Abstract
Scleroderma is an autoimmune disease characterized by extracellular matrix deposition and inflammation. Topical vitamin D analogs have been reported as effective treatments for scleroderma. We previously reported that a matricellular protein, periostin (POSTN), contributes to pathogenesis of scleroderma as POSTN knockout mice were resistant to bleomycin (BLM)-induced scleroderma. We investigated whether a vitamin D analog affects the expression of POSTN in dermal fibroblasts and in a BLM-induced scleroderma model. The vitamin D analog, maxacalcitol (22-oxacalcitriol [OCT]), was applied to dermal fibroblasts and POSTN expression was measured. The effect of OCT on Th2 cytokine- and TGFβ-induced POTSN and Collagen 1 α 1 (Col1A1) expression was also assessed. In vivo, OCT was administered to BLM-induced scleroderma model and outcomes were determined by dermal thickness, collagen density and POSTN expression. Treatment with OCT significantly decreased POSTN expression in dermal fibroblasts. Th2 cytokine- and TGFβ-induced expression of POSTN and Col1A1 was also suppressed by OCT. In vivo, OCT administration decreased the density of collagen bundles and POSTN expression in a BLM-induced scleroderma model. In addition to the previously reported immunosuppressive effect, the vitamin D analog OCT might be effective to treat scleroderma, in part through inhibition of Th2 cytokine- and TGFβ-induced POSTN expression.
Keywords: bleomycin, interleukin-6, interleukin-4, periostin, scleroderma, vitamin D
Abbreviations
- LS
localized scleroderma
- SSc
systemic sclerosis
- 25OH-D3
25-hydroxyvitamin D3
- POSTN
periostin
- BLM
bleomycin
- OCT
Maxacalcitol (1α, 25-(OH 2-22-oxavitaminD3)
- NHDFs
normal human dermal fibroblasts
Introduction
Scleroderma is an autoimmune disease characterized by extracellular matrix deposition and inflammation. Scleroderma may be further sub-categorized into localized scleroderma (LS), limited cutaneous systemic sclerosis, diffuse cutaneous systemic sclerosis, and systemic sclerosis (SSc).1 The etiologies of scleroderma are not completely understood. However, a combination of genetics and environmental factors is thought to cause the disease.2
Low serum levels of 25-hydroxyvitamin D3 (25OH-D3) have been reported in patients with SSc.3-6 The average concentration of 25OH-D3 was significantly lower in patients with a high degree of skin involvement (Rodnan skin score > 10) compared with patients with low degree of skin involvement (Rodnan skin score ≤ 10).3 The effects of systemic vitamin D supplementation in SSc have been evaluated in some studies, however, the results vary between studies.7-9
The effects of topical vitamin D analogs have also been reported to improve scleroderma in LS patients.10-13 In one study, calcipotriol, a derivative of vitamin D, inhibited the proliferation of fibroblasts derived from LS patients.14 These data suggest that inhibition of fibroblast proliferation and collagen synthesis is one mechanism by which vitamin D acts to treat LS. Recently, we reported that periostin (POSTN), a matricellular protein, plays an important role in scleroderma.15 The expression of POSTN was increased in the skin of SSc patients. In addition, POSTN knockout mice were resistant to bleomycin (BLM)-induced scleroderma.15 Serum POSTN levels are also reported to correlate with progressive skin sclerosis in patients with SSc.16 Furthermore, histamine is reported to induce collagen expression through POSTN.17 Finally, the Th2 cytokines play an important role as inducers of POSTN in chronic allergic inflammation and in subepithelial fibrosis of bronchial asthma.18,19
In this study, we investigated the effect of a vitamin D3 analog, maxacalcitol (22-oxacalcitriol [OCT]), on Th2 cytokine-induced fibrosis. OCT is the most used vitamin D3 analog in Japan, which displays approximately 10 times greater efficacy at suppressing keratinocyte proliferation in vitro compared with calcipotriol and tacalcitol.20 We found that OCT significantly suppressed the expression of IL-4 and IL-13-induced POSTN in dermal fibroblasts. We hypothesize that decreased POSTN expression might be one of the mechanisms by which topical treatment with a vitamin D analog is effective in scleroderma, especially in LS.
Material and Methods
Cell culture
NHDFs were purchased from DS Pharma Biomedical (Osaka, Japan). NHDFs were cultured in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% fetal bovine serum (FBS). Isolation and culture of mouse fibroblasts were carried out as previously described.21 Full-thickness skin harvested from day 2 to day 4 newborn mice was treated with 4 mg/ml of dispase (Gibco; Invitrogen, Paisley, UK) for 1 h at 37°C. Next, the epidermis was peeled from the dermis. The dermis was placed in DMEM+0.05% type-1 collagenase (Sigma- Aldrich, St Louis, MO, USA) and incubated at 37°C for 30 min with vigorous agitation to prepare single cells. After filtration, cells were centrifuged at 200 g for 10 min, resuspended in DMEM+10% FBS, and then incubated at 37°C and 5% CO2. The fibroblasts used for experiments came from the first or second passages.
