Abstract
Background
Whether heart rate upon discharge following hospitalization for heart failure is associated with long‐term adverse outcomes and whether this association differs between patients with sinus rhythm (SR) and atrial fibrillation (AF) have not been well studied.
Methods and Results
We conducted a retrospective cohort study from clinical registry data linked to Medicare claims for 46 217 patients participating in Get With The Guidelines®–Heart Failure. Cox proportional‐hazards models were used to estimate the association between discharge heart rate and all‐cause mortality, all‐cause readmission, and the composite outcome of mortality/readmission through 1 year. For SR and AF patients with heart rate ≥75, the association between heart rate and mortality (expressed as hazard ratio [HR] per 10 beats‐per‐minute increment) was significant at 0 to 30 days (SR: HR 1.30, 95% CI 1.22 to 1.39; AF: HR 1.23, 95% CI 1.16 to 1.29) and 31 to 365 days (SR: HR 1.15, 95% CI 1.12 to 1.20; AF: HR 1.05, 95% CI 1.01 to 1.08). Similar associations between heart rate and all‐cause readmission and the composite outcome were obtained for SR and AF patients from 0 to 30 days but only in the composite outcome for SR patients over the longer term. The HR from 0 to 30 days exceeded that from 31 to 365 days for both SR and AF patients. At heart rates <75, an association was significant for mortality only for both SR and AF patients.
Conclusions
Among older patients hospitalized with heart failure, higher discharge heart rate was associated with increased risks of death and rehospitalization, with higher risk in the first 30 days and for SR compared with AF.
Keywords: heart failure, heart rate, mortality
Introduction
Heart rate has served as a marker for health and disease in humans for centuries. Prospective cohort studies and retrospective observational studies over the past several decades have contributed to a growing evidence base supporting an association between increased resting heart rate and adverse all‐cause and cardiovascular outcomes.1–2 Recent randomized clinical trial data from patients with heart failure (HF) have implicated heart rate as a potentially modifiable risk factor.3–4 Most of these prior studies examined patients with chronic stable HF and reduced ejection fraction (EF) participating in a clinical trial.4–10 The prognostic importance of discharge heart rate in unselected patients after hospitalization for HF has been less well studied. Few studies have included HF patients with preserved EF or those with atrial fibrillation (AF), or had sufficient power to evaluate potential time‐dependent differences in the relationship between heart rate and outcomes.
Given this growing awareness as well as potential therapeutic import of an association between resting heart rate and outcomes in patients with HF,11 we analyzed the relationship between heart rate at the time of hospital discharge and mortality and rehospitalization through 1 year in patients hospitalized for HF. We included patients in sinus rhythm (SR) as well as those in AF given the prevalence and prognostic importance of the latter in patients with HF.12–13 Our specific objectives were to (1) examine baseline patient characteristics across the distribution of heart rates recorded at discharge in hospitalized patients with a primary discharge diagnosis of HF; (2) examine the association between discharge heart rate and all‐cause mortality through 1 year in patients with SR and AF; and (3) examine the association between discharge heart rate and all‐cause hospital readmission and the composite outcome of all‐cause mortality or all‐cause readmission through 1 year in patients with SR and AF.
Methods
Data Sources
The Get With The Guidelines®–Heart Failure (GWTG‐HF) program is among the largest quality‐improvement initiatives focusing on patients hospitalized with clinician‐confirmed HF.14 The design of the program has been previously described.15–16 Hospitals participating in the registry use a web‐based patient management tool (PMT, Quintiles) to collect data for consecutive patients admitted with HF and to receive recommendations for qualitative improvement in medical management. Patients hospitalized with new or worsening HF as primary diagnosis or patients who developed significant HF symptoms such that HF was the primary discharge diagnosis were included in the registry starting January 1, 2005. Patients were enrolled into the program regardless of their left ventricular function. Hospitals from all regions of the United States are represented and a variety of institutions participate, from community hospitals to large tertiary medical centers. Data collected for each HF patient include demographics, medical/surgical history including any history of AF, admission medications, admission and discharge vital signs, physical examination, rhythm at time of admission, serum laboratory tests, pharmacological and nonpharmacologic interventions, in‐hospital outcomes, and discharge information. Trained hospital personnel enter the data by using standardized definitions. All participating hospitals were required to submit the GWTG‐HF protocol to their institutional review board for approval. Because data collected were used for qualitative performance improvement, sites were granted a waiver of informed consent under the common rule. Quintiles is the data collection coordination center for the American Heart Association/American Stroke Association Get With the Guidelines® programs. The Duke Clinical Research Institute serves as the data analysis center and has an agreement to analyze de‐identified data for research purposes.
We obtained clinical data from the GWTG‐HF registry and Medicare claims data from the Centers for Medicare and Medicaid Services. The Medicare data include inpatient claims and corresponding denominator files for 2006 through 2011. The inpatient files contain hospitalization claims covered under Medicare Part A. The denominator files include date of death and information about program eligibility and enrollment. We linked data from the GWTG‐HF registry to the research identifiable inpatient claims data with the use of indirect identifiers: admission date, discharge date, sex, and age or date of birth.17 Combinations of these identifiers are almost always unique, enabling identification of registry hospitalizations in the Medicare claims data. For patients with multiple linked hospitalizations in the registry, we selected the first hospitalization for analysis.
Study Population
SR group
From January 1, 2005, to December 31, 2011, there were 65 032 admissions for patients ≥65 years of age with HF, at 292 sites fully participating in GWTG‐HF. From these, we excluded (1) 2473 (3.8%) patients who were not enrolled in fee‐for‐service Medicare; (2) 2685 (4.1%) patients with missing EF information; and (3) 13 657 (21%) patients without discharge heart rate recorded. Finally, 20 197 patients without AF (defined as fitting none of the criteria for AF, ie, history of AF, AF at presentation or during hospitalization, or new‐onset of AF) comprised the SR group. The final sample size for the SR group was 26 020 patients from 271 sites.
AF group
Above exclusions 1 to 3 were employed along with the exclusion of the 26 020 SR patients. The final sample size for the AF group was 20 197 patients from 262 sites.
Outcome measures
The primary outcome was all‐cause mortality rate by 1 year. Secondary outcomes were all‐cause readmission rate by 1 year and all‐cause readmission or mortality rate by 1 year.
Heart rate and rhythm determination
Heart rhythm was electrocardiographically determined. Heart rate was determined in conformance with local protocol for obtaining vital signs. For SR patients, heart rate was determined by palpation or telemetry (depending on patient location). For AF patients, heart rate was electrocardiographically or telemetrically determined.
EF determination
EF in GWTG‐HF patients is determined by 2‐dimensional transthoracic echocardiography, gated scintigraphy, or contrast left ventriculography.
Statistical Analysis
Baseline patient characteristics were compared across heart rate tertiles for SR and AF patients. Medians and (25th to 75th) percentiles were determined for continuous variables and percentages for categorical variables. Chi‐square tests were used to compare categorical variables across tertiles and Wilcoxon rank‐sum statistics were used to compare continuous variables across tertiles. Kaplan–Meier survival estimates are plotted by tertile and log rank statistics assessed the difference in survival across the tertiles.
We fit models separately in SR and AF patients to allow for different relationships between patient and hospital characteristics and outcome(s) in each subgroup. The association of heart rate with each outcome for SR patients was assessed using unadjusted and adjusted Cox proportional‐hazards regression models for 1‐year follow‐up. The functional form of heart rate was assessed by first comparing a linear fit to the fit of a restricted cubic spline. Evidence of a nonlinear relationship was identified and linear splines with and without truncation were considered. Several knot points were assessed, and the final transformation selected was the one that maximized model likelihood. The final linear spline was compared to the restricted cubic spline and suggested no lack of fit. Proportional hazards assumptions were assessed using Schoenfeld residuals. There was evidence of a nonproportional relationship over 1 year for each end point, but only for the upper portion of the linear spline (heart rate ≥75 beats per minute [bpm]). Subsequently, we determined that fitting a time‐varying hazard held proportional on the interval 0 to 30 days and from 31 to 365 days fit the data well. Although risk does not change suddenly at any 1 point in time, this approach attempts to compromise between model fit and intuitive interpretation. A model assuming proportional hazards throughout 1‐year follow‐up is also reported. Adjusted models include age, gender, race (white versus other), insurance (none, Medicare, Medicaid, other), EF, history of atrial flutter, history of chronic obstructive pulmonary disease (COPD) or asthma, history of diabetes, history of hypertension, history of hyperlipidemia, history of peripheral vascular disease, prior myocardial infarction, prior stroke or transient ischemic attack, history of anemia, history of chronic renal insufficiency, smoking, US census‐based geographic region, academic or teaching hospital, rural location, hospital size, and defect‐free compliance score (defined as the frequency of patients with 100% compliance with all GWTG‐HF‐defined performance measures). Single imputation was used to reduce missingness in models. Missing values for categorical variables were imputed to the most likely category.
To determine whether cardiac resynchronization therapy (CRT) might serve as an effect modifier of the heart‐rate‐outcome relationship, an interaction term was included in adjusted models. The analysis plan specified that if significant, the relationship between heart rate and outcome would be described within CRT and no CRT subgroups; if not significant, the interaction term was dropped and the model was simply adjusted for CRT. Effect modification by EF group (EF≤0.4 versus EF>0.4) was explored in a similar manner.
A sensitivity analysis was performed to determine whether the association between heart rate and the primary outcome (all‐cause mortality at 1 year) might be biased by missing EF data. An indicator variable was defined reflecting the presence or absence of EF in each subject and an interaction term, heart rate×EF missing status, was added to the model and a formal test for interaction was performed.
The analysis was repeated in similar fashion for AF patients. A 2‐sided P value of <0.05 was considered statistically significant for each test. Analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC).
Results
Overall Sample
The overall sample (Table 1) comprised 46 217 patients from 273 sites with a primary discharge diagnosis of HF. The median (interquartile range) age was 80 (12) years with a slight female preponderance (54.3% female versus 45.7% male). Fifty‐four percent had a past medical history of HF. Discharge heart rate, the primary variable of interest, appeared normally distributed. The difference between discharge and admission heart rates was statistically significant (mean [SD] difference, −8.8 [20]; P<0.0001). Vital signs at discharge, medications at discharge, and hospital characteristics are reported in Table S1.
Table 1.
