Abstract
Pulmonary complications are frequently observed after transthoracic oesophagectomy. These complications may be reduced by sparing the vagus nerve branches to the lung. However, current descriptions of the regional anatomy are insufficient. Therefore, we aimed to provide a highly detailed description of the course of the pulmonary vagus nerve branches. In six fixed adult human cadavers, bilateral microscopic dissection of the vagus nerve branches to the lungs was performed. The level of branching and the number, calibre and distribution of nerve branches were described. Nerve fibres were identified using neurofilament immunohistochemistry, and the nerve calibre was measured using computerized image analysis. Both lungs were supplied by a predominant posterior and a smaller anterior nerve plexus. The right lung was supplied by 13 (10–18) posterior and 3 (2–3) anterior branches containing 77% (62–100%) and 23% (0–38%) of the lung nerve supply, respectively. The left lung was supplied by a median of 12 (8–13) posterior and 3 (2–4) anterior branches containing 74% (60–84%) and 26% (16–40%) of the left lung nerve supply, respectively. During transthoracic oesophagectomy with en bloc lymphadenectomy and transection of the vagus nerves at the level of the azygos vein, 68–100% of the right lung nerve supply and 86–100% of the inferior left lung lobe nerve supply were severed. When vagotomy was performed distally to the last large pulmonary branch, 0–8% and 0–13% of the nerve branches to the right middle/inferior lobes and left inferior lobe, respectively, were lost. In conclusion, this study provides a detailed description of the extensive pulmonary nerve supply provided by the vagus nerves. During oesophagectomy, extensive mediastinal lymphadenectomy denervates the lung to a great extent; however, this can be prevented by performing the vagotomy distal to the caudalmost large pulmonary branch. Further research is required to determine the feasibility of sparing the pulmonary vagus nerve branches without compromising the completeness of lymphadenectomy.
Keywords: autonomic nervous system, lung, oesophageal surgery, pulmonary nerves
Background
Annually, 456 000 people are newly diagnosed with oesophageal cancer and 400 000 people die from this disease, making it the eighth most common cancer and the sixth most common cause of cancer-related death worldwide (Ferlay et al. 2015). The cornerstone of treatment is resection of the oesophagus including all regional lymph nodes. Such a resection is best performed via a combined abdominal and transthoracic approach, which is called a transthoracic oesophagectomy, and is considered one of the most complex and invasive surgical procedures (Markar et al. 2012).
Postoperatively, pulmonary complications are very frequently observed and are associated with an increased need for readmission to the intensive care unit (36 vs. 9%) and an increased length of stay (22 vs. 15 days), both of which significantly contribute to postoperative morbidity (Sluis et al. 2014). Pneumonia is the most frequently encountered pulmonary complication, but acute lung injury, acute respiratory distress syndrome, atelectasis and sputum stasis also occur. The incidence of pulmonary complications remains problematically high (47 and 59% in patients without and with neoadjuvant chemoradiotherapy, respectively) despite many recent improvements such as minimally invasive surgery, fast track recovery and care centralization (van de Poll-Franse et al. 2011; Biere et al. 2012; Markar et al. 2012; Bosch et al. 2014). