Abstract
Background
N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) has been associated with important risk factors for contrast‐induced nephropathy (CIN). However, few studies have investigated the predictive value of NT‐proBNP itself. This study investigated whether levels of preprocedural NT‐proBNP could predict CIN after elective coronary angiography as effectively as the Mehran CIN score.
Methods and Results
We retrospectively observed 2248 patients who underwent elective coronary angiography. The predictive value of preprocedural NT‐proBNP for CIN was assessed by receiver operating characteristic and multivariable logistic regression analysis. The 50 patients (2.2%) who developed CIN had higher Mehran risk scores (9.5±5.1 versus 4.8±3.8), and higher preprocedural levels of NT‐proBNP (5320±7423 versus 1078±2548 pg/mL, P<0.001). Receiver operating characteristic analysis revealed that NT‐proBNP was not significantly different from the Mehran CIN score in predicting CIN (C=0.7657 versus C=0.7729, P=0.8431). An NT‐proBNP cutoff value of 682 pg/mL predicted CIN with 78% sensitivity and 70% specificity. Multivariable analysis suggested that, after adjustment for other risk factors, NT‐proBNP >682 pg/mL was significantly associated with CIN (odds ratio: 4.007, 95% CI: 1.950 to 8.234; P<0.001) and risk of death (hazard ratio: 2.53; 95% CI: 1.49 to 4.30; P=0.0006).
Conclusions
Preprocedural NT‐proBNP >682 pg/mL was significantly associated with the risk of CIN and death. NT‐proBNP, like the Mehran CIN score, may be another useful and rapid screening tool for CIN and death risk assessment, identifying subjects who need therapeutic measures to prevent CIN.
Keywords: contrast‐induced nephropathy, coronary angiography, N‐terminal pro‐brain natriuretic peptide
Introduction
Contrast‐induced nephropathy (CIN) is the third most common cause of hospital‐acquired kidney injury, which contributes to decreased mobility and motility, prolonged hospitalization, and increased healthcare costs.1–2 Identifying patients at risk of CIN easily and accurately would allow the administration of prophylactic interventions to those at high risk.3 Several risk‐score models reflecting the cumulative risk of several periprocedural predictors, such as the Mehran CIN score and BMC2 CIN score, have been established and have proven useful for both bedside clinical decision‐making and risk assessment.4–5 However, although these score models include a multitude of qualitative risk factors, they lack quantitative biomarkers that are associated with multiple organ function and might serve as predictors in risk‐score models.
Thus, there is a need for a more objective identification tool, readily available at hospital admission, before patients are subjected to contrast exposure during coronary angiography or percutaneous coronary intervention (PCI). N‐terminal pro‐brain natriuretic peptide (NT‐proBNP), an easily available biomarker, is associated with advanced age, kidney dysfunction, anemia, heart failure, and diabetes, which are risk factors for CIN.6–9 Recent data suggest that measurement of serum BNP at hospital admission may help identify patients with ST‐segment elevation myocardial infarction who are at risk for developing CIN after primary PCI.10 In the present study, our objective was to investigate the predictive value of objective preprocedural NT‐proBNP for CIN in patients undergoing selective coronary angiography.
Methods
Patients
We conducted a prospective observational study at the Guangdong Cardiovascular Institute of Guangdong General Hospital, Guangdong Academy of Medical Sciences, between October 2008 and December 2012. All consecutive patients aged >18 years who underwent coronary angiography or PCI were eligible for enrollment. The exclusion criteria included pregnancy, lactation, intravascular administration of a contrast medium within the previous 7 days or 3 days postoperation (n=83), cardiovascular surgery or endovascular repair (n=382), end‐stage renal disease or renal replacement (n=7), missing preoperative or postoperative creatinine (n=61), and malignancy (n=3). Patients undergoing emergent coronary intervention (n=406) and others who had no preprocedural evaluation of NT‐proBNP (n=691) were also excluded. Finally, 2248 patients were analyzed in the study. The study was approved by an institutional review committee and the subjects gave informed consent.
Protocol
Once the patients had been admitted to the hospital, preprocedural NT‐proBNP was measured using electrochemiluminescence immunoassay (Roche Diagnostics, Germany). Serum creatinine concentration was assessed in all patients, as a part of standard clinical care, at the time of hospital admission and daily for 3 days after contrast administration, as well as upon hospital discharge. Estimated glomerular filtration rate (eGFR) was evaluated using the level‐modified Modification of Diet in Renal Disease equation: 186.3×serum creatinine−1.154×(age in years)−0.203×1.212 (if patient was black)×0.742 (if patient was female).11 Coronary angiography or PCI was performed using standard techniques.12 The contrast type and dose were left to the discretion of the interventional cardiologist, according to the patient's need. The use of adrenergic blocking agents, angiotensin‐converting enzyme inhibitors, diuretics, intra‐aortic balloon pump support, or inotropic drugs was left to the discretion of the interventional cardiologist and the physicians responsible for the patients. Patients received intravenous normal (0.9%) saline at a rate of 1 mL/kg per hour, 2 to 12 hours before and 6 to 24 hours after the administration of contrast medium. In patients with a left ventricular ejection fraction (LVEF) <40% or overt heart failure, the hydration rate was reduced to 0.5 mL/kg per hour.
End Point and Clinical Definitions
The primary end point of the study was the development of CIN, defined as an increase in serum creatinine of >0.5 mg/dL over the baseline value within 48 to 72 hours after the administration of contrast medium.13 Additional end points recorded during the study included major adverse clinical events and CIN requiring renal replacement therapy. Major adverse clinical events included death, renal replacement therapy, target vessel revascularization, rehospitalization, and stroke.
