Abstract
Background
Androgen deprivation therapy (ADT) is a standard treatment for patients with aggressive prostate cancer. Although ADT improves survival, it increases the risk of diabetes. Emerging evidence suggests that ADT increases adverse cardiovascular events as early as 3 months after initiation in patients with cardiovascular disease, but the mechanism is unknown. We hypothesized that ADT may impair endothelium‐dependent vasodilation due to increases in lipids and insulin resistance and may provide a link for heightened cardiovascular risk in this population.
Methods and Results
We prospectively evaluated conduit artery endothelium‐dependent and ‐independent vasodilation, lipids, and insulin resistance in 16 consecutively treated men (mean age 66±7 years; 25% with diabetes) with prostate cancer before and after 3 months of ADT. High‐resolution B‐mode ultrasound was used to assess flow‐mediated (endothelium‐dependent) and nitroglycerine‐mediated (endothelium‐independent) brachial artery vasodilation. ADT significantly increased insulin resistance, total cholesterol, HDL, and LDL. Endothelium‐dependent vasodilation was greater at 3 months than at baseline (10.8% [interquartile range: 7.7% to 14.6%] versus 8.9% [interquartile range: 4.0% to 12.6%], respectively; P=0.046, allometric P=0.037). Nitroglycerine‐mediated vasodilation did not change from baseline (P>0.2). The subset of participants on ADT for 6 months returned for reevaluation at 1 year. In this group, endothelium‐dependent vasodilation increased from baseline to 3 months and returned to baseline 6 months after ADT withdrawal (9.4% [interquartile range: 6.9% to 10.9%], 11.6% [interquartile range: 7.9% to 15.2%], and 9.0% [interquartile range: 5.1% to 12.5%], respectively; P=0.05).
Conclusions
In contrast to our expectation, ADT improved endothelium‐dependent vasodilation and its cessation returned endothelium‐dependent vasodilation to baseline. Determining the mechanism of this change requires further investigation.
Keywords: androgen deprivation therapy, endothelial function, inflammation, insulin resistance, prostate cancer
Introduction
Androgen deprivation therapy (ADT) has been a mainstay of the treatment of prostate cancer since the observation by Huggins of improved outcomes after orchiectomy.1 In the past decade, 2 risks of ADT have become apparent: metabolic derangement and cardiovascular events. The first has translated into increased total and LDL cholesterol, increased insulin resistance, and loss of lean body mass. The second has been associated with increased cardiovascular morbidity and mortality.
Keating and colleagues, using the Surveillance, Epidemiology, and End Results (SEER)–Medicare database, reported a 44% increased risk of incident diabetes, a 16% increase in coronary heart disease, an 11% increase in myocardial infarction, and a 16% increase in sudden cardiac death in men with prostate cancer treated with a gonadotropin‐releasing hormone agonist.2 The risk of incident coronary heart disease began 3 months after ADT was initiated. Recently, a nationwide Danish population‐based cohort study similarly noted a 31% increased risk of myocardial infarction and a 19% increased risk of stroke in men treated with ADT.3 This and other work prompted a science advisory from the American Heart Association, the American Cancer Society, and the American Urological Association, with endorsement by the American Society for Radiation Oncology,4 raising fear of the use of an effective treatment in high‐risk patients.
A strong candidate link among ADT, the observed metabolic changes, and cardiovascular events is impairment of vascular endothelial function. Increases in fat mass, lipids, and insulin resistance have all been demonstrated to cause endothelial dysfunction with reduction in the bioavailability of endothelium‐derived nitric oxide. We hypothesized that therapeutic ADT would impair endothelium‐dependent vasodilation in men with prostate cancer at 3 months to match the timing of the increase in cardiovascular events.
Methods
Participants
Men with high‐risk but not metastatic prostate cancer were recruited prior to initiation of ADT. No patient had received prior ADT, and no patient underwent surgery. ADT was to be applied per the treating attending physician. All participants received combined androgen blockade consisting of 50 mg oral bicalutamide daily plus an injection of 22.5 mg leuprolide acetate every 3 months for a minimum of 6 months. All participants underwent screening medical history, physical examination, and laboratory analysis, including serum electrolytes, glucose, blood urea nitrogen, creatinine, and total and LDL cholesterol. Men with acute coronary syndrome, stroke, and coronary or peripheral artery intervention within 1 year were excluded. The protocol was approved by the human research committees of the Dana‐Farber Cancer Institute and the Brigham and Women's Hospital. All participants provided informed consent prior to participation.
Protocol
The effect of ADT on endothelium‐dependent and ‐independent vasodilation of the brachial artery was studied using a single‐arm trial of men at baseline and after 3 months of ADT. We chose 3 months because this time point is the earliest an increase in diabetes and coronary heart disease has been reported.2 All participants were studied in the morning in the postabsorptive state, fasting after the previous midnight. Participants also underwent evaluation of fasting lipids, insulin sensitivity, inflammation, and testosterone and estradiol levels at each time period. In addition, participants who received 6 months of ADT were invited to return at 12 months, 6 months after ADT was discontinued, for reevaluation.