Baseline Patient Characteristics, Overall and by Heart Rate Tertiles
| Variable | Level | Overall (N=46 217) | T1 (32 to 68 bpm) (N=16 273) | T2 (69 to 79 bpm) (N=14 574) | T3 (80 to 168 bpm) (N=15 370) | P Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||||
| Age | Median | 46 217 | 80 | 16 273 | 81 | 14 574 | 81 | 15 370 | 80 | <0.0001 |
| 25th | 74 | 74 | 74 | 73 | ||||||
| 75th | 86 | 86 | 86 | 86 | ||||||
| Gender | Female | 25 088 | 54.3 | 8816 | 54.2 | 7734 | 53.1 | 8538 | 55.5 | <0.0001 |
| Male | 21 129 | 45.7 | 7457 | 45.8 | 6840 | 46.9 | 6832 | 44.4 | ||
| Race | Other (includes UTD) | 1062 | 2.3 | 385 | 2.4 | 332 | 2.3 | 345 | 2.3 | 0.0761 |
| Asian | 582 | 1.3 | 234 | 1.4 | 183 | 1.3 | 165 | 1.1 | ||
| Hispanic (any race) | 2211 | 4.8 | 803 | 4.9 | 704 | 4.9 | 704 | 4.6 | ||
| Black | 4474 | 9.8 | 1562 | 9.7 | 1374 | 9.5 | 1538 | 10.1 | ||
| White | 37 478 | 81.8 | 13 141 | 81.5 | 11 867 | 82.1 | 12 470 | 81.9 | ||
| Missing | 410 | 0.9 | 148 | 0.9 | 114 | 0.8 | 148 | 0.9 | ||
| Medical history | ||||||||||
| Chronic or recurrent atrial fib | Yes | 17 277 | 37.7 | 5749 | 35.6 | 5474 | 37.9 | 6054 | 39.7 | <0.0001 |
| Atrial flutter | Yes | 1020 | 2.2 | 370 | 2.3 | 302 | 2.1 | 348 | 2.3 | 0.4140 |
| COPD or asthma | Yes | 13 223 | 28.8 | 4174 | 25.8 | 4065 | 28.1 | 4984 | 32.7 | <0.0001 |
| Diabetes—insulin treated | Yes | 7632 | 16.6 | 2858 | 17.7 | 2425 | 16.8 | 2349 | 15.4 | <0.0001 |
| Diabetes—noninsulin treated | Yes | 10 775 | 23.5 | 3869 | 23.9 | 3401 | 23.5 | 3505 | 22.9 | 0.1267 |
| Hyperlipidemia | Yes | 21 761 | 47.5 | 8160 | 50.5 | 6833 | 47.3 | 6768 | 44.4 | <0.0001 |
| Hypertension | Yes | 35 584 | 77.6 | 12 908 | 79.9 | 11 197 | 77.5 | 11 479 | 75.3 | <0.0001 |
| PVD | Yes | 6490 | 14.1 | 2403 | 14.9 | 2021 | 13.9 | 2066 | 13.5 | 0.0026 |
| CAD | Yes | 23 915 | 52.2 | 8921 | 55.2 | 7719 | 53.4 | 7275 | 47.7 | <0.0001 |
| Prior MI | Yes | 8463 | 18.5 | 3185 | 19.7 | 2727 | 18.9 | 2551 | 16.7 | <0.0001 |
| CVA/TIA | Yes | 7590 | 16.5 | 2809 | 17.4 | 2409 | 16.7 | 2372 | 15.6 | <0.0001 |
| ICD | Yes | 3407 | 7.4 | 1212 | 7.5 | 1231 | 8.5 | 964 | 6.3 | <0.0001 |
| Heart failure | Yes | 24 717 | 53.9 | 8755 | 54.2 | 7807 | 54.0 | 8155 | 53.5 | 0.4049 |
| Anemia | Yes | 9043 | 19.7 | 3231 | 20.0 | 2844 | 19.7 | 2968 | 19.5 | 0.4767 |
| Pacemaker | Yes | 6552 | 14.3 | 2406 | 14.9 | 2408 | 16.7 | 1738 | 11.4 | <0.0001 |
| CRT‐P (CRT‐pacing only) | Yes | 323 | 0.7 | 126 | 0.8 | 107 | 0.7 | 90 | 0.6 | 0.1087 |
| CRT‐D (CRT with ICD) | Yes | 885 | 1.9 | 277 | 1.7 | 365 | 2.5 | 243 | 1.6 | <0.0001 |
| Dialysis (chronic) | Yes | 1319 | 2.9 | 402 | 2.5 | 388 | 2.7 | 529 | 3.5 | <0.0001 |
| Renal insufficiency | Yes | 8927 | 19.5 | 3356 | 20.8 | 2779 | 19.2 | 2792 | 18.3 | <0.0001 |
| Depression | Yes | 4711 | 10.3 | 1627 | 10.1 | 1489 | 10.3 | 1595 | 10.5 | 0.5264 |
| Prior PCI | Yes | 4679 | 10.2 | 1770 | 10.9 | 1523 | 10.5 | 1386 | 9.1 | <0.0001 |
| Prior CABG | Yes | 6787 | 14.8 | 2620 | 16.2 | 2194 | 15.2 | 1973 | 12.9 | <0.0001 |
| Valvular heart disease | Yes | 6692 | 14.6 | 2314 | 14.3 | 2102 | 14.5 | 2276 | 14.9 | 0.3188 |
| CABG/PCI undetermined | Yes | 6108 | 13.3 | 2261 | 14.0 | 2032 | 14.1 | 1815 | 11.9 | <0.0001 |
| Smoking | Yes | 4188 | 9.1 | 1353 | 8.4 | 1271 | 8.8 | 1564 | 10.3 | <0.0001 |
| Diagnosis | ||||||||||
| Cardiac diagnosis | Heart failure with CAD | 20 740 | 44.96 | 7734 | 47.60 | 6681 | 45.93 | 6325 | 41.23 | <0.0001 |
| Confirmed AMI—non‐STEMI | 113 | 0.24 | 42 | 0.26 | 33 | 0.23 | 38 | 0.25 | ||
| Confirmed AMI—STEMI | 10 | 0.02 | 1 | 0.01 | 3 | 0.02 | 6 | 0.04 | ||
| Confirmed AMI—STEMI/non‐STEMI unspecified | 13 | 0.03 | 3 | 0.02 | 7 | 0.05 | 3 | 0.02 | ||
| Other | 670 | 1.45 | 242 | 1.49 | 188 | 1.29 | 240 | 1.56 | ||
| Peripheral vascular disease | 38 | 0.08 | 15 | 0.09 | 10 | 0.07 | 13 | 0.08 | ||
| Cerebral vascular disease | 9 | 0.02 | 2 | 0.01 | 4 | 0.03 | 3 | 0.02 | ||
| Unstable angina | 52 | 0.11 | 23 | 0.14 | 6 | 0.04 | 23 | 0.15 | ||
| Coronary artery disease | 90 | 0.20 | 31 | 0.19 | 22 | 0.15 | 37 | 0.24 | ||
| Heart failure, no CAD | 24 398 | 52.89 | 8156 | 50.19 | 7591 | 52.19 | 8651 | 56.40 | ||
| Missing | 84 | 0.18 | 24 | 0.15 | 29 | 0.20 | 31 | 0.20 | ||
| Vital signs at admission | ||||||||||
| Heart rate, bpm | Median | 45 668 | 80 | 16 050 | 72 | 14 417 | 80 | 15 201 | 89 | <0.0001 |
| 25th | 69 | 63 | 70 | 78 | ||||||
| 75th | 94 | 85 | 92 | 103 | ||||||
| Systolic blood pressure, mm Hg | Median | 45 664 | 139 | 16 059 | 143 | 14 409 | 139 | 15 196 | 134 | <0.0001 |
| 25th | 120 | 124 | 120 | 117 | ||||||
| 75th | 159 | 164 | 159 | 153 | ||||||
| Diastolic blood pressure, mm Hg | Median | 45 692 | 73 | 16 072 | 72 | 14 416 | 73 | 15 204 | 74 | <0.0001 |
| 25th | 63 | 62 | 63 | 64 | ||||||
| 75th | 85 | 84 | 85 | 85 | ||||||
| Labs at admission | ||||||||||
| BNP <100 | Yes | 2002 | 5.9 | 653 | 5.5 | 588 | 5.5 | 761 | 6.8 | <0.0001 |
| No | 31 670 | 94.1 | 11 237 | 94.5 | 10 018 | 94.5 | 10 415 | 93.2 | ||
| Missing | 12 545 | 27.1 | 4383 | 26.9 | 3968 | 27.2 | 4194 | 27.3 | ||
| Serum creatinine, mg/dL | Median | 41 899 | 1.3 | 14 667 | 1.3 | 13 229 | 1.3 | 14 003 | 1.3 | <0.0001 |
| 25th | 1.0 | 1.0 | 1.0 | 1.0 | ||||||
| 75th | 1.8 | 1.8 | 1.8 | 1.8 | ||||||
| BUN, mg/dL | Median | 41 563 | 25 | 14 564 | 26 | 13 120 | 25 | 13 879 | 25 | <0.0001 |
| 25th | 18 | 18 | 18 | 18 | ||||||
| 75th | 37 | 37 | 37 | 37 | ||||||
| Troponin, ng/dL | Median | 34 666 | 0.05 | 12 262 | 0.05 | 10 875 | 0.05 | 11 529 | 0.05 | 0.0731 |
| 25th | 0.03 | 0.03 | 0.03 | 0.03 | ||||||
| 75th | 0.10 | 0.10 | 0.10 | 0.11 | ||||||
| Ejection fraction | Median | 46 217 | 45 | 16 273 | 50 | 14 574 | 45 | 15 370 | 45 | <0.0001 |
| 25th | 30 | 30 | 30 | 30 | ||||||
| 75th | 55 | 57 | 55 | 55 | ||||||
AMI indicates acute myocardial infarction; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CABG, coronary artery bypass graft; CAD indicates coronary artery disease; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; CVA/TIA, cerebrovascular accident/transient ischemic attack; ICD, implantable cardiac defibrillator; MI, myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; STEMI, ST‐segment elevation myocardial infarction; UTD, unable to determine.
SR Patients
There were 26 020 HF patients in the SR group. Discharge heart rates appeared normally distributed with a median of 72 bpm and interquartile range of 18 bpm.
Characteristics of SR Patients
Younger patients, women, and nonwhite patients were more likely to have a higher heart rate (Table 2). A higher heart rate and lower systolic blood pressure on both admission and discharge were more frequent in the highest discharge heart rate tertile. Patients with higher discharge heart rate were more likely to have a history of COPD and smoking but less likely to have a history of diabetes, hypertension, peripheral vascular disease, prior myocardial infarction, prior stroke, and renal insufficiency. For SR patients with a history of COPD or asthma, and eligible for β‐blocker therapy, 91.6% were prescribed β‐blockers at discharge and 8.4% were not. For patients with a history of COPD or asthma, and eligible for evidence‐based β‐blocker therapy, 80.5% were prescribed evidence‐based β‐blockers at discharge and 19.5% were not. The quality of care composite measure, defect‐free care, was lowest in the highest heart rate tertile. Importantly, this measure includes the use of β‐blockers. Vital signs at discharge, medications at discharge, and hospital characteristics are reported in Table S2.
Table 2.
