Abstract
Background
Nationally representative data evaluating recent trends and future projections of vascular risk factor treatment and control rates in secondary prevention of ischemic heart disease are sparse.
Methods and Results
We evaluated sex‐ and race‐stratified cholesterol, blood pressure, and hemoglobin A1c levels and risk factor treatment and control rates in 1580 individuals who self‐reported a history of myocardial infarction from The National Health and Nutrition Examination Surveys (NHANES) 1999 to 2012. We used weighted linear regression to estimate time trends and created forward linear projections to 2020. Participants were 30% to 41% women, 73% to 85% white, and had a mean age of 63 to 66 years. Cholesterol treatment rates increased and reached above 80% in men and women by 2011–2012, with significant increases in control rates (as then defined) in men to 85% in 2011–2012, with projections to reach 100% by 2020. Cholesterol treatment rates significantly increased in non‐Hispanic whites and Hispanics. Statin use increased significantly to 73% of myocardial infarction survivors by 2011–2012, and aspirin use increased significantly but only to 28% by 2011–2012. There were no changes in blood pressure treatment or control rates by sex, and hypertension treatment increased only in non‐Hispanic blacks. Projected hypertension control rates remained suboptimal.
Conclusions
While temporal trends suggest improvements in cholesterol treatment, unchanged treatment and control of blood pressure and persistently low aspirin use represent missed opportunities. Urgent action is needed to improve secondary prevention rates projected by 2020 to reduce recurrent events in this high‐risk group.
Keywords: myocardial infarction, secondary prevention, trends
Introduction
Cardiovascular diseases, including heart disease and stroke, are leading causes of morbidity and mortality in the United States.1 Recurrent myocardial infarction (MI) events represent one fourth of the ≈915 000 coronary events that occur in the United States each year,2 and ≈7% of nondiabetic US adults self‐reported a history of MI from 1999 to 2001.3 In individuals who have experienced an MI, treatment and control of risk factors are essential to reduce the risk of recurrent MI, other nonfatal cardiovascular disease events, and death. Secondary prevention guidelines have recommended intensive treatment and control of vascular risk factors such as hypertension, dyslipidemia, diabetes mellitus, and smoking over the past decade.4–6 Initiatives such as Million Hearts and Healthy People 2020 include increasing secondary prevention coverage as targets among their objectives.7–8 Recently, the American Heart Association established its Strategic Impact Goal to improve the cardiovascular health of all Americans by 20% and to reduce deaths from cardiovascular diseases (CVD) by 20% by 2020, through targeting these modifiable health factors and health behaviors.9 It is unclear whether recent trends in risk factor treatment and control in secondary prevention will support achievement of these goals.
National‐level data on adherence to secondary prevention guidelines are limited, and available only through 2002.10–11 Although updated cholesterol and blood pressure guidelines were released in 2013,12–13 recent trends of risk factor management occurred under the preceding secondary prevention guidelines. Based on these gaps and using goal risk factor levels established under contemporaneous iterations of prevention guidelines, we evaluated nationally representative risk factor treatment and control trends in American adults who self‐reported a history of MI from 1999 to 2012, and projected these trends to 2020 to evaluate progress toward the American Heart Association's 2020 Strategic Impact Goal.
Methods
Study Sample
We analyzed recent cross‐sectional data of all adults >20 years of age from the National Health and Nutrition Examination Survey (NHANES).14 The NHANES examinations represent serial cross‐sectional estimates of the prevalence of diseases, conditions, and medication use in the general noninstitutionalized US population. Data were available for seven 2‐year cycles between 1999 and 2012. We analyzed data from the 1580 individuals who self‐reported a history of MI and who participated in both the interview and mobile NHANES examinations. NHANES was approved by the National Center for Health Statistics Ethics Review Board, and subjects gave informed consent.
Study Variables
Methods for obtaining medical history, physical examination, and laboratory measurements (including blood pressure, total cholesterol, high‐density lipoprotein [HDL] cholesterol, low‐density lipoprotein [LDL] cholesterol, fasting plasma glucose, and hemoglobin A1c), and changes in measurement methods during the study period have been previously reported.14 Non‐HDL cholesterol was calculated as total cholesterol minus HDL cholesterol. Medical history was based on self‐report of a participant being told by a health professional that he/she had a condition. Time since MI was calculated as current age minus age at which patient had an MI. Treatment rates were calculated based on either self‐report of pharmacotherapy for hypertension, hyperlipidemia, or diabetes mellitus, or record of a medication for these conditions during interviewer review of participants’ prescription medication bottles. Aspirin, statin, angiotensin‐converting enzyme (ACE) inhibitor, angiotensin II receptor blocker (ARB), and β‐blocker use information were also obtained during review of participant prescription medication bottles. Based on previous guidelines for CVD prevention, target risk factor levels that defined control were a systolic blood pressure <140 mm Hg and a diastolic blood pressure <90 mm Hg for hypertension, total cholesterol <200 mg/dL for hyperlipidemia, or hemoglobin A1c <7% for diabetes mellitus. Total cholesterol, rather than LDL cholesterol, was used to calculate hyperlipidemia control rates because LDL data were available only on a subset of individuals (n=690).
Statistical Analyses
We conducted statistical analyses using SAS v9.3 (SAS Institute, Cary, NC), and accounted for the complex weighted sampling design of NHANES in all analyses. We used the full sample 2‐year mobile examination center weight, except for analysis of fasting plasma glucose, where we used the fasting subsample 2‐year weight. We calculated means and rates using PROC surveymeans and surveyfreq, accounting for stratum and cluster information when allowed by sample size. Data are reported as mean and standard error for continuous variables, or frequency and percentage for categorical variables, and are based on sampling weight. In subgroups where estimates did not converge due to limited sample size, we removed stratum and cluster information from analyses to obtain an estimate of linear trends. To estimate trends from 1999 to 2012, we created weighted linear regression models with estimated mean values or percentages as dependent variables and survey time as independent variables. We used reciprocals of variances as weights. We report regression β coefficients that represent the slope or rate of change per year within and among study variables across the study period. We evaluated potential nonlinear trends by including a quadratic term for time in the linear model where appropriate and allowed by sample size. Given the minimal differences seen in the quadratic models, we used linear trends for simplicity.
Employing previously published methods to create forward, linear projections,15 we created projections to 2020 under the assumption that previous trends would continue at a similar rate in a linear fashion, based on prior findings that document linear changes in parameters of interest across time.16–17 A 2‐sided P value <0.05 defined statistical significance.
Results
Characteristics of participants reporting prior MI from 1999 to 2012 are summarized in Table 1. There were no significant differences in participant age, sex, and race/ethnicity over time. Mean age ranged from 63 to 66 years. The majority of participants reporting prior MI were men. The most common racial/ethnic group was non‐Hispanic white (73% to 85%), followed by non‐Hispanic black (6% to 13%). Mean time since MI was ≈10 years across the study period. Over the study period, the prevalence of diabetes trended upwards, but there were no significant changes in the prevalence of other co‐morbid conditions. Smoking rates remained unchanged. Aspirin use increased from 4% in 1999–2000 to 28% in 2011–2012 (P<0.01). Statin use increased from 36% to 73% (P=0.04). Use of ACE inhibitors or ARBs increased by 2011–2012 to 46% (P=0.03). β‐Blocker use did increase overall, but the trend was not significant. Body mass index trended upwards, up to 30 kg/m2 (P=0.07). Overall mean total cholesterol decreased (P=0.01), non‐HDL decreased (P=0.01), and LDL decreased (P=0.01). Overall trends of other lipid components, blood pressure, and glucose were not significant.
Table 1.