Mice
Eight-week-old male Hos: HR-1 mice (hairless mice) were obtained from Japan SLC, Inc. (Osaka, Japan). Animal care was in strict accordance with the institutional guidelines of Osaka University. All of the animal experiments were carried out with the approval of the Animal Experiments Committee of Osaka University (#20-003-0). Bleomycin (Nippon Kayaku, Tokyo, Japan) was dissolved in phosphate-buffered saline (PBS) at a concentration of 1 mg/ml. Injections of 100 μl of BLM or PBS were administered to subcutaneously to the dorsal area 3 times per week for 3 weeks. One day after final BLM injection, 100 μl of 10−7 M OCT diluted in ethanol, which was further diluted with PBS (1:1000), were subcutaneously injected to BLM-induced sclerotic skin per day for continuous 5 d. As a vehicle control, ethanol diluted in PBS (1:1000) was injected. OCT was kindly provided by Chugai Pharma, Ltd (Tokyo, Japan).
Histopathological analysis
The dorsal skin was removed one day after the final injection of OCT. The skin pieces were fixed in 10% formaldehyde for 24 h, then embedded in paraffin for microtome sectioning. Sections (4 μm thick) were stained with hematoxylin and eosin (H&E). The amount of collagen was evaluated after tissue staining with the Masson's trichrome technique. For immunohistochemical analysis, sections were hydrated by passage through xylene and graded ethanol. Next, slides were blocked with 2% bovine serum albumin for 10 min, and then stained with primary antibody for 60 min (rabbit anti-POSTN 1:2000 dilution, Abcam, Cambridge, UK). After washing with TBS containing 0.05% Triton-X100 (TBST), slides were developed using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA) followed by counterstaining with hematoxylin. Rabbit IgG was used as the isotype control.
Western blot analysis
Cells or skin samples crushed in liquid nitrogen were solubilized at 4°C in lysis buffer (0.5% sodium deoxycholate, 1% Nonidet P40, 0.1% sodium dodecyl sulfate, 100 μg/ml phenylmethylsulphonyl fluoride, and protease inhibitor cocktail). Five micrograms of protein were separated on SDS-polyacrylamide gels and transferred onto polyvinylidine fluoride membranes (Bio-Rad, Hercules, CA USA). Non-specific antibody binding was blocked by incubating the membranes in 5% w/v non-fat milk powder in TBS-T (50 mM Tris-HCl, pH 7.6, 150 mM NaCl, and 0.1% v/v Tween-20). The membranes were incubated with rabbit anti-POSTN antibody (Abcam, Cambridge, UK) diluted 1:1000 overnight at 4°C or with mouse anti-β-actin antibody (Sigma- Aldrich, St Louis, MO, USA) diluted 1:10,000 overnight at 4°C. Then, the membranes were washed 3 times in TBS-T for 5 min. Finally, the membranes were incubated with either HRP-conjugated anti-mouse or anti-rabbit at a dilution of 1:10,000 for 60 min at room temperature. Protein bands were detected using the ECL Plus kit (Thermo Scientific, Rockford, IL USA). The intensity of the bands was quantified using NIH image J software.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from cells using the SV Total RNA Isolation System (Promega). The product was reverse-transcribed into first-strand cDNA (cDNA). qRT-PCR was performed on a ABI 7000 Prism (Applied Biosystems) using the Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's protocol. The cycling conditions were as follows: 40 cycles of denaturation at 92°C for 15 sec and annealing at 60°C for 60 sec. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene control. Sequence-specific primers were designed as follows: mouse Col1A1, sense 5′-gagccctcgcttccgtactc-3′, antisense 5′-tgttccctactcagccgtctgt-3′; mouse POSTN, sense 5′-aaccaaggacctgaaacacg-3′, antisense 5′- caaagagcgtgaagtgacca-3′; mouse IL-6, sense 5′- ctgatgctggtgacaaccac-3′, antisense 5′- cagaattgccattgcacaac-3′; human POSTN, sense 5′- gtgatccatggagagccaat-3′, antisense 5′- aacttcctcacgggtgtgtc-3′; mouse GAPDH, sense 5′- tgtcatcatacttggcaggtttct-3′, antisense 5′-catggccttccgtgttccta-3′; human GAPDH, sense 5′- gcaacaatatccactttaccagagttaa-3′, antisense 5′-ggagtcaacggatttggtcgta-3′.
