Abstract
Gastric diverticulum (GD) is a pouch protruding from the gastric wall. It is rare and in the majority of cases, asymptomatic. Usually GDs are detected incidentally by gastrointestinal study. The current study reports one case and a literature review of GD mimicking an adrenal mass. A 49-year-old male presented with a low-density lesion in the left adrenal area, but no symptoms of an adrenal adenoma. The lesion was identified as a gastric diverticulum in the fundus of the stomach following enhancement of computed tomography (CT) imaging using an oral contrast agent. Although the majority of previously reported cases utilized upper gastrointestinal barium studies to confirm the presence of GD, the current study demonstrated that CT scanning enhanced with oral contrast material may aid in the differentiation of adrenal masses from other gastrointestinal abnormalities.
Keywords: gastric diverticulum, adrenal incidentaloma, left adrenal mass
Introduction
Gastric diverticulum (GD) is a rare condition, with a prevalence of 0.04 and 0.02% in upper gastrointestinal and autopsy studies, respectively (1). GD is characterized by the presence of a pouch protruding from the gastric wall (1,2), however, the majority of GD cases are asymptomatic and are diagnosed incidentally during routine examinations (1). GDs are classified into congenital and acquired types, of which, congenital types are more common. Congenital GDs, located in the retroperitoneal space, may be explained by embryogenesis. Acquired GDs are pseudodiverticula and are typically located in the gastric antrum. Acquired GDs are usually associated with gastrointestinal diseases, such as, peptic ulcers, gastric outlet obstruction or other malignancies (1). Although there is currently no specific treatment for asymptomatic GD, symptomatic GD requires appropriate management, which depends on the underlying pathology and the severity of the presentation. In symptomatic patients, typical symptoms include upper abdominal and epigastric pain, anorexia, nausea and dysphagia (3). The first line therapy includes a comprehensive examination for underlying pathology and medical treatment, such as protein pump inhibitors, antacids, or antispasmodics (4). When the diverticulum is large, has not responded to medical therapy, or is complicated by bleeding, perforation or other malignancies, surgical resectioning is recommended (5). With prompt management, GDs may be treated successfully without further complications.
Computed tomography (CT) imaging may detect the presence of GDs as thin-walled cystic masses in the left adrenal area. Conducting scans with the patient in a prone position may further aid diagnosis, by forcing gastric air into the diverticulum cavity, leading to the formation of an air-fluid level in the mass (6). However, sole reliance on CT imaging has been demonstrated to lead to the misdiagnosis of gastric diverticula as adrenal tumors (7–15). The current study reports the case of a 49-year-old male patient whose initial abdominal CT scan indicated the presence of an adrenal mass. A systematic literature review was also performed to investigate the reasons for incorrect interpretation, and how this may be avoided in future cases. Written informed consent was obtained from the patient.
Case report
A 49-year-old male was admitted to The First Affiliated Hospital, Zhejiang University School of Medicine (Hangzhou, China) for further assessment of a left adrenal lesion which had been identified incidentally at a routine physical examination one month previously. The patient underwent an upper abdominal CT scan with intravenous contrast. A low-density mass measuring 2.3 cm in diameter was observed in the area of the left adrenal gland (Fig. 1). However, the patient did not exhibit any clinical features and symptoms typically associated with adrenal adenomas, which include hypertension, hypokalemia and symptoms of hypercortisolism. Furthermore, laboratory tests revealed that the basal levels of plasma adrenocorticotropin, cortisol, aldosterone, renin, testosterone, adrenaline, noradrenaline and dopamine were within the normal range. A moderate elevation of urinary free cortisol (345.4 µg/day; normal, 24–268 µg/day) was detected, which decreased by >50% following treatment with low dose dexamethasone. The results of all other laboratory examinations were normal.
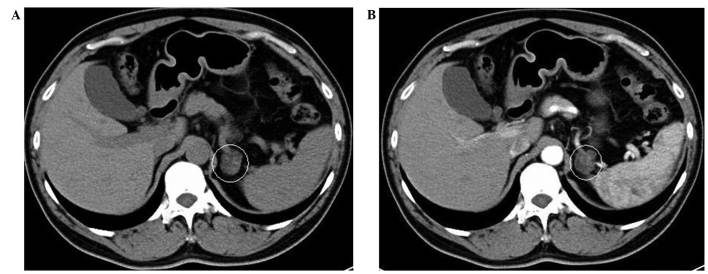
Figure 1.
(A) Abdominal computed tomography imaging revealed a low-density mass measuring 2.3 cm in diameter in the area of the left adrenal gland. (B) Following administration of intravenous contrast, the mass was not remarkably enhanced.