Baseline Patient Characteristics for Patients With Normal Sinus Rhythm, Overall and by Heart Rate Tertiles
| Variable | Level | Overall (N=26 020) | T1 (33 to 67 bpm) (N=8544) | T2 (68 to 78 bpm) (N=8793) | T3 (79 to 148 bpm) (N=8683) | P Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||||
| Age | Median | 26 020 | 79 | 8544 | 80 | 8793 | 79 | 8683 | 79 | <0.0001 |
| 25th | 72 | 73 | 73 | 72 | ||||||
| 75th | 85 | 86 | 85 | 85 | ||||||
| Gender | Female | 14 448 | 55.5 | 4763 | 55.7 | 4806 | 54.7 | 4879 | 56.2 | 0.1104 |
| Male | 11 572 | 44.5 | 3781 | 44.2 | 3987 | 45.3 | 3804 | 43.8 | ||
| Race | White | 19 799 | 76.9 | 6531 | 77.2 | 6689 | 76.8 | 6579 | 76.5 | 0.0011 |
| Black | 3326 | 12.9 | 1024 | 12.1 | 1103 | 12.7 | 1199 | 13.9 | ||
| Hispanic (any race) | 1586 | 6.2 | 534 | 6.3 | 567 | 6.5 | 485 | 5.6 | ||
| Asian | 366 | 1.4 | 135 | 1.6 | 132 | 1.5 | 99 | 1.15 | ||
| Other (includes UTD) | 682 | 2.6 | 232 | 2.7 | 217 | 2.5 | 233 | 2.71 | ||
| Missing | 261 | 1.0 | 88 | 1.0 | 85 | 1.0 | 88 | 1.0 | ||
| Medical history | ||||||||||
| Chronic or recurrent atrial fib | No | 25 667 | 100 | 8435 | 100 | 8671 | 100 | 8561 | 100 | |
| Atrial flutter | Yes | 325 | 1.3 | 113 | 1.3 | 105 | 1.2 | 107 | 1.2 | 0.7430 |
| COPD or asthma | Yes | 7262 | 28.3 | 2105 | 24.9 | 2327 | 26.8 | 2830 | 33.1 | <0.0001 |
| Diabetes—insulin treated | Yes | 4770 | 18.6 | 1690 | 20.0 | 1624 | 18.7 | 1456 | 17.0 | <0.0001 |
| Diabetes—non‐insulin‐treated | Yes | 6446 | 25.1 | 2118 | 25.1 | 2196 | 25.3 | 2132 | 24.9 | 0.8153 |
| Hyperlipidemia | Yes | 12 284 | 47.9 | 4282 | 50.8 | 4221 | 48.7 | 3781 | 44.2 | <0.0001 |
| Hypertension | Yes | 20 076 | 78.2 | 6813 | 80.8 | 6829 | 78.8 | 6434 | 75.2 | <0.0001 |
| PVD | Yes | 3692 | 14.4 | 1254 | 14.9 | 1276 | 14.7 | 1162 | 13.6 | 0.0311 |
| CAD | Yes | 13 628 | 53.1 | 4759 | 56.4 | 4729 | 54.5 | 4140 | 48.4 | <0.0001 |
| Prior MI | Yes | 4902 | 19.1 | 1759 | 20.8 | 1706 | 19.7 | 1437 | 16.8 | <0.0001 |
| CVA/TIA | Yes | 3973 | 15.5 | 1390 | 16.5 | 1378 | 15.9 | 1205 | 14.1 | <0.0001 |
| ICD | Yes | 1770 | 6.9 | 574 | 6.8 | 663 | 7.7 | 533 | 6.2 | 0.0011 |
| Heart failure | Yes | 12 722 | 49.6 | 4242 | 50.3 | 4327 | 49.9 | 4153 | 48.5 | 0.0504 |
| Anemia | Yes | 5011 | 19.5 | 1674 | 19.8 | 1687 | 19.5 | 1650 | 19.3 | 0.6300 |
| Pacemaker | Yes | 2786 | 10.8 | 980 | 11.6 | 1063 | 12.3 | 743 | 8.7 | <0.0001 |
| CRT‐P (CRT‐pacing only) | Yes | 94 | 0.4 | 36 | 0.4 | 31 | 0.4 | 27 | 0.3 | 0.4789 |
| CRT‐D (CRT with ICD) | Yes | 435 | 1.7 | 137 | 1.6 | 183 | 2.1 | 115 | 1.3 | 0.0004 |
| Dialysis (chronic) | Yes | 940 | 3.7 | 266 | 3.1 | 286 | 3.3 | 388 | 4.5 | <0.0001 |
| Renal insufficiency | Yes | 5245 | 20.4 | 1810 | 21.5 | 1775 | 20.5 | 1660 | 19.4 | 0.0037 |
| Depression | Yes | 2594 | 10.1 | 815 | 9.7 | 905 | 10.4 | 874 | 10.2 | 0.2259 |
| Prior PCI | Yes | 2711 | 10.6 | 939 | 11.1 | 975 | 11.2 | 797 | 9.3 | <0.0001 |
| Prior CABG | Yes | 3564 | 13.9 | 1329 | 15.8 | 1216 | 14.0 | 1019 | 11.9 | <0.0001 |
| Valvular heart disease | Yes | 2857 | 11.1 | 940 | 11.1 | 946 | 10.9 | 971 | 11.3 | 0.6651 |
| CABG/PCI undetermined | Yes | 3745 | 14.6 | 1289 | 15.3 | 1366 | 15.7 | 1090 | 12.7 | <0.0001 |
| Smoking | Yes | 2751 | 10.7 | 816 | 9.6 | 894 | 10.3 | 1041 | 12.1 | <0.0001 |
| Diagnosis | ||||||||||
| Cardiac diagnosis | Heart failure with CAD | 11 556 | 44.50 | 4050 | 47.48 | 4008 | 45.65 | 3498 | 40.39 | <0.0001 |
| Confirmed AMI—non‐STEMI | 81 | 0.31 | 24 | 0.28 | 29 | 0.33 | 28 | 0.32 | ||
| Confirmed AMI—STEMI | 8 | 0.03 | 1 | 0.01 | 2 | 0.02 | 5 | 0.06 | ||
| Confirmed AMI—STEMI/non‐STEMI unspecified | 11 | 0.04 | 1 | 0.01 | 6 | 0.07 | 4 | 0.05 | ||
| Other | 432 | 1.66 | 140 | 1.64 | 136 | 1.55 | 156 | 1.80 | ||
| Peripheral vascular disease | 24 | 0.09 | 7 | 0.08 | 10 | 0.11 | 7 | 0.08 | ||
| Cerebral vascular disease | 6 | 0.02 | 2 | 0.02 | 2 | 0.02 | 2 | 0.02 | ||
| Unstable angina | 35 | 0.13 | 18 | 0.21 | 3 | 0.03 | 14 | 0.16 | ||
| Coronary artery disease | 58 | 0.22 | 23 | 0.27 | 14 | 0.16 | 21 | 0.24 | ||
| Heart failure, no CAD | 13 759 | 52.98 | 4264 | 49.99 | 4569 | 52.04 | 4926 | 56.88 | ||
| Missing | 50 | 0.19 | 14 | 0.16 | 14 | 0.16 | 22 | 0.25 | ||
| Vital signs at admission | ||||||||||
| Heart rate, bpm | Median | 25 681 | 80 | 8426 | 71 | 8687 | 80 | 8568 | 88 | <0.0001 |
| 25th | 69 | 62 | 70 | 78 | ||||||
| 75th | 93 | 84 | 91 | 101 | ||||||
| Systolic blood pressure, mm Hg | Median | 25 666 | 142 | 8419 | 147 | 8680 | 141 | 8567 | 137 | <0.0001 |
| 25th | 122 | 127 | 123 | 118 | ||||||
| 75th | 163 | 169 | 163 | 157 | ||||||
| Diastolic blood pressure, mm Hg | Median | 25 692 | 73 | 8430 | 72 | 8688 | 73 | 8574 | 73 | 0.0069 |
| 25th | 63 | 62 | 63 | 63 | ||||||
| 75th | 85 | 84 | 85 | 85 | ||||||
| Labs at admission | ||||||||||
| BNP <100 pg/mL | Yes | 1183 | 6.3 | 316 | 5.1 | 404 | 6.4 | 463 | 7.4 | <0.0001 |
| No | 17 626 | 93.7 | 5865 | 94.9 | 5951 | 93.6 | 5810 | 92.6 | ||
| Missing | 7211 | 27.7 | 2363 | 27.7 | 2438 | 27.7 | 2410 | 27.8 | ||
| Serum creatinine, mg/dL | Median | 23 562 | 1.3 | 7671 | 1.4 | 7972 | 1.3 | 7919 | 1.3 | <0.0001 |
| 25th | 1.0 | 1.0 | 1.0 | 1.0 | ||||||
| 75th | 1.8 | 1.9 | 1.8 | 1.8 | ||||||
| BUN, mg/dL | Median | 23 352 | 25 | 7613 | 26 | 7903 | 25 | 7836 | 25 | <0.0001 |
| 25th | 18 | 18 | 18 | 17 | ||||||
| 75th | 37 | 37 | 37 | 37 | ||||||
| Troponin, ng/dL | Median | 19 600 | 0.05 | 6454 | 0.05 | 6595 | 0.05 | 6551 | 0.06 | <0.0001 |
| 25th | 0.03 | 0.03 | 0.03 | 0.03 | ||||||
| 75th | 0.12 | 0.10 | 0.12 | 0.13 | ||||||
| Ejection fraction | Median | 26 020 | 45 | 8544 | 50 | 8793 | 45 | 8683 | 40 | <0.0001 |
| 25th | 30 | 30 | 30 | 26 | ||||||
| 75th | 55 | 58 | 55 | 55 | ||||||
AMI indicates acute myocardial infarction; BNP, brain natriuretic peptide; bpm, beats per minute; BUN, blood urea nitrogen; CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; CVA/TIA, cerebrovascular accident/transient ischemic attack; ICD, implantable cardiac defibrillator; MI, myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; STEMI, ST‐segment elevation myocardial infarction; UTD, unable to determine.
Study Outcomes in SR Patients
Overall all‐cause mortality at 1 year was 30.1%, and the rate of all‐cause readmission or mortality at 1 year was 70.0% (Table 3). There were significant differences in each outcome by 1 year across the discharge heart rate tertiles, with higher mortality and the composite outcome of mortality or all‐cause readmission in the highest tertile (Table 3). Survival by 1 year was significantly different across the tertiles, with the lowest survival rate occurring in the highest tertile (log rank P<0.0001) (Figure 1). A similar pattern was observed for the composite outcome of readmission/mortality by 1 year.
Table 3.
Frequency of 1‐Year Outcomes by Heart Rate Tertiles (Sinus Rhythm Patients)
| Variable | Level | Overall (N=26 020) | T1 (33 to 67 bpm) (N=8544) | T2 (68 to 78 bpm) (N=8793) | T3 (79 to 148 bpm) (N=8683) | P Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mortality | Yes | 7820 | 30.05 | 2283 | 26.72 | 2512 | 28.57 | 3025 | 34.84 | <0.0001 |
| All‐cause readmission | Yes | 16 154 | 62.09 | 5277 | 61.76 | 5500 | 62.56 | 5377 | 61.93 | 0.5236 |
| Mortality or all‐cause readmission | Yes | 18 219 | 70.02 | 5835 | 68.29 | 6116 | 69.56 | 6268 | 72.20 | <0.0001 |
Figure 1.
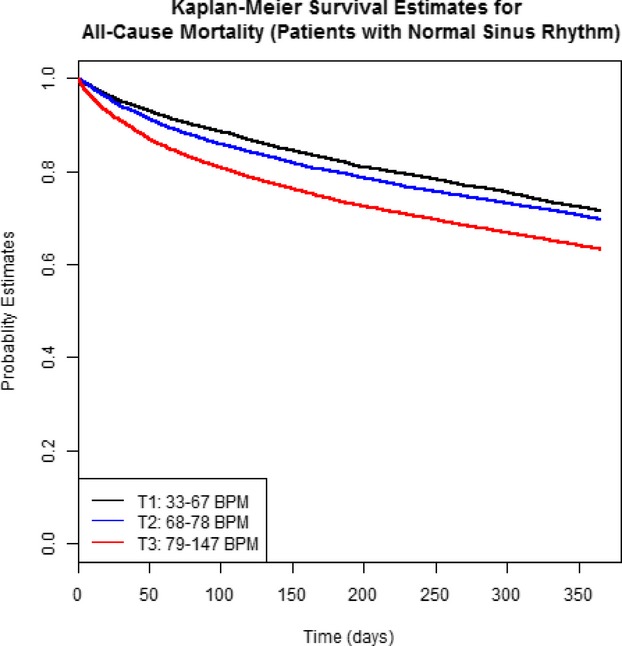
Kaplan–Meier plot of event‐free survival by 1 year in patients with sinus rhythm (n=26 020). There was a significant difference across the discharge heart rate tertiles (log rank P<0.0001) with survival highest in the lowest tertile (see text for details). BPM indicates beats per minute.
