Abstract
Human beings have considerably expanded cognitive abilities compared with all other species and they also have a relatively larger cerebral cortex compared with their body size. But is a bigger brain the only reason for higher cognition or have other features evolved in parallel? Humans have more and different types of GABAergic interneurons, found in different places, than our model species. Studies are beginning to show differences in function. Is this expanded repertoire of functional types matched by an evolution of their developmental origins? Recent studies support the idea that generation of interneurons in the ventral telencephalon may be more complicated in primates, which have evolved a large and complex outer subventricular zone in the ganglionic eminences. In addition, proportionally more interneurons appear to be produced in the caudal ganglionic eminence, the majority of which populate the superficial layers of the cortex. Whether or not the cortical proliferative zones are a source of interneurogenesis, and to what extent and of what significance, is a contentious issue. As there is growing evidence that conditions such as autism, schizophrenia and congenital epilepsy may have developmental origins in the failure of interneuron production and migration, it is important we understand fully the similarities and differences between human development and our animal models.
Keywords: evolution, ganglionic eminences, inhibitory interneurons, telencephalon
Introduction
The vast majority of interneurons in the cerebral cortex express the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) and have aspiny dendrites (Markram et al. 2004) but with a role that goes far beyond the simple blockade of neurotransmission. This includes generation of oscillatory firing patterns by neuronal assemblies (Buzsaki & Wang, 2012) and even excitatory effects depending on the nature of the particular synapse (Szabadics et al. 2006: Sauer et al. 2012). Similarly, a wide range of morphologies, physiology and gene expression is displayed. The morphology of an interneuron’s axons and dendrites, combined with its cortical layer position, can be combined with information of the synaptic targets and physiological properties of these cells, and the expression of certain neuropeptides and calcium binding proteins, to provide a fairly comprehensive, if not perfect, categorisation of these interneurons (Markram et al. 2004; Ascoli et al. 2008; Rudy et al. 2011; De Felipe et al. 2013; Zaitsev, 2013). For instance, most large basket cells express parvalbumin and have axons that contribute to ‘baskets’ of GABAergic synaptic terminals around the cell bodies of pyramidal neurons. These are fast spiking cells that can fire rapid trains of action potentials. Chandelier cells are another class of fast-spiking cells that can express parvalbumin or calbindin and synapse on the axon initial segment of pyramidal neurons (Inan et al. 2012; Taniguchi et al. 2013). These cells can have a depolarising or hyperpolarising effect depending on the membrane potential of the target axon (Szabadics et al. 2006). Other interneurons, expressing other markers, for instance Calb2 (calretinin), and showing different electrophysiological properties, target dendrites of pyramidal cells or other interneurons (Fig.1 for a fuller description). To a large extent the organisation and function of interneurons is shared between species; however, it is known that primates have a higher proportion of cortical interneurons (25–34% of cortical neurons) than rodents (15–25%, Gabbott & Bacon, 1996; Gabbott et al. 1997; De Felipe, 2011). Studies are now beginning to show functional differences between species and, importantly, between rodents and primates, including humans (Molnár et al. 2008; Povysheva et al. 2013).
Figure 1.
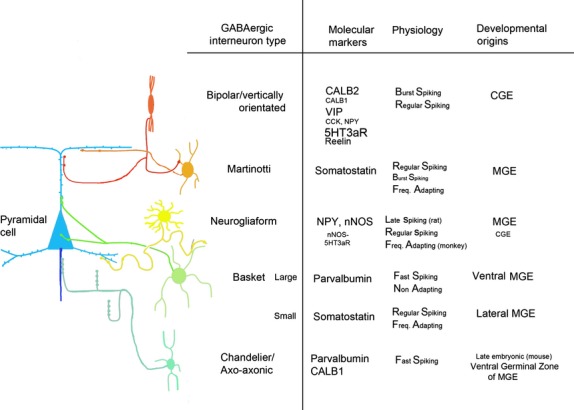
The morphology and axonal connections of the major types of GABAergic interneuron are illustrated on the left, with extra information provided in table form on the right. This is a simplified version of all the possible phenotypic sub-types that have been described. Size of text indicates proportion of interneurons expressing a particular molecular marker. Multiple expression of markers is common. There is variation between species (see text). CALB1 and CALB2 are also known as calbindin and calretinin, 5HT3aR is a subtype of ionotropic serotonin receptor, NPY is neuropeptide Y, nNOS is neuronal nitric oxide synthase. Diagram adapted with permission from Zaitsev (2013) with additional information from Wang et al. (2004), Markram et al. (2004), Tricoire et al. (2010), Rudy et al. (2011), Taniguchi et al. (2013) and Zaitsev (2013).
An enhanced role for GABAergic neurons in the human cerebral cortex
When searching for a substrate for the increased cognitive power of the human cerebral cortex, the greater interconnectedness of a greater number of functional modules than in other species springs to mind. However, interconnectivity does not only depend on the physical presence of axon pathways and synapses, but also on synchronicity between neural activity in the cortical areas that are communicating with each other, binding together outputs of all neurons coding for certain features of a sensory input (Singer & Gray, 1995). Electrical activity in groups of neurons synchronises, giving rise to oscillations around characteristic frequencies. Gamma rhythms (30–80 Hz) are a common feature of neuronal population activity in the mammalian cortex and it has been suggested that they are essential to higher order cognitive processing (Whittington et al. 2011). Their dynamic properties permit the synchronisation of neuronal responses to sensory input within spatially distributed networks (Burchell et al. 1998) the transient formation of local neuronal\cell assemblies (Harris et al. 2003) and coherent response patterns essential for intercortical regional communication (Fries, 2009). All forms of physiological gamma rhythm are dependent on the activity of GABAergic interneurons, particularly ‘fast spiking’ interneurons, which usually express the calcium binding protein parvalbumin (Whittington et al. 1995; Hájos et al. 2004; Buzsaki & Wang, 2012). Interestingly, there are species differences; monkey basket cells in the prefrontal cortex are more excitable than rat basket cells and lack the quiescent periods during the firing of spike trains seen in rat. These differences may contribute to the differential patterns of neuronal activation observed in rats and monkeys performing working-memory tasks (Povysheva et al. 2008). Patterns of distribution of parvalbumin interneurons and their axons between cortical layers are also quite different between species, including human (Simon et al., in press).