Statistical analysis
The data are expressed as mean values ± standard deviations (SD). The Student's t-test was used to determine the level of significance of differences between the 2 groups. Analysis of variance for the groups was performed by ANOVA, followed by the Bonferroni-Dunn for multiple comparisons to allow pairwise testing for significant differences between groups. Statistical significance was defined as P < 0.05.
Results
OCT decreased the expressions of POSTN and IL-6 in mouse dermal fibroblasts in a dose-dependent manner
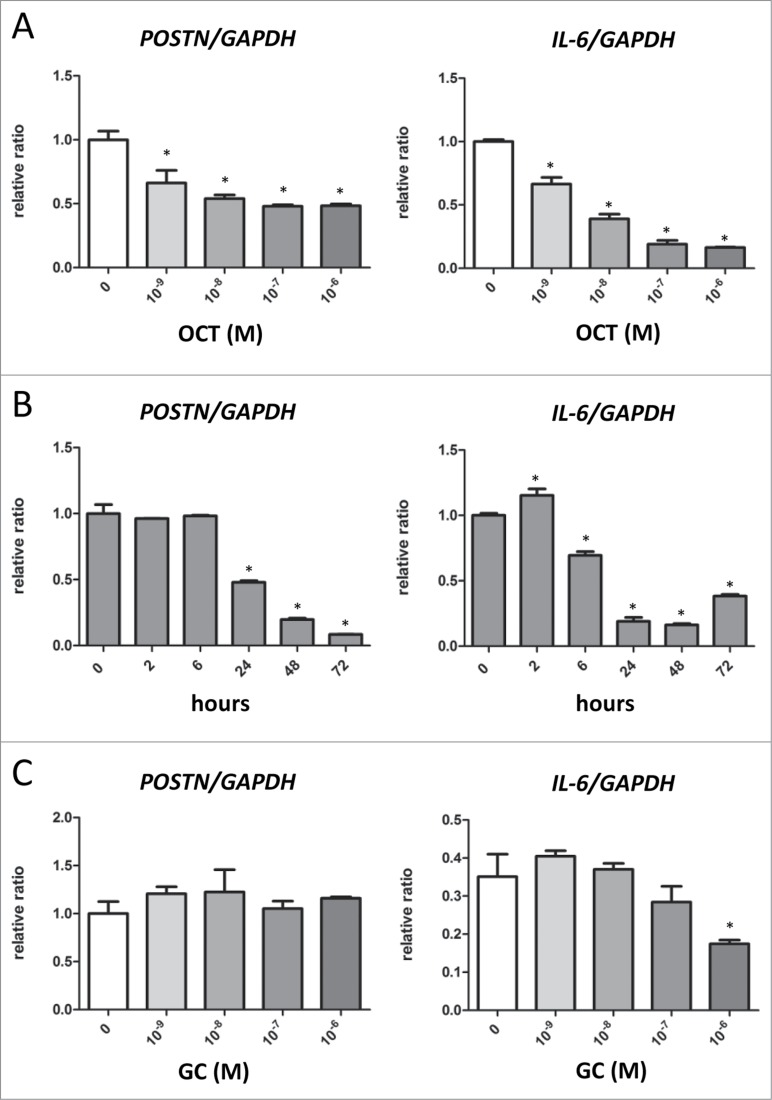
First, we evaluated the expressions of POSTN and IL-6 in mouse dermal fibroblasts treated with OCT. The doses of OCT treatment ranged from 10−9 M to 10−6 M. We observed a dose-dependent downregulation of POSTN and IL-6 in response to OCT. (Fig. 1A). Additionally, treatment with 10−7 M of OCT caused a time dependent decrease in the expression of POSTN from 24 h and IL-6 from 6 h (Fig. 1B). In contrast, treatment with cortisol ranging in doses from 10−9 M to 10−6 M did not alter the expression of POSTN (Fig. 1C). In contrast, cortisol at dose of 10−6 M decreased the expression of IL-6 (Fig. 1C).
Figure 1.
OCT decreases POSTN and IL-6 expressions in mouse dermal fibroblasts. (A and C) Mouse dermal fibroblasts were treated with indicated doses of OCT (A) or cortisol (GC) (C) for 24 h. Expressions of POSTN and IL-6 was measured by RT-PCR. GAPDH served as an internal control. Bars indicate the mean ± SD. N = 4; *P < 0.0001, one-way ANOVA followed by the Bonferroni-Dunn test for multiple comparisons. (B) Mouse dermal fibroblasts were treated with 10−7 M of OCT for the indicated lengths of time. Expressions of POSTN and IL-6 was measured by RT-PCR. GAPDH served as an internal control. Bars indicate the mean ± SD. N = 4; *P < 0.0001, one-way ANOVA followed by the Bonferroni-Dunn test for multiple comparisons.