Statistical Analysis
Demographics and traditional risk factors were compared between patients who developed CIN and those who did not, and the clinical outcomes and incidence of CIN were compared among NT‐proBNP quartiles. Comparisons between normally distributed continuous variables, expressed as mean±SD, were performed using 1‐way analysis of variance; non‐normally distributed continuous variables, presented as median and interquartile range, were analyzed using Kruskal–Wallis tests. The Pearson χ2 or Fisher exact tests were used, as appropriate, for categorical data, expressed as percentages. Analyses of receiver operating characteristic curves were conducted and the Youden index was used to determine the cutoff value of NT‐proBNP for predicting CIN. The area under receiver operating characteristic curves of NT‐proBNP and Mehran CIN score for predicting CIN were compared using the nonparametric approach of DeLong et al.14 The final model included the significant baseline characteristics. Multivariable logistic regression analysis was performed to identify the independent risk factors for CIN. Higher NT‐proBNP group, congestive heart failure, age >75 years, eGFR <60 mL/min per 1.73 m2, diabetes mellitus, and contrast dose >200 mL were included in the multivariable logistic regression analysis for CIN. Univariate analyses of mortality were performed using a log‐rank test for patients categorized by NT‐proBNP. Multivariable Cox regression analyses were also performed. All data analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). A 2‐sided P‐value <0.05 was considered significant.
Results
Baseline Characteristics
A total of 2248 consecutive patients were enrolled in this study. All underwent elective PCI or diagnostic coronary angiography. Their average age was 63.5±10.7 years and 571 (25.4%) were female. Baseline serum creatinine was 90.2±39.1 μmol/L, and mean eGFR was 82.2±25.2 mL/min per 1.73 m2. Of the total patient population, 565 (25.2%) had diabetes, and 324 (14.6%) had congestive heart failure.
Tables 1 and 2 show the univariate analysis of the baseline and procedural characteristics, including the traditional risk factors for CIN between the patients with and without CIN. Patients who developed CIN were more likely to be older, hypotensive, and smokers, as well as have anemia, congestive heart failure, lower LVEF and hemoglobin values, and higher levels of serum creatinine, uric acid, low‐density lipoprotein cholesterol, and high‐sensitivity C‐reactive protein values. They presented with longer procedure durations or more multivessel disease, and they were more frequently treated with diuretics. Furthermore, these patients were more likely to have a higher Mehran risk score (9.53±5.14 versus 4.75±3.76) and higher NT‐proBNP levels (5320±7423 versus 1078±2548 pg/mL). Quartiles of NT‐proBNP for the present study population were Q1 (<65 pg/mL), Q2 (65 to 247.5 pg/mL), Q3 (247.5 to 976 pg/mL), and Q4 (≥976 pg/mL).
Table 1.
Baseline Characteristics for Patients With and Without CIN
| CIN (n=50) | No CIN (n=2198) | P Value | |
|---|---|---|---|
| Age, y | 71.2±8.5 | 63.3±10.7 | <0.001 |
| Age >75 years, % | 19 (38.0) | 281 (12.8) | <0.001 |
| Female, % | 16 (32.0) | 555 (25.3) | 0.278 |
| Weight, kg | 60.0±8.8 | 64.8±10.8 | 0.002 |
| eGFR, mL/min per 1.73 m2 | 55.0±26.1 | 82.8±24.9 | <0.001 |
| SBP at admission, mm Hg | 133.7±22.8 | 130.6±19.3 | 0.259 |
| DBP at admission, mm Hg | 74.5±10.6 | 76.7±11.8 | 0.191 |
| LVEF, % | 52.6±15.5 | 58.8±12.6 | 0.009 |
| Congestive heart failure | 20 (40.0%) | 304 (14.0%) | <0.001 |
| Hypertension | 15 (30.0%) | 894 (40.7%) | 0.128 |
| Diabetes mellitus | 13 (26.0%) | 552 (25.1%) | 0.889 |
| CABG | 0 (0.0%) | 18 (0.8%) | 0.521 |
| Hyperlipidemia | 6 (12.0%) | 321 (14.6%) | 0.606 |
| Anemia | 31 (63.3%) | 747 (34.4%) | <0.001 |
| Prior MI | 4 (8.0%) | 253 (11.5%) | 0.440 |
| Smoking | 11 (22.0%) | 833 (37.9%) | 0.022 |
| Serum creatinine, mmol/L | 135.5±66.7 | 89.2±37.9 | <0.001 |
| CrCl, mL/min | 43.0±19.9 | 72.4±26.4 | <0.001 |
| NT‐proBNP | 5320±7423 | 1078±2548 | <0.001 |
| Log (NT‐proBNP) | 7.5±1.8 | 5.6±1.8 | <0.001 |
| LDL‐C, mmol/L | 2.3±1.0 | 2.9±1.1 | 0.047 |
| CHO, mmol/L | 4.5±1.7 | 4.3±1.5 | 0.516 |
| TG, mmol/L | 1.6±0.9 | 1.5±1.2 | 0.760 |
| ALB, g/L | 31.0±5.0 | 35.7±7.8 | <0.001 |
| High‐sensitivity C‐reactive protein, mg/L | 30 (81.1%) | 826 (51.9%) | <0.001 |
| HGB, g/L | 115.5±22.8 | 132.5±16.0 | <0.001 |
| Hematocrit, % | 0.35±0.07 | 0.39±0.09 | <0.001 |
| HbA1c, % | 6.5±1.1 | 6.5±1.4 | 0.971 |
| URI | 435±145 | 381±109 | 0.022 |
| Urinary PH | 5.7±0.8 | 6.6±16.9 | 0.038 |
| Mahran score | 9.5±5.1 | 4.8±3.8 | <0.001 |
Values are mean±SD or n (%). ALB indicates albumin; CABG, coronary artery bypass grafting; CHO, cholesterol; CIN, contrast‐induced nephropathy; CrCl, creatinine clearance; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HGB, hemoglobin; LDL‐C, low‐density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; SBP, systolic blood pressure; TG, triglyceride; URI, uric acid.
Table 2.