Characteristics of NHANES Participants Reporting Prior MI in Biennial Examinations From 1999–2012
| 1999–2000 | 2001–2002 | 2003–2004 | 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | P (Trend) | R 2 | |
|---|---|---|---|---|---|---|---|---|---|
| Demographics, N (%) | 187 | 211 | 256 | 208 | 267 | 258 | 193 | ||
| Women | 61 (30.5) | 69 (37.6) | 98 (40.3) | 66 (40.4) | 90 (38.0) | 76 (29.7) | 73 (41.0) | 0.88 | 0.01 |
| Age in y, mean (SE) | 63.7 (1.2) | 65.2 (1.3) | 66.0 (1.2) | 65.7 (0.9) | 65.0 (0.9) | 63.1 (1.2) | 65.5 (0.8) | 0.98 | 0.01 |
| Race | |||||||||
| Non‐Hispanic white | 114 (81.9) | 135 (81.5) | 179 (82.1) | 140 (84.6) | 162 (72.9) | 161 (78.5) | 101 (77.4) | 0.18 | 0.32 |
| Non‐Hispanic black | 27 (6.4) | 43 (12.6) | 33 (8.8) | 44 (9.1) | 47 (10.9) | 42 (10.6) | 40 (8.2) | 0.29 | 0.22 |
| Hispanic | 31 (2.0) | 28 (3.3) | 32 (2.7) | 16 (2.2) | 21 (3.0) | 36 (6.0) | 12 (3.4) | 0.25 | 0.25 |
| Medical history, N (%) | |||||||||
| Years since MI, mean (SE) | 9.1 (0.8) | 9.0 (0.7) | 10.6 (0.8) | 8.9 (0.7) | 10.6 (0.5) | 10.2 (0.5) | 11.5 (0.8) | 0.05 | 0.55 |
| Stroke | 32 (13.4) | 39 (16.9) | 36 (13.8) | 43 (17.4) | 46 (16.7) | 48 (19.8) | 43 (17.0) | 0.11 | 0.42 |
| Heart failure | 58 (29.9) | 66 (30.1) | 82 (29.5) | 78 (34.0) | 90 (33.3) | 73 (28.2) | 72 (33.0) | 0.41 | 0.14 |
| Malignant neoplasm | 37 (18.5) | 47 (20.1) | 52 (21.7) | 42 (18.7) | 60 (25.3) | 67 (26.9) | 34 (16.3) | 0.50 | 0.09 |
| Current smoker | 37 (23.9) | 41 (23.2) | 50 (24.3) | 41 (21.7) | 63 (21.6) | 66 (24.0) | 49 (28.9) | 0.68 | 0.04 |
| Diabetes | 49 (19.7) | 58 (27.0) | 69 (23.5) | 66 (28.4) | 94 (31.5) | 76 (27.4) | 70 (29.7) | 0.07 | 0.51 |
| Aspirin use | 7 (4.0) | 16 (6.6) | 37 (13.9) | 40 (20.9) | 43 (15.7) | 52 (21.4) | 43 (28.0) | <0.01 | 0.90 |
| Statin use | 62 (36.3) | 111 (56.5) | 113 (46.1) | 114 (56.4) | 130 (48.6) | 146 (54.6) | 129 (72.9) | 0.04 | 0.59 |
| ACE inhibitor or ARB use | 66 (32.9) | 81 (39.9) | 108 (41.9) | 88 (41.1) | 138 (47.5) | 120 (43.8) | 102 (46.3) | 0.03 | 0.65 |
| β‐Blocker use | 51 (27.2) | 89 (47.0) | 106 (41.7) | 131 (63.3) | 136 (51.2) | 152 (57.0) | 117 (56.6) | 0.12 | 0.41 |
| Physical exam/laboratory measures, mean (SE) | |||||||||
| BMI, kg/m2 | 28.7 (0.5) | 30.0 (0.6) | 29.2 (0.5) | 29.6 (0.5) | 29.9 (0.5) | 31.1 (0.6) | 30.1 (0.5) | 0.07 | 0.52 |
| Systolic BP, mm Hg | 129.0 (2.4) | 129.2 (1.7) | 131.8 (2.0) | 126.8 (2.2) | 129.5 (1.3) | 122.8 (1.2) | 127.9 (1.5) | 0.25 | 0.25 |
| Diastolic BP, mm Hg | 69.1 (1.2) | 66.6 (1.2) | 67.8 (0.7) | 66.1 (1.0) | 68.5 (0.9) | 65.8 (1.2) | 67.1 (1.1) | 0.34 | 0.18 |
| Total cholesterol, mg/dL | 201.9 (3.3) | 191.3 (6.0) | 200.1 (3.8) | 180.0 (4.1) | 180.4 (3.4) | 178.5 (3.2) | 172.7 (2.7) | 0.01 | 0.81 |
| HDL, mg/dL | 47.5 (1.7) | 46.8 (1.4) | 51.0 (1.4) | 50.9 (1.7) | 49.9 (1.2) | 46.7 (1.1) | 47.9 (1.8) | 0.99 | 0.00 |
| Non‐HDL, mg/dL | 154.4 (3.9) | 144.5 (6.1) | 149.1 (3.7) | 129.2 (4.1) | 130.6 (3.7) | 131.8 (3.1) | 124.7 (2.5) | 0.01 | 0.81 |
| LDL, mg/dL* | 121.6 (3.9) | 116.2 (7.2) | 106.0 (4.1) | 94.5 (4.6) | 101.9 (3.0) | 99.9 (2.7) | 94.7 (3.7) | 0.01 | 0.75 |
| Fasting glucose, mg/dL | 119.3 (5.5) | 115.9 (8.6) | 120.0 (6.7) | 112.7 (2.5) | 116.4 (3.8) | 114.9 (2.5) | 118.3 (2.8) | 0.57 | 0.07 |
| HbA1c, % | 5.8 (0.1) | 5.8 (0.1) | 6.2 (0.2) | 5.7 (0.1) | 6.1 (0.1) | 6.1 (0.1) | 6.1 (0.1) | 0.23 | 0.27 |
| Optimal regimen, N (%)* | 4 (2.7) | 13 (4.9) | 33 (12.9) | 35 (18.2) | 35 (13.8) | 48 (19.6) | 40 (23.5) | <0.01 | 0.89 |
Rates and means calculated based on sample weights. ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MI, myocardial infarction; NHANES, National Health and Nutrition Examination Survey.
LDL only measured on a subset of participants.
Optimal regimen defined as treated with aspirin, a cholesterol‐lowering drug, and a blood pressure–lowering drug.
Cholesterol
Trends and projections for cholesterol treatment and cholesterol levels by sex are listed in Table 2. From 1999–2000 to 2011–2012, cholesterol treatment rates increased in men from 47% to 80% (P=0.03), with large increases seen particularly between 1999–2000 and 2001–2002, and between 2009–2010 and 2011–2012. Among those men treated, control rates increased from 69% to 85% (P=0.01). Projections based on these trends showed that treatment and control rates would reach 100% by 2020. These increasing treatment and control rates corresponded to decreases in total cholesterol (P<0.01) and LDL cholesterol (P=0.02). In women, treatment rates trended upwards (P=0.06) but remained lower than treatment rates in men, until 2011–2012 when treatment rates reached 82%. Mean total cholesterol in treated women decreased across time, from 194 to 173 mg/dL (P=0.01).
Table 2.
