Abstract
The functions of microRNAs (miRNA/miR) in the development of cervical cancer remain largely undefined. The present study investigated the role of miR-195 in cervical cancer development. The expression of miR-195 mimics in the cervical cancer HeLa cell line significantly decreased the cell proliferation, migration and invasion capacities in vitro. Using miRNA target prediction algorithms and reporter assays, cyclin D2 (CCND2) and v-myb avian myeloblastosis viral oncogene homolog (MYB) were identified as direct targets of miR-195. Moreover, miR-195 repressed the expression of CCND2 and MYB in the HeLa cells at the mRNA and protein levels. Finally, the expression of miR-195 was downregulated in cervical cancer tissues compared with normal tissues. Together, these data suggest that miR-195 is a tumor suppressor in cervical cancer.
Keywords: microRNA-195, cervical cancer, proliferation, migration, invasion
Introduction
As the third most commonly diagnosed cancer and the fourth leading cause of cancer mortality in females globally, cervical cancer accounts for 9% of the total new cases of cancer and 8% of the total cancer-related fatalities among females in 2008. In total, >85% of these cases and fatalities occur in developing countries (1,2). It has been reported that human papillomavirus (HPV) infection is involved in the development of >90% of cases (3). However, people who have had HPV infections do not necessarily develop cervical cancer, indicating that other risk factors also contribute to the development of cervical cancer (3). Despite a number of studies and resources aimed at elucidating the molecular mechanisms of cervical cancer, the exact initiation and progression processes remain unclear.
microRNAs (miRNA/miR) are 20–25-nucleotide RNAs that regulate gene expression at the post-transcriptional level by binding to target mRNAs for translational repression or mRNA cleavage (4). Recent studies have shown that miRNAs are grossly deregulated in a variety of human cancers, including cervical cancer, and are vital in the processes of cancer initiation, progression and metastasis (5,6). Studies have shown that miR-195 acts as a putative tumor suppressor in a variety of cancers, including hepatocellular carcinoma (7,8), non-small cell lung cancer (9), human glioblastoma (10), breast cancer (11) and colorectal cancer (12). miR-195 has been reported to be downregulated in the tumor tissues and serum of cancer patients. miR-195 is involved in multiple processes during cancer development, including cell proliferation, migration, invasion and metastasis (7–12). However, the function of miR-195 in cervical cancer development is unknown.
In the present study, the expression of miR-195 was detected in cervical cancer tissues and the functions of miR-195 in the cervical cancer HeLa cell line was studied in order to investigate the role of miR-195 in cervical cancer.
Materials and methods
Cell culture
HeLa cells were obtained from the American Type Culture Collection and maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Invitrogen Life Technologies). The cells were cultured at 37°C in an atmosphere containing 5% CO2 and 100% humidity.
Tissue samples
The cervical tissue specimens and adjacent normal cervical tissue specimens were obtained from 10 patients at the Women and Children's Health Care Hospital of Linyi (Linyi, Shandong, China) following surgical resection. The study was performed with the approval of the Medical Ethics Committee of the Women and Children's Health Care Hospital of Linyi and written informed consent was obtained from all patients.
Transfections
The HeLa cells were transiently transfected with 10 nM of the chemically synthesized miR-195 mimics (sense sequence, 5′-UAGCAGCACAGAAAUAUUGGC-3′) or negative control RNA (NC; sense sequence, 5′-UUCUCC GAACGUGUCACGU-3′) (Genepharma, Shanghai, China) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The cells were treated for further experiments 24 h after transfection.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from lung tissues using TRIzol reagent (Invitrogen Life Technologies) according to the manufacturer's instructions. RT-qPCR assays were performed using the TaqMan miRNA Assay (Life Technologies, Grand Island, NY, USA) according to the manufacturer's instructions. The expression levels of miR-195 were normalized to the expression level of U6 small nuclear RNA.
Cell proliferation assay
The cells were transfected with NC or miR-195. At 24 h post-transfection, the cells were seeded in 6-well plates at the density of 2×105 cells per well. Cell number was directly counted using a blood cell counting plate (XB-K25; Shanghai Qiujing Biochemical Reagent & Instrument Co., Ltd., Shanghai, China) at the indicated time.