Procedural Characteristics for Patients With and Without CIN
| CIN (n=50) | No CIN (n=2198) | P Value | |
|---|---|---|---|
| Periprocedural medications | |||
| ACEI/ARB | 42 (84.0%) | 1948 (88.6%) | 0.310 |
| β‐blocker | 39 (78.0%) | 1915 (87.2%) | 0.057 |
| CCB | 9 (18.8%) | 408 (18.6%) | 0.977 |
| Diuretic | 18 (36.0%) | 338 (15.4%) | <0.001 |
| Procedural variables | |||
| Number of lesions | 2.53±1.19 | 1.93±1.16 | <0.001 |
| Number of stents | 1.83±1.20 | 1.56±1.30 | 0.162 |
| Stent length | 47.4±37.1 | 38.3±34.5 | 0.081 |
| Total fluoroscopy time, min | 81.3±47.6 | 68.1±45.9 | 0.049 |
| Contrast volume, mL | 132.6±77.5 | 123.9±68.0 | 0.372 |
| VCrClratio | 4.04±3.73 | 2.02±1.70 | <0.001 |
| HVWratio, mL/kg | 19.9±13.2 | 11.8±7.1 | <0.001 |
| Preprocedural hypotension | 4 (8.5%) | 20 (0.9%) | <0.001 |
| Postprocedural IABP | 7 (14.0%) | 35 (1.6%) | <0.001 |
Values are mean±SD or n (%). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; CIN, contrast‐induced nephropathy; HVWratio, hydration volume‐to‐weight; IABP, intra‐aortic balloon pump; VCrClratio, volume of contrast agent‐to‐creatinine clearance rate.
NT‐proBNP and In‐Hospital Outcomes
Individuals with high preprocedural NT‐proBNP concentrations were more likely to develop CIN (Q1, Q2, Q3, Q4: 0.5%, 1.2%, 1.2%, 5.9%; P<0.001). The differences were consistent with a different definition of CIN, including an absolute increase of >0.3 mg/dL and/or a relative increase of >50% in serum creatinine from baseline within 72 hours after contrast administration (Table 3). The patients with elevated NT‐proBNP levels were more likely to have major adverse clinical events, including overall death (0.2%, 0%, 0.2%, 1.8%; P<0.001) and a need for renal replacement therapy (Q1, Q2, Q3, Q4: 0.0%, 0.4%, 0.2%, 1.2%, P=0.023).
Table 3.
CIN Incidence and Clinical Outcomes According to NT‐proBNP Quartiles
| Outcomes | NT‐proBNP Quartiles | P Value | |||
|---|---|---|---|---|---|
| Q1 (n=562) (<65 pg/mL) | Q2 (n=562) (65 to 247.5 pg/mL) | Q3 (n=562) (247.5 to 976 pg/mL) | Q4 (n=562) (≥976 pg/mL) | ||
| CIN | |||||
| Scr increase ≥0.5 mg/dL or ≥25%, n (%) | 40 (7.1) | 41 (7.3) | 45 (8.0) | 74 (13.2) | <0.001 |
| Scr increase ≥0.5 mg/dL, n (%) | 3 (0.5) | 7 (1.2) | 7 (1.2) | 33 (5.9) | <0.001 |
| Scr increase ≥0.3 mg/dL, n (%) | 9 (1.6) | 18 (3.2) | 22 (3.9) | 57 (10.3) | <0.001 |
| Scr increase ≥0.3 mg/dL or ≥50%, n (%) | 9 (1.6) | 18 (3.2) | 22 (3.9) | 57 (10.3) | <0.001 |
| Death, n (%) | 1 (0.2) | 0 (0.0) | 1 (0.2) | 10 (1.8) | <0.001 |
| Require RRT, n (%) | 0 (0.0) | 2 (0.4) | 1 (0.2) | 7 (1.2) | 0.023 |
CIN indicates contrast‐induced nephropathy; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; RRT, renal replacement therapy; Scr, serum creatinine.
Multivariable Factors for Predicting CIN
Receiver operating characteristic curve analysis indicated that a cutoff value of 682 pg/dL for NT‐proBNP could predict CIN with a sensitivity of 78% and a specificity of 70% (area under the curve=0.766, P<0.001) (Figure 1). NT‐proBNP was not significantly different from the Mehran CIN score with respect to predicting CIN (C=0.7657 versus C=0.7729, P=0.8431).
Figure 1.
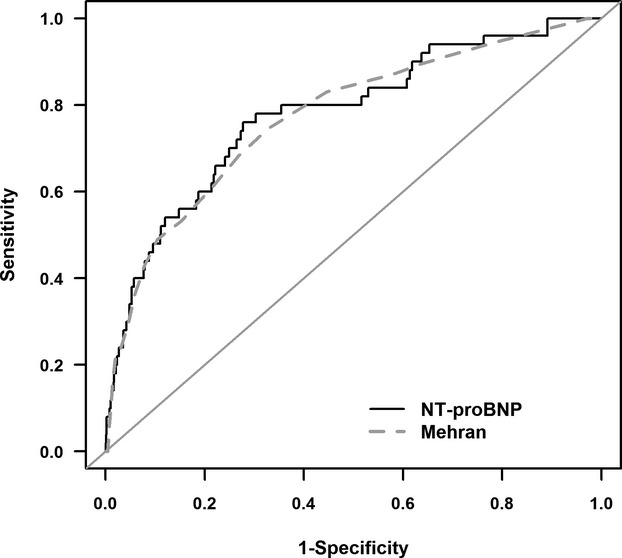
Receiver operating characteristic curve analysis for N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) and Mehran scores in the prediction of contrast‐induced nephropathy (C=0.7657 vs C=0.7729; P=0.8431).
When analyzing all of the patients, we observed a significant positive correlation between NT‐proBNP and the patients' age and Mehran CIN score. A significant negative correlation was demonstrated between NT‐proBNP and the values of LVEF and hemoglobin (Figure 2).
Figure 2.