OCT inhibits IL-4-, IL-13-, TGFβ-induced expression of POSTN and Collagen 1 α 1 in mouse dermal fibroblasts
IL-4, IL-13 and TGFβ are known to induce the expression of POSTN and reported to associate with fibrosis and remodeling.18 We examined whether OCT could inhibit IL-4-, IL-13-, TGFβ-induced POSTN expression. Indeed, the addition of IL-4, IL-13 and TGFβ increased the expression of POSTN as previously reported (Fig. 2A). When cells were treated with OCT prior to any of these inducing agents, POSTN expression was significantly decreased, as shown by RT-PCR (Fig. 2A) and western blot (Figs. 2B and C). Moreover, Collagen 1 α 1 (Col1A1) expression in the culture supernatant was decreased in groups treated with OCT (Figs. 2D and E).
Figure 2.
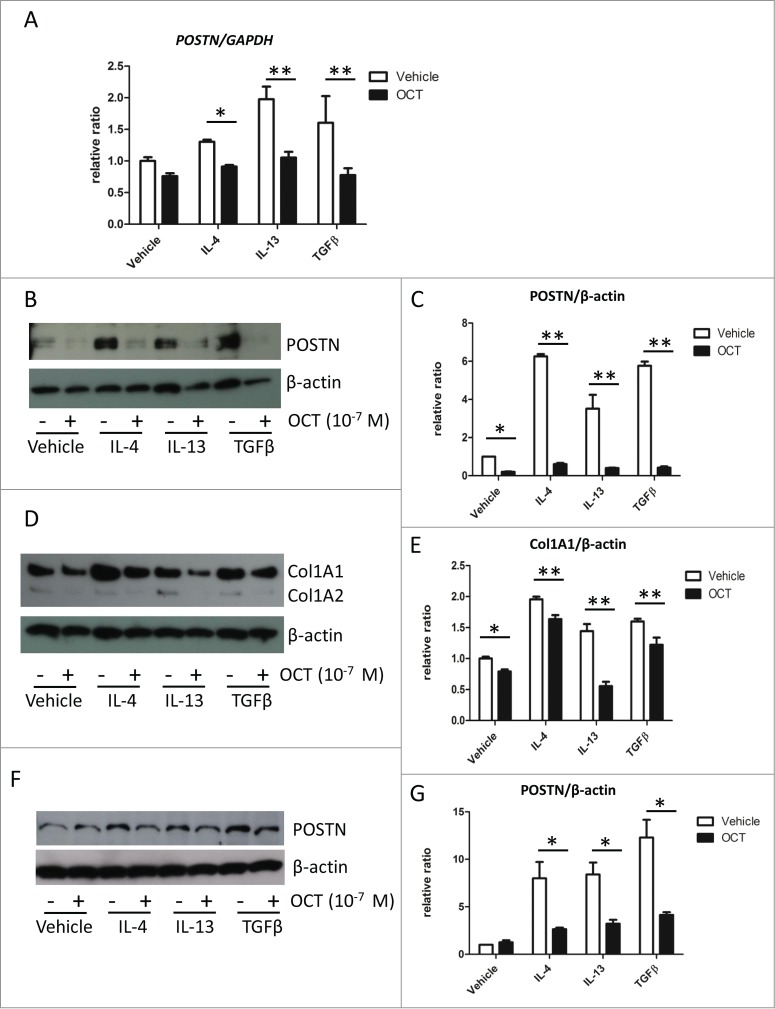
OCT causes downregulation of IL-4-, IL-13-, and TGFβ-induced POSTN and Col1A1 expressions in fibroblasts. (A) Mouse dermal fibroblasts were pre-treated with 10−7 M of OCT for 2 h and treated with IL-4 (10 ng/ml), IL-13 (10 ng/ml), or TGFβ (10 ng/ml) for additional 24 h. Bars indicate the mean ± SD. N = 4; *P < 0.01, **P < 0.0001, 2-way ANOVA followed by the Bonferroni-Dunn test for multiple comparisons. (B) Mouse dermal fibroblasts were treated as in Figure 2A. The expression of POSTN in cell lysates was measured by western blot analysis. β-actin served as an internal control. (C) Bars show the results of densitometric analysis of POSTN relative to β-actin. Mean ± SD of each group are shown. N = 3; *P < 0.0001, 2-way ANOVA followed by the Bonferroni-Dunn test for multiple comparisons. (D) Mouse dermal fibroblasts were treated as in Figure 2A. Expression of Col1A1 and Col1A2 in culture medium was measured by protein gel blot analysis. β-actin served as an internal control. (E) Bars show the densitometric analysis of Col1A1 relative to β-actin. The mean ± SD is shown for each group. N = 3; *P < 0.05, **P < 0.001, 2-way ANOVA followed by the Bonferroni-Dunn test for multiple comparisons. (F) Normal Human dermal fibroblasts were pre-treated with 10−7 M of OCT for 2 h and treated with IL-4 (10 ng/ml), IL-13 (10 ng/ml), or TGFβ (10 ng/ml) for an additional 24 h. Expression of POSTN was measured in cell lysates by western blot analysis. β-actin served as an internal control. (G) Bars show the densitometric analysis of POSTN relative to β-actin. The mean ± SD of each group is shown. N = 3; *P < 0.0001, 2-way ANOVA followed by the Bonferroni-Dunn test for multiple comparisons.
