formula (eg, BSA=0.007184×[weight0.425]×[height0.725]). From the LA volumes, the LA emptying fractions (EF) were calculated as follows19: (1) LA total EF=(Vmax−Vmin)×100%/Vmax, (2) LA passive EF=(Vmax−VpreA)×100%/Vmax, and (3) LA active EF=(VpreA−Vmin)×100%/VpreA.
Reproducibility
Intra‐ and interobserver reproducibility for the LA parameters were examined in a group of 20 randomly selected patients by 1 investigator who made 2 independent measurements, and by 2 other investigators who were unaware of the other investigator's measurements and of the study time point. The bias (mean difference) and limits of agreement (1.96 SD of difference) between the first and second measurements were determined by the Bland‐Altman method. Intraclass correlation coefficients were also assessed to evaluate reproducibility.
Statistical Analysis
Continuous variables were presented as the means±SD and categorical variables as frequencies and percentages. The participants’ baseline data and LA parameters were compared between the stroke and the control groups using Student t test for continuous variables and χ2 test for categorical variables. Univariable and multivariable logistic regression analyses were performed to evaluate the association between clinical variables and stroke/TIA. Model 1 reflected (univariable) unadjusted relations of LA and LV measurements to stroke/TIA. Model 2 was adjusted for the CHA2DS2‐VASc score prior to stroke/TIA to incorporate a history of Cardiac failure, Hypertension, Age, Diabetes, Stroke/TIA, Vascular disease, and Sex. In Model 3, an additional adjustment was made for the LA Vmin. The incremental value for assessing the risk of stroke was studied by calculating the improvement in the global χ2. Data were analyzed with JMP version 10.0 (SAS Institute, Inc, Cary, NC) and MedCalc version 13.3 (MedCalc Software, Inc, Mariakerke, Belgium). A 2‐sided P‐value of <0.05 was considered statistically significant.
Results
Patient Demographics
Clinical characteristics of the patients are summarized in Table 1. A total of 169 patients (59±10 years, 74% male, 29% persistent AF) were included in the analysis. Compared to the control group, patients in the stroke group were significantly older (P=0.02). Other clinical characteristics, including the CHADS2 score and CHA2DS2‐VASc scores, did not show any significant difference between the stroke and control groups. Three of 18 patients (16.7%) in the stroke group and 18 of 151 patients (11.9%) in the control group underwent cardioversion before pre‐ablation CMR (P=0.84). There was no significant difference in AF duration before cardioversion (3.3±3.2 versus 1.2±1.3 years, P=0.15) and the time from cardioversion to CMR (59.7±42.7 versus 52.2±42.7 days, P=0.80).
Table 1.
Patient Demographics
| Stroke (n=18) | Control (n=151) | P Value | |
|---|---|---|---|
| Age, y | 65.0±8.2 | 58.5±10.6 | 0.02* |
| Sex, male | 12 (66.7) | 113 (74.8) | 0.50 |
| Body mass index, kg/m2 | 27.8±2.4 | 28.0±5.3 | 0.94 |
| Type of AF (persistent) | 5 (27.8) | 44 (29.1) | 0.91 |
| Coronary artery disease | 4 (22.2) | 16 (10.6) | 0.14 |
| Hypertension | 7 (38.9) | 60 (39.7) | 0.94 |
| Heart failure | 2 (11.1) | 12 (7.9) | 0.63 |
| Diabetes mellitus | 3 (16.7) | 18 (11.9) | 0.55 |
| CHADS2 score before stroke | 0.94±0.90 | 0.74±0.90 | 0.20 |
| CHA2DS2‐VASc score before stroke | 2.00±1.32 | 1.45±1.49 | 0.07 |
| Medications | |||
| β‐Blockers | 10 (55.5) | 73 (48.3) | 0.70 |
| Ca‐channel blockers | 6 (33.3) | 33 (21.9) | 0.44 |
| ACE inhibitors/ARBs | 6 (33.3) | 49 (32.5) | 0.96 |
| Statins | 7 (38.9) | 62 (41.1) | 0.86 |
| Number of antiarrhythmic drugs | 1.4±0.9 | 1.6±0.9 | 0.60 |
Data are expressed as the means±SD, or as n (%). ACE indicates angiotensin‐converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker.