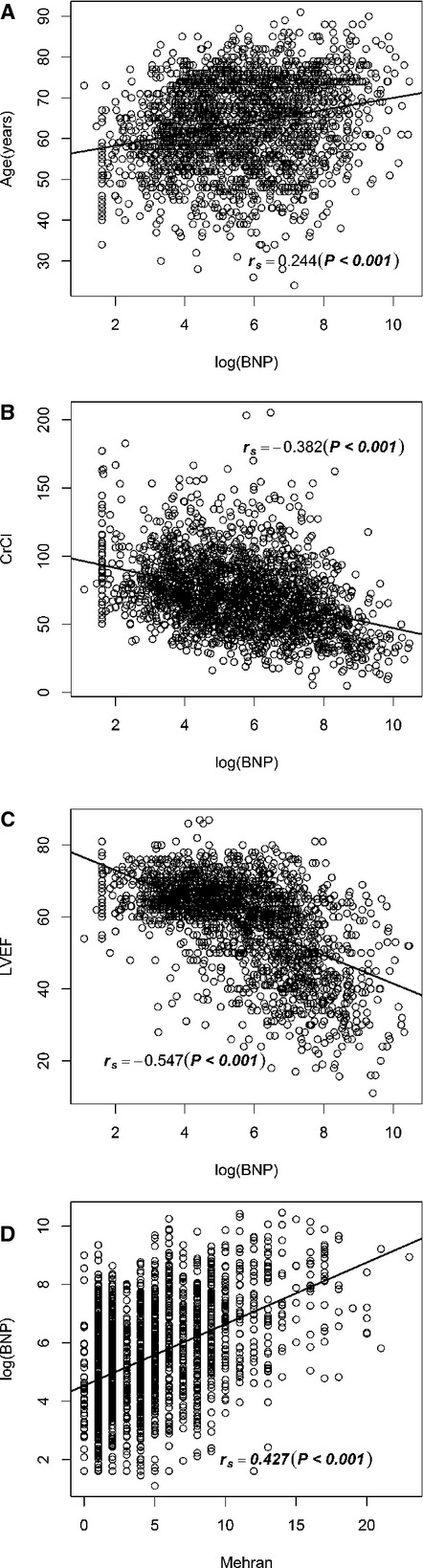
Correlation between N‐terminal pro‐brain natriuretic peptide (BNP) and age (A), creatinine clearance (CrCl) (B), left ventricular ejection fraction (LVEF) (C), and Mehran contrast‐induced nephropathy score (D).
Univariate logistic regression found that NT‐proBNP >682 pg/dL was significantly associated with CIN (odds ratio: 8.16; 95% CI: 4.15 to 16.02, P<0.001). Multivariable logistic regression analysis showed that BNP >682 pg/dL was an independent predictor of CIN (odds ratio: 4.007; 95% CI: 1.950 to 8.234; P<0.001), even after adjustment for other established risk factors, including congestive heart failure, age >75 years, eGFR <60 mL/min per 1.73 m2, diabetes mellitus, and dose >200 mL (Table 4).
Table 4.
Univariate and Multivariable Logistic Regression Analysis
| Risk Factors | Univariate Logistic Regression | Multivariate Logistic Regression | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| NT‐proBNP >682 pg/mL | 8.156 | 4.151 to 16.022 | 0.000 | 4.007 | 1.950 to 8.234 | 0.0002 |
| Age >75 years | 4.182 | 2.331 to 7.503 | 0.000 | 1.932 | 1.039 to 3.591 | 0.0373 |
| eGFR <60 mL/min per 1.73 m2 | 10.428 | 4.871 to 22.325 | 0.000 | 5.121 | 2.272 to 11.540 | 0.0001 |
| Congestive heart failure | 4.101 | 2.299 to 7.315 | 0.000 | 1.918 | 1.035 to 3.556 | 0.0386 |
| Diabetes mellitus | 1.046 | 0.552 to 1.983 | 0.889 | 0.816 | 0.421 to 1.583 | 0.5483 |
| Dose >200 mL | 1.274 | 0.647 to 2.510 | 0.483 | 1.233 | 0.611 to 2.485 | 0.5588 |
eGFR indicates estimated glomerular filtration rate; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; OR, odds ratio.
NT‐proBNP and Long‐Term Clinical Outcomes
We assessed the predictive value of different NT‐proBNP cutoffs (682 pg/dL). After adjusting for baseline clinical and other procedural variables, a higher NT‐proBNP group remained an independent predictor of long‐term clinical outcomes (Figures 3 and 4); the adjusted mortality was higher for patients with NT‐proBNP >682 pg/dL (hazard ratio: 2.53; 95% CI: 1.49 to 4.30; P=0.0006).
Figure 3.
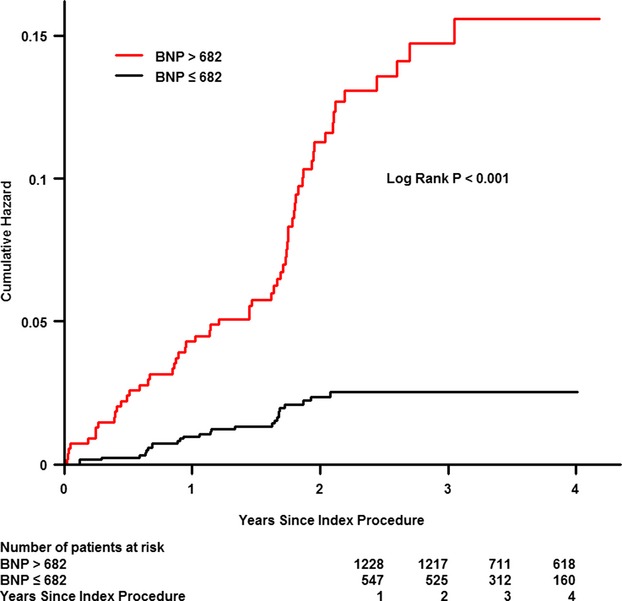
Cumulative mortality for patients according to the cutoff value for N‐terminal pro‐brain natriuretic peptide (BNP) (682 ng/mL).
Figure 4.