1999–2012 Cholesterol Trends and Projection to 2020 in NHANES Participants Reporting Prior MI, by Sex
| 1999–2000 | 2001–2002 | 2003–2004 | 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | β (SE) | P (Trend) | R 2 | 2020 (Projected) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Men, N (total) | 126 | 142 | 158 | 142 | 177 | 182 | 120 | ||||
| Treated, N (% of total) | 56 (46.6) | 89 (66.4) | 94 (59.1) | 98 (72.3) | 128 (74.3) | 126 (65.7) | 94 (80.2) | 1.84 (0.58) | 0.03 | 0.67 | 100% |
| Controlled, N (% of treated) | 35 (68.8) | 59 (66.8) | 66 (69.3) | 79 (81.5) | 101 (84.3) | 102 (80.5) | 79 (85.1) | 1.51 (0.40) | 0.01 | 0.74 | 100% |
| Total cholesterol, M (SE) | 185.9 (3.5) | 178.1 (6.3) | 180.4 (4.7) | 171.0 (7.5) | 162.7 (4.1) | 166.4 (3.5) | 162.7 (3.0) | −1.98 (0.34) | <0.01 | 0.87 | 142.8 |
| HDL, M (SE) | 39.7 (1.8) | 41.9 (1.1) | 45.7 (1.3) | 47.6 (1.5) | 44.8 (0.9) | 45.1 (2.2) | 44.2 (1.7) | 0.34 (0.22) | 0.19 | 0.32 | 49.2 |
| LDL, M (SE) | 108.9 (3.5) | 109.0 (7.6) | 91.2 (2.6) | 88.2 (2.8) | 88.8 (4.7) | 90.7 (4.0) | 87.2 (5.1) | −1.81 (0.56) | 0.02 | 0.68 | 67.7 |
| Untreated, N (% of total) | 70 (53.4) | 53 (33.6) | 64 (40.8) | 44 (27.7) | 50 (25.7) | 56 (34.3) | 26 (19.8) | −1.84 (0.58) | 0.03 | 0.67 | 0% |
| At goal, N (% of untreated) | 32 (44.3) | 29 (62.9) | 31 (43.9) | 21 (43.2) | 33 (42.1) | 34 (63.7) | 11 (31.2) | −1.26 (0.91) | 0.23 | 0.27 | 32% |
| Total cholesterol, M (SE) | 208.0 (6.8) | 206.1 (13.1) | 214.6 (9.6) | 198.6 (5.8) | 209.7 (4.6) | 197.3 (5.0) | 206.7 (6.2) | −0.47 (0.59) | 0.46 | 0.11 | 198.8 |
| HDL, M (SE) | 48.4 (1.6) | 43.3 (3.0) | 47.8 (2.2) | 44.3 (1.1) | 51.7 (4.9) | 46.6 (1.1) | 59.3 (8.6) | 0.77 (0.44) | 0.14 | 0.38 | 60.3 |
| LDL, M (SE) | 126.0 (3.6) | 120.5 (4.3) | 113.4 (8.5) | 128.8 (8.9) | 128.8 (1.7) | 108.2 (7.3) | 119.5 (0.3) | −0.51 (0.78) | 0.54 | 0.08 | 113.0 |
| Women, N (total) | 61 | 69 | 98 | 66 | 90 | 76 | 73 | ||||
| Treated, N (% of total) | 24 (38.8) | 37 (62.7) | 53 (60.3) | 39 (60.2) | 48 (55.9) | 42 (55.7) | 53 (81.7) | 2.35 (0.94) | 0.06 | 0.55 | 100% |
| Controlled, N (% of treated) | 14 (56.6) | 22 (58.6) | 22 (41.3) | 28 (80.7) | 33 (76.5) | 27 (66.0) | 37 (72.3) | 1.94 (1.51) | 0.25 | 0.25 | 100% |
| Total cholesterol, M (SE) | 194.4 (3.2) | 190.5 (5.1) | 208.2 (4.7) | 169.9 (2.0) | 175 (5.3) | 179.7 (6.5) | 173.1 (3.8) | −2.73 (0.67) | 0.01 | 0.77 | 152.8 |
| HDL, M (SE) | 47.0 (2.2) | 51.9 (1.4) | 57.9 (2.4) | 54.9 (1.9) | 53.9 (2.5) | 48.5 (1.1) | 48.3 (2.1) | −0.12 (0.41) | 0.78 | 0.02 | 49.9 |
| LDL, M (SE) | 112.9 (0.0) | 117.6 (2.9) | 121.8 (6.4) | 82.0 (6.2) | 95.3 (6.4) | 103.2 (6.8) | 92.2 (2.0) | −2.10 (1.19) | 0.14 | 0.38 | 72.1 |
| Untreated, N (% of total) | 37 (61.2) | 32 (37.3) | 45 (39.7) | 27 (39.8) | 42 (44.1) | 34 (44.3) | 20 (18.3) | −2.35 (0.94) | 0.06 | 0.55 | 0% |
| At goal, N (% of untreated) | 12 (26.9) | 12 (32.9) | 23 (48.5) | 12 (50.6) | 19 (46.1) | 20 (65.5) | 8 (55.7) | 1.19 (1.04) | 0.31 | 0.21 | 86% |
| Total cholesterol, M (SE) | 222.3 (7.4) | 213.4 (10.5) | 212.0 (6.2) | 203.6 (9.5) | 212.4 (8.4) | 185.4 (6.1) | 189.9 (17.3) | −2.11 (1.07) | 0.11 | 0.44 | 164.6 |
| HDL, M (SE) | 59.5 (4.5) | 59.5 (2.9) | 59.2 (2.4) | 60.9 (3.3) | 58.4 (5.6) | 50.1 (4.4) | 55.3 (4.1) | −0.58 (0.29) | 0.10 | 0.45 | 48.9 |
| LDL, M (SE) | 151.5 (9.1) | 123.4 (8.6) | 118.7 (4.8) | 103.2 (11.7) | 126.0 (4.9) | 103.2 (4.1) | 136.9 (0.0) | −1.37 (1.69) | 0.45 | 0.12 | 102.7 |
Cholesterol listed in mg/dL. Untreated individuals at goal had a total cholesterol level <200 mg/dL. β coefficients represent the change in treatment or control prevalence or risk factor level per year. HDL indicates high‐density lipoprotein; LDL, low‐density lipoprotein; MI, myocardial infarction; NHANES, National Health and Nutrition Examination Survey.
Cholesterol treatment and control by race/ethnic group are listed in Table S1. Treatment rates increased in non‐Hispanic whites from 44% in 1999–2000 to 84% in 2011–2012 (P=0.02), with increases in control up to 82% in 2011–2012 (P=0.05). Treatment rates were projected to reach 100% in non‐Hispanic white and Hispanic individuals by 2020, although the projected treatment and control rate for non‐Hispanic black individuals remained suboptimal. These increases corresponded to decreases in total cholesterol (P=0.01) and LDL cholesterol (P=0.03) in non‐Hispanic whites across the study period. In Hispanic/Latinos, cholesterol treatment rates increased from 36% to 67% (P=0.03), although no change in control rates or cholesterol levels were observed. There were no changes in cholesterol treatment, control, or cholesterol levels across the study period in non‐Hispanic blacks.
Blood Pressure
There was no significant trend in blood pressure treatment or control in men or women from 1999–2012 (Table 3), and projections to 2020 indicate stable rates of treatment, particularly for women. There was also no significant trend in systolic blood pressure or diastolic blood pressure, in either treated or untreated men or women. By race/ethnic group, blood pressure treatment rates increased in non‐Hispanic blacks from 69% in 1999–2000 to 93% in 2011–2012 (P=0.05) with a projected treatment rate of 100% by 2020 (Table S2). There was no significant trend in blood pressure treatment in non‐Hispanic whites or Hispanic/Latinos, and no significant change in blood pressure control rates in any racial/ethnic group, corresponding to low projected treatment and control in these groups. Across the study period there was no significant change in systolic or diastolic blood pressure in any race/ethnic group.
Table 3.