The patient underwent several ultrasonography examinations to visualize and evaluate the presumptive adrenal mass, with negative results. A subsequent thorough review of the data, including all prior CT images, revealed that the abnormality was only visible in a small number of the CT imaging slices, and appeared to be associated with the stomach. Oral contrast was administered during a repeat abdominal CT scan in order to assess whether the mass originated from the gastrointestinal tract. Visualization of the mass was enhanced by the oral contrast material shortly after staining the stomach and duodenum (Fig. 2). The results showed the presence of a diverticulum in the fundus of the stomach. No treatment was administered as the patient was asymptomatic. The patient was followed up regularly and underwent an annual upper gastrointestinal (GI) barium study. At the time of writing the patient had been followed for more than three years and their condition was stable.
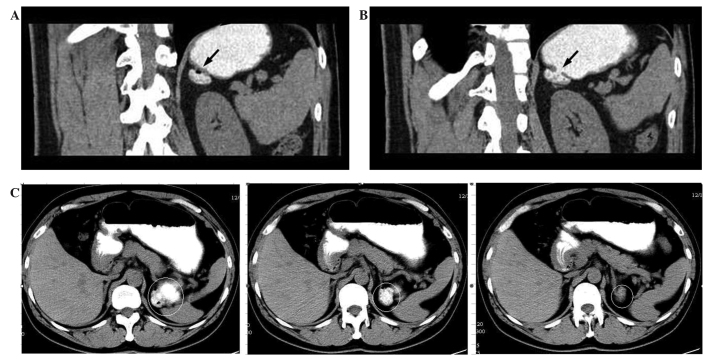
Figure 2.
Abdominal CT scan following administration of oral contrast. (A) The adrenal mass was enhanced by oral contrast material shortly after staining the stomach and duodenum, and a bubble/shadow was observed within the mass (arrow). (B) Another CT image showed a connection between the adrenal mass and the stomach (arrow). (C) Transverse view following administration of oral contrast agent shortly after staining the stomach and duodenum. CT, computed tomography.
Discussion
In the present case of gastric diverticulum, a misdiagnosis was initially determined on the basis of a hypodense component observed in the initial CT images. However, laboratory findings were normal and ultrasonography examinations did not detect an adrenal mass. Oral contrast material was administered during a repeat abdominal CT scan, subsequently revealing the presence of a diverticulum in the fundus of the stomach.
Reliance on CT scans in the absence of upper gastrointestinal examinations may lead to misdiagnosis in cases of GD, as this disorder, in addition to a number of normal structures, anatomic variations, and lesions of adjacent organs (including hepatic tumors, fluid-filled colon, splenic lobulation, tortuous or dilated splenic arteries or veins, exophytic upper pole renal mass, suprarenal fat, thickening of the diaphragm crura and pancreatic tail masses) may mimic adrenal tumors. It is important to be cautious when diagnosing masses in the region of the left adrenal gland that are visualized on CT images (16).
A search of Pubmed, Medline, Cochrane, EMBASE, and Google Scholar databases was conducted, identifying a total of 15 studies that described similar cases of misdiagnosed GD. Following the exclusion of four non-relevant studies and four non-English articles, a systematic review was conducted on seven studies, including one article in Chinese (7,9–14) (Table I). All studies were case reports of male patients with GD that simulated masses in the left adrenal region. Of the seven studies, six used an initial CT scan to visualize the lesion in the left adrenal area. Approximately 5% of GDs are overlooked, even when upper gastrointestinal tests are conducted (17). These studies emphasize the importance of careful analysis of clinical data in order to eliminate the possibility of pseudotumors and reduce unnecessary surgical procedures. Only one case reported the use of magnetic resonance imaging for initial diagnosis, based on which the patient was diagnosed with an adrenal cyst (10). In this patient, a signal void was observed the region three years later, and a retrospective study of a previous CT images revealed the presence of a previously unnoticed GD (10).
Table 1.