P<0.05.
LA Function and Stroke
The time course of LA volume, strain, and strain rate in representative patients with and without stroke are shown in Figure 3. The CMR parameters in the stroke and the control groups are shown in Table 2. In the stroke group, the LA volumes (Vmax, VpreA, and Vmin) were significantly higher, the LA EFs (total, passive, and active) were lower, the LA longitudinal Smax and SpreA were lower, and the absolute values of the LA SRs and SRe were lower than in the control group. There was no significant difference regarding LA wall LGE between the 2 groups (stroke group versus control group: 29.3±17.6% versus 27.1±16.1%, respectively, P=0.75). LV parameters, including mass index, ejection fraction, end‐diastolic volume index, and longitudinal strain, did not show any significant difference between the stroke and control groups.
Figure 3.
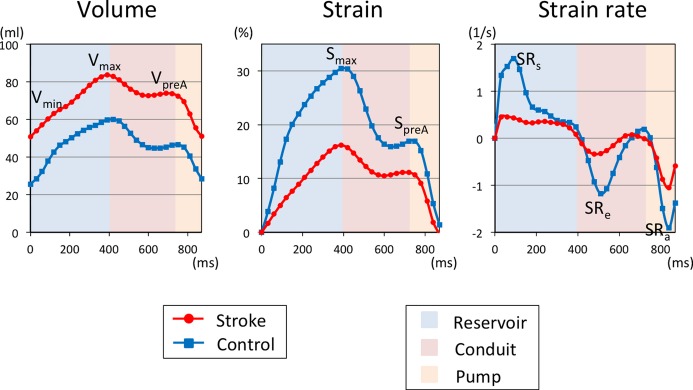
LA measurements by tissue‐tracking CMR in patients with and without stroke. A, The LA volume, (B) LA global longitudinal strain, and (C) LA strain rate in a patient with stroke (red line) and without stroke (blue line). The patient with stroke has a larger LA volume and smaller strain and strain rate. The LA serves as a reservoir during LV systole, as a conduit during LV early diastole, and as an active pump during late diastole. CMR indicates cardiac magnetic resonance; LA, left atrial; LV, left ventricular; Smax, maximum strain; SpreA, pre‐atrial contraction strain; SRa, strain rate at atrial contraction; SRe, strain rate at LV early diastole; SRs, maximum strain rate; Vmax, maximum indexed volume; Vmin, minimum indexed volume; VpreA, pre‐atrial contraction indexed volume.
Table 2.
Comparison of CMR Measurements Between the Stroke and Control Groups
| Stroke (n=18) | Control (n=151) | P Value | |
|---|---|---|---|
| LA Vmax, mL/m2 | 52.2±16.2 | 44.2±12.9 | 0.024* |
| LA VpreA, mL/m2 | 44.8±14.5 | 35.7±11.7 | 0.005* |
| LA Vmin, mL/m2 | 35.1±15.8 | 24.6±10.7 | <0.001* |
| LA total EF, % | 34.6±13.5 | 45.6±11.8 | <0.001* |
| LA passive EF, % | 14.1±5.6 | 19.7±7.8 | 0.005* |
| LA active EF, % | 23.8±15.8 | 32.7±10.9 | 0.004* |
| LA Smax, % | 19.4±9.2 | 28.6±10.6 | <0.001* |
| LA SpreA, % | 10.1±6.6 | 15.0±7.1 | 0.010* |
| LA SRs, 1/s | 0.81±0.37 | 1.15±0.47 | 0.005* |
| LA SRe, 1/s | −0.78±0.41 | −1.12±0.62 | 0.033* |
| LA SRa, 1/s | −1.14±0.55 | −1.52±0.84 | 0.071 |
| LV mass index, g/m2 | 71.0±16.1 | 65.6±14.6 | 0.177 |
| LV ejection fraction, % | 54.4±14.6 | 57.3±9.5 | 0.281 |
| LV end‐diastolic volume index, mL/m2 | 78.7±26.7 | 71.7±12.4 | 0.292 |
| LV longitudinal strain, % | −16.5±5.2 | −18.2±4.4 | 0.149 |
Data are expressed as the means±SD. CMR indicates cardiac magnetic resonance; EF, emptying fraction; LA, left atrial; LV, left ventricular; Smax, maximum strain; SpreA, pre‐atrial contraction strain; SRa, strain rate at atrial contraction; SRe, strain rate at LV early diastole; SRs, maximum strain rate; Vmax, maximum indexed volume; Vmin, minimum indexed volume; VpreA, pre‐atrial contraction indexed volume.