1999–2012 Blood Pressure Trends and Projection to 2020 in NHANES Participants Reporting Prior MI, by Sex
| 1999–2000 | 2001–2002 | 2003–2004 | 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | β (SE) | P (Trend) | R 2 | 2020 (Projected) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Men, N (total) | 126 | 142 | 158 | 142 | 177 | 182 | 120 | ||||
| Treated, N (% of total) | 86 (60.4) | 108 (73.8) | 124 (76.1) | 119 (84.5) | 150 (85.4) | 145 (74.0) | 103 (81.7) | 0.65 (0.81) | 0.45 | 0.12 | 100% |
| Controlled, N (% of treated) | 53 (67.8) | 55 (60.9) | 69 (58.7) | 85 (70.3) | 95 (67.7) | 99 (72.4) | 68 (68.2) | 0.46 (0.39) | 0.29 | 0.22 | 80% |
| Systolic BP, M (SE) | 127.9 (2.0) | 128.9 (3.1) | 129.3 (2.3) | 124.0 (2.5) | 127.4 (1.6) | 122.8 (1.2) | 127.6 (2.6) | −0.27 (0.23) | 0.29 | 0.22 | 122.8 |
| Diastolic BP, M (SE) | 68.8 (1.1) | 67.7 (1.7) | 67.9 (1.5) | 68.1 (1.4) | 67.8 (1.4) | 64.5 (1.2) | 68.1 (1.0) | −0.15 (0.13) | 0.29 | 0.22 | 65.3 |
| Untreated, N (% of total) | 40 (39.6) | 34 (26.2) | 34 (23.9) | 23 (15.5) | 27 (14.6) | 37 (26.0) | 17 (18.3) | −0.65 (0.81) | 0.45 | 0.12 | 0% |
| At goal, N (% of untreated) | 10 (32.1) | 15 (57.9) | 8 (18.0) | 6 (38.9) | 7 (33.2) | 12 (27.4) | 4 (34.7) | −0.35 (1.16) | 0.77 | 0.02 | 20% |
| Systolic BP, M (SE) | 125.9 (3.1) | 118.7 (1.1) | 131.2 (2.9) | 124.2 (3.3) | 125.5 (1.2) | 123.2 (3.2) | 124.0 (4.0) | −0.04 (0.39) | 0.91 | 0.002 | 124.0 |
| Diastolic BP, M (SE) | 76.0 (1.9) | 70.1 (1.9) | 72.5 (1.0) | 67.6 (2.4) | 75.1 (1.7) | 74.1 (2.8) | 73.0 (4.0) | 0.03 (0.31) | 0.94 | 0.002 | 73.0 |
| Women, N (total) | 61 | 69 | 98 | 66 | 90 | 76 | 73 | ||||
| Treated, N (% of total) | 47 (76.5) | 57 (85.3) | 80 (77.9) | 54 (78.4) | 71 (76.6) | 65 (79.8) | 68 (86.2) | −0.21 (0.46) | 0.66 | 0.04 | 87% |
| Controlled, N (% of treated) | 18 (48.4) | 31 (60.0) | 34 (39.7) | 37 (54.8) | 38 (56.1) | 43 (73.8) | 37 (58.3) | 1.73 (1.11) | 0.18 | 0.33 | 85% |
| Systolic BP, M (SE) | 137.2 (4.9) | 131.0 (4.4) | 136.4 (3.6) | 133.4 (2.8) | 132.9 (3.1) | 124.8 (3.7) | 131.5 (3.3) | −0.59 (0.33) | 0.13 | 0.39 | 123.6 |
| Diastolic BP, M (SE) | 64.5 (2.2) | 62.3 (2.2) | 64.7 (2.2) | 62.1 (2.0) | 65.5 (1.8) | 63.3 (1.8) | 64.3 (2.1) | 0.04 (0.13) | 0.78 | 0.02 | 64.4 |
| Untreated, N (% of total) | 14 (23.5) | 12 (14.7) | 18 (22.1) | 12 (21.6) | 19 (23.4) | 11 (20.2) | 5 (13.8) | 0.21 (0.46) | 0.66 | 0.04 | 13% |
| At goal, N (% of untreated) | 5 (62.9) | 2 (12.2) | 2 (5.8) | 6 (50.4) | 6 (22.9) | 6 (55.5) | 2 (33.5) | 3.97 (4.23) | 0.40 | 0.18 | 41% |
| Systolic BP, M (SE) | 121.1 (8.5) | 153.8 (12.0) | 130.4 (1.6) | 121.7 (1.6) | 136.0 (6.1) | 113.7 (5.1) | 117.0 (0.0) | −1.55 (1.25) | 0.27 | 0.24 | 104.4 |
| Diastolic BP, M (SE) | 59.4 (6.2) | 70.8 (3.4) | 68.8 (1.0) | 67.8 (0.7) | 76.6 (2.2) | 63.7 (5.3) | 63.0 (0.0) | 0.08 (0.59) | 0.90 | 0.003 | 68.3 |
Blood pressure listed in mm Hg. Untreated individuals at goal had a blood pressure <120/80 mm Hg. β coefficients represent the change in treatment or control prevalence or risk factor level per year. BP indicates blood pressure; MI, myocardial infarction; NHANES, National Health and Nutrition Examination Survey.
Glucose
Trends in blood glucose measures by sex are listed in Table 4. Blood glucose treatment rates increased in men from 17% in 1999–2000 to 25% in 2011–2012 (P=0.01), with increases in control from 27% to 55% (P=0.03). Treatment and control rates were projected to reach 36% and 82%, respectively, by 2020. Mean fasting glucose trended downwards in treated men (P=0.08), but there was no significant change in HbA1c levels. Although there was no significant change in blood glucose treatment in women, control levels decreased from 99% in 1999–2000 to 92% in 2011–2012 (P=0.04) and were projected to reach 82%. There was no significant change in fasting glucose or HbA1c levels in treated or untreated women across time.
Table 4.
1999–2012 Blood Glucose Trends and Projection to 2020 in NHANES Participants Reporting Prior MI, by Sex
| 1999–2000 | 2001–2002 | 2003–2004 | 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | β (SE) | P (Trend) | R 2 | 2020 (Projected) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Men, N (total) | 126 | 142 | 158 | 142 | 177 | 182 | 120 | ||||
| Treated, N (% of total) | 28 (17.4) | 33 (22.3) | 34 (19.0) | 42 (23.5) | 52 (23.5) | 52 (25.3) | 41 (25.1) | −0.62 (0.15) | 0.01 | 0.78 | 36% |
| Controlled, N (% of treated) | 9 (27.3) | 20 (44.8) | 17 (43.9) | 20 (59.7) | 24 (42.0) | 25 (50.4) | 19 (54.7) | 2.13 (0.73) | 0.03 | 0.63 | 82% |
| Fasting glucose, M (SE) | 220.4 (0.0) | 189.2 (5.4) | 122.0 (21.5) | 136.2 (0.0) | 151.9 (2.6) | 147.5 (3.7) | 131.5 (6.2) | −5.72 (2.61) | 0.08 | 0.49 | 71.1 |
| HbA1c, M (SE) | 8.2 (0.0) | 7.3 (0.1) | 7.8 (0.1) | 6.8 (0.0) | 7.6 (0.1) | 7.6 (0.2) | 7.0 (0.3) | −0.06 (0.04) | 0.23 | 0.28 | 6.6 |
| Untreated, N (% of total) | 98 (82.6) | 109 (77.8) | 124 (81.0) | 100 (76.5) | 125 (76.5) | 130 (74.8) | 79 (74.9) | −0.62 (0.15) | 0.01 | 0.78 | 65% |