Cell cycle analysis
For cell cycle analysis, the HeLa cells transfected with NC or miR-195 were fixed, stained with propidium iodide and examined with a flow cytometer (Beckman Coulter, Inc., Brea, CA, USA), and DNA histograms were analyzed.
Wound healing assay
The HeLa cells transfected with control RNA or miR-195 were plated in 6-well plates. When the cells grew to full confluence, a line was scratched using a pipette tip. The cells were then washed with serum-free medium and incubated with DMEM. Images of the wound were captured at the time-points of 0 h and 12 h.
Cell invasion assay
Cell invasion was measured using Transwell chambers (Millipore Corporation, Billerica, MA, USA). Transwell inserts were coated with Matrigel (Invitrogen Life Technologies). The HeLa cells transfected with NC or miR-195 were seeded into the upper chambers in DMEM with FBS. The same medium was also placed in the lower wells. After 24 h, the cells migrating to the lower surface of the Transwell membrane were fixed and stained with 0.2% crystal violet solution. Images were captured under a wide-field microscope (Eclipse TS100; Nikon, Melville, NY, USA). Crystal violet was resolved in 10% acetic acid and the absorption at 590 nm was then measured. The absorption of the control group was normalized to 1.0.
Protein extraction and immunoblotting
The whole cell lysate was extracted using RIPA lysis buffer [100 mm Tris (pH 8.0), 1% Triton X-100, 100 mm NaCl and 0.5 mm EDTA], and cleared by centrifugation at 14,000 × g for 10 min. Equal amounts of proteins were subjected to SDS-PAGE electrophoresis and transferred to a nitrocellulose membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membrane was blocked with 5% milk in Tris-buffered saline with Tween 20 [20 mm Tris-HCl (pH 8.0), 150 mm NaCl and 0.05% Tween 20]. The membrane was then incubated with antibodies targeting cyclin D2 (CCND2; rabbit anti-human polyclonal; cat no. sc-181; 1:500 dilution), v-myb avian myeloblastosis viral oncogene homolog (MYB; rabbit anti-human polyclonal; cat no. sc-517; 1:500 dilution) or α-β-actin (mouse anti-human monoclonal; cat no. sc-8432; 1:1,000 dilution; all Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight. Subsequent to being washed 3 times, the membrane was incubated with horseradish-conjugated polyclonal goat anti-rabbit (cat. no. sc-2004; 1:1,000) or anti-mouse (cat. no. sc-2005; 1:1,000) IgG secondary antibodies (Santa Cruz Biotechnology, Inc.) and expression was detected with the SuperSignal Protein Detection kit (EMD Millipore, Billerica, MA, USA).
Plasmids construction
The 3′-untranslated regions (3′-UTRs) of the CCND2 and MYB genes containing predicted miR-195 target sites were amplified by PCR. The 3′-UTR fragments were cloned downstream of the firefly luciferase coding region into the XbaI site of the pGL3-control plasmid (Promega Corporation, Madison, WI, USA).
Luciferase assays
The HeLa cells in 24-well plates were transfected with 100 ng firefly luciferase reporter plasmid, 10 ng of pRL-TK plasmid (Promega Corporation) and 10 pmol mir-195 mimic or NC. The cells were harvested at 48 h post-transfection, and firefly luciferase activity was measured and normalized to Renilla signals.
Statistical analysis
miR-195 expression in the tissue samples was analyzed using the Mann-Whitney U test. Other data are expressed as the mean ± standard deviation and were evaluated with a double-sided Student's t-test. P<0.05 was used to indicate a statistically significant difference.