Vascular Reactivity Studies
Brachial (conduit) artery vascular function was assessed according to standard methods and as we have previously performed.5–8 All participants were studied in the morning in the postabsorptive state, fasting after the previous midnight. Cyclooxygenase inhibitors, alcohol, and caffeine were prohibited for 24 hours before the study. Participants were studied in a quiet, temperature‐controlled, dimly lit room after resting supine for a minimum of 5 minutes. High‐resolution B‐mode ultrasonography of the brachial artery was performed with a 7.5‐MHz linear array probe (GE VIVID 7; GE Healthcare). The brachial artery was imaged longitudinally just proximal to the antecubital fossa. The transducer position was adjusted to obtain optimal images of the near and far walls of the intima. The video output and electrocardiographic signal of the ultrasound machine were connected to a computer equipped with a Data Translation Frame‐Grabber video card, (Dataviz). The R wave on the electrocardiogram served as a trigger to acquire frames at end diastole. After baseline image acquisition, a sphygmomanometric cuff placed on the upper arm was inflated to suprasystolic pressure (200 mm Hg) for 5 minutes. On cuff release, reactive hyperemia caused flow to increase through the brachial artery subserving the forearm. Flow‐induced endothelium‐dependent vasodilation of the brachial artery was determined by acquiring images from 60 to 70 seconds after cuff deflation. Flow‐mediated vasodilation at this time point is largely endothelium dependent and nitric oxide mediated and can be inhibited by administration of the nitric oxide synthase antagonist NG‐monomethyl l‐arginine.5 Ten minutes after cuff release, the brachial artery was imaged again to reestablish basal conditions. Subsequently, to determine endothelium‐independent vasodilation, participants received 0.4 mg nitroglycerin sublingually. The brachial artery was imaged 3 minutes later. Brachial artery blood flow velocity was determined via velocity–time integral measurement. Nitroglycerin was not administered if the systolic blood pressure was <100 mm Hg or if the participant refused nitroglycerin, usually to avoid a severe headache during the second visit.
Laboratory Analyses
All chemistry and immunoassay testing with the exception of insulin was performed using the Cobas 6000 analyzer (Roche Diagnostics). Total and HDL cholesterol, triglycerides, and blood glucose were measured by the standard colorimetric assays CHOL2, HDLC3, TRIGL, and GLUC3. LDL cholesterol levels were measured directly, using the LDL‐Cholesterol plus second generation (LDL‐C) assay. High‐sensitivity C‐reactive protein concentrations were tested using the immunoturbidimetric CRPHS assay. This method has been standardized against the reference preparation of the Institute for Reference Materials and Measurements BCR470/CRM470. Testosterone and estradiol were measured on the e601 module of Cobas 6000 using immunoassays with electrochemiluminescent detection Estradiol II and Testosterone II. Insulin concentrations were measured on the Centaur XP immunoanalyzer using the Insulin IRI assay (Siemens Healthcare Diagnostics).
Homeostatic model assessment insulin resistance was calculated as fasting glucose (mg/dL) times fasting insulin (μIU/mL) divided by 405.6 Homeostatic model assessment β cell function was calculated as (20×fasting insulin)/(fasting glucose−3.5).
Statistical Methods
Descriptive and anthropomorphic measures are reported as mean±SD. Experimental measures are reported as median (interquartile range [IQR]). Descriptive measures were compared by paired 2‐tailed t test. Experimental measures were initially tested for normality using Shapiro–Wilks testing and found to be nonnormally distributed. Wilcoxon signed rank tests were used for comparison of experimental measures, including vascular function and laboratory measures. Changes in flow‐mediated vasodilation were also assessed using the allometric method of comparison to account for changes in baseline diameter.9–11 Correlations of anthropomorphic characteristics and the change in flow‐mediated vasodilation were tested using Spearman's ρ because of the nonnormal distribution of data. Prior to study initiation, we determined the sample size needed to find a significant decrease in flow‐mediated endothelium‐dependent vasodilation with ADT. Based on our previous work in diabetes,6–7 assuming a test value for flow‐mediated endothelium‐dependent vasodilation of 8% at entry with a decrement to 5.5% at 3 months, SD of the sample of 3%, and an α error level of 5%, 15 participants were needed to achieve power of 89%. Statistical significance was accepted at the 95% confidence level (P<0.05). All statistics were run on SPSS Base 22 (IBM).
Results
Baseline characteristics of the recruited participants are shown in Table 1. The participants were 66±7 years, 12 of 16 participants (75%) were white, the majority carried the diagnosis of hyperlipidemia, 4 had type 2 diabetes, and none smoked within 1 year of study entry. Ten participants carried the diagnosis of hypertension, but blood pressure prior to the administration of androgen deprivation was controlled on medication. Five participants took angiotensin receptor blockers or angiotensin‐converting enzyme inhibitors, 8 took statins, and 8 took aspirin. No participant had a history of myocardial infarction, stroke, or peripheral artery disease. The median Gleason grade of the enrolled participants was 7 (IQR: 6 to 8). During the course of the study, there were no changes in medications, no participants had a medical procedure or surgery, and there were no hospitalizations.
Table 1.
Baseline Demographics
| Characteristic | N=16 |
|---|---|
| Age | 66±7 |
| BMI | 28.8±4.8 |
| SBP | 131±19 |
| DBP | 75±8 |
| HR | 61±12 |
| White | 75% |
| Diabetes | 25% |
Data are presented as mean±SD. BMI indicates body mass index; DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure.