OCT inhibits IL-4-, IL-13-, TGFβ-induced POSTN expression in NHDFs
Next, we evaluated the effects of OCT in normal human dermal fibroblasts (NHDF). Similar to mouse dermal fibroblasts, prior treatment with OCT decreased the IL-4-, IL-13-, TGFβ-induced POSTN expression in NHDF (Figs. 2F and G). However, in contrast to mouse dermal fibroblasts, OCT treatment did not affect basal POSTN level in NHDFs (Fig. 2F).
OCT inhibits BLM-induced scleroderma
Finally, we evaluated the effects of OCT in BLM-induced skin sclerosis model. Subcutaneous injection of BLM induced dermal thickening and thickening of collagen bundles (Figs. 3A and B). We calculated the dermal area as a means of evaluating dermal thickness. While treatment with OCT did not cause a significant decrease in dermal area, collagen bundles were thicker and more densely packed in vehicle-treated mice compared with OCT-treated mice, as observed by Masson's trichrome staining (Figs. 3B and C). Accordingly, collagen area was significantly lower in OCT-treated group compared with vehicle-treated group (Fig. 3D).
Figure 3.
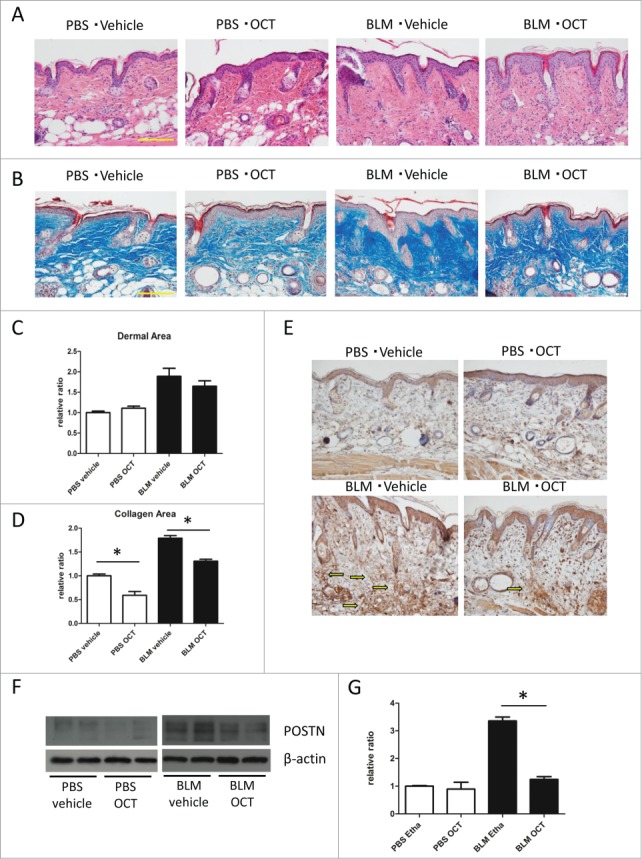
(See previous page). OCT inhibits BLM-induced scleroderma and POSTN induction. (A) Representative H&E staining of back skin treated with PBS or BLM. Bar = 100 μm. (B) Representative Masson's trichrome staining (low magnification and high magnification) of back skin treated with PBS or BLM. Bar = 100 μm. (C) Dermal area of skin sections treated with PBS or BLM. The dermal area in each mouse was measured using Image J. Bars show the mean dermal area ± SD (N = 6). (D) Collagen area of skin sections treated with PBS of BLM. The collagen area in each mouse was measured using Olympus cellSens Dimension software. Bars show the mean dermal area ± SD (N = 6) (E) Representative POSTN staining of back skin treated with PBS of BLM. Arrows show matricellular staining of POSTN. (F) Western blot analysis of POSTN expression in mouse skin treated with either PBS or BLM. (G) Bars show the densitometric analysis of POSTN relative to the β-actin loading control. Mean ± SD of each group are shown. N = 4; *P < 0.0001, one-way ANOVA followed by the Bonferroni-Dunn test for multiple comparisons.