Summary of reviewed articles describing cases where gastric diverticula were initially misdiagnosed as adrenal tumors.
| Author (ref) | Age, y/ gender | Medical history | Assessment/ imaging finding | Initial diagnosis and treatment | Assessment/ imaging finding not supporting initial diagnosis | Confirmation of gastric diverticulum |
|---|---|---|---|---|---|---|
| Nogurea et al (10) | N/A | N/A | MRI: A small signal void in ventral area interpreted as ferritin and hemosiderin. | Left adrenal cyst. Treatment: N/A |
Retrospective viewing of previous CT: Small bubble of gas in ventral area of diverticulum (initially overlooked). | CT scan |
| Kodera et al (9) | 46/M | Four-month history of hypertension | CT: 2.5 cm tumor located in left adrenal region, observable with homogeneously low (15HU) CT density. MRI: Tumor clearly isolated from other tissues in suprarenal region and exhibited high-intensity area on T2-weighted image. |
Left adrenal cystic and/or degenerated tumor. Treatment: None | Review of previous CT: Small bubble shadow was observed in ventral aspect of left adrenal tumor only in single slice of a CT scan. Consecutive views of repeated thin-sliced CT scan did not detect air-like inclusion in the tumor. | CT with oral contrast gastrografin and upper GI barium study |
| Araki et al (7) | 47/M | Hypertension/primary aldosteronism | CT: Two masses in left upper (3 cm) and lower (1.5 cm) adrenal portion. | Two left adrenal adenomas. Treatment: Transperitoneal laparoscopic left adrenalectomy | Laparoscopic surgical exploration: Surface of 3 cm mass observed to be continuous with stomach. Reexamination of previous CT scan: Small bubble at ventral aspect of 3 cm mass; supplied by a branch of splenic artery. |
CT with more concentrated oral contrast medium |
| Chasse et al (11) | 42/M | Two-month history of left lumbar pain, asthenia, and frequent headaches. | CT: 4.5 cm necrotic mass close to left adrenal gland on upper abdominal CT scan. | Left adrenal mass. Treatment: Surgical exploration |
Surgical exploration: No mass identified in vicinity of kidney, adrenal gland or tail of pancreas. Repeated CT: Mass adjacent to left adrenal gland containing fluid and circular hyperdense images consistent with tablets. |
Upper GI barium study |
| Silverman (12) | 46/M | Seven-month history of hoarseness | CT: Failed intention for mediastinal mass, discovered soft tissue mass posterior to gastric fundus. | Left adrenal mass. Treatment: N/A |
Upper GI barium study: Gastric diverticulum extending posteriorly from fundus of stomach. barium study | Selective CT following upper GI |
| Schwartz et al (13) | 63/M | Abdominal aortic aneurysm | CT: Thin-walled cystic mass with air-fluid level adjacent to left adrenal gland. | Cystic left adrenal mass. Treatment: N/A |
Repeat CT in 10 mm sections with oral contrast: Barium-fluid-air level with mass, indicating communication with GI tract. | Upper GI barium study |
| Jing and Huang (14) | 67/M | Hypertension, CVD, pulmonary emphysema | CT: 3.4 cm necrotic mass close to left adrenal gland on upper abdominal CT scan. | Left adrenal tumor mass Treatment: N/A |
Repeat CT revealed mass adjacent to left adrenal gland containing bubble. Upper GI barium study confirmed 3 cm gastric diverticulum extending posteriorly from fundus of stomach. |
Repeat CT following upper GI barium study |
N/A, not available; y, years; M, male; MRI, magnetic resonance image; CT, computed tomography; GI, gastrointestinal; CVD, cardiovascular disease.
Following an initial misdiagnosis as an adrenal cyst or tumor, five of the seven studies used an upper GI barium test, which confirmed the presence of GD in these patients. In one of these cases, a left adrenal tumor was incidentally discovered in a 46-year-old male with hypertension who had been subjected to an abdominal CT scan. Based on the presence of a bubble shadow in the ventral section of the mass, oral contrast gastrografin was used in a repeat CT scan to enhance the mass, and an upper GI barium study was used to determine the diagnosis of GD (9). A 42-year-old male who presented with left lumbar pain, asthenia and headaches was subjected to an upper abdominal CT scan which indicated a necrotic mass in the area of the left adrenal gland. However, surgical exploration revealed no mass in the kidney, adrenal gland or tail of the pancreas, and the presence of a GD was determined following an upper GI barium study (11). Similarly, in a previous case report in Japan, GD was not confirmed until postoperative X-rays of the upper GI were evaluated (15).
Only one study exhibited similar characteristics to the current study in the use of oral contrast material to diagnose GD without performing an upper GI barium test (7). In this case, reported by Araki et al (7), the presence of two left adrenal adenomas in a 47-year-old male was indicated by an initial CT scan. Laparoscopic left adrenalectomy revealed one adrenal tumor, while the second mass was located between the spleen and stomach and was continuous with the stomach. A repeat CT scan using oral contrast material confirmed that the second mass was a GD. Thus, in the case reported by Araki et al (7), oral contrast material was used to diagnose GD without performing an upper GI barium test, which was consistent with that of the present case.