P<0.05.
Univariable and Multivariable Analyses
The univariable and multivariable analyses regarding the association between the CMR‐measured parameters and stroke are summarized in Table 3. In Model 1, a univariable analysis identified larger LA volumes (Vmax, VpreA, and Vmin), lower EFs (total, active, and passive EF), lower strains (Smax and SpreA), and lower absolute values of SR (SRs and SRe) as significant contributors to stroke, indicating that all of the LA parameters that differed significantly between the stroke and control groups in Table 2 remained significant and were associated with stroke. In Model 2, larger VpreA and Vmin, lower EFs (total, active, and passive EF), lower strains (Smax and SpreA), and lower SRs were significantly associated with stroke after adjusting for the CHA2DS2‐VASc score. In Model 3, only the LA total EF, Smax, and SRs, which reflect the LA reservoir function (Figures 2 and 3), remained significant after additionally adjusting for the LA Vmin.
Table 3.
Univariable and Multivariable Analyses of the Associations Between CMR Measurements and Stroke
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| LA Vmax | 1.04 | 1.01 to 1.08 | 0.030* | 1.04 | 0.99 to 1.08 | 0.062 | — | ||
| LA VpreA | 1.06 | 1.02 to 1.10 | 0.008* | 1.05 | 1.01 to 1.10 | 0.019* | — | ||
| LA Vmin | 1.07 | 1.03 to 1.11 | 0.002* | 1.06 | 1.02 to 1.11 | 0.005* | — | ||
| LA total EF | 0.93 | 0.89 to 0.97 | 0.002* | 0.93 | 0.89 to 0.97 | 0.002* | 0.94 | 0.89 to 0.99 | 0.030* |
| LA passive EF | 0.89 | 0.81 to 0.96 | 0.007* | 0.89 | 0.81 to 0.96 | 0.020* | 0.92 | 0.83 to 1.00 | 0.063 |
| LA active EF | 0.94 | 0.90 to 0.98 | 0.006* | 0.94 | 0.90 to 0.98 | 0.008* | 0.96 | 0.91 to 1.00 | 0.074 |
| LA Smax | 0.90 | 0.83 to 0.96 | 0.002* | 0.90 | 0.83 to 0.95 | 0.002* | 0.91 | 0.84 to 0.97 | 0.018* |
| LA SpreA | 0.89 | 0.80 to 0.97 | 0.012* | 0.89 | 0.80 to 0.97 | 0.016* | 0.92 | 0.82 to 1.01 | 0.091 |
| LA SRs | 0.10 | 0.02 to 0.44 | 0.006* | 0.11 | 0.02 to 0.50 | 0.009* | 0.17 | 0.02 to 0.94 | 0.042* |
| LA SRe | 3.11 | 1.14 to 8.72 | 0.039* | 2.88 | 1.04 to 9.69 | 0.055 | 2.21 | 0.72 to 7.49 | 0.175 |
| LA SRa | 2.21 | 1.02 to 5.50 | 0.066 | 2.08 | 0.94 to 5.24 | 0.095 | 1.48 | 0.66 to 3.94 | 0.391 |
| LV mass index | 1.03 | 0.99 to 1.06 | 0.189 | 1.02 | 0.98 to 1.06 | 0.224 | 1.02 | 0.98 to 1.06 | 0.321 |
| LV ejection fraction | 0.98 | 0.93 to 1.03 | 0.301 | 0.98 | 0.93 to 1.03 | 0.347 | 0.98 | 0.93 to 1.06 | 0.547 |
| LV EDVI | 1.02 | 0.99 to 1.05 | 0.253 | 0.98 | 0.95 to 1.01 | 0.262 | 0.98 | 0.95 to 1.01 | 0.303 |
| LV longitudinal strain | 1.08 | 0.97 to 1.21 | 0.151 | 1.07 | 0.96 to 1.20 | 0.223 | 1.05 | 0.94 to 1.18 | 0.399 |
Model 1: unadjusted. Model 2: adjusted for CHA2DS2‐VASc score. Model 3: additionally adjusted for the LA Vmin. CMR indicates cardiac magnetic resonance; EDVI, end‐diastolic volume index; EF, emptying fraction; LA, left atrial; LV, left ventricular; OR, odds ratio; Smax, maximum strain; SpreA, pre‐atrial contraction strain; SRa, strain rate at atrial contraction; SRe, strain rate at LV early diastole; SRs, maximum strain rate; Vmax, maximum indexed volume; Vmin, minimum indexed volume; VpreA, pre‐atrial contraction indexed volume.