OCT inhibits BLM-induced POSTN induction
The expression of POSTN was induced in BLM-treated scleroderma skin as previously reported.15 We found that the expression of POSTN was significantly decreased in mice treated with OCT as shown by immunohistochemistry (Fig. 3E) and protein gel blot (Fig. 3F and G).
Discussion
The effect of vitamin D in BLM-induced dermal sclerosis has been reported previously.22 In that study, metabolites of vitamin D, such as 20S-hydroxyvitamin D, markedly suppressed BLM-induced fibrogenesis in mice.22 TGFβ-induced collagen and hyaluronan production was inhibited by 20(OH)D3 and 1,25(OH)2D3 in vitro.22 This study, as well as others, implicates vitamin D as an effective treatment for sclerodermas.
In addition to TGFβ, the Th2 cytokines play an important role in fibrogenesis in SSc. Th1 and Th17 cells are predominant in the early stages of inflammation, and Th2 cells are predominant in the late fibrosing stage,.23 IL-4, IL-13, and IL-6, which are secreted by Th2 cells, work as profibrotic mediators.24 IL-6 produced from dermal fibroblasts also contribute to fibrosis by activating fibroblasts to myofibroblasts, which are known to produce collagen.25 In this study, we found that OCT significantly suppressed the expression of not only TGFβ-induced POSTN expression, but also IL-4 and IL-13-induced POSTN expression in dermal fibroblasts. As POSTN is an essential matricellular protein in skin sclerosis of SSc patients and in a BLM-induced scleroderma model,15 we hypothesize that OCT suppresses dermal sclerosis in part by downregulating the expression of POSTN.
In BLM-induced skin sclerosis model, OCT did not markedly affected the thickness of the dermis, however, thinning of the collagen fiber bundles represented by decreased collagen area was observed. These histological findings are very similar with previous findings in scleroderma patients treated with tocilizumab.26 These patients showed softening of the skin with reductions of Vesmeter hardness, and similar histological changes.26 Thickness of the collagen fiber bundles but not dermal thickness may be important in skin softening in scleroderma.
Besides inhibiting fibroblast proliferation and collagen synthesis, vitamin D has immunosuppressive effects. Vitamin D decreases expression of toll-like receptors (TLRs) and suppresses TLR-mediated inflammation in monocytes.27 In peripheral blood mononuclear cells, vitamin D decreases proinflammatory cytokine release.28 Vitamin D also inhibits TNFα- and /or IFNγ-induced IL-8 and IL-6 production in keratinocytes.29,30 Thus, in addition to its effect of suppressing POSTN, topical OCT might be effective on local scleroderma by inhibiting inflammatory cytokine expression.
In this study, we also evaluated the effect of cortisol, the frequently used topical immunosuppressive ointment to treat local sclerosis, on POSTN expression in dermal fibroblasts (Fig. 1C). In contrast to OCT, cortisol did not alter the expression of POSTN in dermal fibroblasts, suggesting that effect of cortisol on sclerosis might not mediate POSTN.
In conclusion, we found that the vitamin D3 analog, OCT, suppressed expression of POSTN that is normally induced by Th2 cytokines and TGFβ in dermal fibroblasts. Because POSTN plays a key role in scleroderma, the effectiveness of OCT in scleroderma might be due to suppression of POSTN expression. We hypothesis that this suppressive effect on POSTN may work synergistically with previously reported immunosuppressive and anti-collagen synthesis effects of vitamin D analogs to improve fibrogenesis in scleroderma.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Mr. Kenju Nishida, Ms. Yumiko Fujii, and Ms. Eriko Nobuyoshi for research assistance.
Funding
This work was supported in part by a Grant-in-Aid for Young Scientists (A) (No. 24689045).