| At goal, N (% of untreated) | 90 (90.8) | 103 (95.9) | 113 (95.4) | 91 (88.9) | 117 (94.3) | 122 (97.3) | 73 (97.0) | 0.35 (0.21) | 0.15 | 0.36 | 100% |
| Fasting glucose, M (SE) | 114.4 (6.8) | 105.4 (1.1) | 123.7 (4.6) | 108.7 (1.3) | 110.6 (1.3) | 106.9 (1.9) | 109.6 (2.1) | −0.26 (0.32) | 0.46 | 0.11 | 106.0 |
| HbA1c, M (SE) | 5.7 (0.2) | 5.5 (0.04) | 5.7 (0.1) | 5.6 (0.04) | 5.7 (0.04) | 5.8 (0.1) | 5.7 (0.1) | 0.01 (0.01) | 0.30 | 0.21 | 5.8 |
| Women, N (total) | 61 | 69 | 98 | 66 | 90 | 76 | |||||
| Treated, N (% of total) | 40 (75.8) | 46 (66.6) | 75 (80.7) | 51 (77.8) | 59 (67.5) | 51 (67.6) | 45 (60.8) | 0.88 (0.92) | 0.38 | 0.15 | 51% |
| Controlled, N (% of treated) | 39 (98.6) | 44 (98.5) | 68 (92.7) | 44 (90.1) | 52 (89.9) | 48 (94.7) | 40 (92.0) | −0.61 (0.23) | 0.04 | 0.59 | 82% |
| Fasting glucose, M (SE) | 143.5 (3.1) | 125.7 (0.0) | 192.5 (0.0) | 127.0 (0.0) | 118.8 (9.9) | 139.3 (2.1) | 179.9 (8.2) | 1.12 (2.91) | 0.72 | 0.03 | 163.4 |
| HbA1c, M (SE) | 7.1 (0.2) | 6.6 (0.0) | 8.2 (0.0) | 6.0 (0.0) | 6.4 (0.1) | 6.7 (0.0) | 7.7 (0.4) | 0.01 (0.08) | 0.95 | <0.01 | 7.0 |
| Untreated, N (% of total) | 21 (24.2) | 23 (33.4) | 23 (19.3) | 15 (22.2) | 31 (32.5) | 25 (32.4) | 28 (39.1) | −0.88 (0.92) | 0.38 | 0.15 | 49% |
| At goal, N (% of untreated) | 6 (24.1) | 12 (61.6) | 7 (29.6) | 9 (66.5) | 18 (61.5) | 11 (42.7) | 9 (32.4) | 1.50 (1.44) | 0.35 | 0.18 | 53% |
| Fasting glucose, M (SE) | 106.7 (2.1) | 98.6 (1.5) | 112.7 (7.4) | 104.5 (1.0) | 108.8 (4.1) | 103.9 (2.0) | 100.4 (1.8) | −0.22 (0.49) | 0.67 | 0.04 | 101.8 |
| HbA1c, M (SE) | 5.5 (0.1) | 5.5 (0.03) | 6.0 (0.3) | 5.4 (0.1) | 5.8 (0.1) | 5.7 (0.04) | 5.7 (0.1) | 0.01 (0.02) | 0.57 | 0.07 | 5.9 |
Fasting glucose listed in mg/dL. HbA1c listed as percent. Untreated individuals at goal had an HbA1c <7%. β coefficients represent the change in treatment or control prevalence or risk factor level per year. HbA1c indicates hemoglobin A1c; MI, myocardial infarction; NHANES, National Health and Nutrition Examination Survey.
Blood glucose treatment and control trend by race/ethnic group are listed in Table S3. Treatment rates trended upwards in non‐Hispanic whites, from 18% in 1999–2000 to 29% in 2011–2012 (P=0.06) with projection to 39% in 2020, although there was no change in control levels, fasting glucose, or HbA1c levels across time. Blood glucose treatment rates increased significantly in Hispanic/Latinos, from 29% in 1999–2000 to 37% in 2011–2012 (P<0.01) with projected 61% treated in 2020, although there was no change in control levels, and no change in fasting glucose or HbA1c levels during the same time period. There was no significant change in blood glucose levels, treatment, or control across the study period in non‐Hispanic blacks.
Individual Medication Use
Trends in statin, aspirin, ACE inhibitor, ARB, and β‐blocker use are illustrated in Figures 1 and 2 and detailed in Table S4. From 1999–2000 to 2011–2012, statin use increased primarily in men, from 37% to 72% (P=0.01) with projection to 87% by 2020. The increasing trend in women was not statistically significant, although statin use reached 74% in women by 2011–2012. Increasing trends in statin use were significant in non‐Hispanic blacks (P=0.05) and Hispanics (P=0.02) and reached 52% (with projection to 71%) and 67% (with projection to 85%) of individuals, respectively. While the trend in statin use in non‐Hispanic whites was not statistically significant, statin use reached 77% by 2011–2012.
Figure 1.
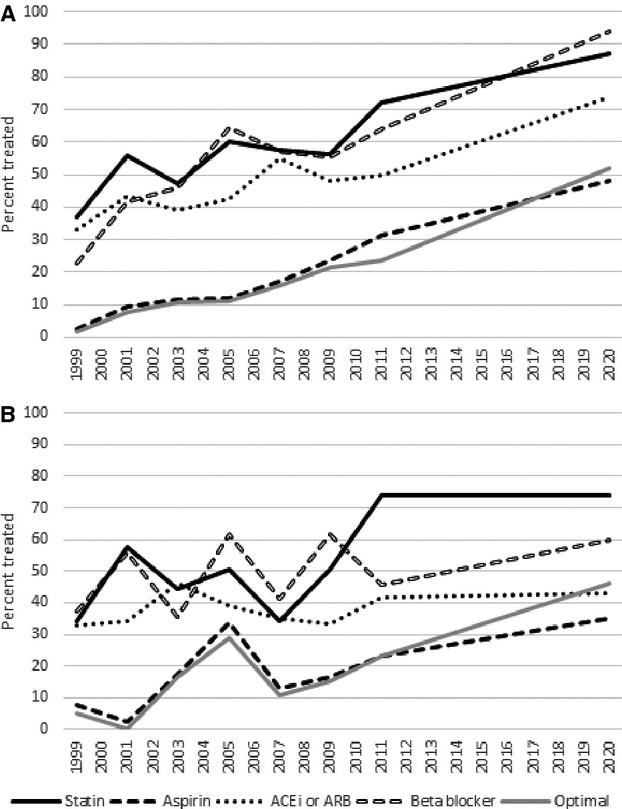
Treatment rates and projected treatment rates in 2020 by medication type in (A) men and (B) women. Optimal regimen indicates treatment with a blood pressure–lowering medication, a cholesterol‐lowering medication, and aspirin. Corresponding data are listed in Table S1. P for trend <0.05 in men for statin, aspirin, ACEi or ARB, β‐blocker, and optimal regimen; in women for optimal regimen. ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Figure 2.
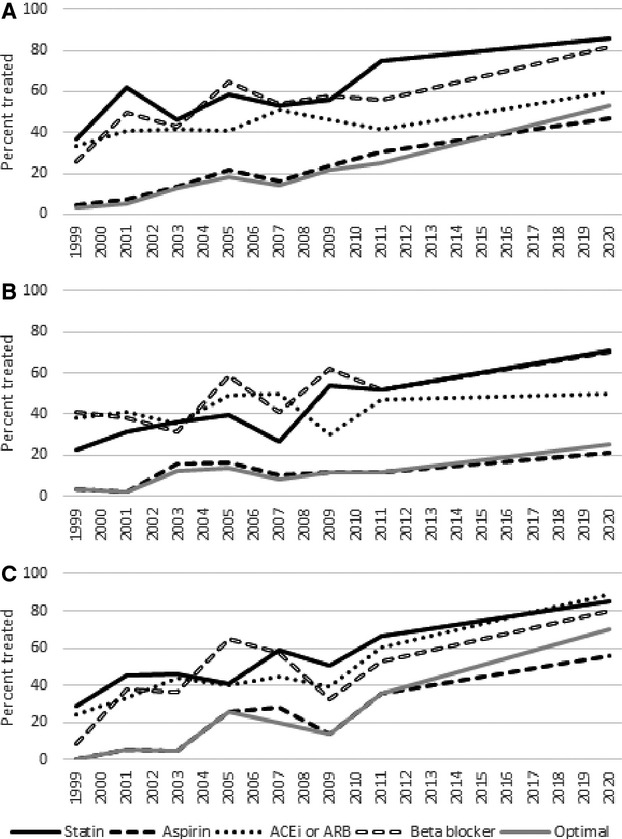
Treatment rates and projected treatment rates in 2020 by medication type in (A) non‐Hispanic whites, (B) non‐Hispanic blacks, and (C) Hispanic/Latinos. Optimal regimen indicates treatment with a blood pressure–lowering medication, a cholesterol‐lowering medication, and aspirin. Corresponding data are listed in Table S1. P for trend <0.05 in non‐Hispanic white for aspirin, optimal regimen; in non‐Hispanic black for statin, aspirin, β‐blocker, and optimal regimen; in Hispanic/Latino for statin, aspirin, ACEi or ARB, β‐blocker, and optimal regimen. ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Aspirin use increased for all individuals from 1999 to 2012 (Table 1, P<0.01) and increasing trends were significant in all groups except for women. Aspirin use reached 31% in men with projection to 48% (P for trend <0.001) and 23% in women by 2011–2012 (P=0.07). In non‐Hispanic whites, aspirin use reached 31% (P=0.001) and in Hispanic/Latinos aspirin use reached 36% (P<0.001) by 2011–2012. Aspirin was taken by 12% of non‐Hispanic blacks by 2011–2012 (P=0.05).