P<0.05.
Incremental Value of LA Function as a Marker of Stroke
Additionally, we found that adding the CMR‐measured LA function significantly improved the statistics of the model on the basis of the conventional risk stratification of strokes (Figure 4). The LA Vmin provided incremental value over the CHA2DS2‐VASc score, and the diagnostic value was further improved by adding the global LA Smax (P=0.017 and 0.009, respectively).
Figure 4.
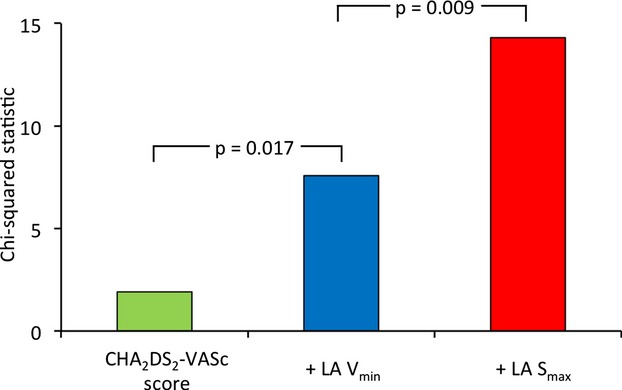
Incremental value of left atrial (LA) strain for diagnosis of stroke. The addition of the LA minimum volume (Vmin) to the model on the basis of the CHA2DS2‐VASc score resulted in significant improvement in the diagnostic value for stroke. The value was further increased by adding the LA global longitudinal maximum strain (Smax).
Reproducibility of LA Analysis by Tissue‐Tracking CMR
The intra‐observer intraclass correlation coefficient was between 0.88 and 0.99, and the interobserver intraclass correlation coefficient was between 0.89 and 0.99 for all measured LA parameters from tissue‐tracking CMR (Table 4).
Table 4.
Reproducibility of the LA Analysis by Tissue‐Tracking CMR
| Intra‐Observer | Inter‐Observer | |||||
|---|---|---|---|---|---|---|
| Bias | Limits of Agreement | ICC | Bias | Limits of Agreement | ICC | |
| LA Vmax, mL/m2 | −0.57 | 4.50 | 0.96 | −1.25 | 4.08 | 0.99 |
| LA VpreA, mL/m2 | −0.93 | 3.42 | 0.97 | −0.34 | 4.75 | 0.99 |
| LA Vmin, mL/m2 | −0.83 | 3.68 | 0.98 | −1.61 | 4.31 | 0.98 |
| LA total EF, % | 1.94 | 7.75 | 0.94 | 1.67 | 4.78 | 0.89 |
| LA passive EF, % | 0.82 | 5.41 | 0.98 | −0.92 | 7.81 | 0.92 |
| LA active EF, % | 2.47 | 10.78 | 0.88 | 3.11 | 0.51 | 0.90 |
| LA Smax, % | −0.75 | 3.19 | 0.94 | 1.26 | 2.88 | 0.90 |
| LA SpreA, % | −1.20 | 2.99 | 0.96 | 1.64 | 2.17 | 0.98 |
| LA SRs, 1/s | 0.10 | 0.43 | 0.91 | 0.11 | 0.18 | 0.95 |
| LA SRe, 1/s | −0.04 | 0.24 | 0.98 | −0.15 | 0.23 | 0.94 |
| LA SRa, 1/s | −0.05 | 0.28 | 0.99 | −0.12 | 0.27 | 0.97 |
CMR indicates cardiac magnetic resonance; EF, emptying fraction; ICC, intraclass correlation coefficient; LA, left atrial; Smax, maximum strain; SpreA, pre‐atrial contraction strain; SRa, strain rate at atrial contraction; SRe, strain rate at LV early diastole; SRs, maximum strain rate; Vmax, maximum indexed volume; Vmin, minimum indexed volume; VpreA, pre‐atrial contraction indexed volume.