Even at the level of a few neurons in a microcircuit, it has been shown that a subset of connections from pyramidal cells to fast-spiking interneurons is selectively strengthened in the human cortical circuit. Individual action potentials in presynaptic pyramidal cells initiate long-lasting sequences of events in a network lasting an order of magnitude longer than detected in other species. This helps human cortical networks to pass on longer sequences of information. The functioning of chandelier cells is crucial to this process (Molnár et al. 2008). These cells are found in other mammals, but in the neocortex complex axo-axonic terminal arrangements involving several boutons from one interneuron axon are rare in the mouse but relatively abundant in the human, especially in association cortex (Inda et al. 2007, 2009) further suggesting this synaptic arrangement has evolved ‘hand in hand’ with cognitive processing.
In addition to fast spiking interneurons, there are many other classes of cortical interneuron (see above). It has been reported that primates, and especially humans, have larger numbers of interneurons than rodents, in particular calbindin and Calb2, which are expressed by interneurons in the superficial layers of the neocortex (Hendry et al. 1987; Beaulieu et al. 1992; del Río & De Felipe, 1996; Gabbott & Bacon, 1996). This includes particular cell types such as the double-bouquet cell that are more abundant in primates than in other species (Ballesteros Yáñez et al. 2005). This suggests an enhanced function for interneurons in higher species. Neurogliaform cells have different electrophysiological properties in rodent and monkey (Povysheva et al. 2007). Neurogliaform cells in monkey prefrontal cortex are more excitable than those in rat and lack the ‘late spiking’ phenotype described for rat neurogliaform interneurons.
In vitro experiments in rat entorhinal cortex have shown that two anatomically distinct subclasses of local circuit interneuron, parvalbumin positive basket cells and calb2-positive goblet cells, in the superficial layers, compete for control over gamma rhythm generation (Middleton et al. 2008). The degree of involvement of each cell type dictates the frequency of the network rhythm within the gamma band. This ability to switch between frequencies opens up the possibility for a group of neurons to bind with different neuronal assemblies depending on which frequency channel is in operation. In addition, coincident expression of multiple types of gamma rhythm possibly provides a mechanism for combining rate and temporal codes of different neuronal assemblies according to stimulus strength, providing a substrate for learning (Ainsworth et al. 2011). Potentially, the greater the repertoire of interneurons present, the greater the potential for generating multiple, layer-specific gamma rhythms. Is it possible that the new sources of interneurons have evolved alongside the evolution of interneuron function to enhance the cognitive abilities of the human cerebral cortex? Failures in proliferation and migration of specific classes of GABAergic interneurons have been implicated in diverse conditions including autism, epilepsy and schizophrenia (De Felipe, 1999; Lewis et al. 2005; Uhlhaas & Singer, 2010; Marín, 2012). Although tremendous progress is being made through the study of mouse models of these neurodevelopmental conditions by exploiting transgenic techniques (Penagarikano et al. 2011; del Pino et al. 2013) we must be careful not to assume that mouse–human extrapolations are always appropriate (Jones, 2009; Clowry et al. 2010).
Developmental origins of inhibitory interneurons
A huge weight of experimental evidence has established the originally surprising finding that, in rodents, GABAergic interneurons are born almost entirely outside the neocortex (dorsal pallium) in the ganglionic eminences and associated structures such as the septum and preoptic area (subpallium), from which they migrate tangentially into the cortex (De Carlos et al. 1996; Parnavelas, 2000; Marín & Rubenstein, 2003; Welagen & Anderson, 2011). The subpallium is divided into three main neurogenic domains, the lateral-, medial- and caudal-ganglionic eminences (LGE, MGE, and CGE). The LGE is the birthplace of the striatal projection neurons and a small population of olfactory bulb interneurons that migrate rostrally (Waclaw et al. 2009). The MGE and the CGE are the major sites of cortical interneurogenesis (Xu et al. 2004; Butt et al. 2005). The CGE is usually considered to be a caudal extension of the LGE and the MGE as the three tissues share many common gene expression profiles (Flames et al. 2007). The MGE and the ventral CGE are defined by the expression of Nkx2.1, which is absent from the LGE and the dorsal CGE (Sussel et al. 1999). Nkx2.1 induces expression of Lhx6, required for specification and migration of MGE-derived GABAergic interneurons (Liodis et al. 2007; Du et al. 2008). After migrating to the cortex, these Nkx2.1/Lhx6-patterned progenitors mature in situ into parvalbumin- and somatostatin-expressing cortical interneurons. Somatostatin-expressing interneurons originate in the more lateral MGE, whereas parvalbumin-expressing interneurons are derived from the more ventral MGE domain (Wonders et al. 2008; Xu et al. 2004; Inan et al. 2012). The MGE can also produce other cell types, including striatal interneurons, cholinergic cells and oligodendrocytes, that display distinct gene expression profiles.
Thus, in the rodent, parvalbumin- and somatostatin-positive GABAergic neurons arise from ventral and dorsal parts of the MGE, respectively, initially entering the neocortex by tangential migration into the frontal two-thirds of the neocortex. However, Calb2-positive interneurons arise from the CGE initially entering more caudal regions before populating the whole neocortex (Xu et al. 2004; Butt et al. 2005; Wonders & Anderson, 2005; Ghanem et al. 2007; Faux et al. 2012). The CGE is characterised by expression of a separate set of transcription factors including Gsx2, Cux2 and Nr2f2 (also known as CouptfII) and so Calb2-positive interneurons remain unaffected in Nkx2.1 Knock-out mice (Sussel et al. 1999). It has also been shown that nearly all cortical interneurons that do not express parvalbumin or somatostatin express the 5HT3a receptor, and that over 90% of these 5HT3a receptor-expressing interneurons derive from the CGE (Lee et al. 2010; Vucurovic et al. 2010; Rudy et al. 2011).
It was originally proposed, from studies of MGE-derived interneurons, that the time an interneuron was born determines the cortical layer it populates in the same fashion as for pyramidal cells, that is, in an ‘inside–out’ arrangement (Miyoshi et al. 2007; Rymar & Sadikot, 2007). It has since been established that this is not the rule for CGE-derived interneurons. The CGE steadily produces 75% superficial layer/25% deep layer destined interneurons throughout the time-course of cell proliferation (Miyoshi et al. 2010). Neither is it so for MGE-derived chandelier cells, which are late-born and thus preferentially target layer 2, but also substantially populate layers 5 and 6 (Taniguchi et al. 2013).
To what extent have these developmental programmes been preserved in primates? The greatly expanded relative size of the primate cerebral cortex means that increased proliferation of interneuron precursors is necessary. This may be brought about by changes to the ganglionic eminences (Hansen et al. 2013) or by the evolution of new sources of neurogenesis such as the proliferative zones of the pallium (Letinic et al. 2002; Zecevic et al. 2011), which, in the light of new evidence, has become a controversial proposal (Molnár & Butt, 2013).