Crude and adjusted measures of the association between discharge heart rate (expressed as a continuous variable) and each outcome are reported in Table 4. For patients with a discharge heart rate ≥75 bpm, there were worse outcomes with increasing heart rate. The slope of this relationship changed for patients with heart rate <75 bpm (Figure 2). After adjustment for differences in clinical characteristics, the risk for all‐cause mortality at heart rate <75 increased by 6.0% (hazard ratio [HR] 1.060, 95% CI 1.018, 1.103; P=0.0047) per 10‐bpm increment over the 1 year of follow‐up. At heart rate ≥75 bpm, the risk for all‐cause mortality increased by 18.5% (HR 1.185, 95% CI 1.149, 1.222; P<0.0001) per 10‐bpm increment over the 1 year of follow‐up. When the data were analyzed by time interval to better meet proportionality assumptions, there was a 19.2% higher risk for mortality (HR 1.192, 95% CI 1.075, 1.322; P=0.0008) per 10‐bpm increment in heart rate <75 bpm over the first 30 days. There was a 30.0% higher risk for mortality (HR 1.300, 95% CI 1.219, 1.386; P<0.0001) per 10‐bpm increment in heart rate ≥75 bpm over the first 30 days. Beyond that and through 365 days, the risk for all‐cause mortality was not significant for heart rates <75 bpm but was 15.5% higher (HR 1.155, 95% CI 1.116, 1.196; P<0.0001) per 10‐bpm increase in heart rate ≥75 bpm. Qualitatively similar patterns were observed for the association between discharge heart rate and all‐cause readmission as well as the composite of all‐cause mortality or readmission (Table 4). For all outcomes, the HR over the first 30 days was significantly different from 1.0 at heart rates <75 bpm but was not significantly different from 1.0 over the interval from 31 to 365 days.
Table 4.
Hazard Ratios (HR) for Heart Rate per 10 bpm (Sinus Rhythm Patients, n=26 020)
| Outcome | Unadjusted Model | Adjusted Model (Patient and Hospital Characteristics) | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Mortality | ||||||
| Heart rate <75 bpm* | 1.054 | 1.015, 1.094 | 0.0061 | 1.060 | 1.018, 1.103 | 0.0047 |
| Heart rate <75 bpm | ||||||
| [0, 30] days | 1.125 | 1.034, 1.224 | 0.0061 | 1.192 | 1.075, 1.322 | 0.0008 |
| [31, 365] days | 1.045 | 1.002, 1.089 | 0.0417 | 1.040 | 0.995, 1.086 | 0.0794 |
| Heart rate ≥75 bpm* | 1.211 | 1.179, 1.245 | <0.0001 | 1.185 | 1.149, 1.222 | <0.0001 |
| Heart rate ≥75 bpm | ||||||
| [0, 30] days | 1.385 | 1.320, 1.454 | <0.0001 | 1.300 | 1.219, 1.386 | <0.0001 |
| [31, 365] days | 1.146 | 1.109, 1.184 | <0.0001 | 1.155 | 1.116, 1.196 | <0.0001 |
| All‐cause readmission | ||||||
| Heart rate <75 bpm* | 1.025 | 1.000, 1.052 | 0.0514 | 1.019 | 0.993, 1.046 | 0.1519 |
| Heart rate <75 bpm | ||||||
| [0, 30] days | 1.056 | 1.011, 1.102 | 0.0141 | 1.045 | 0.999, 1.092 | 0.0530 |
| [31, 365] days | 1.013 | 0.982, 1.045 | 0.4309 | 1.009 | 0.977, 1.042 | 0.5787 |
| Heart rate ≥75 bpm* | 1.054 | 1.031, 1.077 | <0.0001 | 1.063 | 1.039, 1.088 | <0.0001 |
| Heart rate ≥75 bpm | ||||||
| [0, 30] days | 1.104 | 1.067, 1.142 | <0.0001 | 1.128 | 1.089, 1.168 | <0.0001 |
| [31, 365] days | 1.020 | 0.991, 1.050 | 0.1777 | 1.020 | 0.990, 1.051 | 0.1872 |
| Composite readmission/mortality | ||||||
| Heart rate <75 bpm* | 1.024 | 1.000, 1.049 | 0.0541 | 1.023 | 0.998, 1.049 | 0.0769 |
| Heart rate <75 bpm | ||||||
| [0, 30] days | 1.051 | 1.010, 1.094 | 0.0148 | 1.052 | 1.008, 1.097 | 0.0191 |
| [31, 365] days | 1.014 | 0.984, 1.044 | 0.3771 | 1.011 | 0.980, 1.042 | 0.4931 |
| Heart rate ≥75 bpm* | 1.098 | 1.077, 1.120 | <0.0001 | 1.082 | 1.059, 1.105 | <0.0001 |
| Heart rate ≥75 bpm | ||||||
| [0, 30] days | 1.171 | 1.137, 1.206 | <0.0001 | 1.148 | 1.111, 1.186 | <0.0001 |
| [31, 365] days | 1.044 | 1.016, 1.072 | 0.0018 | 1.037 | 1.008, 1.067 | 0.0125 |
Ignoring violation of proportional hazards assumption (see text for details), model adjusted for the following covariates: age, gender, race (white vs other), insurance (none, Medicare, Medicaid, other), ejection fraction, history of atrial flutter, history of chronic obstructive pulmonary disease or asthma, history of diabetes, history of hyperlipidemia, history of hypertension, history of peripheral vascular disease, prior myocardial infarction, prior stroke or transient ischemic attack, history of anemia, history of chronic renal insufficiency, pacemaker, smoking, geographic region, academic or teaching hospital, rural location, hospital size, and defect‐free compliance score.
Figure 2.
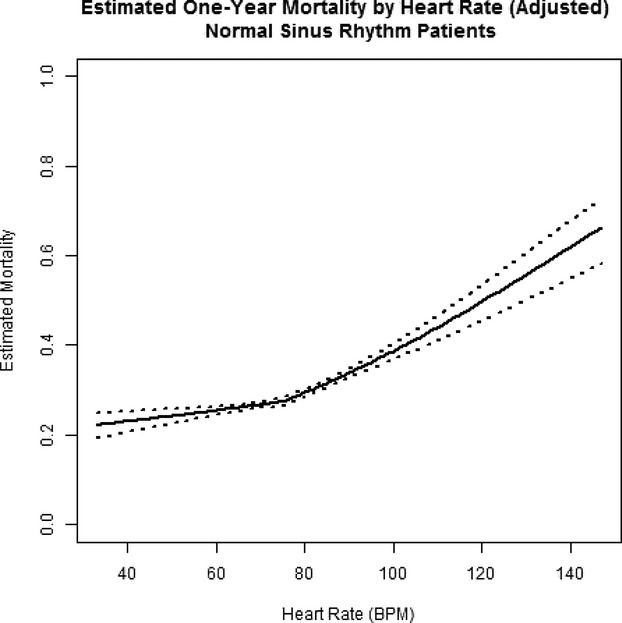
Estimated mortality at 1 year in patients with sinus rhythm (n=26 020). The inflection point represents a single linear spline at 75 bpm (see text for details). Risk of mortality rises steadily with heart rate. BPM indicates beats per minute.
There was no evidence for effect modification by CRT in models for mortality (interaction P=0.374), all‐cause readmission (P=0.952), or composite readmission/mortality (P=0.981). Including CRT in the adjusted model did not meaningfully change the HRs or 95% CIs for all‐cause mortality or all‐cause readmission at heart rate ≥75 bpm at either 0 to 30 days or 31 to 365 days. However, the HRs for heart rate <75 bpm at 0 to 30 days and heart rate ≥75 bpm at 31 to 365 days were no longer significant for the composite of all‐cause mortality/readmission. There was no evidence of effect modification by EF in adjusted models for mortality (interaction P=0.292), all‐ cause readmission (P=0.054), or composite readmission/mortality (P=0.187).
EF data were missing in 6.06% of SR patients. There was no significant interaction between heart rate and the presence or absence of missing EF data (P for interaction 0.87), indicating no difference in the degree of association between heart rate and all‐cause mortality between those with missing and nonmissing EF data.
AF Patients
There were 20 197 patients (43.7%) with either a history of AF or AF documented during the index hospitalization. Similar to the SR patients, discharge heart rates appeared normally distributed with a median of 74 bpm and interquartile range of 19 bpm.
Characteristics of AF Patients
Women and white patients were more likely to have higher heart rate (Table 5). A higher heart rate and lower systolic blood pressure on both admission and discharge were more frequent in the highest discharge heart rate tertile. Patients with higher discharge heart rates were more likely to have a history of COPD and smoking but less likely to have a history of diabetes, hypertension, peripheral vascular disease, prior myocardial infarction, prior stroke, and renal insufficiency. EF significantly varied by discharge heart rate tertile. For AF patients with a history of COPD or asthma, and eligible for β‐blocker therapy, 91.4% were prescribed β‐blockers at discharge, and 8.7% were not. For patients with a history of COPD or asthma, and eligible for evidence‐based β‐blockers, 78.8% were prescribed evidence‐based β‐blockers at discharge, and 21.2% were not. Defect‐free care was lowest in the highest discharge heart rate tertile. Importantly, this measure includes the use of β‐blockers. Vital signs at discharge, medications at discharge, and hospital characteristics are reported in Table S3.
Table 5.