Laboratory Analyses
Anthropomorphic findings are noted in Table 2. The effect of ADT on our participants yielded the expected metabolic changes (Table 3). ADT reduced serum testosterone and estradiol levels significantly. Fasting insulin levels and fasting glucose levels and homeostatic model assessment insulin resistance increased significantly from baseline to 3 months, respectively. Each component of the lipid profile except triglycerides increased significantly with ADT. Finally, there was no change in systemic inflammation as measured by high‐sensitivity C‐reactive protein.
Table 2.
Effect of Androgen Deprivation Therapy on Anthropomorphic Data
| Parameter | Baseline | 3 Months | P Value |
|---|---|---|---|
| Height, cm | 174±9 | 174±9 | >0.2 |
| Weight, kg | 86.0±13.1 | 86.9±11.8 | >0.2 |
| BMI | 28.6±4.9 | 28.9±4.5 | >0.2 |
| SBP, mm Hg | 131±19 | 129±13 | >0.2 |
| DBP, mm Hg | 76±8 | 78±7 | >0.2 |
| HR, bpm | 61±12 | 60±11 | >0.2 |
Data are presented as mean±SD. BMI indicates body mass index; bpm, beats per minute; DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure.
Table 3.
Effect of Androgen Deprivation Therapy on Metabolism
| Measure | Baseline | 3 Months | P Value |
|---|---|---|---|
| Insulin, μIU/mL | 5.6 (4.6 to 8.6) | 10.0 (6.5 to 14.8) | 0.005 |
| Glucose, mg/dL | 96 (85 to 99) | 100 (95 to 123) | 0.037 |
| HOMAIR | 1.3 (1.1 to 1.7) | 2.6 (1.6 to 3.6) | 0.005 |
| HOMAB | 65 (53 to 112) | 76 (57 to 127) | >0.2 |
| Total cholesterol, mg/dL | 159 (131 to 194) | 200 (158 to 219) | 0.005 |
| HDL cholesterol, mg/dL | 45 (39 to 53) | 59 (39 to 53) | 0.028 |
| LDL cholesterol, mg/dL | 101 (70 to 125) | 126 (87 to 149) | 0.005 |
| Triglycerides, mg/dL | 78 (69 to 104) | 95 (71 to 118) | >0.2 |
| hs‐CRP | 0.4 (0.2 to 1.7) | 0.8 (0.5 to 1.6) | >0.2 |
| Estradiol, pg/mL | 22.9 (19.3 to 40) | 5.0 (5.0 to 12.2) | <0.005 |
| Testosterone, ng/dL | 451 (317 to 794) | 6 (3 to 14) | <0.005 |
Data are presented as median (interquartile range). HOMAB indicates homeostatic model assessment β cell function; HOMAIR, homeostatic model assessment insulin resistance; hs‐CRP, high‐sensitivity C‐reactive protein.
Androgen and Vascular Function
Baseline brachial artery diameter decreased with androgen deprivation, but ADT did not significantly change the reactive hyperemic stimulus or shear rate (Table 4). At baseline, flow‐mediated endothelium‐dependent vasodilation was 8.9% (IQR: 4.0% to 12.6%), and it increased to 10.8% (IQR: 7.7% to 14.6%) after 3 months of ADT (P=0.046). When flow‐mediated endothelium‐dependent vasodilation was compared using an allometric method that accommodated for the change in baseline diameter, the change remained statistically significant (allometric P=0.037).9–10 Similarly, there was a significant increase in absolute diameter size with the reactive hyperemic stimulus. In contrast, nitroglycerin‐mediated endothelium‐independent vasodilation did not change significantly with ADT. There was no correlation between any anthropomorphic characteristic and the change in flow‐mediated endothelium‐dependent vasodilation (P>0.2 for age, body mass index, and blood pressure).
Table 4.
Effect of Androgen Deprivation Therapy on Vascular Function
| Parameter | Baseline | 3 Months | P Value |
|---|---|---|---|
| Baseline arterial diameter, mm | 3.93 (3.39 to 4.36) | 3.79 (3.45 to 4.12) | 0.031 |
| Reactive hyperemic stimulus, fold increase (VTI) | 4.3 (2.6 to 6.8) | 6.0 (3.8 to 7.1) | 0.18 |
| Diameter increase, mm | 0.34 (0.14 to 0.48) | 0.36 (0.29 to 0.55) | 0.047 |
| Flow‐mediated vasodilation, % | 8.9 (4.0 to 12.6) | 10.8 (7.7 to 14.6) | 0.046 |
| Nitroglycerin‐mediated vasodilation, % | 16.7 (12.8 to 25.9) | 19.2 (13.9 to 25.7) | >0.2 |
| Diameter increase, mm | 0.70 (0.56 to 0.82) | 0.67 (0.59 to 0.88) | >0.2 |
Data are presented as median (interquartile range), N=16. VTI indicates velocity–time integral.