The human subpallium
At seven post-conceptional weeks, just prior to the formation of the cortical plate, the same neurogenic domains of the subpallium defined by gene expression patterns seen in the rodent are also observed in the human (Pauly et al. 2013). A boundary between the pallium and subpallium (PSB) is clearly defined by a sharp reduction in DLX2 expression in proliferative zones dorsally, and reduced expression of PAX6 ventrally, with the dorsal LGE, a major source of olfactory bulb interneurons in mouse (Stenman et al. 2003) defined by mixed expression of DLX2 and PAX6 in the ventricular zone (VZ). The MGE is characterised by high expression of NKX2.1. As maturation progresses, the primate subpallium diverges from the rodent model. These neurogenic domains are retained with age (Ma et al. 2013) but the ganglionic eminences expand markedly in size and cell number between PCW8 and PCW14 (Hansen et al. 2013). Neuroepithelial progenitors diminish over this period, with VZ thickness in the MGE decreasing by approximately 50%. In contrast, the subventricular zone (SVZ) greatly expands during this interval and becomes densely populated with cells expressing SOX2 and ASCL1, markers for progenitor cells. These observations suggest that the majority of MGE-derived inhibitory neurons are produced by non-ventricular progenitors in the SVZ of the MGE (Hansen et al. 2013). The overall configuration of proliferative zones in the human and macaque ganglionic eminences resembles that found in developing mouse ganglionic eminences (Petryniak et al. 2007) with three compartments being distinguishable by their cellular components. First, the VZ consisted primarily of cells that express markers (e.g. SOX2) of radial glial (neural stem) cells. Secondly, the inner SVZ of the ganglionic eminences (ISVZ, equivalent to the rodent SVZ1,) was enriched with cells that express the markers (e.g. DLXs) of intermediate progenitors undergoing differentiation to the neuronal lineage. Third, the outer SVZ (OSVZ) was populated by both of these cell types, in addition to newborn and migrating neurons. The OSVZ of the human ganglionic eminences may share evolutionary origins with the rodent SVZ2 but there are certain distinctions, chiefly that it contains a far higher proportion of progenitor cells and is much increased in size, being 50 times the thickness of the VZ rather of three times thicker (Hansen et al. 2013).
The human MGE, in particular, is enriched with neural stem cells. In the mouse MGE, Sox5 is expressed in ventricular radial glia and is down-regulated in MGE-derived neurons (Azim et al. 2009). In the human MGE, SOX5 expression was not confined to the ventricular zone, but was highly expressed throughout the OSVZ (Hansen et al. 2013). Therefore, the human MGE OSVZ supports a large reservoir of undifferentiated cells expressing SOX5 and other early progenitor cell markers (SOX2, OLIG2) that can sustain the production of intermediate progenitors (expressing ASCL1, DLX2) sufficient to meet the needs of interneuron production for the larger human neocortex (Hansen et al. 2013).
Only approximately 30% of cortical interneurons in the rodent cortex originate in the CGE (Miyoshi et al. 2010), yet two recent studies have estimated that 40% (Ma et al. 2013) or more than 50% (Hansen et al. 2013) of GABAergic interneurons in the human cortex come from the CGE (and dorsal LGE) based on the post-mitotic expression of NR2F2 and SP8. As these neurons will largely differentiate into CALB2-positive interneurons (Ma et al. 2013) it is possible that the higher proportion of CALB2 interneurons observed in the superficial layers of the cortex result from a relative expansion of the CGE as a source of cortical interneurons in primates. One of the less satisfactory aspects of this model is that there is a higher ratio of NR2F2/SP8/CALB2-positive neurons to SOX6/PV-positive neurons in the anterior cortex, compared with posterior cortex (Ma et al. 2013) despite the proposed origin of SOX6/PV cells, the MGE, being more rostral than the supposed origin of NR2F2/SP8/CALB2 cells in the CGE.
Generation of interneurons in the pallium
Two recent exhaustive studies in both monkey and human came to the conclusion that interneurogenesis in the primate cerebral cortex, as in the rodent, is extremely rare and if it does happen at all, it comes from progenitor cells derived from the subpallium undergoing further divisions once they have migrated into the pallium (Hansen et al. 2013; Ma et al. 2013) as happens in mouse in the later stages corticoneurogenesis (Wu et al. 2011) to provide a small number of interneurons for the neocortex and larger numbers of cells for the olfactory bulb. This contradicts a number of studies in the past 12 years that provide evidence for GABAergic interneuron production within the primate cerebral cortex (Rakic & Zecevic, 2003, Jakovcevski et al. 2011, Zecevic et al. 2011; Al-Jaberi et al. 2013) beginning with a highly cited study that suggested as many as 65% of cortical interneurons are generated intrinsically (Letinic et al. 2002). This conclusion relied on the double expression of ASCL1 and DLX2 to define cells as of cortical origin, as Letinic et al. (2002) observed ASCL1 to be down-regulated in migrating interneurons before they leave the MGE in organotypic cultures of human fetal forebrain. The reliability of ASCL1 as a marker for interneurons of cortical origin has been questioned on two grounds (Hansen et al. 2013). First, ASCL1 may be expressed by progenitors of glutamatergic neurons in the cortex and is not an exclusive marker for GABAergic neurons (Britz et al. 2006; Wilkinson et al. 2013). Secondly, interneurons originating from the CGE, now recognised to be a major source of interneurons, may continue to co-express ASCL1 and DLX2 as they migrate into the cortex (Miyoshi et al. 2010). The first argument, although true, downplays the fact that ASCL1 expression in glutamatergic neuron precursors is confined to the progenitor stage (Britz et al. 2006) and persistent expression of ASCL1 is an indicator of a GABAergic fate determination (Parras et al. 2002; Yun et al. 2002; Parras et al. 2004). The second argument is more challenging, as it offers an explanation for the presence of ASCL1-expressing cells in post-mitotic zones such as the cortical plate.
We have studied GABAergic gene expression in the early developing human cortex (8–12 PCW) by qPCR, ISH and IHC (Al-Jaberi et al. 2013) and by analysis of RNAseq data deposited at the Brainspan website (http://brainspan.org/rnaseq/search/index.html; see Supporting Information Table S1 and Fig.2). What is clear is that genes expressed by GABAergic neurons and their progenitors are expressed throughout the cortex, although sometimes at much lower levels than in the ganglionic eminences, and are expressed to different degrees in different cortical regions. For instance, ASCL1 is expressed at very high levels across the ganglionic eminences but still shows substantial expression in the cortex, generally higher in the anterior/lateral/ventral regions and lower in the posterior/medial and dorsal regions. This is the opposite of the pattern of NGN2 expression, a marker for glutamatergic pyramidal neurons, which is lowest in the ganglionic eminences and highest in the posterior/medial and dorsal cortex.