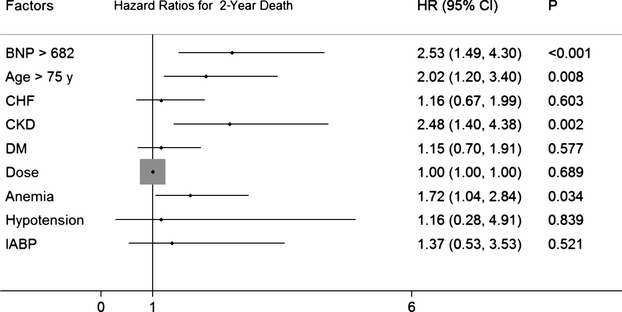
Hazard ratios for patients' 2‐year death according to the cutoff value for BNP (682 ng/mL). BNP indicates N‐terminal pro‐brain natriuretic peptide; CHF, congestive heart failure; CKD, chronic kidney disease; DM, diabetes mellitus; HR, hazard ratio; IABP, intra‐aortic balloon pump.
Discussion
The present study showed that elevation of preprocedural NT‐proBNP, an easily available biomarker, was significantly associated with an increased risk of CIN. After adjustment for other traditional confounders, including congestive heart failure, a level of preprocedural NT‐proBNP >682 pg/mL, the best cutoff point, was a strong and independent predictor of CIN and long‐term death; thus, besides the Mehran CIN score, preprocedural NT‐proBNP could become another objective and rapidly available tool for risk stratification and/or a modifiable target for prevention in patients undergoing coronary angiography.
NT‐proBNP may be a promising and timely tool for predicting the risk of CIN and adverse events in patients undergoing coronary angiography. The predictive value of the preprocedural NT‐proBNP level was not significantly different from that of the Mehran CIN score (P=0.8431), including 8 periprocedural risk factors, some of which were related to the patients' medical histories and were often unavailable to the cardiologists before the procedure.4 NT‐proBNP, a qualitative and more objective risk stratification tool compared with the Mehran score system, may potentially help cardiologists to identify high‐risk patients (with high NT‐proBNP), who may then receive timely preventive measures. At our institute, ≈80% patients who were undergoing elective coronary angiography and who were suspected to be at risk of heart failure also underwent NT‐proBNP testing; this NT‐proBNP test is routinely performed at our facility. In addition, most of the risk factors included in the Mehran score were associated with elevated NT‐proBNP levels in our study. Therefore, this simple and readily available test may be a better tool for predicting CIN than the existing Mehran CIN score system.
CIN is highly prevalent in patients with congestive heart failure and chronic kidney disease (CKD) undergoing coronary interventions.2,15–17 One study showed that LVEF <40% was significantly associated with CIN (odds ratio: 4.52; 95% CI: 1.30 to 15.71; P=0.02) in patients without congestive heart failure.18 Another study conducted in Denmark showed that using a heart failure diagnosis requiring elevated NT‐proBNP reduces the prevalence of heart failure with preserved ejection fraction and results in a survival rate similar to that of heart failure with reduced ejection fraction; the finding supported the use of NT‐proBNP for the selection of high‐risk patients having heart failure with preserved ejection fraction in future interventional clinical trials.19 In 1 recent study, NT‐proBNP also had strong associations with left ventricular systolic dysfunction,20 even in patients without clinical heart failure; the study evaluated the cross‐sectional associations of NT‐proBNP with cardiac structural and functional abnormalities in patients with CKD (n=3232). NT‐proBNP significantly reclassified participants' likelihood of having left ventricular systolic dysfunction (net reclassification improvement 0.28, 95% CI: 0.27 to 0.30; P<0.001). In addition, point‐of‐care tests are available in which BNP concentrations are available within 20 minutes, making it a potentially useful candidate for early prognostication. Therefore, preprocedural NT‐proBNP may be a more objective and rapidly available tool for the estimation of the risk of worsening renal function after coronary angiography.
Measurement of NT‐proBNP on admission has been a routine procedure for patients with suspected coronary heart disease who are to undergo coronary angiography. Natriuretic peptides are a family of hormones synthesized in myocytes and secreted increasingly in response to the elevation of any chamber wall stress.21–22 Consequently, as the inactive metabolite of BNP, NT‐proBNP has diagnostic and prognostic capabilities across a variety of conditions involving heart dysfunction. Elevated NT‐proBNP levels have been shown to be a powerful predictor of short‐term and long‐term outcomes.23–26 In the general population, elevated NT‐proBNP levels predict poor outcomes in both asymptomatic and dyspneic subjects, irrespective of renal function.27–28 CKD itself also amplifies the cardiovascular risk.29 Even medical care supported by heart failure treatment that is guided by BNP/NT‐proBNP as an adjunct to standard clinical management is superior to standard care.30 Therefore, recent clinical practice guidelines recommended NT‐proBNP for guiding heart failure management.31
In the present study, the data suggested that elevation of preprocedural NT‐proBNP was also an independent risk factor for CIN in patients undergoing selected cardiac catheterization. One recent study,10 a substudy of the HORIZONS‐AMI trial, focused on the correlation between BNP and the risk of CIN in patients with acute ST‐segment elevation myocardial infarction (odds ratio: 1.29, 95% CI: 1.10 to 1.51; P<0.001); however, BNP and serial creatinine levels suitable for complete measurement were only available in 979 of the 3602 patients enrolled (27.2%) and were not sufficient to determine an optimal BNP cutoff concentration for the prediction of CIN. Another study indicated that preoperative BNP level is associated with postoperative acute kidney injury in high‐risk patients undergoing cardiac surgery. BNP was linearly associated with the risk of at least mild acute kidney injury, whereas for evaluating severe acute kidney injury, a risk threshold was observed above the intermediate levels of BNP.32 Our results are in accordance with the observations of a recent study, showing that patients with acute heart failure who develop acute kidney injury within 48 hours after admission have significantly higher initial BNP concentrations. According to our findings, an NT‐proBNP level >682 pg/mL was a strong and independent predictor of CKD patients presenting with acute onset or worsening of symptoms; in contrast, the optimal cutoff value of NT‐proBNP for diagnosing heart failure in unselected patients is 300 pg/mL.31 Because all the subjects presented with renal dysfunction, the cutoff value of NT‐proBNP for predicting CIN was strikingly higher than that for diagnosing heart failure.