Results at 1 Year
Of the 16 enrolled participants, 3 were treated with long‐term ADT, and 2 decided to end participation after 3 months, but 11 participants returned for assessment at 1 year. In this group, there were no changes in anthropomorphic variables from 3 to 12 months. The discontinuation of ADT resulted in return to baseline for laboratory analyses such that there were no statistical differences between the baseline and 1‐year time points (Table 5). In these participants, there was no significant difference in baseline arterial diameter or reactive hyperemic stimulus at any time point (Table 6). In contrast, flow‐mediated vasodilation was 9.4% (IQR: 6.9% to 10.9%) at baseline, 11.6% (IQR: 7.9% to 15.2%) at 3 months (P=0.05), and 9.0% (IQR: 5.1% to 12.5%) at 1 year (Figure). Nitroglycerine‐mediated vasodilation was similar at all 3 time points.
Table 5.
Effect of Androgen Deprivation Therapy in a Subset on Laboratory Analysis
| Measure | Baseline | 3 Months | 1 Year |
|---|---|---|---|
| Insulin, μIU/mL | 8.5 (3.5 to 11.3) | 14.4* (8.4 to 19.7) | 7.2.0 to 12.3) |
| Glucose, mg/dL | 96 (79 to 118) | 101* (99 to 133) | 96 (81 to 102) |
| HOMAIR | 1.4 (0.7 to 1.7) | 3.5* (2.0 to 3.9) | 1.7 (1.2 to 2.4) |
| Total cholesterol, mg/dL | 148 (113 to 201) | 215* (135 to 220) | 163 (110 to 191) |
| HDL cholesterol, mg/dL | 41 (32 to 58) | 51 (43 to 64) | 41 (38 to 59) |
| LDL cholesterol, mg/dL | 95 (54 to 127) | 138* (69 to 152) | 110 (57 to 123) |
| Triglycerides, mg/dL | 75 (72 to 144) | 103 (85 to 124) | 93 (71 to 115) |
| hs‐CRP, mg/dL | 0.3 (0.2 to 1.3) | 1.1* (0.4 to 1.8) | 0.6 (0.4 to 2.2) |
| Estradiol, pg/mL | 23.1 (16.7 to 31.3) | 5.0* (5.0 to 14.9) | 27.8 (17.2 to 31.1) |
| Testosterone, ng/dL | 346 (158 to 738) | 13* (4 to 23) | 462 (353 to 565) |
Data are presented as median (interquartile range). HOMAIR indicates homeostatic model assessment insulin resistance; hs‐CRP, high‐sensitivity C‐reactive protein.
P<0.05, compared with baseline.
P=0.068, compared with baseline.
Table 6.
Effect of Androgen Deprivation Therapy in a Subset on Vascular Function
| Parameter | Baseline | 3 Months | 1 Year |
|---|---|---|---|
| Baseline arterial diameter, mm | 3.92 (3.35 to 4.07) | 3.77 (3.39 to 3.92) | 3.79 (3.41 to 4.01) |
| Reactive hyperemic stimulus, fold increase (VTI) | 5.7 (3.3 to 8.0) | 6.2 (4.0 to 9.5) | 6.4 (4.5 to 7.9) |
| Flow‐mediated vasodilation, % | 9.4 (6.9 to 10.9) | 11.6* (7.9 to 15.2) | 9.0 (5.1 to 12.5) |
| Nitroglycerin‐mediated vasodilation, % | 17.4 (15.0 to 26.7) | 17.7 (14.0 to 24.9) | 17.5 (11.4 to 22.5) |
Data are presented as median (interquartile range), n=11. VTI indicates velocity–time integral.
P=0.05 compared with baseline.
Figure 1.
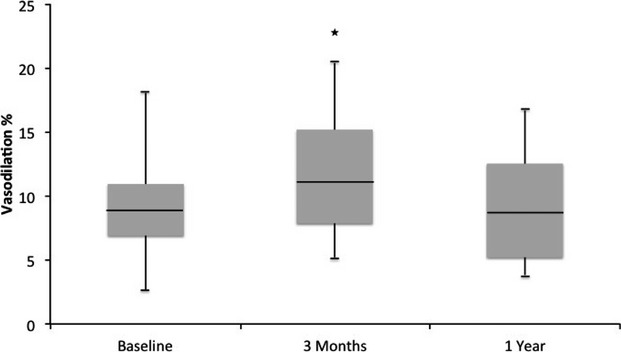
Flow‐mediated vasodilation of the brachial artery. Flow‐mediated vasodilation at baseline, after 3 months of androgen deprivation therapy (ADT), and 6 months after ADT cessation (n=11). Androgen deprivation increased flow‐mediated vasodilation (*P=0.05 compared with baseline) at 3 months and returned to baseline after cessation.
Discussion
In contrast to our hypothesis, gonadotropin‐releasing hormone agonist–mediated ADT increased conduit artery flow‐mediated vasodilation in men with prostate cancer between baseline and 3 months and returned to baseline 6 months after ADT cessation. These results suggest that the mechanism of increased cardiovascular events that occurs within weeks to months of ADT initiation is not related to reductions in the bioavailability of endothelium‐derived nitric oxide. To our knowledge, this report is the first in intact ambulatory humans demonstrating conduit artery function before, during, and after the application of ADT.