Figure 2.
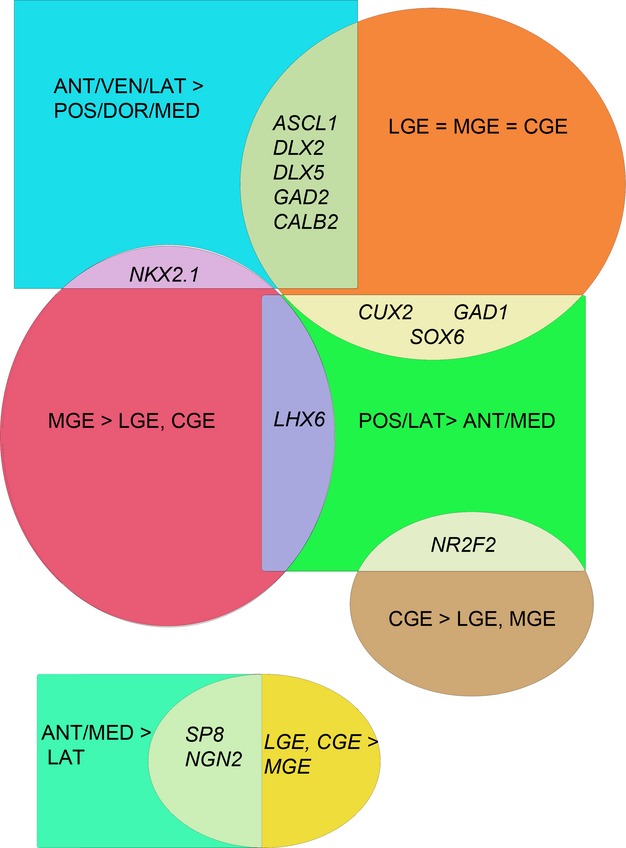
Expression of 13 genes in 18 neocortical regions, and the three divisions of the ganglionic eminences was obtained from RNA seq data, accessed via the database at http://brainspan.org/rnaseq/search/index.html from human fetal tissue aged 8 or 9 post-conceptional weeks; see Table S1 for more details. This figure is a summary of expression patterns observed. For the genes analysed, broadly speaking, expression patterns in the cortex fell into three categories represented by three coloured rectangles; higher expression anteriorly, laterally and ventrally (blue) or higher expression posteriorly and laterally (green) or higher expression anteriorly and medially compared to laterally (turquoise). Four patterns of expression were observed in the ganglionic eminences; equal expression across the ganglionic eminences (orange) higher expression in the MGE (dark red) or higher expression in the CGE (dark yellow). The resulting Venn diagram divides the expression of 13 genes (in italics) between five points of overlap. We propose that a component of the higher expression of some GABAergic genes in the anterior/lateral/ventral cortex derives from a subset of GABAergic precursors generated in the pallium specified by antero-ventral signalling, whereas higher expression of some cortical GABAergic genes in lateral and posterior regions likely derives entirely from inwardly migrating cells (see text for more details). The expression pattern of NGN2 and SP8 is quite different from the rest reflecting their primary roles in determination of glutamatergic identity (NGN2, Wilkinson et al. 2013) and anterior/medial identity (SP8, O’Leary & Sahara, 2008) in the pallium at this stage of development.
Studies in rodents show that Ngn 1/2 are predominantly expressed in the pallium, whereas Ascl1 expression shows less exclusivity, being expressed in both dorsal and ventral domains. Within the subpallium, Ascl1 is highly expressed in VZ/SVZ progenitors across the ganglionic eminences (Casarosa et al. 1999) with transcripts and protein persisting in post-mitotic neurons (Parras et al. 2004; Dixit et al. 2011). In the pallium, Ascl1 is also expressed in a subset of cortical VZ and SVZ progenitors which co-express neurogenin1/2 (Britz et al. 2006). Commitment to a glutamatergic or GABAergic lineage may require the destabilisation of Neurog2–Ascl1 co-expression in cortical progenitors (Wilkinson et al. 2013). The stable expression of Neurog2 may be initiated by Wnt/b-catenin signalling in early cortical progenitors (Gunhaga et al. 2003; Israsena et al. 2004; Backman et al. 2005) which is derived from the dorsomedial cortical hem signalling centre (O’Leary & Sahara, 2008; Rakic, 2009), while FGF signallng has been shown to block Neurog2 expression and promote Ascl1 expression (Hack et al. 2004; Abematsu et al. 2006), which is derived from anterior signalling centres in the forebrain (Monuki, 2007; O’Leary & Sahara, 2008; Rakic, 2009). The number of cortical progenitors exhibiting co-expression decreases with time, and thus it may be a mechanism for maintaining cells in proliferating state prior to lineage commitment (Wilkinson et al. 2013). But it also gives progenitors the potential to commit to GABAergic neuron production in response to anterior/ventral signals. What is interesting is that a number of GABAergic genes share a similar areal expression profile to ASCL1 namely DLX2, DLX5, GAD2 and CALB2 (Table S1, Fig.2; Al-Jaberi et al. 2013), suggesting co-expression. Anterior/ventral signalling may have an influence on a proportion of dorsal progenitors in ventral, lateral and anterior regions of the cerebral cortex, committing them to a GABAergic cell-fate.
NKX2.1 also displayed this gradient of expression in the 8–9 PCW cortex, albeit at very low levels of expression (Table S1, Fig.2), but with a high expression restricted to the MGE (as would be predicted from other studies, Hansen et al. 2013; Pauly et al. 2013). The only other gene to show the highest expression in the MGE at this stage is LHX6 (Fig.2), which is downstream of NKX2.1, but in the cortex this gene was preferentially expressed in the posterior and lateral cortex, a pattern of expression shared with NR2F2 (CGE marker), SOX6 (MGE marker) CUX2 (CGE and upper cortical layer glutamatergic neuron marker) and GAD1 (Fig.2). One hypothesis might be that, at this stage of development, intracortical interneurogenesis is induced to some extent in the anterior/lateral/ventral cortex under the influence of anterior signalling, producing neurons expressing NKX2.1 and other markers, whereas migrating interneurons from the ventral telencephalon carry expression of LHX6 or NR2F2 and other markers and preferentially target the posterior and lateral cortex.
More evidence is required to support this hypothesis but some already exists. NKX2.1 has been detected in the human VZ/SVZ (Rakic & Zecevic, 2003). At later stages of gestation, NKX2.1/calretinin-positive cells have been observed in cortical VZ/SVZ that co-express the proliferative marker Ki-67 (Jakovcevski et al. 2011). DLX2 (Al-Jaberi et al. 2013). NR2F2 (Reinchisi et al. 2012) has also been shown to have co-expression with Ki67 in the cortical VZ/SVZ. That such cells are found in the VZ differentiates them from the migratory GABAergic progenitors observed in mouse (Wu et al. 2011), which are only seen to undergo division in the SVZ.