Discussion
Main Findings
We found that greater LA volumes, lower LA EFs, lower strain, and lower peak SR were associated with a prior history of stroke. We also found that LA total EF, LA Smax, and SRs, representing the LA reservoir function, are independently associated with stroke or TIA after adjusting for potential confounders and clinical risk factors. These results are consistent with the previous reports that the reduced LA reservoir function assessed using transthoracic echocardiography in the absence of AF is an independent predictor of clinical stroke/TIA4 as well as subclinical cerebrovascular events detected by brain MRI.5 Our findings are particularly important because, to our knowledge, this is the first report to demonstrate the significant contribution of the LA reservoir function to stroke in AF patients during sinus rhythm. In contrast to studies that showed a significant association between stroke and the LA reservoir function with echocardiography during AF,7–8 the sinus rhythm in our study allowed us to assess all the LA function components, including the reservoir function, conduit function, and booster pump function, and to successfully determine that only the LA reservoir function is significantly associated with stroke. This important finding further supports the concept that the underlying atrial fibrosis and subsequent LA dysfunction is mechanistically contributing to cerebrovascular events in AF patients.
Depressed LA Reservoir Function as a Mechanism of Thromboembolic Events in AF Patients
The atrial function consists of 3 components: a reservoir function for pulmonary venous return during LV systole, the conduit function for pulmonary venous return during LV early diastole, and the booster pump function that augments LV filling during LV late diastole (Figure 3). LA total EF, Smax, and SRs represent LA compliance and reservoir function, reflecting the passive stretch of the LA during LV systole. The mechanism as to how the depressed LA reservoir function leads to thromboembolic events is unclear. It is possible that the depressed LA reservoir function results in blood flow stasis in the LA and subsequent thrombus formation. Studies have also shown that low LA strains are associated with low flow velocities and thrombi in the LA appendage,20 which is the most common site of intracardiac thrombus.21 Of note, since none of the LV indices—mass, ejection fraction, end‐diastolic volume, and longitudinal strain—were significantly associated with stroke/TIA, the role of the LA reservoir function in the pathogenesis of intracardiac thrombosis is not due to the indirect consequence of LV function. The possibility that cardioversion‐induced atrial stunning could have confounded our findings is low because (1) cardioversion was performed in only a minority of patients in both groups; (2) there was no significant difference in the fraction of patients who underwent cardioversion between both groups; and (3) CMR was performed ≈8 weeks after cardioversion on average, while cardioversion‐induced atrial stunning usually recovers within 4 weeks.22 In our data set, there was no significant difference in the extent of the LA fibrosis quantified by LGE MRI between the stroke group and the control group. This finding is not consistent with a previous report,23 but this may be due to a small sample size since only a small number of patients underwent LGE (85 out of 169 patients).
LA Measurements by Tissue‐Tracking CMR
CMR has been established as a highly accurate and reproducible imaging modality, and is considered a standard clinical technique for measuring LA dimensions and volumes.24–25 We analyzed LA volume and function with tissue‐tracking CMR, which has been used to measure multiple LA parameters with excellent intra‐ and interobserver reproducibility in healthy subjects.10–11 Consistent with these previous reports, our results showed excellent inter‐ and intraobserver reproducibility for LA strain analysis with tissue‐tracking CMR, which is similar or superior to those of speckle‐tracking echocardiography26 Additionally, tissue‐tracking CMR can be performed with a routine cine CMR examination and does not require separate image acquisitions (eg, tagged MRI or displacement encoding with stimulated echoes (DENSE)27) or contrast media, although contrast media might be used in clinical settings to identify LA wall fibrosis in LGE and LA appendage thrombus as a sign of possible impending stroke in CMR angiography before AF ablations.28 Another strength of tissue‐tracking CMR is that it is user friendly. The images can be analyzed within minutes per patient by an operator without prior experience in image analysis, in contrast to LA fibrosis quantification with LGE that require hours of analysis per patient in the hands of an expert.