The reasons for the strong association between elevated preprocedural NT‐proBNP levels and a higher risk of CIN were not clear; however, the potential mechanisms by which high BNP concentrations are associated with CIN may include the following. First, elevated BNP concentrations are also related to other risk factors, which are themselves directly associated with the development of CIN, such as worse heart and renal dysfunction, advanced age, and diabetes mellitus. Higher levels of NT‐proBNP were significantly associated with a higher risk of developing end‐stage renal disease in diabetic patients with CKD after adjustment for other risk factors.33 Second, the elevated NT‐proBNP in patients with CKD may be due to decreased renal clearance, or decreased renal responsiveness to BNP. Since previous studies found that plasma concentrations of NT‐proBNP increase as GFR declines in patients with or without apparent cardiac dysfunction,34–36 the decreased clearance ability of NT‐proBNP due to impaired renal function may play an important role. Elevated NT‐proBNP concentrations may reflect cardiac involvement in patients with CKD, especially those undergoing catheterization. Third, BNP, the source of NT‐proBNP, reduces the effects of catecholamines, and potentiates the generation of nitric oxide, which in turn is a known inhibitor of myocardial contractility, thereby possibly resulting in systemic vasodilation and renal hypoperfusion.37–38
CIN is regarded as a specific subtype of cardiorenal syndrome type 1, which is defined as worsening renal function attributed to acute heart dysfunction that may exacerbate the condition of both cardiac and renal impairment.39–40 It is associated with increased cardiovascular mortality and morbidity, increased stroke risk, longer hospitalization, and a higher readmission rate. Although there is growing recognition of the frequency of cardiorenal syndrome, its underlying pathophysiology is not yet well understood. There is no doubt that renal hypoperfusion and passive congestion can play important roles. Vascular factors such as nitric oxide, prostaglandin, natriuretic peptides, and endothelin may modulate renal perfusion independently of cardiac hemodynamics.41 Recently, researchers have focused on the role of inflammatory markers as links between cardiovascular and kidney disease. The natriuretic peptides are established biomarkers in heart failure and, since elevated natriuretic peptide levels can be caused by renal dysfunction as well as congestive heart failure, they probably also reflect renal injury.42–43,40 Thus, natriuretic peptides are emerging as pleiotropic biomarkers that are useful in the settings of both cardiac and renal dysfunction and have the potential to serve as a valuable diagnostic tool in the diagnosis of type 1 cardiorenal syndrome.41 Moreover, BNP has been shown to cause natriuresis and diuresis in animals, healthy men, and patients with congestive heart failure.44–45 Recombinant human BNP significantly reduced the incidence of CIN, reduced serum creatinine, and improved eGFR in a population of patients with congestive heart failure and ST‐elevation myocardial infarction.46 The hormone may be especially beneficial in patients with acute myocardial infarction complicated by congestive heart failure, because of its inhibitory effect on the neuroendocrine system47 and its diuretic and natriuretic action.
The cutoff value of NT‐proBNP, 682 pg/mL in the present study, may provide guidance for carrying out fluid management, as do the 35% or 40% limits for LVEF. It may be reasonable to limit the hydration speed and volume, or even to consider the use of diuretics, in patients with NT‐proBNP >682 pg/mL who are undergoing cardiac catheterization. Patients with very high levels of NT‐proBNP may become optimal target subjects for receiving therapeutic measures to prevent CIN in future randomized trials.
Limitations
The present study had several limitations. It was a single‐center, observational study without long‐term follow‐up for the systematic measurement of creatinine concentrations, such as at 3 months or 1 year. Patients undergoing primary PCI were excluded because they lacked preprocedural NT‐proBNP measurements; this might have caused selection bias. Variations in the measurement times may have led to missed peak levels of creatinine after the procedure. Failure to perform systematic measurements at optimal times for determining peak creatinine concentrations may have led to an underestimation of the true incidence of CIN.
Conclusions
Besides the Mehran CIN score, measurement of serum NT‐proBNP at hospital admission may also help identify patients who are at high risk for developing CIN after cardiac catheterization. The data may provide a modifiable target for therapeutic measures to prevent CIN in future randomized trials, where patients with NT‐proBNP >682 pg/mL may be the optimal target subjects.
Sources of Funding
This study was supported by Science and Technology Planning Project of Guangdong Province (grant no.: 2008A030201002), Guangdong Cardiovascular Institute; and Guangdong Provincial Cardiovascular Clinical Medicine Research Fund (grant no.: 2009X41), Guangzhou, China.
Disclosures
None.