These observations of dividing GABAergic precursors in human cortex are in stark contrast to Hansen et al. (2013), who found co-localisations between GABAergic markers and markers of cell division to be extremely rare and occurring only close to the border with the lateral ganglionic eminence. However, Jakovcevski et al. (2011) found such cells in the medial wall, far from the lateral ganglionic evidence. The observations of Hansen et al. (2013) also contradict observations that GABergic gene expression in monkey and human can be lower on the pallial side of the pallial/subpallial boundary, compared with the ganglionic eminences and more dorsal or anterior parts of the cortex at certain stages of development (Petanjek et al. 2009; Al-Jaberi et al. 2013). However, LHX6-positive cells found in the cortical VZ/SVZ were concluded to have migrated there from the ganglionic eminences, as no dividing LHX6-postive cells were found (Jakovcevski et al. 2011).
Evidence for generation of GABAergic cells in organotypic tissue culture or dissociated cell culture from human or monkey fetal cortex has been reported to be virtually non-existent in some studies (Hansen et al. 2010, 2013; Ma et al. 2013). However, in organotypic SVZ slices from human fetal cortex, cells expressing NKX2.1 and DLX2 were shown to take up BrdU, indicating their local proliferation (Zecevic et al. 2005). Furthermore, Yu & Zecevic (2011) reported generation of cells immunoreactive first for NKX2.1 and DLX and the CALB2 and GABA from radial glia cells of human cortical origin at mid-term (but not from mouse radial glial cells taken at E16). Similarly, radial glia-like cells derived from human embryonic stem cells, co-cultured with fetal human forebrain tissue, produce neurons, up to 20% of which are GABAergic (Reinchisi et al. 2013). We have made primary cultures from cortical fetal tissue at an earlier stage (8–12 PCW). These cultures consist of mainly MAP2-expressing neurons and neuroblasts (60–80%) and a minority of GFAP-expressing cells, presumably radial glial cells (Ip et al. 2010). We found expression of DLX2 and GAD1 in around 30% of cells, compared with expression of pallial genes (EMX2, TBR1) in approximately 40–60% of cells (Fig.3, Ip et al. 2010).
Figure 3.
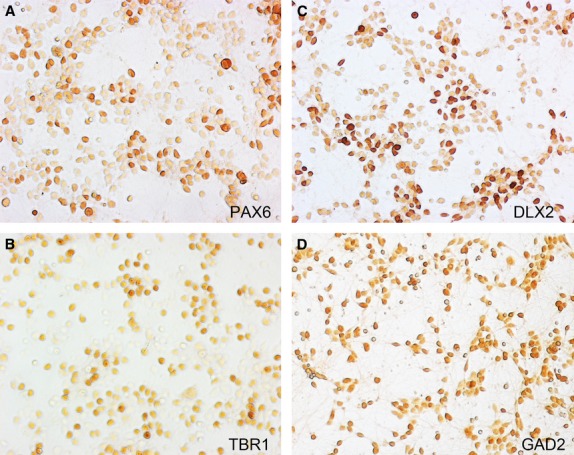
Primary cultures of early human fetal cortical cells, prepared according to Ip et al. (2010) after 3 days in vitro are a mixture of cells expressing a variety of markers including TBR1; post-mitotic glutamatergic cortical neurons (A) PAX6; mitotic radial glia-like cells (B) DLX2 and GAD2; markers for intermediate progenitors and neurons committed to the GABAergic lineage (C,D). Protein expression was revealed by immunoperoxidase cytochemistry. Micrographs were kindly provided by Dr Nahidh Al-Jaberi.
It should be remembered that even mouse cortical progenitors prepared at E13 can generate up to 30% GAD-expressing neurons when differentiated in culture, compared with 90% from progenitor cells derived from the mouse medial ganglionic eminence (He et al. 2001). A cloned neurogenic progenitor cell line produced from embryonic rat cortex generated interneurons in response to the ventral signalling molecule sonic hedgehog (Shh); however, this is suppressed by the dorsal signalling molecule Bmp2 (Li et al. 2008). In vivo, it may be that cortical neural stem cells initially have some intrinsic ability to produce GABAergic neurons in response to the appropriate signals, but this is down-regulated in rodents once cortical plate formation begins. Perhaps in human, this multipotency is retained for longer.
The current uncertainties can lead to differing interpretations of data gained from studies of neurodevelopmental conditions. For instance, Fertuzinhos et al. (2009) carried out a study of brains of human fetuses or infants with holoprosencephaly that suffered developmental malformation of the ganglionic eminences. The decrease in cortical interneurons expressing a number of markers including somatostatin, neuropeptide Y and neuronal nitric oxide synthase, but no reduction in CALB2 interneurons, was interpreted as showing loss of ganglionic eminence-derived interneurons, but preservation of pallium-derived interneurons (Fertuzinhos et al. 2009; Zecevic et al. 2011). However, in these brains, only the NKX2.1-expressing proliferative region was totally ablated, and some DLX2 and ASCL1 expression was still observed in the extant subpallium. Thus, Hansen et al. (2013) suggest an alternative interpretation that MGE-derived interneurons were lost, while CGE-derived interneurons were largely unaffected. However, only a small striatothalamic eminence along the ventral midline was observed in the severe holoprosencephalic cases, with relatively sparse DLX2 and ASCL1 expression (Fertuzinhos et al. 2009) and this appears insufficient to produce a normal population of CALB2 interneurons in the cortex. Furthermore, any induction of NKX2.1 in the pallium by anterior/ventral signallng would also be affected by this condition, as it is known that genes implicated in this condition, e.g. FGFR1 and FGFR2 (Monuki, 2007; Fernandes & Hébert, 2008) are also expressed in the human fetal neocortex (Ip et al. 2010).
Conclusion
The increase in relative size and complexity of the human cerebral cortex during evolution may have been brought about by a number of mechanisms, including a vastly increased number of neurons, which may have depended on the elaboration of the cortical subventricular zone to include new and expanded classes of progenitor cell (Bayatti et al. 2008; Hansen et al. 2010; Fietz et al. 2010; Lui et al. 2011). This has gone ‘hand in hand’ with increased connectivity between an expanded number of functional modules, which may, in turn, be dependent an enhanced function of GABAergic neurotransmission in synchronising this connectivity, provided by an expanded repertoire of GABAergic interneuron subtypes. It appears that the primate ganglionic eminences have also evolved an expanded outer subventricular zone, ‘keeping pace’ with cortical expansion in order to provide sufficient interneurons. Furthermore, an increasing contribution from the CGE may account for the increasing proportion of superficial layer interneurons of a certain phenotype.