References
- 1. Fett N. Scleroderma: nomenclature, etiology, pathogenesis, prognosis, and treatments: facts and controversies. Clin Dermatol 2013; 31:432-7; PMID:23806160; http://dx.doi.org/ 10.1016/j.clindermatol.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 2. Yamamoto T. Chemokines and chemokine receptors in scleroderma. Int Arch Allergy Immunol 2006; 140:345-56; PMID:16804319; http://dx.doi.org/ 10.1159/000094242 [DOI] [PubMed] [Google Scholar]
- 3. Arnson Y, Amital H, Agmon-Levin N, Alon D, Sanchez-Castanon M, Lopez-Hoyos M, Matucci-Cerinic M, Szucs G, Shapira Y, Szekanecz Z, et al. . Serum 25-OH vitamin D concentrations are linked with various clinical aspects in patients with systemic sclerosis: a retrospective cohort study and review of the literature. Autoimmun Rev 2011; 10:490-4; PMID:21320645; http://dx.doi.org/ 10.1016/j.autrev.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 4. Gambichler T, Chrobok I, Hoxtermann S, Kreuter A. Significantly decreased serum 25-hydroxyvitamin d levels in a large german systemic sclerosis cohort. J Rheumatol 2011; 38:2492-3; author reply 4; PMID:22045936; http://dx.doi.org/ 10.3899/jrheum.110695 [DOI] [PubMed] [Google Scholar]
- 5. Vacca A, Cormier C, Mathieu A, Kahan A, Allanore Y. Vitamin D levels and potential impact in systemic sclerosis. Clin Exp Rheumatol 2011; 29:1024-31; PMID:22011638 [PubMed] [Google Scholar]
- 6. Caramaschi P, Dalla Gassa A, Ruzzenente O, Volpe A, Ravagnani V, Tinazzi I, Barausse G, Bambara LM, Biasi D. Very low levels of vitamin D in systemic sclerosis patients. Clin Rheumatol 2010; 29:1419-25; PMID:20454816; http://dx.doi.org/ 10.1007/s10067-010-1478-3 [DOI] [PubMed] [Google Scholar]
- 7. Antico A, Tampoia M, Tozzoli R, Bizzaro N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun Rev 2012; 12:127-36; PMID:22776787; http://dx.doi.org/ 10.1016/j.autrev.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 8. Hulshof MM, Bouwes Bavinck JN, Bergman W, Masclee AA, Heickendorff L, Breedveld FC, Dijkmans BA. Double-blind, placebo-controlled study of oral calcitriol for the treatment of localized and systemic scleroderma. J Am Acad Dermatol 2000; 43:1017-23; PMID:11100017; http://dx.doi.org/ 10.1067/mjd.2000.108369 [DOI] [PubMed] [Google Scholar]
- 9. Caca-Biljanovska NG, Vlckova-Laskoska MT, Dervendi DV, Pesic NP, Laskoski DS. Treatment of generalized morphea with oral 1,25-dihydroxyvitamin D3. Adv Exp Med Biol 1999; 455:299-304; PMID:10599359; http://dx.doi.org/ 10.1007/978-1-4615-4857-7_44 [DOI] [PubMed] [Google Scholar]
- 10. Kieffer MA. Topical vitamin D analogs. Dermatol Nurs 2004; 16:89-90, 3, 100; PMID:15022510 [PubMed] [Google Scholar]
- 11. Tay YK. Topical calcipotriol ointment in the treatment of morphea. J Dermatolog Treat 2003; 14:219-21; PMID:14660267; http://dx.doi.org/ 10.1080/09546630310015449 [DOI] [PubMed] [Google Scholar]
- 12. Cunningham BB, Landells ID, Langman C, Sailer DE, Paller AS. Topical calcipotriene for morphea/linear scleroderma. J Am Acad Dermatol 1998; 39:211-5; PMID:9704831; http://dx.doi.org/ 10.1016/S0190-9622(98)70077-5 [DOI] [PubMed] [Google Scholar]
- 13. Koeger AC, Rozenberg S, Fautrel B. Effectiveness of topical calcitriol for localized scleroderma. J Rheumatol 1999; 26:239-40; PMID:9918276 [PubMed] [Google Scholar]
- 14. Bottomley WW, Jutley J, Wood EJ, Goodfield MD. The effect of calcipotriol on lesional fibroblasts from patients with active morphoea. Acta Derm Venereol 1995; 75:364-6; PMID:8615053 [DOI] [PubMed] [Google Scholar]
- 15. Yang L, Serada S, Fujimoto M, Terao M, Kotobuki Y, Kitaba S, Matsui S, Kudo A, Naka T, Murota H, et al. . Periostin facilitates skin sclerosis via PI3K/Akt dependent mechanism in a mouse model of scleroderma. PloS one 2012; 7:e41994; PMID:22911870; http://dx.doi.org/ 10.1371/journal.pone.0041994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamaguchi Y, Ono J, Masuoka M, Ohta S, Izuhara K, Ikezawa Z, Aihara M, Takahashi K. Serum periostin levels are correlated with progressive skin sclerosis in patients with systemic sclerosis. The British journal of dermatology 2013; 168:717-25; PMID:23110679; http://dx.doi.org/ 10.1111/bjd.12117 [DOI] [PubMed] [Google Scholar]