Clinical Implications
The CHADS2 and CHA2DS2‐VASc scores are the most widely accepted and validated models to estimate the risk of stroke in AF patients.13–14 In addition, an increased LA volume is also associated with a higher risk of stroke.29 Our results demonstrate that the LA reservoir function is significantly associated with a prior history of stroke/TIA independent of the CHA2DS2‐VASc score and the LA volume (Table 3). Our results offer a basis for a prospective study to determine the role of LA reservoir function by tissue‐tracking CMR in predicting stroke or TIA. This may improve the current risk stratification strategy for stroke and potentially allow for early identification of subjects at risk of stroke, with or without a history of AF. Aggressive clinical management, such as early ablation or pharmacotherapy for AF to improve LA remodeling30–31 and early anticoagulation may reduce the risk of stroke in subjects with a high risk of stroke. In addition, further studies are warranted to investigate whether the risk of stroke decreases if LA function improves by these therapies.
Study Limitations
This study represents a single‐center, retrospective, cross‐sectional analysis. Therefore, there is a non‐negligible chance of selection bias. For example, the analysis may be missing patients who died of stroke. In addition, CMR was not performed at the time of stroke/TIA, and the time from stroke to CMR could not be determined from the records. When evaluating the predictive value of the LA parameters for stroke, baseline LA strain analysis should ideally be assessed before stroke. Moreover, the stroke mechanism in each patient is unclear from the records. For the deformation analysis, we used only 2‐ and 4‐chamber cine CMR, which was included in a routine image‐acquisition protocol. Therefore, it is possible that our analysis underestimated the degree of dysfunction by missing regional dysfunction that was not covered by those 2 views. Since the strain was 2‐D and was obtained only in the in‐plane direction, the strain values may have been underestimated compared with those in 3‐D strains. In addition, the endo‐ and epicardial contours included the ostium of pulmonary veins and the LA appendage in a small number of patients. This could have led to underestimation of strain values; however, 2 previous studies used the same approach and validated the results.10–11 Despite these potential causes of underestimation, our analysis demonstrated a significant association between LA dysfunction and a prior history of stroke with excellent reproducibility (Table 4). Therefore, we believe that the advantage of our approach outweighs the disadvantage of including more views (eg, multiple short‐axis LA slices) and of excluding the ostia area to assess the whole LA deformation, which would increase the scan time and postprocessing burden.
Conclusions
CMR measurements indicate that lower LA total EF, Smax, and SRs, representing LA reservoir function, are significantly associated with a prior history of stroke/TIA in patients with AF. Our results offer a basis for a prospective study to determine the role of depressed LA reservoir function by tissue‐tracking CMR in predicting stroke or TIA for early detection of the population at risk of developing stroke.
Acknowledgments
We thank Elzbieta Chamera for excellent technical assistance and Yoshiaki Ohyama for the valuable advice on statistical analysis.
Supplementary Material
Appendix Supplementary Video S1.
Appendix Supplementary Video S2.
Sources of Funding
This work was supported by research grants from Philips Healthcare (to Ashikaga), the Magic That Matters Fund for Cardiovascular Research (to Ashikaga), the Uehara Memorial Foundation, Japan (to Inoue), a Boston Scientific Corporation Fellowship Grant (to Berger), the Grunwald Endowment (to Calkins), the Roz and Marvin H. Weiner and Family Foundation (to Calkins), and the Chiaramonte Family Foundation (to Calkins).
Disclosures
None.