However, it remains an intriguing possibility that some regions of cortex, possibly prefrontal or medial structures far removed from the ganglionic eminences, retain some intrinsic ability to produce GABAergic interneurons at certain stages of development under the influence of local signalling. This may turn out to be small scale in terms of the number of interneurons produced, but on the other hand may produce specialised interneuron subtypes. Thus it is worthy of further study, as deficiencies in the production and migration of interneurons are likely to play a role in the aetiology of neurodevelopmental conditions, and a lack of knowledge of origins of inhibitory interneurons may lead to misinterpretations of data.
Conflict of interest
The author has no conflict of interests to declare.
Acknowledgments
The author is grateful to the Wellcome Trust and the Iraqi Ministry of Higher Education and Scientific Research for funding his research into the topic of this review carried out in collaboration with colleagues at Newcastle University, in particular Prof. Susan Lindsay, Dr Nahidh Al-Jaberi and Dr Nadhim Bayatti. The human embryonic and fetal material was provided by the Joint MRC/Wellcome Trust Human Developmental Biology Resource.
Supporting Information
Table S1. Human GABAergic interneuron gene expression in the forebrain at 8-9 post-conceptional weeks.
References
- Abematsu M, Kagawa T, Fukuda S, et al. Basic fibroblast growth factor endows dorsal telencephalic neural progenitors with the ability to differentiate into oligodendrocytes but not gamma-aminobutyric acidergic neurons. J Neurosci Res. 2006;83:731–743. doi: 10.1002/jnr.20762. [DOI] [PubMed] [Google Scholar]
- Ainsworth M, Lee S, Cunningham MO, et al. Rates and rhythms: a synergistic view of frequency and temporal coding in neuronal networks. Neuron. 2011;75:572–583. doi: 10.1016/j.neuron.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Al-Jaberi N, Lindsay S, Sarma S. The early fetal development of human neocortical GABAergic interneurons. Cereb Cortex. 2013 doi: 10.1093/cercor/bht254. et al. (, epub, ahead of print doi: 10.1093/cercor/bht254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim E, Jabaudon D, Fame RM, et al. Sox6 controls dorsal progenitor identity and interneuron diversity during neocortical development. Nat Neurosci. 2009;12:1238–1247. doi: 10.1038/nn.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman M, Machon O, Mygland L, et al. Effects of canonical Wnt signaling on dorso-ventral specification of the mouse telencephalon. Dev Biol. 2005;279:155–168. doi: 10.1016/j.ydbio.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Ballesteros Yáñez I, Muñoz A, Contreras J, et al. Double bouquet cell in the human cerebral cortex and a comparison with other mammals. J Comp Neurol. 2005;486:344–360. doi: 10.1002/cne.20533. [DOI] [PubMed] [Google Scholar]
- Bayatti N, Moss JA, Sun L, et al. A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb Cortex. 2008;18:1536–1548. doi: 10.1093/cercor/bhm184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C, Kisvarday Z, Somogyi P, et al. Quantitative distribution of GABA-immunopositive and -immunonegative neurons and synapses in the monkey striate cortex (area 17) Cereb Cortex. 1992;2:295–309. doi: 10.1093/cercor/2.4.295. [DOI] [PubMed] [Google Scholar]
- Britz O, Mattar P, Nguyen L, et al. A role for proneural genes in the maturation of cortical progenitor cells. Cereb Cortex. 2006;16(Suppl. 1):138–151. doi: 10.1093/cercor/bhj168. [DOI] [PubMed] [Google Scholar]
- Burchell TR, Faulkner HJ, Whittington MA. Gamma frequency oscillations gate temporally coded afferent inputs in the rat hippocampal slice. Neurosci Lett. 1998;255:151–154. doi: 10.1016/s0304-3940(98)00676-4. [DOI] [PubMed] [Google Scholar]
- Butt SJB, Fuccillo M, Nery S, et al. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarosa S, Fode C, Guillemot F. Mash1 regulates neurogenesis in the ventral telencephalon. Development. 1999;126:525–534. doi: 10.1242/dev.126.3.525. [DOI] [PubMed] [Google Scholar]
- Clowry G, Molnár Z, Rakic P. Renewed focus on the developing human neocortex. J Anat. 2010;217:276–288. doi: 10.1111/j.1469-7580.2010.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carlos JA, López-Mascaraque L, Valverde F. Dynamics of cell migration from the lateral ganglionic eminence in the rat. J Neurosci. 1996;16:6146–6156. doi: 10.1523/JNEUROSCI.16-19-06146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felipe J. Chandelier cells and epilepsy. Brain. 1999;122:1807–1822. doi: 10.1093/brain/122.10.1807. [DOI] [PubMed] [Google Scholar]
- De Felipe J. The evolution of the brain, the human nature of cortical circuits, and intellectual creativity. Front Neuroanat. 2011;5:29. doi: 10.3389/fnana.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felipe J, Lopez-Cruz PL, Benvides-Piccione R. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Neurosci. 2013;14:202–216. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R, Zimmer C, Waclaw RR, et al. Ascl1 participates in Cajal-Retzius cell development in the neocortex. Cereb Cortex. 2011;21:2599–2611. doi: 10.1093/cercor/bhr046. [DOI] [PubMed] [Google Scholar]
- Du T, Xu Q, Ocbina PJ, et al. Nkx2.1 specifies cortical interneuron fate by activating Lhx6. Development. 2008;135:1559–1567. doi: 10.1242/dev.015123. [DOI] [PubMed] [Google Scholar]
- Faux C, Rakic S, Andrews W, et al. Neurons on the move: migration and lamination of cortical interneurons. Neurosignals. 2012;20:168–189. doi: 10.1159/000334489. [DOI] [PubMed] [Google Scholar]
- Fernandes M, Hébert JM. The ups and downs of holoprosencephaly: dorsal versus ventral patterning forces. Clin Genet. 2008;73:413–423. doi: 10.1111/j.1399-0004.2008.00994.x. [DOI] [PubMed] [Google Scholar]
- Fertuzinhos S, Krsnik E, Kawasawa YI, et al. Selective depletion of molecularly defined cortical interneurons in human holoprosencephaly with severe striatal hypoplasia. Cereb Cortex. 2009;19:2196–2207. doi: 10.1093/cercor/bhp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietz SA, Kelava I, Vogt J, et al. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci. 2010;13:690–699. doi: 10.1038/nn.2553. [DOI] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, et al. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a, b, c, 25 and 32) in the monkey: II. Quantitative areal and laminar distributions. J Comp Neurol. 1996;364:609–636. doi: 10.1002/(SICI)1096-9861(19960122)364:4<609::AID-CNE2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Dickie BG, Vaid RR. Local-circuit neurones in the medial prefrontal cortex (areas 25, 32 and 24b) in the rat: morphology and quantitative distribution. J Comp Neurol. 1997;377:465–499. doi: 10.1002/(sici)1096-9861(19970127)377:4<465::aid-cne1>3.0.co;2-0. et al. ( [DOI] [PubMed] [Google Scholar]