In conclusion, given the risk of severe complications in cases of GD, which includes bleeding, perforation, and potential for malignant transformation, determining the correct diagnosis is imperative (1). The current case and data collected from the literature review suggests that CT imaging enhanced by oral contrast material is an effective technique which may aid in differentiating GD from an adrenal mass. With the current advances in imaging technology, several non-invasive modalities are available, such as CT image reconstruction. Further investigations are required in order to evaluate their diagnostic use in GD cases and to establish whether they could be used to differentiate GDs from adrenal gland tumors.
References
- 1.Rashid F, Aber A, Iftikhar SY. A review on gastric diverticulum. World J Emerg Surg. 2012;7:1. doi: 10.1186/1749-7922-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen JH, Su WC, Chang CY, Lin H. Education and imaging. Gastrointestinal: bleeding gastric diverticulum. J Gastroenterol Hepatol. 2008;23:336. doi: 10.1111/j.1440-1746.2007.05301.x. [DOI] [PubMed] [Google Scholar]
- 3.DuBois B, Powell B, Voeller G. Gastric diverticulum: “a wayside house of ill fame” with a laparoscopic solution. JSLS. 2012;16:473–477. doi: 10.4293/108680812X13462882736330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer ED. Gastric diverticulosis. Am Fam Physician. 1973;7:114–117. [PubMed] [Google Scholar]
- 5.Rodeberg DA, Zaheer S, Moir CR, Ishitani MB. Gastric diverticulum: a series of four pediatric patients. J Pediatr Gastroenterol Nutr. 2002;34:564–567. doi: 10.1097/00005176-200205000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Verbeeck N, De Geeter T. Suprarenal mass due to a gastric diverticulum. J Belge Radiol. 1994;77:119–120. [PubMed] [Google Scholar]
- 7.Araki A, Shinohara M, Yamakawa J, Tanaka M, Natsui S, Izumi Y. Gastric diverticulum preoperatively diagnosed as one of two left adrenal adenomas. Int J Urol. 2006;13:64–66. doi: 10.1111/j.1442-2042.2006.01236.x. [DOI] [PubMed] [Google Scholar]
- 8.Velanovich V. Gastric diverticulum. Endoscopic and radiologic appearance. Surg Endosc. 1994;8:1338–1339. doi: 10.1007/BF00188296. [DOI] [PubMed] [Google Scholar]
- 9.Kodera R, Otsuka F, Inagaki K, et al. Gastric diverticulum simulating left adrenal incidentaloma in a hypertensive patient. Endocr J. 2007;54:969–974. doi: 10.1507/endocrj.K07E-025. [DOI] [PubMed] [Google Scholar]
- 10.Noguera JJ, Benito A, HernandezSastre C, Cano D, Vivas I, Gonzalez-Crespo I. Gastric diverticulum mimicking cystic lesion in left adrenal gland. Urology. 2009;73:997–998. doi: 10.1016/j.urology.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Chasse E, Buggenhout A, Zalcman M, Jeanmart J, Gelin M, El Nakadi I. Gastric diverticulum simulating a left adrenal tumor. Surgery. 2003;133:447–448. doi: 10.1067/msy.2003.47. [DOI] [PubMed] [Google Scholar]
- 12.Silverman PM. Gastric diverticulum mimicking adrenal mass: CT demonstration. J Comput Assist Tomogr. 1986;10:709–710. doi: 10.1097/00004728-198607000-00039. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz AN, Goiney RC, Graney DO. Gastric diverticulum simulating an adrenal mass: CT appearance and embryogenesis. AJR Am J Roentgenol. 1986;146:553–554. doi: 10.2214/ajr.146.3.553. [DOI] [PubMed] [Google Scholar]
- 14.Jing SL, Huang GZ. One case of gastric diverticulum misdiagnosed as tumor in left adrenal gland. Zhongguo Xian Dai Yi Xue Za Zhi. 2007;17:2432. (In Chinese) [Google Scholar]
- 15.Inaba Y, Maeda H, Umezu K. A case of gastric diverticulum difficult to differentiate from left adrenal tumor. Hinyokika Kiyo. 1993;39:553–555. (In Japanese) [PubMed] [Google Scholar]
- 16.Gokan T, Ohgiya Y, Nobusawa H, Munechika H. Commonly encountered adrenal pseudotumours on CT. Br J Radiol. 2005;78:170–174. doi: 10.1259/bjr/18362306. [DOI] [PubMed] [Google Scholar]
- 17.Meeroff M, Gollán JR, Meeroff JC. Gastric diverticulum. Am J Gastroenterol. 1967;47:189–203. [PubMed] [Google Scholar]