- 17. Yang L, Murota H, Serada S, Fujimoto M, Kudo A, Naka T, Katayama I. Histamine contributes to tissue remodeling via periostin expression. J Invest Dermatol 2014; 134(8):2105-13; PMID:24577408; http://dx.doi.org/ 10.1038/jid.2014.120 [DOI] [PubMed] [Google Scholar]
- 18. Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, Toda S, Inagaki N, Kurihara Y, Hayashida S, et al. . Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest 2012; 122:2590-600; PMID:22684102; http://dx.doi.org/ 10.1172/JCI58978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol 2006; 118:98-104; PMID:16815144; http://dx.doi.org/ 10.1016/j.jaci.2006.02.046 [DOI] [PubMed] [Google Scholar]
- 20. Barker JN, Ashton RE, Marks R, Harris RI, Berth-Jones J. Topical maxacalcitol for the treatment of psoriasis vulgaris: a placebo-controlled, double-blind, dose-finding study with active comparator. Br J Dermatol 1999; 141:274-8; PMID:10468799; http://dx.doi.org/ 10.1046/j.1365-2133.1999.02975.x [DOI] [PubMed] [Google Scholar]
- 21. Terao M, Murota H, Kitaba S, Katayama I. Tumor necrosis factor-alpha processing inhibitor-1 inhibits skin fibrosis in a bleomycin-induced murine model of scleroderma. Exp Dermatol 2010; 19:38-43; PMID:19758314; http://dx.doi.org/ 10.1111/j.1600-0625.2009.00973.x [DOI] [PubMed] [Google Scholar]
- 22. Slominski A, Janjetovic Z, Tuckey RC, Nguyen MN, Bhattacharya KG, Wang J, Li W, Jiao Y, Gu W, Brown M, et al. . 20S-hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. J Clin Endocrinol Metab 2013; 98:E298-303; PMID:23295467; http://dx.doi.org/ 10.1210/jc.2012-3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kurzinski K, Torok KS. Cytokine profiles in localized scleroderma and relationship to clinical features. Cytokine 2011; 55:157-64; PMID:21536453; http://dx.doi.org/ 10.1016/j.cyto.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O'Reilly S, Hugle T, van Laar JM. T cells in systemic sclerosis: a reappraisal. Rheumatology (Oxford) 2012; 51:1540-9; PMID:22577083; http://dx.doi.org/ 10.1093/rheumatology/kes090 [DOI] [PubMed] [Google Scholar]
- 25. Kitaba S, Murota H, Terao M, Azukizawa H, Terabe F, Shima Y, Fujimoto M, Tanaka T, Naka T, Kishimoto T, et al. . Blockade of interleukin-6 receptor alleviates disease in mouse model of scleroderma. Am J Pathol 2012; 180:165-76; PMID:22062222; http://dx.doi.org/ 10.1016/j.ajpath.2011.09.013 [DOI] [PubMed] [Google Scholar]
- 26. Shima Y, Kuwahara Y, Murota H, Kitaba S, Kawai M, Hirano T, Arimitsu J, Narazaki M, Hagihara K, Ogata A, et al. . The skin of patients with systemic sclerosis softened during the treatment with anti-IL-6 receptor antibody tocilizumab. Rheumatology (Oxford) 2010; 49:2408-12; PMID:20819796; http://dx.doi.org/ 10.1093/rheumatology/keq275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, Zugel U, Steinmeyer A, Pollak A, Roth E, et al. . Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol 2006; 36:361-70; PMID:16402404; http://dx.doi.org/ 10.1002/eji.200425995 [DOI] [PubMed] [Google Scholar]
- 28. Khoo AL, Chai LY, Koenen HJ, Sweep FC, Joosten I, Netea MG, van der Ven AJ. Regulation of cytokine responses by seasonality of vitamin D status in healthy individuals. Clin Exp Immunol 2011; 164:72-9; PMID:21323660; http://dx.doi.org/ 10.1111/j.1365-2249.2010.04315.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koizumi H, Kaplan A, Shimizu T, Ohkawara A. 1,25-Dihydroxyvitamin D3 and a new analogue, 22-oxacalcitriol, modulate proliferation and interleukin-8 secretion of normal human keratinocytes. J Dermatol Sci 1997; 15:207-13; PMID:9302649; http://dx.doi.org/ 10.1016/S0923-1811(97)00609-9 [DOI] [PubMed] [Google Scholar]
- 30. Komine M, Watabe Y, Shimaoka S, Sato F, Kake K, Nishina H, Ohtsuki M, Nakagawa H, Tamaki K. The action of a novel vitamin D3 analogue, OCT, on immunomodulatory function of keratinocytes and lymphocytes. Arch Dermatol Res 1999; 291:500-6; PMID:10541880; http://dx.doi.org/ 10.1007/s004030050444 [DOI] [PubMed] [Google Scholar]