Results
miR-195 inhibits HeLa cell proliferation
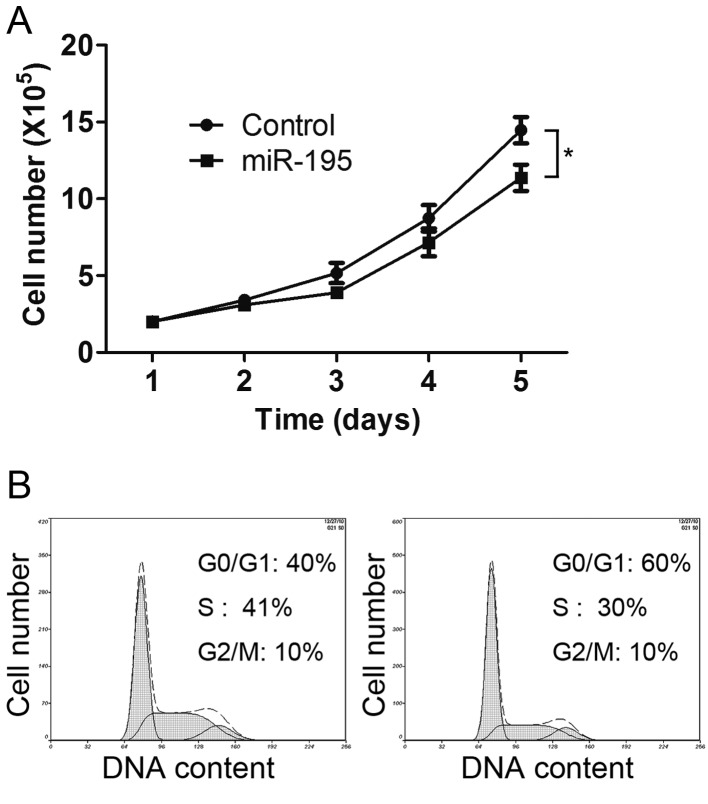
To investigate the function of miR-195 on cervical cancer cell behavior, HeLa cells were used. First, the HeLa cells were transfected with miR-195 and the proliferation rates were detected. Compared with the control group, the proliferation rate was significantly decreased in the cells transfected with miR-195 (Fig. 1A), indicating that miR-195 inhibits HeLa cell proliferation.
Figure 1.
miR-195 inhibits the proliferation of HeLa cells. (A) HeLa cells were transfected with the negative control RNA or miR-195, and their cell proliferation rates were assessed by direct cell counting at the indicated time-points. Data is presented as the mean ± standard deviation of three independent experiments. *P<0.05. (B) Cell cycle distributions of HeLa cells transfected with the miR-195 mimics were analyzed by flow cytometry. miR, microRNA.
Cell proliferation is tightly controlled by the cell cycle. Therefore, cell cycle distribution was detected using flow cytometry. The data showed that the HeLa cells transfected with miR-195 had increased numbers of cells in the G0/G1 phase and decreased numbers of cells in the S phase (Fig. 1B). These data suggested that miR-195 induces G0/G1 arrest in HeLa cells.
miR-195 suppresses the migration and invasion of HeLa cells
Furthermore, the effects of miR-195 on the migration and invasion of HeLa cells, which are two essential steps for malignant progression and metastasis, were detected. The HeLa cells were transfected with miR-195 and cell migration was evaluated using wound healing assays. The data showed that the migration capacity was significantly decreased in the miR-195-expressing cells (Fig. 2).
Figure 2.
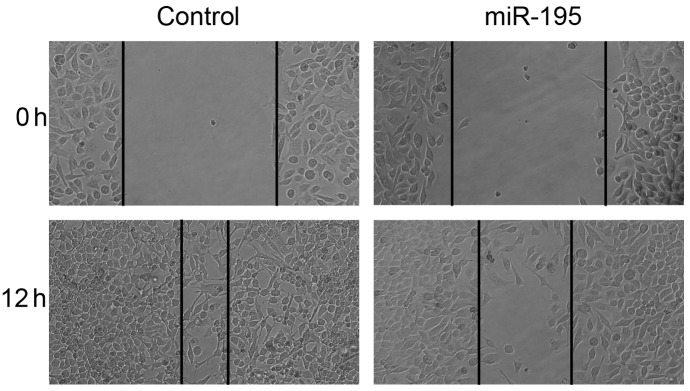
miR-195 suppresses the migration and invasion of HeLa cells. HeLa cells transfected with negative control RNA or miR-195 were subjected to wound healing assays and images were captured at 0 h and 12 h. miR, microRNA.