References
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres J, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015; 131:e29-e322. [DOI] [PubMed] [Google Scholar]
- Hart RG. Atrial fibrillation and stroke prevention. N Engl J Med. 2003; 349:1015-1016. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995; 92:835-841. [DOI] [PubMed] [Google Scholar]
- Wong JM, Welles CC, Azarbal F, Whooley MA, Schiller NB, Turakhia MP. Relation of left atrial dysfunction to ischemic stroke in patients with coronary heart disease (from the Heart and Soul Study). Am J Cardiol. 2014; 113:1679-1684. [DOI] [PubMed] [Google Scholar]
- Russo C, Jin Z, Liu R, Iwata S, Tugcu A, Yoshita M, Homma S, Elkind MS, Rundek T, Decarli C, Wright CB, Sacco RL, Di Tullio MR. LA volumes and reservoir function are associated with subclinical cerebrovascular disease: the CABL (Cardiovascular Abnormalities and Brain Lesions) study. JACC Cardiovasc Imaging. 2013; 6:313-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi M, Lima JA, Khurram IM, Zimmerman SL, Zipunnikov V, Fukumoto K, Spragg D, Ashikaga H, Rickard J, Marine JE, Calkins H, Nazarian S. Association of left atrial function and left atrial enhancement in patients with atrial fibrillation: cardiac magnetic resonance study. Circ Cardiovasc Imaging. 2015; 8:e002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih JY, Tsai WC, Huang YY, Liu YW, Lin CC, Huang YS, Tsai LM, Lin LJ. Association of decreased left atrial strain and strain rate with stroke in chronic atrial fibrillation. J Am Soc Echocardiogr. 2011; 24:513-519. [DOI] [PubMed] [Google Scholar]
- Obokata M, Negishi K, Kurosawa K, Tateno R, Tange S, Arai M, Amano M, Kurabayashi M. Left atrial strain provides incremental value for embolism risk stratification over CHA(2)DS(2)‐VASc score and indicates prognostic impact in patients with atrial fibrillation. J Am Soc Echocardiogr. 2014; 27:709-716.e704. [DOI] [PubMed] [Google Scholar]
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014; 130:e199-e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi M, Chahal H, Opdahl A, Gjesdal O, Helle‐Valle TM, Heckbert SR, McClelland R, Wu C, Shea S, Hundley G, Bluemke DA, Lima JA. Association of CMR‐measured LA function with heart failure development: results from the MESA study. JACC Cardiovasc Imaging. 2014; 7:570-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M, Ambale Venkatesh B, Samiei S, Donekal S, Habibi M, Armstrong AC, Heckbert SR, Wu CO, Bluemke DA, Lima JA. Multi‐Ethnic Study of Atherosclerosis: association between left atrial function using tissue tracking from cine MR imaging and myocardial fibrosis. Radiology. 2014; 273:703-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba Y, Yuda S, Kobayashi N, Hashimoto A, Uno K, Nakata T, Tsuchihashi K, Miura T, Ura N, Shimamoto K. Strain rate imaging for noninvasive functional quantification of the left atrium: comparative studies in controls and patients with atrial fibrillation. J Am Soc Echocardiogr. 2005; 18:729-736. [DOI] [PubMed] [Google Scholar]
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke. JAMA. 2001; 285:2864-2870. [DOI] [PubMed] [Google Scholar]
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach. Chest. 2010; 137:263-272. [DOI] [PubMed] [Google Scholar]
- Rivard L, Hocini M, Rostock T, Cauchemez B, Forclaz A, Jadidi AS, Linton N, Nault I, Miyazaki S, Liu X, Xhaet O, Shah A, Sacher F, Derval N, Jais P, Khairy P, Macle L, Nattel S, Willems S, Haissaguerre M. Improved outcome following restoration of sinus rhythm prior to catheter ablation of persistent atrial fibrillation: a comparative multicenter study. Heart Rhythm. 2012; 9:1025-1030. [DOI] [PubMed] [Google Scholar]
- Khurram IM, Beinart R, Zipunnikov V, Dewire J, Yarmohammadi H, Sasaki T, Spragg DD, Marine JE, Berger RD, Halperin HR, Calkins H, Zimmerman SL, Nazarian S. Magnetic resonance image intensity ratio, a normalized measure to enable interpatient comparability of left atrial fibrosis. Heart Rhythm. 2014; 11:85-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujino K, Barnes ME, Cha SS, Langins AP, Bailey KR, Seward JB, Tsang TS. Two‐dimensional echocardiographic methods for assessment of left atrial volume. Am J Cardiol. 2006; 98:1185-1188. [DOI] [PubMed] [Google Scholar]