ADT and Endothelial Function
The relevance of androgens to vascular function stems from the association of heightened mortality in states of androgen deficiency.12–13 In the InCHIANTI study, decline of testosterone levels was an independent predictor of mortality over 6 years of follow‐up.14 Similarly a nested case–control study with 6‐ to 10‐year follow‐up showed an association with testosterone decline and increased mortality.15 Longer follow‐up, studied in the Massachusetts Male Aging Study, attenuates the relationship.16 Possible mechanisms for increased mortality stem from the association of diminished androgens and increased levels of inflammation and fat mass.16–17 Pertinent to this work, androgen deficiency has been associated with impaired conduit artery endothelium‐dependent vasodilation;18 however, the relationship is not straightforward. Testosterone replacement has been shown to improve vascular stiffness19 but also has been shown to worsen endothelium‐dependent vasodilation in hypogonadal men.20 It is likely that the etiology of decreased androgens plays an important role beyond the change in hormone levels per se in determining vascular health. Testosterone may be a biomarker of illness in this setting as well as a contributor to vascular function.
In contrast to illness or aging states, therapeutic androgen deprivation is applied to men without underlying cause for androgen deficiency. The effect of androgen deprivation has been evaluated by 2 groups with conflicting results. Herman and colleagues compared endothelium‐dependent vasodilation in men with prostate cancer treated with orchiectomy or androgen blockade for >6 months, in healthy controls, and in men in remission from nonprostate cancers.21 The men with prostate cancer had testosterone concentrations of 23 ng/dL compared with 548 ng/dL for healthy controls and 461 ng/dL for men in remission from nonprostate cancers. Brachial artery endothelium‐dependent vasodilation was significantly higher in the men with ADT (6.2%) compared with healthy controls (2.7%) or men in remission from nonprostate cancers (2.0%). In contrast, Gilbert and colleagues studied men with prostate cancer treated with ADT and controls matched for sex, age, and body mass index.22 Similarly, the ADT‐treated men had significantly lower testosterone levels compared with controls (12 versus 533 ng/dL). In this study, however, brachial artery endothelium‐dependent vasodilation was lower in men with prostate cancer treated with ADT (3.9%) compared with matched controls (5.9%).
Our investigation in a similarly aged population with a similar reduction in testosterone showed an increase in endothelium‐dependent vasodilation in men treated with ADT compared with baseline. These results remained consistent when controlling for the change in baseline arterial diameter. Several possibilities may explain the variance among the studies. The most obvious difference is the duration of ADT. In our participants who received 3 evaluations, ADT was measured at 3 months. Herman and colleagues studied men with a mean ADT duration of 18 months. In contrast, Gilbert and colleagues studied men with a mean treatment duration of 37 months. The application of long‐term ADT suggests a sicker population and metabolic and vascular changes that may not have been manifest at the time we studied the participants. One advantage to our study is its pre–post design that permits the evaluation of the changes in androgen production, lipids, and insulin resistance, which are not captured in a cross‐sectional analysis. The decrease in size in baseline arterial diameter is suggestive of an adaptation to the change in the vascular environment, similar to that found in severely obese patients who lose significant amounts of weight.23 The change in baseline arterial diameter did not affect our determination because we used an allometric model. As noted by Atkinson and colleagues, “An allometric model involving the flow‐mediated response (on a log scale) as the outcome and Dbase (log‐transformed) as a covariate can completely eradicate the problem of Dbase dependency.”10 Chung and colleagues showed that as the atherosclerotic risk factor profile worsens, resting brachial artery diameter increases.24 The return to baseline for laboratory and vascular assessments demonstrates the impact of ADT and its potential for reversibility when applied for 6 months. Further human and basic biological study is needed to determine the mechanism underlying these changes.
Cardiovascular Risk With ADT
Daskavich and colleagues recently reported long‐term follow‐up of the Prostate Cancer Outcomes Study showing that older men (aged ≥60 years) with multiple comorbid conditions are far more likely to die from causes other than prostate cancer.25 This observation gains in importance when put in the perspective of the increased risk of cardiovascular events resulting specifically from prostate cancer treatment. Several investigators have noted increased rates of cardiovascular events within the first year of ADT.2–3,2–27 In examining the populations more closely, it appears that baseline risk significantly affects the observed cardiovascular risk. We showed, for example, that men with a history of congestive heart failure or myocardial infarction,28 regardless of prior coronary revascularization, had a significantly increased risk of mortality.29 We also showed that patients with a low‐risk profile do not suffer increased cardiovascular events when treated with ADT.30 It is unclear whether the effect is advancing atherogenesis or activating the vulnerable patient and plaque.
The mechanism by which cardiovascular risk is increased can only be surmised at this time. ADT induces a dysmetabolic state by increasing inflammation, LDL levels, triglycerides, and insulin resistance. Offsetting this risk would be the increase in HDL levels. Interestingly, the change in HDL may be enough to mitigate the rest of the changes in toto.31–32 Consequently, standard risk profile components including age, smoking, lipids, blood pressure, and inflammation do not appear to explain the observed significant increase in cardiovascular risk during ADT.