The Matrigel invasion assays showed that the HeLa cells transfected with miR-195 displayed a markedly decreased invasion ability compared with the NC cells (0.65 fold; P<0.01; Fig. 3). These results suggested that miR-195 inhibits HeLa cell motility, including migration and invasion.
Figure 3.
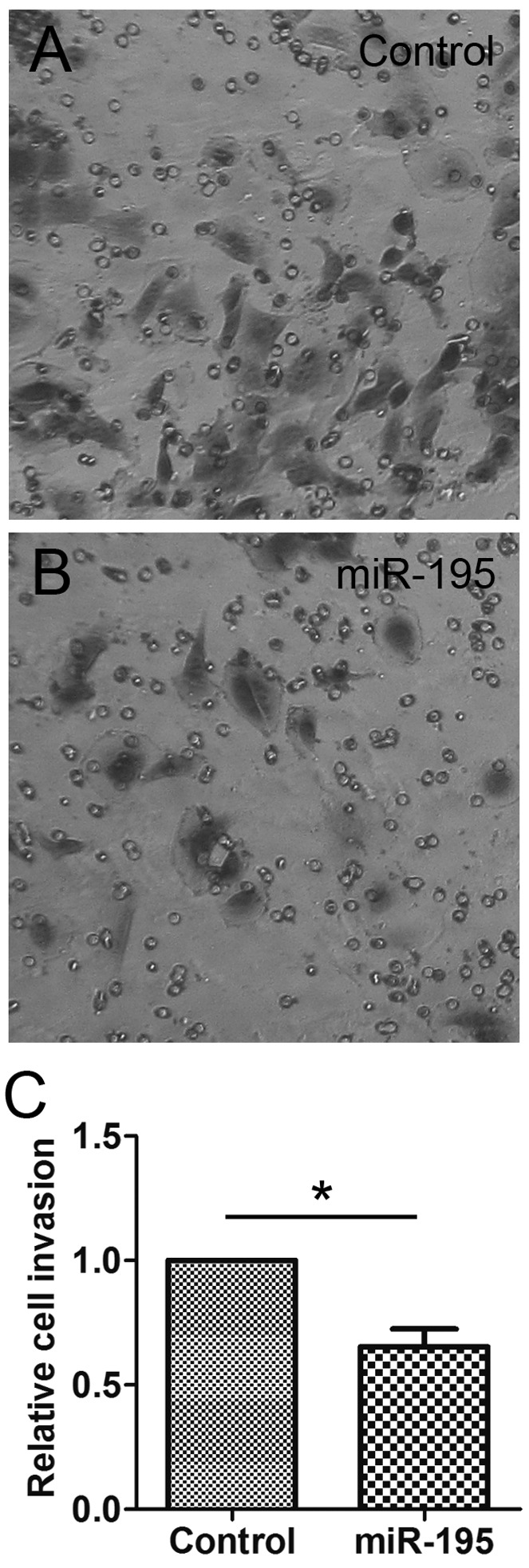
miR-195 suppresses the invasion of HeLa cells. HeLa cells transfected with (A) neagtive control RNA or (B) miR-195 were subjected to Matrigel migration assays. The migrated cells were stained with crystal violet and then images were captured. (C) The absorption at 590 nm was detected. Data are presented as the mean ± standard deviation of three independent experiments. *P<0.05. miR, microRNA.
miR-195 represses the expression of CCND2 and MYB in HeLa cells
Using online bioinformatics tools, including TargetScan (http://www.targetscan.org) and miRanda (http://www.microrna.org), it was found that a series of 3′-UTRs of human genes contained potential miR-195 binding sequences. Two of them, CCND2 (13) and MYB (14), were closely associated with the cell cycle and cell migration. Therefore, these were picked for further analysis. To determine whether CCND2 and MYB are miR-195 target genes in HeLa cells, the HeLa cells were transfected with miR-195 or control RNA, and the expression of CCND2 and MYB was detected. The data showed that the mRNA (Fig. 4A) and protein (Fig. 4B) levels of CCND2 and MYB were significantly decreased in the miR-195-transfected cells. These data demonstrated that miR-195 inhibits the expression of CCND2 and MYB in HeLa cells.