Baseline Patient Characteristics for Patients With Atrial Fibrillation, Overall and by Heart Rate Tertiles
| Variable | Level | Overall (N=20 197) | T1 (32 to 68 bpm) (N=6692) | T2 (69 to 79 bpm) (N=6385) | T3 (80 to 168 bpm) (N=7120) | P Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||||
| Age | Median | 20 197 | 82 | 6692 | 82 | 6385 | 82 | 7120 | 81 | 0.0591 |
| 25th | 75 | 75 | 76 | 75 | ||||||
| 75th | 87 | 87 | 87 | 87 | ||||||
| Gender | Female | 10 640 | 52.7 | 3480 | 52.0 | 3264 | 51.1 | 3896 | 54.7 | <0.0001 |
| Male | 9557 | 47.3 | 3212 | 48.0 | 3121 | 48.9 | 3224 | 45.3 | ||
| Race | White | 17 679 | 88.2 | 5835 | 87.8 | 5642 | 88.9 | 6202 | 87.9 | 0.0489 |
| Black | 1148 | 5.7 | 409 | 6.2 | 329 | 5.2 | 410 | 5.8 | ||
| Hispanic (any race) | 625 | 3.1 | 193 | 2.9 | 186 | 2.9 | 246 | 3.5 | ||
| Asian | 216 | 1.2 | 86 | 1.3 | 60 | 0.95 | 70 | 1.0 | ||
| Other (includes UTD) | 380 | 1.9 | 123 | 1.9 | 126 | 1.99 | 131 | 1.8 | ||
| Missing | 149 | 0.7 | 46 | 0.7 | 42 | 0.66 | 61 | 0.9 | ||
| Medical history | ||||||||||
| Chronic or recurrent atrial fib | Yes | 17 277 | 85.6 | 5749 | 85.9 | 5474 | 85.8 | 6054 | 85.1 | 0.3493 |
| Atrial flutter | Yes | 695 | 3.4 | 245 | 3.7 | 203 | 3.2 | 247 | 3.5 | 0.3191 |
| COPD or asthma | Yes | 5961 | 29.5 | 1796 | 26.8 | 1888 | 29.6 | 2277 | 32.0 | <0.0001 |
| Diabetes—insulin treated | Yes | 2862 | 14.2 | 964 | 14.4 | 926 | 14.5 | 972 | 13.7 | 0.2989 |
| Diabetes—non‐insulin‐treated | Yes | 4329 | 21.5 | 1481 | 22.1 | 1358 | 21.3 | 1490 | 20.9 | 0.2219 |
| Hyperlipidemia | Yes | 9477 | 46.9 | 3368 | 50.3 | 2937 | 46.0 | 3172 | 44.6 | <0.0001 |
| Hypertension | Yes | 15 508 | 76.8 | 5265 | 78.7 | 4879 | 76.5 | 5364 | 75.4 | <0.0001 |
| PVD | Yes | 2798 | 13.9 | 986 | 14.7 | 855 | 13.4 | 957 | 13.5 | 0.0410 |
| CAD | Yes | 10 287 | 51.0 | 3598 | 53.8 | 3334 | 52.3 | 3355 | 47.2 | <0.0001 |
| Prior MI | Yes | 3561 | 17.6 | 1213 | 18.1 | 1158 | 18.1 | 1190 | 16.7 | 0.0431 |
| CVA/TIA | Yes | 3617 | 17.9 | 1252 | 18.7 | 1140 | 17.9 | 1225 | 17.2 | 0.0743 |
| ICD | Yes | 1637 | 8.1 | 569 | 8.5 | 607 | 9.5 | 461 | 6.5 | <0.0001 |
| Heart failure | Yes | 11 995 | 59.4 | 3991 | 59.6 | 3797 | 59.5 | 4207 | 59.2 | 0.8272 |
| Anemia | Yes | 4032 | 20.0 | 1371 | 20.5 | 1254 | 19.7 | 1407 | 19.8 | 0.4329 |
| Pacemaker | Yes | 3766 | 18.7 | 1308 | 19.5 | 1423 | 22.3 | 1035 | 14.5 | <0.0001 |
| CRT‐P (CRT‐pacing only) | Yes | 229 | 1.1 | 84 | 1.3 | 80 | 1.3 | 65 | 0.9 | 0.0921 |
| CRT‐D (CRT with ICD) | Yes | 450 | 2.2 | 128 | 1.9 | 188 | 2.9 | 134 | 1.9 | <0.0001 |
| Dialysis (chronic) | Yes | 379 | 1.9 | 113 | 1.7 | 110 | 1.7 | 156 | 2.2 | 0.0509 |
| Renal insufficiency | Yes | 3682 | 18.2 | 1326 | 19.8 | 1135 | 17.8 | 1221 | 17.2 | 0.0002 |
| Depression | Yes | 2117 | 10.5 | 706 | 10.5 | 658 | 10.3 | 753 | 10.6 | 0.8577 |
| Prior PCI | Yes | 1968 | 9.7 | 716 | 10.7 | 612 | 9.6 | 640 | 9.0 | 0.0030 |
| Prior CABG | Yes | 3223 | 16.0 | 1159 | 17.3 | 1056 | 16.5 | 1008 | 14.2 | <0.0001 |
| Valvular heart disease | Yes | 3835 | 19.0 | 1259 | 18.8 | 1206 | 18.9 | 1370 | 19.3 | 0.7774 |
| CABG/PCI undetermined | Yes | 2363 | 11.7 | 823 | 12.3 | 769 | 12.1 | 771 | 10.8 | 0.0167 |
| Medical history | ||||||||||
| Smoking | Yes | 1437 | 7.2 | 421 | 6.3 | 445 | 7.0 | 571 | 8.1 | 0.0003 |
| Diagnosis | ||||||||||
| Cardiac diagnosis | Heart failure with CAD | 9184 | 45.55 | 3200 | 47.89 | 2983 | 46.81 | 3001 | 42.21 | <0.0001 |
| Confirmed AMI—non‐STEMI | 32 | 0.16 | 12 | 0.18 | 10 | 0.16 | 10 | 0.14 | ||
| Confirmed AMI—STEMI | 2 | 0.01 | 0 | 0.00 | 1 | 0.02 | 1 | 0.01 | ||
| Confirmed AMI—STEMI/non‐STEMI unspecified | 2 | 0.01 | 2 | 0.03 | 0 | 0.00 | 0 | 0.00 | ||
| Other | 238 | 1.18 | 80 | 1.20 | 67 | 1.05 | 91 | 1.28 | ||
| Peripheral vascular disease | 14 | 0.07 | 7 | 0.10 | 1 | 0.02 | 6 | 0.08 | ||
| Cerebral vascular disease | 3 | 0.01 | 0 | 0.00 | 2 | 0.03 | 1 | 0.01 | ||
| Unstable angina | 17 | 0.08 | 4 | 0.06 | 4 | 0.06 | 9 | 0.13 | ||
| Coronary artery disease | 32 | 0.16 | 7 | 0.10 | 9 | 0.14 | 16 | 0.23 | ||
| Heart failure, no CAD | 10 639 | 52.76 | 3370 | 50.43 | 3295 | 51.71 | 3974 | 55.90 | ||
| Missing | 34 | 0.17 | 10 | 0.15 | 13 | 0.20 | 11 | 0.15 | ||
| Vital signs at admission | ||||||||||
| Heart rate, bpm | Median | 19 987 | 81 | 6601 | 73 | 6324 | 80 | 7062 | 90 | <0.0001 |
| 25th | 70 | 63 | 70 | 78 | ||||||
| 75th | 97 | 87 | 93 | 105 | ||||||
| Systolic blood pressure, mm Hg | Median | 19 998 | 135 | 6615 | 138 | 6325 | 135 | 7058 | 132 | <0.0001 |
| 25th | 118 | 120 | 118 | 115 | ||||||
| 75th | 154 | 158 | 154 | 150 | ||||||
| Diastolic blood pressure, mm Hg | Median | 20 000 | 73 | 6616 | 72 | 6326 | 73 | 7058 | 74 | <0.0001 |
| 25th | 63 | 62 | 63 | 64 | ||||||
| 75th | 85 | 83 | 84 | 86 | ||||||
| Labs at admission | ||||||||||
| BNP <100 pg/mL | Yes | 819 | 5.5 | 269 | 5.4 | 242 | 5.1 | 308 | 5.9 | 0.2406 |
| No | 14 044 | 94.5 | 4690 | 94.6 | 4454 | 94.8 | 4900 | 94.1 | ||
| Missing | 5334 | 26.4 | 1733 | 25.9 | 1689 | 26.4 | 1912 | 26.8 | ||
| Serum creatinine, mg/dL | Median | 18 337 | 1.3 | 6062 | 1.3 | 5795 | 1.3 | 6480 | 1.3 | <0.0001 |
| 25th | 1.0 | 1.0 | 1.0 | 1.0 | ||||||
| 75th | 1.7 | 1.7 | 1.7 | 1.7 | ||||||
| BUN, mg/dL | Median | 18 211 | 26 | 6023 | 26 | 5750 | 26 | 6438 | 25 | 0.2853 |
| 25th | 18 | 18 | 18 | 18 | ||||||
| 75th | 37 | 38 | 37 | 37 | ||||||
| Troponin, ng/dL | Median | 15 066 | 0.05 | 5019 | 0.05 | 4737 | 0.05 | 5310 | 0.05 | 0.0117 |
| 25th | 0.03 | 0.03 | 0.03 | 0.02 | ||||||
| 75th | 0.10 | 0.10 | 0.10 | 0.10 | ||||||
| Ejection fraction | Median | 20 197 | 48 | 6692 | 50 | 6385 | 45 | 7120 | 48 | <0.0001 |
| 25th | 30 | 32 | 30 | 30 | ||||||
| 75th | 55 | 57 | 55 | 55 | ||||||
AMI indicates acute myocardial infarction; bpm, beats per minute; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; CVA/TIA, cerebrovascular accident/transient ischemic attack; ICD, implantable cardiac defibrillator; MI, myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; STEMI, ST‐segment elevation myocardial infarction; UTD, unable to determine.
Study Outcomes in AF Patients
Overall all‐cause mortality at 1 year was 35.3% and the rate of all‐cause readmission or mortality at 1 year was 72.4%. There was a significant difference in mortality by 1 year across the tertiles (log rank P<0.0001) (Figure 3). In contrast to the SR patients, the composite outcome of mortality/all‐cause readmission was not significantly different across the discharge heart rate tertiles (Table 6).
Figure 3.
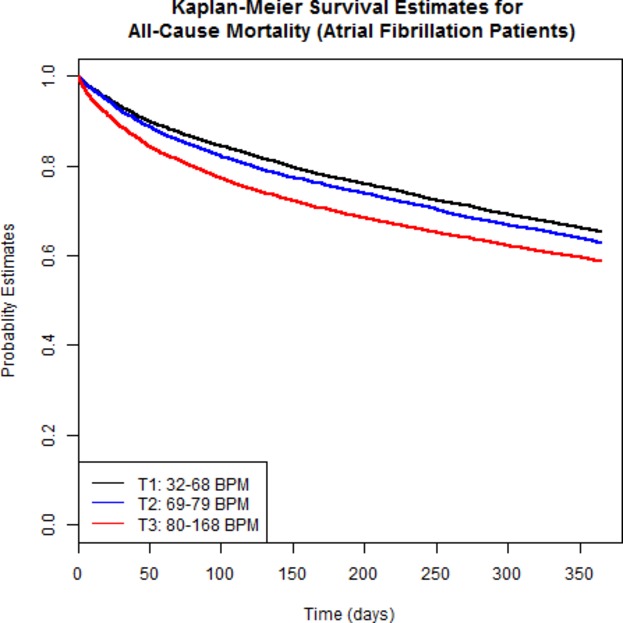
Kaplan–Meier plot of event‐free survival by 1 year in patients with atrial fibrillation (n=20 197). There was a significant difference across the discharge heart rate tertiles (log rank P<0.0001), with survival highest in the lowest tertile (see text for details). BPM indicates beats per minute.
Table 6.