ACE inhibitor and ARB use increased in the total sample. By sex, prevalence in the use of either ACE inhibitors or ARBs increased in men to 50% by 2011–2012 (P=0.04) but remained unchanged in women. By race, ACE inhibitor or ARB use trended upwards in Hispanic/Latinos (to 61%, P=0.05) but did not change in non‐Hispanic whites or non‐Hispanic blacks.
β‐Blocker use increased in men from 23% in 1999–2000 to 64% in 2011–2012 (P=0.04) with projected rise to 94% in 2020 if current trends continue. The trend in β‐blocker use in women was not significant. The increase in β‐blocker use through 2011–2012 was significant in non‐Hispanic blacks (P=0.05) and Hispanic/Latinos (P=0.05), but not in non‐Hispanic whites.
Overall, the proportion of individuals treated with an “optimal regimen” (treated with aspirin, a cholesterol‐lowering drug, and a blood pressure–lowering drug) increased, reaching 24% by 2011–2012 (Table 1, P<0.01).
Discussion
We report nationally representative data of treatment and control of cholesterol, blood pressure, and glucose in American adults who reported a history of MI from 1999 to 2012. Across this time, cholesterol treatment rates increased in men and women, with improvements in control seen in men. Cholesterol treatment rates increased in non‐Hispanic whites and Hispanic/Latinos, while treatment and control rates remained notably unchanged and suboptimal in non‐Hispanic blacks. Blood pressure treatment and control rates did not change in either men or women, and by race treatment rates increased only in non‐Hispanic blacks (in whom it increased markedly, to 93%) with no changes in control in any race group. While glucose treatment and control rates increased in men, treatment remained unchanged in women and control rates decreased. By race, blood glucose treatment increased in Hispanics and non‐Hispanic whites, but no changes in glucose treatment were seen in non‐Hispanic blacks.
Projected treatment and control rates based on recent trends provide an assessment of whether patients and practitioners are on track to attain national goals for health improvement. Both men and women MI patients are projected to achieve cholesterol treatment rate targets of 100% treated, although by race non‐Hispanic black individuals are projected to remain well below optimal levels of treatment for cholesterol. Blood pressure treatment rates are projected to remain stable, particularly in women. While rates of treatment for blood glucose are predicted to increase by 2020, anticipated control rates for those requiring treatment are inadequate. Projected rates of treatment with aspirin are notably low, particularly in non‐Hispanic Black individuals.
The evidence supporting lipid‐lowering therapy in MI secondary prevention is clear.18–19 In meta‐analysis, statins for CVD secondary prevention demonstrated an ≈20% reduction in risk of all‐cause mortality and major vascular events for each 1 mmol/L decrease in LDL cholesterol.20–22 Major clinical trials that demonstrated the benefit of statins for secondary prevention were released prior to our study period, and the notable rise in overall cholesterol treatment rates and in percentage of individuals specifically on statins observed early in our study period, between 1999–2000 and 2001–2002, may reflect the effect of these studies as well as contemporaneous guideline updates5 on clinician adoption of statins for secondary prevention. During the subsequent duration of the study period, cholesterol treatment remained generally unchanged, until a second notable rise in cholesterol treatment rates between 2009–2010 and 2011–2012 that may in part be related to the release of studies in the late 2000s that supported statin use in primary prevention. This increase is evident in the ≈20% rise in statin use seen in both sexes from 2009–2010 and 2011–2012.
In our analysis we noted that treatment rates in women remained notably below those of men, which may have been due to greater emphasis on prevention in men given the known difference in risk between sexes. Nevertheless, by 2011–2012 women's treatment rates surpassed those in men, and treatment and control in both sexes was projected to reach 100% by 2020. The projected rise in treatment rates to 100% by 2020 are encouraging and indicate that cholesterol treatment may reach optimal levels in accordance with American Heart Association goals if the most recent trends continue. These increases in treatment rates corresponded to general improvement in cholesterol levels across the study period, particularly in men and in non‐Hispanic whites. Notably, some cholesterol trends are interpreted with caution in cases of isolated elevated mean levels (eg, 2011–2012 HDL cholesterol in treated men, and 1999–2000 HDL cholesterol in untreated non‐Hispanic blacks) or limited available data (eg, for LDL cholesterol).
The benefits of treating hypertension for the prevention of ischemic heart disease events are also well known23 and include evidence from a recent meta‐analysis that indicated that a blood pressure reduction of 10 mm Hg systolic or 5 mm Hg diastolic in individuals with a history of coronary heart disease results in a 24% reduction in the risk for a recurrent coronary heart disease event.24 In general, treatment is indicated in patients who are hypertensive, although secondary prevention guidelines suggest consideration of ACE inhibitors in MI patients even at normal blood pressure where no contraindications exist.25 Our projections of blood pressure levels and treatment/control rates based on trends under the prevailing hypertension treatment guidelines indicate continued suboptimal blood pressure management, despite the presence of a recommended target blood pressure level of <140/<904 mm Hg during the study period. Nevertheless, blood pressure treatment rates by 2011–2012 were on average higher than earlier in the study period, and comparable across sexes. Across race/ethnic groups, trends in treatment rates were statistically significant only in non‐Hispanic blacks (notably to 93% treated by 2011–2012), though in all race groups treatment rates were higher at the end of the study period compared to the beginning.
The number of untreated individuals at ideal, normotensive blood pressure levels (<120/<80) remained low during our analysis, but mean blood pressures in those not treated were below <140/90 mm Hg, suggesting that by blood pressure criteria an antihypertensive medication would not necessarily be indicated. However, as guidelines still suggest consideration of an ACE inhibitor (or, alternatively, an ARB) in all MI patients,25 treatment with this class of antihypertensive drug may still be indicated for prevention of recurrent events. Overall use of ACE inhibitor or ARBs reached 46% in 2011–2012 subsequent to an increasing trend over the preceding decade. This increase was primarily driven by an increase of ACE inhibitor or ARB use in men. Contraindications and/or relative/absolute hypotension would preclude use of these medications, but overall these data suggest room for improvement in antihypertensive medication use in the prevention of recurrent MI, particularly in the case of ACE inhibitors or ARBs in women.
Secondary prevention guidelines also suggest the use of β‐blockers within 3 years of MI (Class I, Level of Evidence: A recommendation) and suggest continuing β‐blockers beyond 3 years (Class IIa, Level of Evidence: B recommendation).25 β‐Blocker use increased primarily in men, and increases were seen in non‐Hispanic blacks and Hispanic/Latinos. However, notably the mean time since MI in this population was ≈10 years, so persistently low use of β‐blockers may reflect weaker evidence for their use multiple years post‐MI.