Figure 4.
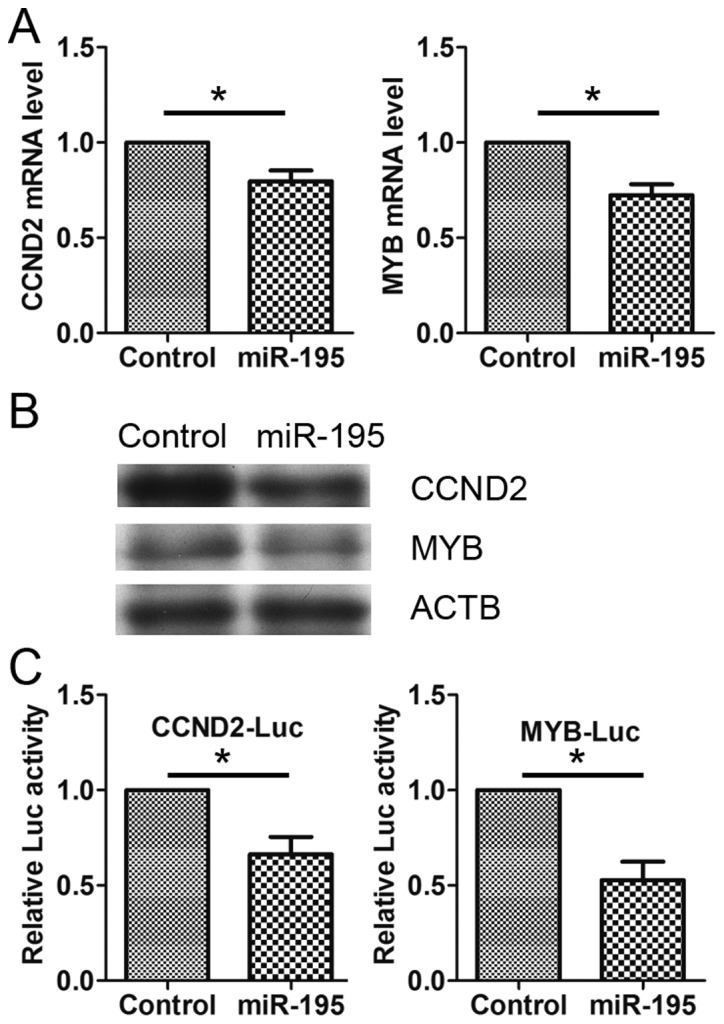
miR-195 represses the expression of CCND2 and MYB. (A) HeLa cells were transfected with negative control RNA (NC) or miR-195. The mRNA levels of CCND2 and MYB were detected by reverse transcription-quantitative polymerase chain reaction. (B) HeLa cells were transfected with NC or miR-195. The cell lysates were blotted with anti-CCND2, anti-MYB and anti-ACTB. (C) HeLa cells were transfected with CCND2-Luc or MYB-Luc, together with miR-195. Luciferase activities were detected at 24 h post-transfection. miR, microRNA; CCND2, cyclin D2; MYB, v-myb avian myeloblastosis viral oncogene homolog; ACTB, α-β-actin; Luc, luciferase.
Next, luciferase assays were used to further investigate whether miR-195 regulates the expression of CCND2 and MYB directly. The 3′-UTRs of the CCND2 and MYB genes were cloned downstream of the coding sequence of luciferase and then the constructs were co-transfected into HeLa cells with miR-195. The results showed that miR-195, but not the NC, specifically decreased the luciferase levels from the reporters (Fig. 4C), suggesting that miR-195 regulates the expression of CCND2 and MYB directly.
miR-195 is downregulated in cervical cancer tissues compared with normal cervical tissues
The aforementioned data suggested that miR-195 is a tumor suppressor in cervical cancer, which led to the suggestion that miR-195 may be aberrantly expressed in cervical cancer. To test this, the expression of miR-195 was compared in cervical cancer and adjacent normal cervical tissues using RT-qPCR. The results demonstrated that the expression levels of miR-195 were significantly decreased in the cervical cancer tissues compared with the normal cervical tissues (P<0.01, Mann-Whitney; Fig. 5). The data indicated that the downregulation of miR-195 was associated with human cervical cancer development.