Frequency of 1‐Year Outcomes by Heart Rate Tertiles (Atrial Fibrillation Patients)
| Variable | Level | Overall (N=20 197) | T1 (32 to 68 bpm) (N=6692) | T2 (69 to 79 bpm) (N=6385) | T3 (80 to 168 bpm) (N=7120) | P Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mortality | Yes | 7138 | 35.34 | 2161 | 32.29 | 2210 | 34.61 | 2767 | 38.86 | <0.0001 |
| All‐cause readmission | Yes | 12 543 | 62.10 | 4212 | 62.94 | 3976 | 62.27 | 4355 | 61.17 | 0.0940 |
| Mortality or all‐cause readmission | Yes | 14 612 | 72.35 | 4795 | 71.65 | 4596 | 71.98 | 5221 | 73.33 | 0.0649 |
Crude and adjusted measures of the association between discharge heart rate (expressed as a continuous variable) and each outcome are reported in Table 7. For patients with a discharge heart rate ≥75 bpm, there were worse outcomes with increasing heart rate. The slope of this relationship changed only slightly for patients with heart rate <75 bpm (Figure 4). After adjustment for differences in clinical characteristics, the risk for all‐cause mortality increased, overall, by 8.4% (HR 1.084, 95% CI 1.039, 1.131; P=0.0002) per 10 bpm increase in heart rate for patients with discharge heart rate <75 bpm and 8.8% (HR 1.088, 95% CI 1.056, 1.120; P<0.0001) in patients with heart rate ≥75 bpm. There was a 22.8% (HR 1.228, 95% CI 1.165, 1.294; P<0.0001) per 10 bpm increment in heart in rate for the first 30 days at heart rate ≥75 bpm. Beyond that, and through 365 days, the risk for all‐cause mortality was 4.9% higher (HR 1.049, 95% CI 1.014, 1.084; P=0.0053) for each 10‐beat increment. Of note is that for heart rate <75 bpm, the risk for mortality increased by 8.7% (HR 1.087, 95% CI 1.042, 1.134; P=0.0024) per 10 bpm increment in heart rate. At heart rates <75 bpm there was no significant association between heart rate and risk for all‐cause readmission or risk for the composite outcome of readmission/mortality. At heart rates ≥75 bpm, there was a 10.7% increase in risk (HR 1.107, 95% CI 1.073, 1.143; P<0.0001) per 10‐beat increment for short‐term (0 to 30 days) all‐cause readmission and a 12.2% increased risk (HR 1.122, 95% CI 1.089, 1.155; P<0.0001) per 10‐beat increment for the short‐term composite outcome readmission/mortality. This increase in risk for the latter outcomes was not, however, present at heart rates ≥75 bpm from 31 to 365 days (Table 7).
Table 7.
Hazard Ratios for Heart Rate, per 10 bpm (Atrial Fibrillation Patients, n=20 197)
| Outcome | Unadjusted Model | Adjusted Model (Patient and Hospital Characteristics) | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Mortality | ||||||
| Heart rate <75 bpm* | 1.074 | 1.033, 1.117 | 0.0003 | 1.084 | 1.039, 1.131 | 0.0002 |
| Heart rate <75 bpm | 1.083 | 1.041, 1.126 | <0.0001 | 1.087 | 1.042, 1.134 | 0.0001 |
| Heart rate ≥75 bpm* | 1.122 | 1.093, 1.151 | <0.0001 | 1.088 | 1.056, 1.120 | <0.0001 |
| Heart rate ≥75 bpm | ||||||
| [0, 30] days | 1.312 | 1.262, 1.364 | <0.0001 | 1.228 | 1.165, 1.294 | <0.0001 |
| [31, 365] days | 1.043 | 1.011, 1.076 | 0.0082 | 1.049 | 1.014, 1.084 | 0.0053 |
| All‐cause readmission | ||||||
| Heart rate <75 bpm* | 1.009 | 0.980, 1.038 | 0.5508 | 1.011 | 0.981, 1.041 | 0.4833 |
| Heart rate <75 bpm | 1.011 | 0.982, 1.040 | 0.4572 | 1.013 | 0.983, 1.043 | 0.4012 |
| Heart rate ≥75 bpm* | 1.026 | 1.004, 1.048 | 0.0224 | 1.033 | 1.010, 1.057 | 0.0046 |
| Heart rate ≥75 bpm | ||||||
| [0, 30] days | 1.094 | 1.062, 1.128 | <0.0001 | 1.107 | 1.073, 1.143 | <0.0001 |
| [31, 365] days | 0.978 | 0.952, 1.006 | 0.1247 | 0.983 | 0.955, 1.012 | 0.2410 |
| Composite readmission/mortality | ||||||
| Heart rate <75 bpm* | 1.008 | 0.982, 1.035 | 0.5531 | 1.012 | 0.984, 1.041 | 0.3902 |
| Heart rate <75 bpm | 1.012 | 0.985, 1.040 | 0.3812 | 1.015 | 0.986, 1.043 | 0.3167 |
| Heart rate ≥75 bpm* | 1.066 | 1.045, 1.086 | <0.0001 | 1.048 | 1.026, 1.071 | <0.0001 |
| Heart rate ≥75 bpm | ||||||
| [0, 30] days | 1.156 | 1.126, 1.186 | <0.0001 | 1.122 | 1.089, 1.155 | <0.0001 |
| [31, 365] days | 0.996 | 0.970, 1.022 | 0.7336 | 0.998 | 0.972, 1.025 | 0.8917 |
bpm indicates beats per minute; HR, hazard ratio.
Ignoring violation of proportional hazards assumption (see text for details), model adjusted for the following covariates: age, gender, race (white vs other), insurance (none, Medicare, Medicaid, other), ejection fraction, history of atrial flutter, history of chronic obstructive pulmonary disease or asthma, history of diabetes, history of hyperlipidemia, history of hypertension, history of peripheral vascular disease, prior myocardial infarction, prior stroke or transient ischemic attack, history of anemia, history of chronic renal insufficiency, pacemaker, smoking, geographic region, academic or teaching hospital, rural location, hospital size, defect‐free compliance score.
Figure 4.
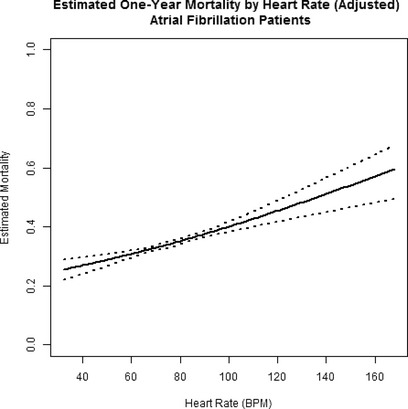
Estimated mortality at 1 year in patients with atrial fibrillation (n=20 197). The inflection point represents a single linear spline at 75 bpm (see text for details). Risk of mortality rises steadily with heart rate, although the slope of this relationship is less steep than the corresponding slope in sinus rhythm patients. BPM indicates beats per minute.
There was no evidence of effect modification by CRT in the model for mortality (interaction P=0.619), and further adjusting for CRT status yielded mostly similar results as presented previously, although the HR for heart rates ≥75 bpm from 31 to 365 days was no longer significant (P=0.2945). There was evidence of effect modification by CRT in the models for all‐cause readmission (P=0.031) and composite readmission/mortality (P=0.033) (Table 8). The interaction term (heart rate×EF) was statistically significant in adjusted models for mortality (interaction P=0.010), all‐cause readmission (P=0.003), and composite readmission/mortality (P=0.019). The association between heart rate and mortality was more pronounced among patients with EF >0.40 compared to EF ≤0.40 (interaction P=0.010). Further evidence of effect modification was observed for all‐cause readmission and composite all‐cause mortality/readmission outcomes (interaction P=0.003, P=0.019, respectively) (Table 9).
Table 8.
Hazard Ratios (HR) for Heart Rate, per 10 bpm Increase (Atrial Fibrillation Patients, n=20 197)
| Outcome | Unadjusted Model | Adjusted Model | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| All‐cause readmission | ||||||
| No CRT | ||||||
| Heart rate <75 bpm | 1.001 | 0.959, 1.046 | 0.9509 | 1.008 | 0.963, 1.055 | 0.7312 |
| Heart rate ≥75 bpm | ||||||
| [0, 30] days | 1.079 | 1.030, 1.130 | 0.0013 | 1.094 | 1.043, 1.147 | 0.0002 |
| [31, 365] days | 0.962 | 0.921, 1.004 | 0.0762 | 0.969 | 0.927, 1.013 | 0.1693 |
| CRT | ||||||
| Heart rate <75 bpm | 1.072 | 0.928, 1.239 | 0.3434 | 1.071 | 0.924, 1.242 | 0.3614 |
| Heart rate ≥75 bpm | ||||||
| [0, 30] days | 1.342 | 1.169, 1.541 | <0.0001 | 1.297 | 1.120, 1.502 | 0.0005 |
| [31, 365] days | 0.909 | 0.762, 1.085 | 0.2910 | 0.880 | 0.736, 1.052 | 0.1608 |
| Composite readmission/mortality | ||||||
| No CRT | ||||||
| Heart rate <75 bpm | 1.010 | 0.970, 1.053 | 0.6211 | 1.015 | 0.972, 1.059 | 0.5070 |
| Heart rate ≥75 bpm | ||||||
| [0, 30] days | 1.118 | 1.073, 1.165 | <0.0001 | 1.098 | 1.050, 1.148 | <0.0001 |
| [31, 365] days | 0.979 | 0.940, 1.019 | 0.2889 | 0.987 | 0.946, 1.028 | 0.5231 |
| CRT | ||||||
| Heart rate <75 bpm | 1.102 | 0.960, 1.266 | 0.1665 | 1.091 | 0.946, 1.260 | 0.2324 |
| Heart rate ≥75 bpm | ||||||
| [0, 30] days | 1.312 | 1.150, 1.497 | <0.0001 | 1.282 | 1.112, 1.478 | 0.0006 |
| [31, 365] days | 0.918 | 0.778, 1.083 | 0.3101 | 0.898 | 0.760, 1.061 | 0.2044 |
bmp indicates beats per minute; CRT, cardiac resynchronization therapy.
Table 9.