References
- Nash K, Hafeez A, Hou S. Hospital‐acquired renal insufficiency. Am J Kidney Dis. 2002; 39:930-936. [DOI] [PubMed] [Google Scholar]
- McCullough PA. Contrast‐induced acute kidney injury. J Am Coll Cardiol. 2008; 51:1419-1428. [DOI] [PubMed] [Google Scholar]
- Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, Almén T, Aspelin P, Bellin MF, Clement O, Heinz‐Peer G. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011; 21:2527-2541. [DOI] [PubMed] [Google Scholar]
- Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast‐induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004; 44:1393-1399. [DOI] [PubMed] [Google Scholar]
- Bartholomew BA, Harjai KJ, Dukkipati S, Boura JA, Yerkey MW, Glazier S, Grines CL, O'Neill WW. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. 2004; 93:1515-1519. [DOI] [PubMed] [Google Scholar]
- Maisel AS, Clopton P, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Steg G, Westheim A, Knudsen CW, Perez A, Kazanegra R, Bhalla V, Herrmann HC, Aumont MC, McCullough PA. Impact of age, race, and sex on the ability of B‐type natriuretic peptide to aid in the emergency diagnosis of heart failure: results from the Breathing Not Properly (BNP) multinational study. Am Heart J. 2004; 147:1078-1084. [DOI] [PubMed] [Google Scholar]
- Takase H, Dohi Y. Kidney function crucially affects B‐type natriuretic peptide (BNP), N‐terminal proBNP and their relationship. Eur J Clin Invest. 2014; 44:303-308. [DOI] [PubMed] [Google Scholar]
- Wu AH, Omland T, Wold Knudsen C, McCord J, Nowak RM, Hollander JE, Duc P, Storrow AB, Abraham WT, Clopton P, Maisel AS, McCullough PA. Relationship of B‐type natriuretic peptide and anemia in patients with and without heart failure: a substudy from the Breathing Not Properly (BNP) Multinational Study. Am J Hematol. 2005; 80:174-180. [DOI] [PubMed] [Google Scholar]
- Pfister R, Sharp S, Luben R, Welsh P, Barroso I, Salomaa V, Meirhaeghe A, Khaw KT, Sattar N, Langenberg C, Wareham NJ. Mendelian randomization study of B‐type natriuretic peptide and type 2 diabetes: evidence of causal association from population studies. PLoS Med. 2011; 8:e1001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarai R, Dangas G, Huber K, Xu K, Brodie BR, Witzenbichler B, Metzger DC, Radke PW, Yu J, Claessen BE, Genereux P, Mehran R, Stone GW. B‐type natriuretic peptide and risk of contrast‐induced acute kidney injury in acute ST‐segment‐elevation myocardial infarction: a substudy from the HORIZONS‐AMI trial. Circ Cardiovasc Interv. 2012; 5:813-820. [DOI] [PubMed] [Google Scholar]
- K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002; 39:S1-S266. [PubMed] [Google Scholar]
- Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2012; 79:453-495. [DOI] [PubMed] [Google Scholar]
- Thomsen HS. European Society of Urogenital Radiology (ESUR) guidelines on the safe use of iodinated contrast media. Eur J Radiol. 2006; 60:307-313. [DOI] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837-845. [PubMed] [Google Scholar]
- Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR., Jr Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002; 105:2259-2264. [DOI] [PubMed] [Google Scholar]
- Taliercio CP, Vlietstra RE, Fisher LD, Burnett JC. Risks for renal dysfunction with cardiac angiography. Ann Intern Med. 1986; 104:501-504. [DOI] [PubMed] [Google Scholar]
- Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, Lansky AJ, Moussa I, Stone GW, Moses JW, Leon MB, Mehran R. Contrast‐induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005; 95:13-19. [DOI] [PubMed] [Google Scholar]
- Rosenstock JL, Gilles E, Geller AB, Panagopoulos G, Mathew S, Malieckal D, DeVita MV, Michelis MF. Impact of heart failure on the incidence of contrast‐induced nephropathy in patients with chronic kidney disease. Int Urol Nephrol. 2010; 42:1049-1054. [DOI] [PubMed] [Google Scholar]
- Carlsen CM, Bay M, Kirk V, Gøtze JP, Køber L, Nielsen OW. Prevalence and prognosis of heart failure with preserved ejection fraction and elevated N‐terminal pro brain natriuretic peptide: a 10‐year analysis from the Copenhagen Hospital Heart Failure Study. Eur J Heart Fail. 2012; 14:240-247. [DOI] [PubMed] [Google Scholar]
- Mishra RK, Li Y, Ricardo AC, Yang W, Keane M, Cuevas M, Christenson R, deFilippi C, Chen J, He J, Kallem RR, Raj DS, Schelling JR, Wright J, Go AS, Shlipak MG. Association of N‐terminal pro‐B‐type natriuretic peptide with left ventricular structure and function in chronic kidney disease (from the Chronic Renal Insufficiency Cohort [CRIC]). Am J Cardiol. 2013; 111:432-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel A, Mueller C, Adams K, Jr, Anker SD, Aspromonte N, Cleland JG, Cohen‐Solal A, Dahlstrom U, DeMaria A, Di Somma S, Filippatos GS, Fonarow GC, Jourdain P, Komajda M, Liu PP, McDonagh T, McDonald K, Mebazaa A, Nieminen MS, Peacock WF, Tubaro M, Valle R, Vanderhyden M, Yancy CW, Zannad F, Braunwald E. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008; 10:824-839. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Burnett JC, Jr, Bermudez EA, Jougasaki M, Bailey KR, Redfield MM. Clinical criteria and biochemical markers for the detection of systolic dysfunction. J Card Fail. 2000; 6:194-200. [DOI] [PubMed] [Google Scholar]
- Masson S, Latini R, Anand IS, Vago T, Angelici L, Barlera S, Missov ED, Clerico A, Tognoni G, Cohn JN. Direct comparison of B‐type natriuretic peptide (BNP) and amino‐terminal proBNP in a large population of patients with chronic and symptomatic heart failure: the Valsartan Heart Failure (Val‐HeFT) data. Clin Chem. 2006; 52:1528-1538. [DOI] [PubMed] [Google Scholar]
- Masson S, Latini R, Anand IS, Barlera S, Angelici L, Vago T, Tognoni G, Cohn JN. Prognostic value of changes in N‐terminal pro‐brain natriuretic peptide in Val‐HeFT (Valsartan Heart Failure Trial). J Am Coll Cardiol. 2008; 52:997-1003. [DOI] [PubMed] [Google Scholar]