Figure 5.
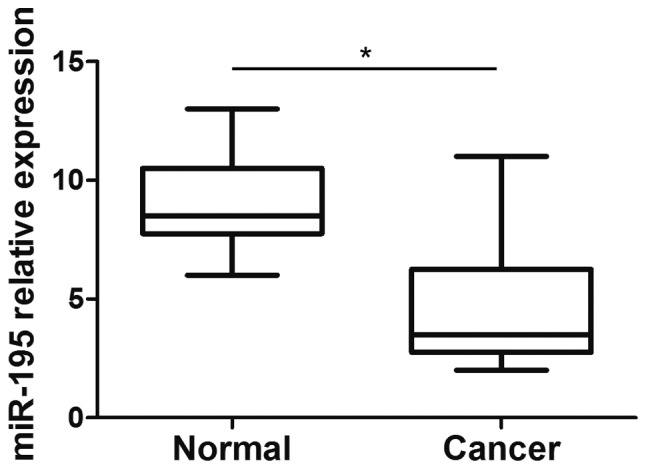
Expression profile of miR-195 in human cervical cancer tissues. The expression levels of miR-195 in 10 cervical cancer tissues and adjacent normal tissues were assessed by reverse transcription-quantitative polymerase chain reaction and normalized to U6 small nuclear RNA (*P<0.05, Mann-Whitney U test). miR, microRNA.
Discussion
In the present study, the function of miR-195 was studied in the development of cervical cancer. The data showed that miR-195 expression was decreased in cervical cancer tissues compared with adjacent normal tissues. Overexpression of miR-195 decreased the proliferation, migration and invasion of HeLa cells. The progression of cervical cancer is a complex process that involves tumor growth and metastasis (1,2). Cell proliferation is associated with tumor growth, while cell migration and invasion are essential for metastasis. The present study data indicated that miR-195 may affect a number of processes in cervical cancer, including tumor growth and metastasis.
Abnormal cell cycle control is not only a vital step in cervical maintenance, but also appears to be an essential early event. Two key classes of regulatory molecules, the cyclins and cyclin-dependent kinases (CDKs), determine the progress of a cell through the cell cycle (15). CCND2 forms a complex with and functions as a regulatory subunit of CDK4 or CDK6, and thus regulates cell cycle G1/S transition (16). In a previous study, CCND2 was shown to be highly expressed in a series of tumors, including ovarian and testicular cancers (17). Knockdown of this gene inhibits cancer cell proliferation (17). In the present study, it was found that miR-195 inhibited cell proliferation and induced G1 arrest in the HeLa cells (Fig. 1). The data also showed that miR-195 inhibited CCND2 expressions (Fig. 5), suggesting that miR-195 regulates cell cycle G1/S transition by downregulating CCND2 expression and therefore inhibits cell proliferation of the HeLa cells. Consistent with this data, Sato et al reported that miR-195 represses CCND2 expression and therefore induces the postnatal quiescence of skeletal muscle stem cells (18).
The transcription factor MYB was originally identified as a cellular homolog of v-myb (19). MYB was later characterized as a transforming oncogene in several types of human cancers (20). MYB affects diverse processes, including proliferation, invasion and migration, through activation of the transcription of target genes, such as cyclooxygenase-2, B-cell lymphoma 2 and c-Myc (20). It has been reported that miR-195 targets MYB in non-small cell lung cancer cells (9). The present study demonstrated that miR-195 directly targeted the 3′-UTR of the MYB gene and inhibited its expression in HeLa cells, suggesting that the deregulation of miR-195-mediated MYB inhibition may serve as a general mechanism in the development of a variety of cancers (Fig. 5).