- Ghanem N, Yu M, Long J, et al. Distinct cis-regulatory elements from the Dlx1/Dlx2 locus mark different progenitor cell populations in the ganglionic eminences and different subtypes of adult cortical interneurons. J Neurosci. 2007;27:5012–5022. doi: 10.1523/JNEUROSCI.4725-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunhaga L, Marklund M, Sjodal M, et al. Specification of dorsal telencephalic character by sequential Wnt and FGF signaling. Nat Neurosci. 2003;6:701–707. doi: 10.1038/nn1068. [DOI] [PubMed] [Google Scholar]
- Hack MA, Sugimori M, Lundberg C, et al. Regionalization and fate specification in neurospheres: the role of Olig2 and Pax6. Mol Cell Neurosci. 2004;25:664–678. doi: 10.1016/j.mcn.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Hájos N, Pálhalmi J, Mann EO, et al. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci. 2004;24:9127–9137. doi: 10.1523/JNEUROSCI.2113-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Parker PR, et al. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Flandin P, et al. Non-epithelial stem cells and cortical interneuron production in the human ganglionic eminences. Nat Neurosci. 2013;16:1576–1587. doi: 10.1038/nn.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Csicsvari J, Hirase H, et al. Organization of cell assemblies in the hippocampus. Nature. 2003;424:552–556. doi: 10.1038/nature01834. [DOI] [PubMed] [Google Scholar]
- He W, Ingraham C, Rising L. Multipotent stem cells from the mouse basal forebrain contribute GABAergic neurons and oligodendrocytes to the cerebral cortex during embryogenesis. J Neurosci. 2001;21:8854–8862. doi: 10.1523/JNEUROSCI.21-22-08854.2001. et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Schwark HD, Jones EG, et al. Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex. J Neurosci. 1987;7:1503–1519. doi: 10.1523/JNEUROSCI.07-05-01503.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan M, Welagen J, Anderson SA. Spatial and temporal bias in the mitotic origins of somatostatin- and parvalbumin-expressing interneuron subgroups and the chandelier subtype in the medial ganglionic eminence. Cereb Cortex. 2012;22:820–827. doi: 10.1093/cercor/bhr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inda MC, De Felipe J, Muñoz A. The distribution of chandelier cell axon terminals that express the GABA plasma membrane transporter GAT-1 in the human neocortex. Cereb Cortex. 2007;17:2060–2071. doi: 10.1093/cercor/bhl114. [DOI] [PubMed] [Google Scholar]
- Inda MC, De Felipe J, Muñoz A. Morphology and distribution of chandelier cell axon terminals in the mouse cerebral cortex and claustroamygdaloid complex. Cereb Cortex. 2009;19:41–54. doi: 10.1093/cercor/bhn057. [DOI] [PubMed] [Google Scholar]
- Ip BK, Wappler I, Peters H, et al. Investigating gradients of gene expression involved in early human cortical development. J Anat. 2010;217:300–311. doi: 10.1111/j.1469-7580.2010.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israsena N, Hu M, Fu W, et al. The presence of FGF2 signaling determines whether beta-catenin exerts effects on proliferation or neuronal differentiation of neural stem cells. Dev Biol. 2004;268:220–231. doi: 10.1016/j.ydbio.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Mayer N, Zecevic N. Multiple origins of human neocortical interneurons are supported by distinct expression of transcription factors. Cereb Cortex. 2011;21:1771–1782. doi: 10.1093/cercor/bhq245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. The origins of cortical interneurons: mouse versus monkey and human. Cereb Cortex. 2009;19:1953–1956. doi: 10.1093/cercor/bhp088. [DOI] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, et al. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010;30:16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory interneurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Li H, Han YR, Bi C, et al. Functional differentiation of a clone resembling embryonic cortical interneuron progenitors. Dev Neurobiol. 2008;68:1549–1564. doi: 10.1002/dneu.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liodis P, Denaxa M, Grigoriou M, et al. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27:3078–3089. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Wang C, Wang L, et al. Subcortical origins of human and monkey neocortical interneurons. Nat Neurosci. 2013;16:1588–1597. doi: 10.1038/nn.3536. [DOI] [PubMed] [Google Scholar]
- Marín O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13:107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- Marín O, Rubenstein JLR. Cell migration in the forebrain. Ann Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Middleton S, Jalics J, Kispersky T, et al. NMDA receptor-dependent switching between different gamma rhythm-generating microcircuits in entorhinal cortex. Proc Natl Acad Sci U S A. 2008;105:18572–18577. doi: 10.1073/pnas.0809302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Butt SJ, Takebayashi H, et al. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Hjerling-Leffler J, Karayannis T, et al. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár Z, Butt SJB. Best laid schemes of interneuron origin for mice and men. Nat Neurosci. 2013;16:1512–1514. doi: 10.1038/nn.3557. [DOI] [PubMed] [Google Scholar]
- Molnár G, Oláh SZ, Komlósi G, et al. Complex events initiated by individual spikes in the human cerebral cortex. PLoS Biol. 2008;6:e222. doi: 10.1371/journal.pbio.0060222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monuki ES. The morphogen signaling network in forebrain development and holoprosencephaly. J Neuropathol Exp Neurol. 2007;66:566–575. doi: 10.1097/nen.0b013e3180986e1b. [DOI] [PubMed] [Google Scholar]
- O’Leary DDM, Sahara S. Genetic regulation of arealisation of the neocortex. Curr Opin Neurobiol. 2008;18:90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnavelas JG. The origin and migration of cortical neurones: New vistas. Trends Neurosci. 2000;23:126–131. doi: 10.1016/s0166-2236(00)01553-8. [DOI] [PubMed] [Google Scholar]
- Parras CM, Schuurmans C, Scardigli R, et al. Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev. 2002;16:324–338. doi: 10.1101/gad.940902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parras CM, Galli R, Britz O, et al. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly M-C, Döbrössy MD, Nikkhah G, et al. Organisation of the human fetal subpallium. Front Neuroanat. 2013;7:54. doi: 10.3389/fnana.2013.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Abrahams BS, Herman EI, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z, Berger B, Esclapez M. Origins of cortical GABAergic neurons in the cynomolgus monkey. Cereb Cortex. 2009;19:249–262. doi: 10.1093/cercor/bhn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryniak MA, Potter GB, Rowitch DH, et al. Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain. Neuron. 2007;55:417–433. doi: 10.1016/j.neuron.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pino I, Garcia-Frigola C, Dehorter N, et al. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like symptoms. Neuron. 2013;79:1152–1168. doi: 10.1016/j.neuron.2013.07.010. [DOI] [PubMed] [Google Scholar]