- Jarai R, Huber K, Bogaerts K, Droogne W, Ezekowitz J, Granger CB, Sinnaeve PR, Ross AM, Zeymer U, Armstrong PW, Van de Werf FJ. Plasma N‐terminal fragment of the prohormone B‐type natriuretic peptide concentrations in relation to time to treatment and Thrombolysis in Myocardial Infarction (TIMI) flow: a substudy of the Assessment of the Safety and Efficacy of a New Treatment Strategy with Percutaneous Coronary Intervention (ASSENT IV‐PCI) trial. Am Heart J. 2010; 159:131-140. [DOI] [PubMed] [Google Scholar]
- de Lemos JA, McGuire DK, Drazner MH. B‐type natriuretic peptide in cardiovascular disease. Lancet. 2003; 362:316-322. [DOI] [PubMed] [Google Scholar]
- Anwaruddin S, Lloyd‐Jones DM, Baggish A, Chen A, Krauser D, Tung R, Chae C, Januzzi JL., Jr Renal function, congestive heart failure, and amino‐terminal pro‐brain natriuretic peptide measurement: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. J Am Coll Cardiol. 2006; 47:91-97. [DOI] [PubMed] [Google Scholar]
- Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N‐terminal pro‐brain natriuretic peptide, C‐reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005; 293:1609-1616. [DOI] [PubMed] [Google Scholar]
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003; 108:2154-2169. [DOI] [PubMed] [Google Scholar]
- Januzzi JL, Troughton R. Are serial BNP measurements useful in heart failure management? Serial natriuretic peptide measurements are useful in heart failure management. Circulation. 2013; 127:500-507. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC, guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012; 33:1787-1847. [DOI] [PubMed] [Google Scholar]
- Patel UD, Garg AX, Krumholz HM, Shlipak MG, Coca SG, Sint K, Thiessen‐Philbrook H, Koyner JL, Swaminathan M, Passik CS, Parikh CR. Preoperative serum brain natriuretic peptide and risk of acute kidney injury after cardiac surgery. Circulation. 2012; 125:1347-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai AS, Toto R, Jarolim P, Uno H, Eckardt KU, Kewalramani R, Levey AS, Lewis EF, McMurray JJ, Parving HH, Solomon SD, Pfeffer MA. Association between cardiac biomarkers and the development of ESRD in patients with type 2 diabetes mellitus, anemia, and CKD. Am J Kidney Dis. 2011; 58:717-728. [DOI] [PubMed] [Google Scholar]
- Tsutamoto T, Wada A, Sakai H, Ishikawa C, Tanaka T, Hayashi M, Fujii M, Yamamoto T, Dohke T, Ohnishi M, Takashima H, Kinoshita M, Horie M. Relationship between renal function and plasma brain natriuretic peptide in patients with heart failure. J Am Coll Cardiol. 2006; 47:582-586. [DOI] [PubMed] [Google Scholar]
- Austin WJ, Bhalla V, Hernandez‐Arce I, Isakson SR, Beede J, Clopton P, Maisel AS, Fitzgerald RL. Correlation and prognostic utility of B‐type natriuretic peptide and its amino‐terminal fragment in patients with chronic kidney disease. Am J Clin Pathol. 2006; 126:506-512. [DOI] [PubMed] [Google Scholar]
- van Kimmenade RR, Januzzi JL, Jr, Bakker JA, Houben AJ, Rennenberg R, Kroon AA, Crijns HJ, van Dieijen‐Visser MP, de Leeuw PW, Pinto YM. Renal clearance of B‐type natriuretic peptide and amino terminal pro‐B‐type natriuretic peptide: a mechanistic study in hypertensive subjects. J Am Coll Cardiol. 2009; 53:884-890. [DOI] [PubMed] [Google Scholar]
- Tsuruda T, Boerrigter G, Huntley BK, Noser JA, Cataliotti A, Costello‐Boerrigter LC, Chen HH, Burnett JC., Jr Brain natriuretic peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res. 2002; 91:1127-1134. [DOI] [PubMed] [Google Scholar]
- Kawakami R, Saito Y, Kishimoto I, Harada M, Kuwahara K, Takahashi N, Nakagawa Y, Nakanishi M, Tanimoto K, Usami S, Yasuno S, Kinoshita H, Chusho H, Tamura N, Ogawa Y, Nakao K. Over expression of brain natriuretic peptide facilitates neutrophil infiltration and cardiac matrix metalloproteinase‐9 expression after acute myocardial infarction. Circulation. 2004; 110:3306-3312. [DOI] [PubMed] [Google Scholar]
- Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P. Cardio‐renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010; 31:703-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008; 52:1527-1539. [DOI] [PubMed] [Google Scholar]
- Forman DE, Bulter J, Wang Y, Abraham WT, O'Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004; 43:61-67. [DOI] [PubMed] [Google Scholar]
- Cruz DN, Bolgan I, Perazella MA, Bonello M, de Cal M, Corradi V, Polanco N, Ocampo C, Nalesso F, Piccinni P, Ronco C. North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS‐AKI): targeting the problem with the RIFLE Criteria. Clin J Am Soc Nephrol. 2007; 2:418-425. [DOI] [PubMed] [Google Scholar]
- Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin AAcute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007; 11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkayam U, Akhter MW, Liu M, Hatamizadeh P, Barakat MN. Assessment of renal hemodynamic effects of nesiritide in patients with heart failure using intravascular Doppler and quantitative angiography. JACC Cardiovasc Imaging. 2008; 1:765-771. [DOI] [PubMed] [Google Scholar]
- Chen HH, Cataliotti A, Schirger JA, Martin FL, Harstad LK, Burnett JC., Jr Local renal delivery of a natriuretic peptide a renal‐enhancing strategy for B‐type natriuretic peptide in overt experimental heart failure. J Am Coll Cardiol. 2009; 53:1302-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger AJ. A review of the renal and neurohormonal effects of B‐type natriuretic peptide. Congest Heart Fail. 2005; 11:30-38. [DOI] [PubMed] [Google Scholar]
- Zhang J, Fu X, Jia X, Fan X, Gu X, Li S, Wu W, Fan W, Su J, Hao G, Jiang Y, Xue L. B‐type natriuretic peptide for prevention of contrast‐induced nephropathy in patients with heart failure undergoing primary percutaneous coronary intervention. Acta Radiol. 2010; 51:641-648. [DOI] [PubMed] [Google Scholar]