In conclusion, the present study found that miR-195 is downregulated in cervical cancer and that this miRNA inhibits cervical cancer cell proliferation and invasion in vitro. These results indicate a suppressive role of miR-195 in cervical cancer development and indicate its potential therapeutic application in cancer.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Dunne EF, Park IU. HPV and HPV-associated diseases. Infect Dis Clin North Am. 2013;27:765–778. doi: 10.1016/j.idc.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.EsquelaKerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 6.Lin PY, Yu SL, Yang PC. MicroRNA in lung cancer. Br J Cancer. 2010;103:1144–1148. doi: 10.1038/sj.bjc.6605901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amer M, Elhefnawi M, ElAhwany E, Awad AF, Gawad NA, Zada S, Tawab FM. Hsa-miR-195 targets PCMT1 in hepatocellular carcinoma that increases tumor life span. Tumour Biol. 2014;35:11301–11309. doi: 10.1007/s13277-014-2445-4. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Li M, Chang S, Wang L, Song T, Gao L, Hu L, Li Z, Liu L, Yao J, Huang C. MicroRNA-195 acts as a tumor suppressor by directly targeting Wnt3a in HepG2 hepatocellular carcinoma cells. Mol Med Rep. 2014;10:2643–2648. doi: 10.3892/mmr.2014.2526. [DOI] [PubMed] [Google Scholar]
- 9.Yongchun Z, Linwei T, Xicai W, Lianhua Y, Guangqiang Z, Ming Y, Guanjian L, Yujie L, Yunchao H. MicroRNA-195 inhibits non-small cell lung cancer cell proliferation, migration and invasion by targeting MYB. Cancer Lett. 2014;347:65–74. doi: 10.1016/j.canlet.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 10.Zhang QQ, Xu H, Huang MB, Ma LM, Huang QJ, Yao Q, Zhou H, Qu LH. MicroRNA-195 plays a tumor-suppressor role in human glioblastoma cells by targeting signaling pathways involved in cellular proliferation and invasion. Neuro Oncol. 2012;14:278–287. doi: 10.1093/neuonc/nor216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo Q, Wei C, Li X, Li J, Chen L, Huang Y, Song H, Li D, Fang L. MicroRNA-195-5p is a potential diagnostic and therapeutic target for breast cancer. Oncol Rep. 2014;31:1096–1102. doi: 10.3892/or.2014.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Chen L, Xu Y, Li R, Du X. MicroRNA-195 promotes apoptosis and suppresses tumorigenicity of human colorectal cancer cells. Biochem Biophys Res Commun. 2010;400:236–240. doi: 10.1016/j.bbrc.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 13.Xiong Y, Menninger J, Beach D, Ward DC. Molecular cloning and chromosomal mapping of CCND genes encoding human D-type cyclins. Genomics. 1992;13:575–584. doi: 10.1016/0888-7543(92)90127-E. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Xu H, Liu J, Zhang C, Leutz A, Mo X. The c-Myb functions as a downstream target of PDGF-mediated survival signal in vascular smooth muscle cells. Biochem Biophys Res Commun. 2007;360:433–436. doi: 10.1016/j.bbrc.2007.06.078. [DOI] [PubMed] [Google Scholar]
- 15.Nigg EA. Cyclin-dependent protein kinases: Key regulators of the eukaryotic cell cycle. Bioessays. 1995;17:471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- 16.Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortega S, Malumbres M, Barbacid M. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim Biophys Acta. 2002;1602:73–87. doi: 10.1016/s0304-419x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 18.Sato T, Yamamoto T, Sehara-Fujisawa A. MiR-195/497 induce postnatal quiescence of skeletal muscle stem cells. Nat Commun. 2014;5:4597. doi: 10.1038/ncomms5597. [DOI] [PubMed] [Google Scholar]
- 19.Roussel M, Saule S, Lagrou C, Rommens C, Beug H, Graf T, Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979;281:452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- 20.Ramsay RG, Gonda TJ. MYB function in normal and cancer cells. Nat Rev Cancer. 2008;8:523–534. doi: 10.1038/nrc2439. [DOI] [PubMed] [Google Scholar]