While diabetes has been found to be an important predictor of recurrent MI and fatal coronary heart disease in both men and women,26 suboptimal diabetes treatment and control may have resulted in part from contemporary uncertainty regarding the efficacy of intensive glycemic control for the reduction of major macrovascular events.27–29 The tenuous link between intensive blood glucose control and risk for recurrent MI may have prompted clinicians to put a lower priority on use of blood glucose–lowering therapy for secondary prevention, even with a goal HbA1c of <7% suggested in secondary prevention guidelines.4,25 It is, however, encouraging that the high rates of untreated individuals meeting goal HbA1c levels suggests that those not being treated did not have an indication for glucose‐lowering therapy. Additionally, increasing trends of glucose treatment and control suggest that MI survivors are being monitored and treated as needed for diabetes. As suboptimal diabetes treatment and control rates have potentially serious implications for the development of co‐morbid microvascular disease in MI survivors, continued rigorous assessment of blood glucose is needed to prevent development of diabetes in MI survivors.
Another recommendation for MI survivors is to take aspirin. Use of aspirin is a class IA recommendation for MI secondary prevention25 when no contraindications exist. Aspirin has been shown to reduce the risk of MI in high‐risk patients,30 and 1 meta‐analysis of aspirin for the secondary prevention of vascular disease demonstrated a 20% reduction in coronary events per year in comparison to control groups.31 Although aspirin use trended upwards in both sexes and across all race groups, our results demonstrate significant room for improvement in treatment with aspirin. Although it is unclear how many participants had a contraindication for aspirin use, projections of aspirin use to 2020 based on recent trends suggest that a substantial proportion of MI survivors will not receive appropriate preventive therapy with aspirin. This result is particularly concerning as many patients in this group likely underwent revascularization as a result of their MI, after which aspirin is an indicated postintervention treatment.
Our results demonstrating promising improvements in cholesterol treatment and control but suboptimal projections of blood pressure treatment and control and aspirin use occurred in the setting of well‐known benefits of therapy for secondary prevention of MI, established MI secondary prevention guidelines, and existing national health improvement initiatives such as Healthy People 2020.4–5,7,23,25,32 Healthy People 2020 does include secondary prevention objectives such as increasing use of aspirin to prevent recurrent cardiovascular events and reducing cholesterol to goal levels in adults with coronary heart disease, although there is no specific objective targeting adequate blood pressure control to prevent recurrent MI. For comparison, recent analysis of treatment trends in MI patients in the United Kingdom during this same time period showed improvements in blood pressure and cholesterol levels as well as treatment rates in coronary heart disease secondary prevention.33
Although treatment decisions must be made individually for each MI patient, the prevalence of a so‐called “optimal regimen” (taking aspirin, a cholesterol‐lowering drug, and a blood pressure–lowering drug) reached only 24% by 2011–2012, suggesting overall inadequate management of MI patients and/or suboptimal adherence to indicated therapies. The overall suboptimal treatment rates we found are consistent with known patterns of low adherence to multidrug regimens for cardiovascular disease prevention within 2 years after initiation of treatment,34–35 particularly given that participants were on average ≈10 years post‐MI in this sample. Patient sex, race, and ethnicity are consistently implicated as predictors of nonadherence, with black individuals and women having higher rates of medication discontinuation.36 Other factors, including complexity of medical therapy in CVD and polypharmacy,37 as well as medication dosing frequency, patient socioeconomic status, poor understanding of medication benefits, co‐morbidities, and intensity of physician follow‐up36 have been shown to influence nonadherence to medication in secondary prevention. Interventions known to improve adherence to medication in patients with CVD, including reduced dosing frequency, use of fixed‐dose combination therapies, and novel financing strategies such as eliminating co‐payments for secondary prevention, may increase patient adherence to secondary prevention regimens. Provider interventions, such as improved patient communication using social media and health information technology, and outpatient quality improvement programs, are also potential avenues to improve management of MI patients.36,38–39 Presumably a combination of clinician care‐improvement strategies and patient interventions to improve adherence are needed to improve MI secondary prevention.
Strengths and Limitations
Strengths of our study include a nationally representative sample with availability of data for treatment and control for major risk factors associated with recurrent MI. Furthermore, we calculated treatment rates using a combination of self‐report and information gathered during actual examination of patient's prescription medication, reducing potential recall bias. However, there are several limitations. First, we relied on self‐reported MI to define the secondary prevention population, which is subject to recall bias. Nevertheless, previous studies calculate sensitivity of self‐report for acute MI to be 97.7% and specificity >99%,40 suggesting that self‐reported MI can reliably be used in analysis. Additionally, data on reasons for medication nonadherence, including contraindications to recommended therapy, are not available in NHANES. However, analysis of secondary prevention after other vascular disease events suggests that contraindications account for only about 5% of medication discontinuation.41
Second, our analysis of cholesterol treatment and control focuses on total cholesterol, although prior guidelines targeted LDL goals. While the limited LDL data in our study precluded robust primary analysis of LDL trends, elevated total cholesterol is also associated with coronary heart disease risk42–43 and has been established as an indicator for monitoring cholesterol‐lowering efforts.44 While participants at the beginning of our study period may have been less healthy than those near the end of the study period, relative risk reduction of secondary prevention medications is similar within all individuals with prior MI, re‐emphasizing the need to improve medication adherence across this entire group.
Third, we did not evaluate trends in coronary revascularization because such data are not available through NHANES, but this may influence rates of medication use, particularly aspirin. Fourth, our sample size is relatively small, which may influence our ability to detect significant trends due to lack of power. Since the United States does not have a national surveillance system to detect nonfatal cardiovascular events, our NHANES analysis provides the available representative estimates of MI secondary prevention. Finally, we are unable to address changes in risk factor treatment and control in MI secondary prevention as a result of updated guidelines and recommendations released subsequent to 2012,12 as NHANES data beyond 2012 are not currently available. The shift in recommendations for treatment of cholesterol may require re‐definition of treatment and control. In our analysis we assess adequacy of MI secondary prevention based on the recommendations in place at the time of data collection, and given the notably suboptimal projections we observed it will be interesting to note whether the new recommendations will help improve treatment and control rates with proven effective therapies.
Conclusions
We present contemporary data from NHANES on MI secondary prevention treatment and control rates for blood pressure, cholesterol, and glucose and use of aspirin therapy. While treatment and control of cholesterol improved over the past decade and is projected to reach previously established goals by 2020, similar results were not found for blood pressure management or aspirin use. These treatment and control gaps occurred under previous iterations of risk factor management guidelines and represent an important focus for targeted efforts to prevent major adverse events in a high‐risk population.
Supplementary Material
Table S1. 1999-2012 cholesterol trends and projection to 2020 in NHANES participants reporting prior MI, by race
Table S2. 1999-2012 blood pressure trends and projection to 2020 in NHANES participants reporting prior MI, by race
Table S3. 1999-2012 blood glucose trends and projection to 2020 in NHANES participants reporting prior MI, by race
Table S4. 1999-2012 cardiovascular medication use within sex and race groups in NHANES participants reporting prior MI
Sources of Funding
Shah was supported by a 2013 Student Scholarship in Cardiovascular Disease from the American Heart Association.
Disclosures
Huffman is supported by a NHLBI Pathway to Independence award (1 R00 HL107749) and has received grant support from the World Heart Federation's Emerging Leaders Program, which is supported by unrestricted educational grants from AstraZeneca, Boehringer Ingelheim, and Bupa Foundation.
References
- Centers for Disease Control and Prevention. Leading causes of death. 2013.
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2014 update. Circulation. 2014; 129:e28-e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin SM, Geiss LS, Pan L, Engelgau MM, Greenlund KJ. Self‐reported heart disease and stroke among adults with and without diabetes—United States, 1999–2001. MMWR. 2003; 58:1065-1070. [PubMed] [Google Scholar]
- Smith SC, Jr, Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, Grundy SM, Hiratzka L, Jones D, Krumholz HM, Mosca L, Pearson T, Pfeffer MA, Taubert KA. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease. J Am Coll Cardiol. 2006; 47:2130-2139. [DOI] [PubMed] [Google Scholar]
- Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001; 285:2486-2497. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003; 42:1206-1252. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Healthy people 2020. 2014.