In our population, we noted an increase of ≈100% in homeostatic model assessment insulin resistance. Although both pre and post levels are below the threshold considered diagnostic for insulin resistance,33–35 the change was significant and may be enough to push at‐risk individuals to a new risk state of either frank insulin resistance or diabetes. Increasing insulin resistance is directly associated with increasing cardiovascular risk in participants with and without type 2 diabetes, although it should be noted that the mechanism of androgen deficiency–mediated insulin resistance remains unknown.36–38
Direct effects of androgens on the vascular wall also may be important. In preclinical models, testosterone has been shown to promote vasodilation through a vascular smooth muscle–mediated mechanism.39 Interestingly, the vasodilatory effect of testosterone is not dependent on activation of androgen receptors.40 In human subcutaneous resistance arteries obtained from men with androgen deficiency studied ex vivo, testosterone was a concentration‐dependent endothelium‐independent vasodilator at the nonphysiological concentration of ≥1 μmol/L.41 In addition, testosterone therapy attenuated the vasodilatory effect of sodium nitroprusside (a vascular smooth muscle vasodilator) and enhanced the contractile response to norepinephrine (an α agonist).41 ADT has been associated with increased large artery stiffness and may participate in adverse cardiovascular outcomes through this mechanism.42–43 Others have postulated a role for a testosterone‐mediated change in oxidative stress as a mechanism for the vascular pathophysiology; however, in humans, higher levels of testosterone have been shown to increase and decrease oxidative stress.44–46 Elucidation of the mechanism underlying androgen deprivation and heightened cardiovascular risk in high‐risk men will require further preclinical and patient‐oriented research.
This study did not address all possible atherogenic mechanisms. ADT has been associated with prothrombotic changes including increases in fibrinogen, plasminogen activator inhibitor 1, and tissue plasminogen activator antigen47–48 that may also explain a reported increase in venous thromboembolism.49 Platelet counts are unaffected by ADT,50 but platelet activation has yet to be characterized. In addition, this study did not include a placebo group because withholding ADT from men with prostate cancer and giving it to men without prostate cancer to enable a placebo group are both unethical because of the risks of morbidity and mortality.
Conclusion
ADT increased insulin resistance, altered the lipid profile, and increased conduit artery flow‐mediated vasodilation in men studied before and after 3 months of therapy. The effect of ADT on laboratory and vascular parameters resolved 6 months after ADT discontinuation. These results suggest that the biology of androgen deficiency, both endogenous and induced, remains unclear and requires further study to elucidate the mechanism of interaction between sex hormones and the vasculature.
Sources of Funding
This study was supported by Watkins Discovery Award, the Prostate Cancer Foundation, Fitz's Cancer Warriors, David and Cynthia Chapin, and a grant from an Anonymous Family Foundation.
Disclosures
Consulting honoraria: Medivations: Dr Nguyen.
References
- Huggins C. Prostatic cancer treated by orchiectomy: the five year results. JAMA. 1946; 131:576-581. [DOI] [PubMed] [Google Scholar]
- Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006; 24:4448-4456. [DOI] [PubMed] [Google Scholar]
- Jespersen CG, Norgaard M, Borre M. Androgen‐deprivation therapy in treatment of prostate cancer and risk of myocardial infarction and stroke: a nationwide Danish population‐based cohort study. Eur Urol. 2014; 65:704-709. [DOI] [PubMed] [Google Scholar]
- Levine GN, D'Amico AV, Berger P, Clark PE, Eckel RH, Keating NL, Milani RV, Sagalowsky AI, Smith MR, Zakai NAmerican Heart Association Council on Clinical C, Council on E, Prevention tACS, the American Urological A. Androgen‐deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. CA Cancer J Clin. 2010; 60:194-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard‐Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial‐dependent flow‐mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002; 39:257-265. [DOI] [PubMed] [Google Scholar]
- Beckman JA, Goldfine AB, Dunaif A, Gerhard‐Herman M, Creager MA. Endothelial function varies according to insulin resistance disease type. Diabetes Care. 2007; 30:1226-1232. [DOI] [PubMed] [Google Scholar]
- Beckman JA, Goldfine AB, Gordon MB, Garrett LA, Keaney JF, Jr, Creager MA. Oral antioxidant therapy improves endothelial function in type 1 but not type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2003; 285:H2392-H2398. [DOI] [PubMed] [Google Scholar]
- Beckman JA, Liao JK, Hurley S, Garrett LA, Chui D, Mitra D, Creager MA. Atorvastatin restores endothelial function in normocholesterolemic smokers independent of changes in low‐density lipoprotein. Circ Res. 2004; 95:217-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson G, Batterham AM, Thijssen DH, Green DJ. A new approach to improve the specificity of flow‐mediated dilation for indicating endothelial function in cardiovascular research. J Hypertens. 2013; 31:287-291. [DOI] [PubMed] [Google Scholar]