Hazard Ratios (HR) for Heart Rate, per 10 bpm Increase (Atrial Fibrillation Patients, n=20 197)
| Outcome | Unadjusted Model | Adjusted Model | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Mortality | ||||||
| LVEF ≤40% | ||||||
| Heart rate <75 bpm | 1.050 | 0.988, 1.117 | 0.1178 | 1.048 | 0.980, 1.120 | 0.1703 |
| Heart rate ≥75 bpm | ||||||
| [0, 30] days | 1.311 | 1.240, 1.387 | <0.0001 | 1.220 | 1.130, 1.316 | <0.0001 |
| [31, 365] days | 0.991 | 0.943, 1.041 | 0.7090 | 1.005 | 0.953, 1.059 | 0.8634 |
| LVEF >40% | ||||||
| Heart rate <75 bpm | 1.101 | 1.046, 1.159 | 0.0002 | 1.117 | 1.057, 1.180 | <0.0001 |
| Heart rate ≥75 bpm | ||||||
| [0, 30] days | 1.315 | 1.252, 1.380 | <0.0001 | 1.234 | 1.156, 1.316 | <0.0001 |
| [31, 365] days | 1.082 | 1.040, 1.125 | <0.0001 | 1.080 | 1.035, 1.126 | 0.0004 |
| All‐cause readmission | ||||||
| LVEF ≤40% | ||||||
| Heart rate <75 bpm | 1.010 | 0.964, 1.058 | 0.6845 | 1.008 | 0.960, 1.057 | 0.7588 |
| Heart rate ≥75 bpm | ||||||
| [0, 30] days | 1.115 | 1.067, 1.165 | <0.0001 | 1.127 | 1.076, 1.180 | <0.0001 |
| [31, 365] days | 0.933 | 0.892, 0.976 | 0.0023 | 0.931 | 0.889, 0.975 | 0.0024 |
| LVEF >40% | ||||||
| Heart rate <75 bpm | 1.011 | 0.975, 1.049 | 0.5601 | 1.017 | 0.980, 1.056 | 0.3806 |
| Heart rate ≥75 bpm | ||||||
| [0, 30] days | 1.081 | 1.039, 1.123 | <0.0001 | 1.094 | 1.051, 1.139 | <0.0001 |
| [31, 365] days | 1.009 | 0.975, 1.044 | 0.6187 | 1.018 | 0.982, 1.055 | 0.3289 |
| Composite readmission/mortality | ||||||
| LVEF ≤40% | ||||||
| Heart rate <75 bpm | 1.009 | 0.967, 1.053 | 0.6788 | 1.006 | 0.962, 1.053 | 0.7826 |
| Heart rate ≥75 bpm | ||||||
| [0, 30] days | 1.172 | 1.129, 1.217 | <0.0001 | 1.141 | 1.093, 1.191 | <0.0001 |
| [31, 365] days | 0.946 | 0.908, 0.985 | 0.0075 | 0.950 | 0.910, 0.991 | 0.0183 |
| LVEF >40% | ||||||
| Heart rate <75 bpm | 1.012 | 0.978, 1.047 | 0.4974 | 1.021 | 0.985, 1.058 | 0.2575 |
| Heart rate ≥75 bpm | ||||||
| [0, 30] days | 1.145 | 1.108, 1.183 | <0.0001 | 1.108 | 1.067, 1.150 | <0.0001 |
| [31, 365] days | 1.030 | 0.997, 1.063 | 0.0729 | 1.031 | 0.997, 1.066 | 0.0707 |
bmp indicates beats per minute; LVEF, left ventricular ejection fraction.
EF data were missing in 4.70% of AF patients. There was no significant interaction between heart rate and the presence or absence of missing EF data (P for interaction 0.81), indicating no difference in the degree of association between heart rate and all‐cause mortality between those with missing and nonmissing EF data.
Discussion
In this analysis of over 46 000 patients discharged alive following treatment for HF in centers participating in the AHA GWTG‐HF program from 2005 to 2011, we observed a significant and independent association of heart rate at the time of discharge on the risks for short‐ (discharge to 30 days) and longer‐term (31 to 365 days) mortality in SR and AF patients. The associations between discharge heart rate and risks of all‐cause readmission and the composite outcome of all‐cause mortality/readmission were significant over the short term in both SR and AF, but only for the composite outcome over the longer term in SR. The magnitude of the association (HR) was greater over the short term and varied according to the cardiac rhythm status. Among patients with AF, the magnitude of association was significantly modified by discharge CRT status (all‐cause readmission and the composite outcome) and by EF (all outcomes).
Our data are in qualitative agreement with conclusions from prior studies regarding an overall positive association between heart rate and adverse cardiovascular outcomes in patients with HF (Table 10). However, important differences exist. Studies derived from randomized clinical trials of specific therapies and which analyzed “baseline” (prerandomization) heart rate as the covariate of interest have been inconsistent in identifying a clinically meaningful association with postdischarge adverse outcomes.5–7 Clinical trials that narrowed heart rate inclusion criteria4 or studies in which post‐hoc defined cut points for the analysis of the heart rate–outcome association3,6 were employed differ methodologically from the present study and enrolled more selective patient populations. Most prior studies excluded patients with AF. Few studies included patients with HF and preserved EF. The majority of prior studies also failed to take into account, or report, the extent of guideline‐based medical and device therapy. Finally, no study recognized, or reported, a time‐dependence of the heart‐rate‐outcome association.
Table 10.
Summary of Recent Clinical Trial and Population‐Based Studies of the Association Between Heart Rate and Clinical Outcomes in Patients With and Without Prevalent HF
| Study (Ref) | Year | Study Design/Patient Population | Specific Outcome(s) | Estimate of Magnitude of Association | Atrial Fibrillation Included? |
|---|---|---|---|---|---|
| BEAUTIFUL3 | 2008 | RCT/CAD,LVD, heart rate ≥60 bpm | CV death HF admission |
Post‐Rx HR 1.08 per 5 bpm Post‐Rx HR 1.16 per 10 bpm |
No |
| SHIFT4 | 2010 | RCT/HFrEF, heart rate ≥70 bpm | Composite (CV death/hospital admission) | Post‐Rx HR 1.16 per 5 bpm | No |
| CHARM5 | 2012 | RCT/HFrEF, HFpEF | All‐cause mortality | “pre‐Rx” HR 1.06 per 10 bpm | Yes |
| EVEREST6 | 2013 | RCT/HFrEF | All‐cause mortality | “baseline” HR 1.05 per 5 bpm* “discharge” HR 1.20 per 5 bpm* |
No |
| Kapoor and Heidenreich18 | 2010 | Prospective cohort/HFpEF | All‐cause mortality | HR 1.47 for heart rate 70 to 90 (compared to rate <60) | No |
| AHA GWTG‐HF19 | 2013 | Prospective, registry design/HFrEF, HFpEF/in‐patient | In‐hospital mortality | Admission HR 1.20 per 10 bpm | Yes |
| FRAMINGHAM20 | 2014 | Prospective cohort, population‐based/no prevalent CV disease | All‐cause mortality | HR 1.17 per 11 bpm | No |
| MESA21 | 2014 | Prospective cohort, population‐based/no prevalent CV disease | Incident HF | HR 1.04 per 1 bpm | No |
| EFFECT‐HF7 | 2014 | Retrospective, population‐based, observational/post‐hospital discharge/HFrEF, HFpEF | All‐cause mortality | OR 1.41 for heart rate >90 (compared to heart rate 40 to 60) | No |
| Cullington, et al22 | 2014 | Retrospective/prospective, community‐based, observational/HFrEF | All‐cause mortality | HR 1.10 per 10 bpm for SR patients only | Yes |
| Present study AHA GWTG‐HF |
2014 | Prospective, registry design/HFrEF, HFpEF/post‐hospital discharge | All‐cause mortality All‐cause mortality/readmission |
See Results | Yes |
bpm indicates beats per minute; CAD, coronary artery disease; CV, cardiovascular; HF, heart failure; HR, hazard ratio; LVD, left ventricular dysfunction; OR, odds ratio; pEF, preserved ejection fraction; RCT, randomized clinical trial; rEF, reduced ejection fraction; SR, sinus rhythm.
P=0.066, heart rate ≥70 bpm.
P<0.001, heart rate ≥70 bpm.
A prior study from the GWTG‐HF program identified an association between the admission heart rate and in‐hospital mortality.19 Although the magnitude of the association in that study depended on the cardiac rhythm, the EF, and the absolute heart rate, there was, overall, a 23% increase in the adjusted odds of in‐hospital death per 10‐bpm increment in heart rate.19 Differences between these 2 studies derive from a number of considerations. First, the latter study analyzed admission heart rate as the covariate of interest, whereas the current study analyzed discharge heart rate and, as such, an association between discharge heart rate and outcome will reflect the impact of treatment and the nature of the association will quite likely differ. Second, the latter study reported crude in‐hospital mortality rates, whereas the current study reports the predicted probability of mortality obtained from the adjusted Cox multivariable model. Adjusted probabilities will likely differ from unadjusted crude rates. Third, the “plateau” in the curve for AF patients in the latter study appears outside of the 5% to 95% percentile distribution of heart rates and may reflect the paucity of data in this region, suggesting limited precision of an estimate. However, both studies agree in that mortality in AF patients exceeds that for SR patients for any heart rate. Finally, “flattening” of the association between heart rate and mortality in AF patients relative to SR patients in both the inpatient study and the present outpatient study are congruent observations.
In the present report from the GWTG‐HF program, we extended the analysis to specifically examine the impact of the heart rate at the time of discharge on longer‐term outcomes. The heart rate at the time of discharge is more likely to reflect the totality of the in‐hospital treatment program including the extent of use of guideline‐based therapies including β‐blockers. Indeed, the average discharge heart rate was lower than that on admission, consistent with such treatment. By including the defect‐free care measure in our adjusted analysis (and thereby accounting for differences in β‐blocker use across the tertiles of heart rate), our findings not only differ from prior studies in this regard but also underscore the powerful, independent, and persistent effect of heart rate on longer‐term outcomes.
In patients with AF, although early (0 to 30 days) and late (31 to 365 days) associations between heart rate and mortality were statistically significant at rates ≥75 bpm, the magnitude of this association decreased over the late term, mirroring the change in HR over time in SR patients. In contrast to the findings in patients in SR, the HRs for the association between discharge heart rate and all‐cause readmission and the composite outcome were not statistically significant and suggest that in patients with AF, heart rate might be of lesser importance over the long term.22 It should be recalled that patients with HF and AF are at intrinsically higher risk for death or readmission compared with patients in SR.12–13 The decreased slope of the overall heart‐rate‐outcome relationship in patients with AF compared to patients with SR may reflect this increased baseline risk in patients with AF, which effectively attenuates a “heart rate” effect. Nevertheless, 50% of the patients in AF had a discharge HR >74 bpm, suggesting additional opportunity for potential benefit of further rate control on outcome.
We identified an early phase (0 to 30 days) HR and a later phase (31 to 365 days) HR for patients with either SR or AF. The HR for the association between heart rate and mortality between 0 and 30 days (early phase) and from 31 to 365 days (later phase) was numerically higher for SR patients compared to AF patients. In addition, in both groups of patients the magnitude of this association diminished over the interval from 31 to 365 days. These findings suggest that there may be potential early postdischarge benefit from lowering heart rate in patients hospitalized with HF in addition to benefit from decreasing the heart rate over the longer term.4 In the present study, 25% of the patients in SR had a discharge heart rate <64 bpm and would likely not be candidates for aggressive attempts at further rate reduction. However, for the 50% of SR patients with a discharge heart rate >72 bpm, further efforts toward rate reduction during this critical time window might be of benefit.
Speculation regarding a fundamental pathophysiologic relationship between higher heart rate and the development20–21 or worsening of HF8–10 has ranged from myocardial energetic considerations23 to favorable alterations in arterial afterload with heart rate reduction.24 By targeting heart rate as a potentially modifiable risk factor in the progression of HF, the SHIFT trial4 has implicated heart rate in the causal pathway of HF progression. Whether benefit from heart rate reduction derives from reduced myocardial oxygen consumption and improved myocardial efficiency, reduced total afterload, or alternative explanations25 remains to be determined.
The present study has a number of limitations that merit discussion. This is a retrospective analysis from a prospectively designed and conducted registry. Data were collected by chart review and are, therefore, dependent on the quality and accuracy of data collection. Hospitals voluntarily participating in GWTG‐HF may not be representative of all hospitals in the United States, although prior study has shown that GWTG‐HF patients and hospitals have characteristics similar to hospitals nationwide. We restricted the analysis to fee‐for‐service Medicare beneficiaries ≥65 years of age in order to allow for assessment of postdischarge outcomes by linkage of GWTG‐HF records with those from Medicare. However, the majority of patients hospitalized with HF in the United States are over the age of 65 years.26 Characteristics and outcomes of Medicare beneficiaries in previous HF registries were similar to the broader Medicare population with HF, suggesting that findings from such registries may be generalizable.27 The extent of missing biomarker data (eg, N‐terminal pro‐brain natriuretic peptide, brain natriuretic peptide) in this registry precludes more objective measures of disease severity. This study was not a prospective randomized trial, and residual measured and unmeasured confounders might have influenced reported outcomes notwithstanding extensive statistical adjusting of the crude rates. Although other medications potentially affecting heart rate (eg, calcium channel blockers, digoxin, amiodarone, and β‐agonists in patients with COPD) are used in patients with HF, these medications are not systematically tracked in this quality assurance program. Consequently, the current models cannot adjust for their use. Attention is drawn, however, to the >90% prevalence of β‐blockers and their dominant effect on heart rate at discharge. This registry does not record provider intention with respect to the management of AF (rate versus rhythm control). Therefore, selection bias and residual confounding due to the use of β‐blockers for rate control in patients with HF and AF is another limitation. As noted above, overall β‐blocker use in AF patients exceeded 90%. The study is also limited in the absence of cause‐specific outcomes. In the absence of independent clinical outcome assessment as would be the case in a clinical trial, we suggest that our choices for all‐cause mortality and all‐cause hospitalization are less subject to bias (eg, misclassification bias), which often limits inferences from observational studies. Increased sensitivity using an all‐cause outcome allows for identification of clinical outcomes in these older subjects that are of broad clinical relevance. We believe that these considerations do not detract from our conclusions regarding the adverse effect of increased heart rate in patients with HF even after accounting for the extent of guideline‐directed medical therapy.
In summary, our observations add to the growing evidence base for a positive association between heart rate and adverse clinical outcomes in patients with HF. Our findings expand on these prior observations and indicate a biphasic nature to the time‐dependent hazard with an early (0 to 30 day) substantial increase in the HR for mortality and a longer‐term (31 to 365 days) lower, albeit persistently and significantly increased, HR. These observations suggest additional opportunities to improve outcomes for HF patients in SR or AF, patients with preserved or depressed left ventricular systolic function, and patients with persistently increased heart rate at the time of hospital discharge.
Supplementary Material
Table S1. Patient Characteristics at Discharge, Overall and by Heart Rate Tertiles
Table S2. Patient Characteristics at Discharge for Patients with Normal Sinus Rhythm, Overall and by Heart Rate Tertiles
Table S3. Patient Characteristics at Discharge for Patients with Atrial Fibrillation, Overall and by Heart Rate Tertiles
Sources of Funding
The GWTG‐HF program is provided by the American Heart Association. GWTG‐HF has been funded in the past through support from Medtronic, GlaxoSmithKline, Ortho‐McNeil, and the American Heart Association Pharmaceutical Roundtable.
Disclosures
Laskey, Alomari, Cox, Schulte, Zhao, Heidenreich, and Yancy have none declared. Hernandez: Research: Amgen, BMS, GSK, Novartis. Honoraria: Amgen, BMS, Novartis, Janssen. Eapen: Advisory Board: Novartis, Cytokinetics. Honoraria: Janssen. Consultant: Amgen. Bhatt: Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Get With The Guidelines® Steering Committee; Data Monitoring Committees: Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Associate Editor; Section Editor, Pharmacology), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor); Research Funding: Amarin, AstraZeneca, Bristol‐Myers Squibb, Eisai, Ethicon, Forest Laboratories, Ischemix, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Unfunded Research: FlowCo, PLx Pharma, Takeda. Fonarow: Research: Agency for Healthcare; Research and Quality, National Institutes of Health; Consulting: Bayer, Gambro, Novartis, Medtronic.
References
- Palatini P, Julius S. Elevated heart rate: a major risk factor for cardiovascular disease. Clin Exp Hypertens. 2004; 26:637-644. [DOI] [PubMed] [Google Scholar]
- Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Sendon JLL, Steg PG, Tardif J‐C, Tavazzi L, Tendera Mfor the Heart Rate Working Group. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007; 50:823-830. [DOI] [PubMed] [Google Scholar]
- Fox K, Steg PG, Tendera M, Robertson M, Ferrari Ron behalf of the BEAUTIFUL Investigators. Heart rate as a prognostic risk factor in patients with coronary artery disease and left‐ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008; 372:817-821. [DOI] [PubMed] [Google Scholar]
- Bohm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost‐Brama A, Lerebours G, Tavazzi Lon behalf of the SHIFT Investigators. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo‐controlled trial. Lancet. 2010; 376:886-894. [DOI] [PubMed] [Google Scholar]
- Castagno D, Skali H, Takeuchi M, Swedberg K, Yusuf S, Granger CB, Michelson EL, Pfeffer MA, McMurray JJV, Solomon SDfor the CHARM Investigators. Association of heart rate and outcomes in a broad spectrum of patients with chronic heart failure. J Am Coll Cardiol. 2012; 59:1785-1795. [DOI] [PubMed] [Google Scholar]
- Greene SJ, Vaduganathan M, Wilcox JE, Harinstein ME, Maggioni AP, Subacius H, Zannad F, Konstam MA, Chioncel O, Yancy CW, Swedberg K, Butler J, Bonow RO, Gheorghiade Mon behalf of the EVEREST Trial Investigators. The prognostic significance of heart rate in patients hospitalized for heart failure with reduced ejection fraction in sinus rhythm. JACC Hear Fail. 2013; 1:488-496. [DOI] [PubMed] [Google Scholar]
- Habal MV, Liu PP, Austin PC, Ross HJ, Newton GE, Wang X, Tu JV, Lee DS. Association of heart rate at hospital discharge with mortality and hospitalizations in patients with heart failure. Circ Heart Fail. 2014; 7:12-20. [DOI] [PubMed] [Google Scholar]
- Lechat P, Hulot J‐S, Escolano S, Mallet A, Leizorovicz A, Werhlen‐Grandjean M, Pochmalicki G, Dargie Hon behalf of the CIBIS II Investigators. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II trial. Circulation. 2001; 103:1428-1433. [DOI] [PubMed] [Google Scholar]
- Metra M, Torp‐Pedersen C, Swedberg K, Cleland JGF, DiLenarda A, Komajda M, Remme WJ, Lutiger B, Scherhag A, Lukas MA, Charlesworth A, Poole‐Wilson PAfor the COMET Investigators. Influnce of heart rate, blood pressure and beta‐blocker dose on outcome and the differences in outcome between carvedilol and metoprolol tartrate in patients with chronic heart failure: results from the COMET trial. Eur Heart J. 2005; 26:2259-2268. [DOI] [PubMed] [Google Scholar]
- Gullestad L, Wikstrand J, Deedwania P, Hjalmarson A, Egstrup K, Elkayam U, Gottlieb S, Rashkow A, Wedel H, Bermann G, Kjekshus Jfor the MERIT‐HF Study Group. What resting heart rate should one aim for when treating patients with heart failure with a beta‐blocker? J Am Coll Cardiol. 2005; 45:252-259. [DOI] [PubMed] [Google Scholar]
- Dobre D, Borer JS, Fox K, Swedberg K, Adams KF, Cleland JGF, Cohen‐Solal A, Gheorghiade M, Gueyffier F, O'Connor CM, Fuizat M, Patak A, Pina IL, Rosano G, Sabbah HN, Tavazzi L, Zannad F. Heart rate: a prognostic factor and therapeutic target in chronic heart failure. The distinct roles of drugs with heart rate‐lowering properties. Eur J Heart Fail. 2014; 16:76-85. [DOI] [PubMed] [Google Scholar]
- Eapen ZJ, Greiner MA, Fonarow GC, Yuan Z, Mills RM, Hernandez AF, Curtis LH. Associations between atrial fibrillation and early outcomes of patients with heart failure and reduced or preserved ejection fraction. Am Heart J. 2014; 167:369-375. [DOI] [PubMed] [Google Scholar]
- Khazanie P, Liang L, Qualls LG, Curtis LH, Fonarow GC, Hammill BG, Hammill SC, Heidenreich PA, Masoudi FA, Hernandez AF, Piccini JP. Outcomes of Medicare beneficiaries with heart failure and atrial fibrillation. JACC Heart Fail. 2014; 2:41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013; 128:e253. [DOI] [PubMed] [Google Scholar]
- Hernandez AF, Fonarow GC, Liang L, Al‐Khatib SM, Curtis LH, LaBresh KA, Yancy CW, Albert NM, Peterson ED. Sex and racial differences in the use of implantable cardioverter‐defibrillators among patients hospitalized with heart failure. JAMA. 2007; 298:1525-1532. [DOI] [PubMed] [Google Scholar]
- Horwich TB, Hernandez AF, Liang L, Albert NM, Labresh KA, Yancy CW, Fonarow GCGet With The Guidelines® Steering Committee and Hospitals. Weekend hospital admission and discharge for heart failure: association with quality of care and clinical outcomes. Am Heart J. 2009; 158:451-458. [DOI] [PubMed] [Google Scholar]
- Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009; 157:995-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor JR, Heidenreich PA. Heart rate predicts mortality in patients with heart failure and preserved systolic function. J Card Fail. 2010; 16:806-811. [DOI] [PubMed] [Google Scholar]
- Bui AL, Grau‐Sepulveda MV, Hernandez AF, Peterson ED, Yancy CW, Bhatt DL, Fonarow GC. Admission heart rate and in‐hospital outcomes in patients hospitalized for heart failure in sinus rhythm and in atrial fibrillation. Am Heart J. 2013; 165:567-574. [DOI] [PubMed] [Google Scholar]
- Ho JE, Larson MG, Ghorbani A, Cheng S, Coglianese EE, Vasan RS, Wang TJ. Long‐term cardiovascular risks associated with an elevated heart rate: the Framingham Heart Study. J Am Heart Assoc. 2014; 3:e00066810.1161/JAHA.113.000668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdahl A, Venkatesh BA, Fernandes VRS, Wu CO, Nasir K, Choi E‐Y, Almeida ALC, Rosen B, Carvalho B, Edvardsen T, Bluemke DA, Lima JAC. Resting heart rate as predictor for left ventricular dysfunction and heart failure: MESA (Multi‐ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2014; 63:1182-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullington D, Goode KM, Zhang J, Cleland JGF, Clark AL. Is heart rate important for patients with heart failure in atrial fibrillation? JACC Heart Fail. 2014; 2:213-220. [DOI] [PubMed] [Google Scholar]
- Levine HJ. Optimum heart rate of large failing hearts. Am J Cardiol. 1988; 61:633-638. [DOI] [PubMed] [Google Scholar]
- Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Change MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992; 86:513-521. [DOI] [PubMed] [Google Scholar]
- Levine HJ. Rest heart rate and life expectancy. J Am Coll Cardiol. 1997; 30:1104-1106. [DOI] [PubMed] [Google Scholar]
- Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure‐associated hospitalizations in the United States. J Am Coll Cardiol. 2013; 61:1259-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich PA, Fonarow GC. Are registry hospitals different? A comparison of patients admitted to hospitals of a commercial heart failure registry with those from national and community cohorts. Am Heart J. 2006; 152:935-939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient Characteristics at Discharge, Overall and by Heart Rate Tertiles
Table S2. Patient Characteristics at Discharge for Patients with Normal Sinus Rhythm, Overall and by Heart Rate Tertiles
Table S3. Patient Characteristics at Discharge for Patients with Atrial Fibrillation, Overall and by Heart Rate Tertiles