- U.S. Department of Health and Human Services. Million hearts. 2014.
- Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010; 121:586-613. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Suri MF, Guterman LR, Hopkins LN. Ineffective secondary prevention in survivors of cardiovascular events in the US population: report from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2001; 161:1621-1628. [DOI] [PubMed] [Google Scholar]
- Muntner P, DeSalvo KB, Wildman RP, Raggi P, He J, Whelton PK. Trends in the prevalence, awareness, treatment, and control of cardiovascular disease risk factors among noninstitutionalized patients with a history of myocardial infarction and stroke. Am J Epidemiol. 2006; 163:913-920. [DOI] [PubMed] [Google Scholar]
- Stone NJ, Robinson J, Lichtenstein AH, Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2013. 10.1161/01.cir.0000437738.63853.7a [Google Scholar]
- James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014; 311:507-520. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. National Health and Nutrition Examination Survey Data. 2011.
- Huffman MD, Capewell S, Ning H, Shay CM, Ford ES, Lloyd‐Jones DM. Cardiovascular health behavior and health factor changes (1988–2008) and projections to 2020: results from the National Health and Nutrition Examination Surveys. Circulation. 2012; 125:2595-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003; 290:199-206. [DOI] [PubMed] [Google Scholar]
- Muntner P, Levitan EB, Brown TM, Sharma P, Zhao H, Bittner V, Glasser S, Kilgore M, Yun H, Woolley JM, Farkouh ME, Rosenson RS. Trends in the prevalence, awareness, treatment and control of high low density lipoprotein‐cholesterol among United States adults from 1999–2000 through 2009–2010. Am J Cardiol. 2013; 112:664-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE, Lewis B, Rifkind BM. The value of lowering cholesterol after myocardial infarction. N Engl J Med. 1990; 323:1112-1119. [DOI] [PubMed] [Google Scholar]
- LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease: a meta‐analysis of randomized controlled trials. JAMA. 1999; 282:2340-2346. [DOI] [PubMed] [Google Scholar]
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005; 366:1267-1278. [DOI] [PubMed] [Google Scholar]
- Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010; 376:1670-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naci H, Brugts JJ, Fleurence R, Tsoi B, Toor H, Ades AE. Comparative benefits of statins in the primary and secondary prevention of major coronary events and all‐cause mortality: a network meta‐analysis of placebo‐controlled and active‐comparator trials. Eur J Prev Cardiol. 2013; 20:641-657. [DOI] [PubMed] [Google Scholar]
- Rosendorff C, Black HR, Cannon CP, Gersh BJ, Gore J, Izzo JL, Jr, Kaplan NM, O'Connor CM, O'Gara PT, Oparil S. Treatment of hypertension in the prevention and management of ischemic heart disease. Circulation. 2007; 115:2761-2788. [DOI] [PubMed] [Google Scholar]
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009; 338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SC, Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd‐Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update. J Am Coll Cardiol. 2011; 58:2432-2446. [DOI] [PubMed] [Google Scholar]
- Leander K, Wiman B, Hallqvist J, Andersson T, Ahlbom A, de Faire U. Primary risk factors influence risk of recurrent myocardial infarction/death from coronary heart disease: results from the Stockholm Heart Epidemiology Program (SHEEP). Eur J Cardiovasc Prev Rehabil. 2007; 14:532-537. [DOI] [PubMed] [Google Scholar]
- Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008; 358:2560-2572. [DOI] [PubMed] [Google Scholar]
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008; 359:1577-1589. [DOI] [PubMed] [Google Scholar]
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009; 360:129-139. [DOI] [PubMed] [Google Scholar]
- Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002; 324:71-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta‐analysis of individual participant data from randomised trials. Lancet. 2009; 373:1849-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). JAMA. 1993; 269:3015-3023. [PubMed] [Google Scholar]
- Hawkins NM, Scholes S, Bajekal M, Love H, O'Flaherty M, Raine R, Capewell S. The UK National Health Service: delivering equitable treatment across the spectrum of coronary disease. Circ Cardiovasc Qual Outcomes. 2013; 6:208-216. [DOI] [PubMed] [Google Scholar]
- Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta‐analysis on 376,162 patients. Am J Med. 2012; 125:882-887.e881. [DOI] [PubMed] [Google Scholar]
- Eagle KA, Kline‐Rogers E, Goodman SG, Gurfinkel EP, Avezum A, Flather MD, Granger CB, Erickson S, White K, Steg PG. Adherence to evidence‐based therapies after discharge for acute coronary syndromes: an ongoing prospective, observational study. Am J Med. 2004; 117:73-81. [DOI] [PubMed] [Google Scholar]
- Desai NR, Choudhry NK. Impediments to adherence to post myocardial infarction medications. Curr Cardiol Rep. 2013; 15:322. [DOI] [PubMed] [Google Scholar]
- Ye S, Rieckmann N, Kronish IM, Harlapur M, Burg MM, Schwartz JE, Chaplin WF, Davidson KW. Polypharmacy is a predictor of medication non‐adherence after acute coronary syndrome: an electronic medication monitoring study. Circulation. 2010; 122:A14790 [Google Scholar]
- Schroeder K, Fahey T, Ebrahim S. Interventions for improving adherence to treatment in patients with high blood pressure in ambulatory settings. Cochrane Database Syst Rev. 2004:CD004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedlbauer A, Davies P, Fahey T. Interventions to improve adherence to lipid lowering medication. Cochrane Database Syst Rev. 2010:CD004371. [DOI] [PubMed] [Google Scholar]
- Machon M, Arriola L, Larranaga N, Amiano P, Moreno‐Iribas C, Agudo A, Ardanaz E, Barricarte A, Buckland G, Chirlaque MD, Gavrila D, Huerta JM, Martinez C, Molina E, Navarro C, Quiros JR, Rodriguez L, Sanchez MJ, Gonzalez CA, Dorronsoro M. Validity of self‐reported prevalent cases of stroke and acute myocardial infarction in the Spanish cohort of the EPIC study. J Epidemiol Community Health. 2013; 67:71-75. [DOI] [PubMed] [Google Scholar]
- Sappok T, Faulstich A, Stuckert E, Kruck H, Marx P, Koennecke HC. Compliance with secondary prevention of ischemic stroke: a prospective evaluation. Stroke. 2001; 32:1884-1889. [DOI] [PubMed] [Google Scholar]
- Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998; 97:1837-1847. [DOI] [PubMed] [Google Scholar]
- Verschuren WM, Jacobs DR, Bloemberg BP, Kromhout D, Menotti A, Aravanis C, Blackburn H, Buzina R, Dontas AS, Fidanza F, Karvonen MJ, Nedelijkovic S, Nissinen A, Toshima H. Serum total cholesterol and long‐term coronary heart disease mortality in different cultures. Twenty‐five‐year follow‐up of the seven countries study. JAMA. 1995; 274:131-136. [PubMed] [Google Scholar]
- Schober SE, Carroll MD, Lacher DA, Hirsch R. High serum total cholesterol—an indicator for monitoring cholesterol lowering efforts: U.S adults, 2005–2006. NCHS Data Brief. 2007; 2:1-7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. 1999-2012 cholesterol trends and projection to 2020 in NHANES participants reporting prior MI, by race
Table S2. 1999-2012 blood pressure trends and projection to 2020 in NHANES participants reporting prior MI, by race
Table S3. 1999-2012 blood glucose trends and projection to 2020 in NHANES participants reporting prior MI, by race
Table S4. 1999-2012 cardiovascular medication use within sex and race groups in NHANES participants reporting prior MI