- Atkinson G, Batterham AM. The percentage flow‐mediated dilation index: a large‐sample investigation of its appropriateness, potential for bias and causal nexus in vascular medicine. Vasc Med. 2013; 18:354-365. [DOI] [PubMed] [Google Scholar]
- Atkinson G, Batterham AM. Allometric scaling of diameter change in the original flow‐mediated dilation protocol. Atherosclerosis. 2013; 226:425-427. [DOI] [PubMed] [Google Scholar]
- Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006; 166:1660-1665. [DOI] [PubMed] [Google Scholar]
- Phillips GB, Pinkernell BH, Jing TY. The association of hypotestosteronemia with coronary artery disease in men. Arterioscler Thromb. 1994; 14:701-706. [DOI] [PubMed] [Google Scholar]
- Maggio M, Lauretani F, Ceda GP, Bandinelli S, Ling SM, Metter EJ, Artoni A, Carassale L, Cazzato A, Ceresini G, Guralnik JM, Basaria S, Valenti G, Ferrucci L. Relationship between low levels of anabolic hormones and 6‐year mortality in older men: the aging in the Chianti Area (INCHIANTI) study. Arch Intern Med. 2007; 167:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Welch A, Day N. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC‐Norfolk) Prospective Population Study. Circulation. 2007; 116:2694-2701. [DOI] [PubMed] [Google Scholar]
- Araujo AB, Kupelian V, Page ST, Handelsman DJ, Bremner WJ, McKinlay JB. Sex steroids and all‐cause and cause‐specific mortality in men. Arch Intern Med. 2007; 167:1252-1260. [DOI] [PubMed] [Google Scholar]
- Basaria S, Lieb J, II, Tang AM, DeWeese T, Carducci M, Eisenberger M, Dobs AS. Long‐term effects of androgen deprivation therapy in prostate cancer patients. Clin Endocrinol (Oxf). 2002; 56:779-786. [DOI] [PubMed] [Google Scholar]
- Akishita M, Hashimoto M, Ohike Y, Ogawa S, Iijima K, Eto M, Ouchi Y. Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res. 2007; 30:1029-1034. [DOI] [PubMed] [Google Scholar]
- Webb CM, Elkington AG, Kraidly MM, Keenan N, Pennell DJ, Collins P. Effects of oral testosterone treatment on myocardial perfusion and vascular function in men with low plasma testosterone and coronary heart disease. Am J Cardiol. 2008; 101:618-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernini G, Versari D, Moretti A, Virdis A, Ghiadoni L, Bardini M, Taurino C, Canale D, Taddei S, Salvetti A. Vascular reactivity in congenital hypogonadal men before and after testosterone replacement therapy. J Clin Endocrinol Metab. 2006; 91:1691-1697. [DOI] [PubMed] [Google Scholar]
- Herman SM, Robinson JT, McCredie RJ, Adams MR, Boyer MJ, Celermajer DS. Androgen deprivation is associated with enhanced endothelium‐dependent dilatation in adult men. Arterioscler Thromb Vasc Biol. 1997; 17:2004-2009. [DOI] [PubMed] [Google Scholar]
- Gilbert SE, Tew GA, Bourke L, Winter EM, Rosario DJ. Assessment of endothelial dysfunction by flow‐mediated dilatation in men on long‐term androgen deprivation therapy for prostate cancer. Exp Physiol. 2013; 98:1401-1410. [DOI] [PubMed] [Google Scholar]
- Hamburg NM, Mott MM, Bigornia SJ, Duess MA, Kluge MA, Hess DT, Apovian CM, Vita JA, Gokce N. Maladaptive enlargement of the brachial artery in severe obesity is reversed with weight loss. Vasc Med. 2010; 15:215-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WB, Hamburg NM, Holbrook M, Shenouda SM, Dohadwala MM, Terry DF, Gokce N, Vita JA. The brachial artery remodels to maintain local shear stress despite the presence of cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2009; 29:606-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskivich TJ, Fan KH, Koyama T, Albertsen PC, Goodman M, Hamilton AS, Hoffman RM, Stanford JL, Stroup AM, Litwin MS, Penson DF. Effect of age, tumor risk, and comorbidity on competing risks for survival in a U.S. population‐based cohort of men with prostate cancer. Ann Intern Med. 2013; 158:709-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alibhai SM, Duong‐Hua M, Sutradhar R, Fleshner NE, Warde P, Cheung AM, Paszat LF. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009; 27:3452-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HK, Chen MH, McLeod DG, Carroll PR, Richie JP, D'Amico AV. Cancer‐specific mortality after radiation therapy with short‐course hormonal therapy or radical prostatectomy in men with localized, intermediate‐risk to high‐risk prostate cancer. Cancer. 2006; 107:2597-2603. [DOI] [PubMed] [Google Scholar]
- Nguyen PL, Chen MH, Beckman JA, Beard CJ, Martin NE, Choueiri TK, Hu JC, Hoffman KE, Dosoretz DE, Moran BJ, Salenius SA, Braccioforte MH, Kantoff PW, D'Amico AV, Ennis RD. Influence of androgen deprivation therapy on all‐cause mortality in men with high‐risk prostate cancer and a history of congestive heart failure or myocardial infarction. Int J Radiat Oncol Biol Phys. 2012; 82:1411-1416. [DOI] [PubMed] [Google Scholar]
- Nguyen PL, Chen MH, Goldhaber SZ, Martin NE, Beard CJ, Dosoretz DE, Katin MJ, Ross R, Salenius SA, D'Amico AV. Coronary revascularization and mortality in men with congestive heart failure or prior myocardial infarction who receive androgen deprivation. Cancer. 2011; 117:406-413. [DOI] [PubMed] [Google Scholar]
- Nguyen PL, Je Y, Schutz FA, Hoffman KE, Hu JC, Parekh A, Beckman JA, Choueiri TK. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta‐analysis of randomized trials. JAMA. 2011; 306:2359-2366. [DOI] [PubMed] [Google Scholar]
- Sniderman AD, Williams K, Contois JH, Monroe HM, McQueen MJ, de Graaf J, Furberg CD. A meta‐analysis of low‐density lipoprotein cholesterol, non‐high‐density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011; 4:337-345. [DOI] [PubMed] [Google Scholar]
- Boekholdt SM, Arsenault BJ, Hovingh GK, Mora S, Pedersen TR, Larosa JC, Welch KM, Amarenco P, Demicco DA, Tonkin AM, Sullivan DR, Kirby A, Colhoun HM, Hitman GA, Betteridge DJ, Durrington PN, Clearfield MB, Downs JR, Gotto AM, Jr, Ridker PM, Kastelein JJ. Levels and changes of HDL cholesterol and apolipoprotein A‐I in relation to risk of cardiovascular events among statin‐treated patients: a meta‐analysis. Circulation. 2013; 128:1504-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedblad B, Nilsson P, Janzon L, Berglund G. Relation between insulin resistance and carotid intima‐media thickness and stenosis in non‐diabetic subjects. Results from a cross‐sectional study in Malmo, Sweden. Diabet Med. 2000; 17:299-307. [DOI] [PubMed] [Google Scholar]
- Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European group for the study of insulin resistance (EGIR). Diabet Med. 1999; 16:442-443. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003; 139:802-809. [DOI] [PubMed] [Google Scholar]
- Bonora E, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, Saggiani F, Poli M, Perbellini S, Raffaelli A, Cacciatori V, Santi L, Targher G, Bonadonna R, Muggeo M. HOMA‐estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care. 2002; 25:1135-1141. [DOI] [PubMed] [Google Scholar]
- Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D'Agostino RB. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006; 91:2906-2912. [DOI] [PubMed] [Google Scholar]
- Jeppesen J, Hansen TW, Rasmussen S, Ibsen H, Torp‐Pedersen C, Madsbad S. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease: a population‐based study. J Am Coll Cardiol. 2007; 49:2112-2119. [DOI] [PubMed] [Google Scholar]
- Yue P, Chatterjee K, Beale C, Poole‐Wilson PA, Collins P. Testosterone relaxes rabbit coronary arteries and aorta. Circulation. 1995; 91:1154-1160. [DOI] [PubMed] [Google Scholar]
- Jones RD, English KM, Jones TH, Channer KS. Testosterone‐induced coronary vasodilatation occurs via a non‐genomic mechanism: evidence of a direct calcium antagonism action. Clin Sci (Lond). 2004; 107:149-158. [DOI] [PubMed] [Google Scholar]
- Malkin CJ, Jones RD, Jones TH, Channer KS. Effect of testosterone on ex vivo vascular reactivity in man. Clin Sci (Lond). 2006; 111:265-274. [DOI] [PubMed] [Google Scholar]
- Dockery F, Rajkumar C, Agarwal S, Waxman J, Bulpitt CJ. Androgen deprivation in males is associated with decreased central arterial compliance and reduced central systolic blood pressure. J Hum Hypertens. 2000; 14:395-397. [DOI] [PubMed] [Google Scholar]
- Smith JC, Bennett S, Evans LM, Kynaston HG, Parmar M, Mason MD, Cockcroft JR, Scanlon MF, Davies JS. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001; 86:4261-4267. [DOI] [PubMed] [Google Scholar]
- Skogastierna C, Hotzen M, Rane A, Ekstrom L. A supraphysiological dose of testosterone induces nitric oxide production and oxidative stress. Eur J Prev Cardiol. 2013; 21:1049-1054. [DOI] [PubMed] [Google Scholar]
- Marin DP, Bolin AP, dos Santos Rde C, Curi R, Otton R. Testosterone suppresses oxidative stress in human neutrophils. Cell Biochem Funct. 2010; 28:394-402. [DOI] [PubMed] [Google Scholar]
- Gardner‐Thorpe D, O'Hagen C, Young I, Lewis SJ. Dietary supplements of soya flour lower serum testosterone concentrations and improve markers of oxidative stress in men. Eur J Clin Nutr. 2003; 57:100-106. [DOI] [PubMed] [Google Scholar]
- Haidar A, Yassin A, Saad F, Shabsigh R. Effects of androgen deprivation on glycaemic control and on cardiovascular biochemical risk factors in men with advanced prostate cancer with diabetes. Aging Male. 2007; 10:189-196. [DOI] [PubMed] [Google Scholar]
- Ziaran S, Goncalves FM, Breza J., Sr Patients with prostate cancer treated by ADT have significantly higher fibrinogenemia than healthy control. World J Urol. 2013; 31:289-292. [DOI] [PubMed] [Google Scholar]
- Ehdaie B, Atoria CL, Gupta A, Feifer A, Lowrance WT, Morris MJ, Scardino PT, Eastham JA, Elkin EB. Androgen deprivation and thromboembolic events in men with prostate cancer. Cancer. 2012; 118:3397-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eri LM, Urdal P, Bechensteen AG. Effects of the luteinizing hormone‐releasing hormone agonist leuprolide on lipoproteins, fibrinogen and plasminogen activator inhibitor in patients with benign prostatic hyperplasia. J Urol. 1995; 154:100-104. [PubMed] [Google Scholar]