- Nacif MS, Barranhas AD, Turkbey E, Marchiori E, Kawel N, Mello RA, Falcao RO, Oliveira AC, Jr, Rochitte CE. Left atrial volume quantification using cardiac MRI in atrial fibrillation: comparison of the Simpson's method with biplane area‐length, ellipse, and three‐dimensional methods. Diagn Interv Radiol. 2013; 19:213-220. [DOI] [PubMed] [Google Scholar]
- Farzaneh‐Far A, Ariyarajah V, Shenoy C, Dorval JF, Kaminski M, Curillova Z, Wu H, Brown KB, Kwong RY. Left atrial passive emptying function during dobutamine stress MR imaging is a predictor of cardiac events in patients with suspected myocardial ischemia. JACC Cardiovasc Imaging. 2011; 4:378-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabay CY, Zehir R, Guler A, Oduncu V, Kalayci A, Aung SM, Karagoz A, Tanboga IH, Candan O, Gecmen C, Erkol A, Esen AM, Kirma C. Left atrial deformation parameters predict left atrial appendage function and thrombus in patients in sinus rhythm with suspected cardioembolic stroke. Echocardiography. 2013; 30:572-581. [DOI] [PubMed] [Google Scholar]
- Stoddard MF, Dawkins PR, Prince CR, Ammash NM. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: a transesophageal echocardiographic study. J Am Coll Cardiol. 1995; 25:452-459. [DOI] [PubMed] [Google Scholar]
- Khan IA. Transient atrial mechanical dysfunction (stunning) after cardioversion of atrial fibrillation and flutter. Am Heart J. 2002; 144:11-22. [DOI] [PubMed] [Google Scholar]
- Daccarett M, Badger TJ, Akoum N, Burgon NS, Mahnkopf C, Vergara G, Kholmovski E, McGann CJ, Parker D, Brachmann J, MacLeod RS, Marrouche NF. Association of left atrial fibrosis detected by delayed‐enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol. 2011; 57:831-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceira AM, Cosin‐Sales J, Roughton M, Prasad SK, Pennell DJ. Reference left atrial dimensions and volumes by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010; 12:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof IE, Velthuis BK, Van Driel VJ, Wittkampf FH, Hauer RN, Loh P. Left atrial volume and function assessment by magnetic resonance imaging. J Cardiovasc Electrophysiol. 2010; 21:1247-1250. [DOI] [PubMed] [Google Scholar]
- Motoki H, Dahiya A, Bhargava M, Wazni OM, Saliba WI, Marwick TH, Klein AL. Assessment of left atrial mechanics in patients with atrial fibrillation: comparison between two‐dimensional speckle‐based strain and velocity vector imaging. J Am Soc Echocardiogr. 2012; 25:428-435. [DOI] [PubMed] [Google Scholar]
- Schmidt EJ, Fung MM, Ciris PA, Song T, Shankaranarayanan A, Holmvang G, Gupta SN, Chaput M, Levine RA, Ruskin J, Reddy VY, D'Avila A, Aletras AH, Danik SB. Navigated DENSE strain imaging for post‐radiofrequency ablation lesion assessment in the swine left atria. Europace. 2014; 16:133-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathi VK, Reddy ST, Anreddy S, Belden W, Yamrozik JA, Williams RB, Doyle M, Thompson DV, Biederman RWW. Contrast‐enhanced CMR is equally effective as TEE in the evaluation of left atrial appendage thrombus in patients with atrial fibrillation undergoing pulmonary vein isolation procedure. Heart Rhythm. 2013; 10:1021-1027. [DOI] [PubMed] [Google Scholar]
- Osranek M, Bursi F, Bailey KR, Grossardt BR, Brown RD, Jr, Kopecky SL, Tsang TS, Seward JB. Left atrial volume predicts cardiovascular events in patients originally diagnosed with lone atrial fibrillation: three‐decade follow‐up. Eur Heart J. 2005; 26:2556-2561. [DOI] [PubMed] [Google Scholar]
- Tsang TSM, Barnes ME, Abhayaratna WP, Cha SS, Gersh BJ, Langins AP, Green TD, Bailey KR, Miyasaka Y, Seward JB. Effects of quinapril on left atrial structural remodeling and arterial stiffness. Am J Cardiol. 2006; 97:916-920. [DOI] [PubMed] [Google Scholar]
- Perea RJ, Tamborero D, Mont L, De Caralt TM, Ortiz JT, Berruezo A, Matiello M, Sitges M, Vidal B, Sanchez M, Brugada J. Left atrial contractility is preserved after successful circumferential pulmonary vein ablation in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2008; 19:374-379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Supplementary Video S1.
Appendix Supplementary Video S2.