- Povysheva NV, Zaitsev AV, Kroner S, et al. Electrophysiological differences between neurogliaform cells from monkey and rat prefrontal cortex. J Neurophysiol. 2007;97:1030–1039. doi: 10.1152/jn.00794.2006. [DOI] [PubMed] [Google Scholar]
- Povysheva NV, Zaitsev AV, Rotaru DC, et al. Parvalbumin-positive basket interneurons in monkey and rat prefrontal cortex. J Neurophysiol. 2008;100:2348–2360. doi: 10.1152/jn.90396.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povysheva NV, Zaitsev AV, Gonzalez-Burgos G, et al. Electrophysiological heterogeneity of fast-spiking interneurons: chandelier versus basket cells. PLoS ONE. 2013;8:e70553. doi: 10.1371/journal.pone.0070553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Rev Nat Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Emerging complexity of layer I in human cerebral cortex. Cereb Cortex. 2003;13:1072–1083. doi: 10.1093/cercor/13.10.1072. [DOI] [PubMed] [Google Scholar]
- Reinchisi G, Ijichi K, Glidden N, et al. COUP-TFII expressing interneurons in human fetal forebrain. Cereb Cortex. 2012;22:2820–2830. doi: 10.1093/cercor/bhr359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinchisi G, Limaye PV, Singh MB, et al. Neurogenic potential of hESC-derived human radial glia is amplified by human fetal cells. Stem Cell Res. 2013;11:587–600. doi: 10.1016/j.scr.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Río MR, De Felipe J. Colocalization of calbindin D-28k, calretinin, and GABA immunoreactivities in neurons of the human temporal cortex. J Comp Neurol. 1996;369:472–482. doi: 10.1002/(SICI)1096-9861(19960603)369:3<472::AID-CNE11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, et al. Three groups of interneurons account for 100% of neocortical interneurons. Dev Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymar VV, Sadikot AF. Laminar fate of cortical GABAergic interneurons is dependent on both birthdate and phenotype. J Comp Neurol. 2007;501:369–380. doi: 10.1002/cne.21250. [DOI] [PubMed] [Google Scholar]
- Sauer JF, Strüber M, Bartos M. Interneurons provide circuit-specific depolarization and hyperpolarization. J Neurosci. 2012;32:4224–4229. doi: 10.1523/JNEUROSCI.5702-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A, Ainsworth M, Clowry GJ. Comparison of parvalbumin expression in the temporal cortex across species. J Anat. et al. (in press), in press. [Google Scholar]
- Singer W, Gray CM. Visual feature correlation and temporal correlation hypothesis. Ann Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Stenman J, Toresson H, Campbell K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci. 2003;23:167–174. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, et al. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnár G. Excitatory effects of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. et al. ( [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Lu J, Huang J. The temporal and spatial origins of chandelier cells in mouse neocortex. Science. 2013;339:70–74. doi: 10.1126/science.1227622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire L, Pelkey KA, Daw MI, et al. Common origins of hippocampal Ivy and nitric oxide synthase expressing neurogliaform cells. J Neurosci. 2010;30:2165–2176. doi: 10.1523/JNEUROSCI.5123-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Vucurovic K, Gallopin T, Ferezou I, et al. Serotonin 3a receptor subtype as an early and protracted marker of cortical interneuron subpopulations. Cereb Cortex. 2010;20:2333–2347. doi: 10.1093/cercor/bhp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waclaw RR, Wang B, Pei Z. Distinct temporal requirements for the homeobox gene Gsx2 in specifying striatal and olfactory bulb neuronal fates. Neuron. 2009;63:451–465. doi: 10.1016/j.neuron.2009.07.015. et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Toledo-Rodriguez M, Gupta A, et al. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J Physiol. 2004;561:65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welagen J, Anderson S. Origins of neocortical interneurons in mice. Dev Neurobiol. 2011;71:10–17. doi: 10.1002/dneu.20857. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Cunningham MO, LeBeau FE, et al. Multiple origins of the cortical gamma rhythm. Dev Neurobiol. 2011;71:92–106. doi: 10.1002/dneu.20814. [DOI] [PubMed] [Google Scholar]
- Wilkinson G, Dennis D, Schuurmans C. Proneural genes in neocortical development. Neuroscience. 2013;253:256–273. doi: 10.1016/j.neuroscience.2013.08.029. [DOI] [PubMed] [Google Scholar]
- Wonders C, Anderson SA. Cortical interneurons and their origins. Neuroscientist. 2005;11:199–205. doi: 10.1177/1073858404270968. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Taylor L, Welagen J. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev Biol. 2008;314:127–136. doi: 10.1016/j.ydbio.2007.11.018. et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Esumi S, Watanabe K, et al. Tangential migration and proliferation of intermediae progenitors of GABAergic neurons in mouse telencephalon. Development. 2011;138:2499–2509. doi: 10.1242/dev.063032. [DOI] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz ED, et al. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Zecevic N. Dorsal radial glial cells have the potential to generate cortical interneurons in human but not in mouse brain. J Neurosci. 2011;31:2413–2420. doi: 10.1523/JNEUROSCI.5249-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun K, Fischman S, Johnson J, et al. Modulation of the notch signalling by Mash1 and Dlx1/2 regulates sequential specification and differentiation of progenitor cell types in the subcortical telencephalon. Development. 2002;129:5029–5040. doi: 10.1242/dev.129.21.5029. [DOI] [PubMed] [Google Scholar]
- Zaitsev AV. Classification and function of GABAergic interneurons of the mammalian cerebral cortex. Biochem (Moscow) Suppl Series A: Membra Cell Biol. 2013;7:253–270. [Google Scholar]
- Zecevic N, Chen Y, Filipovic R. Contributions of cortical subventricular zone to the development of the human cerebral cortex. J Comp Neurol. 2005;491:109–122. doi: 10.1002/cne.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Hu F, Jakovcevski I. Interneurons in the developing human neocortex. Dev Neurobiol. 2011;71:18–33. doi: 10.1002/dneu.20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Human GABAergic interneuron gene expression in the forebrain at 8-9 post-conceptional weeks.


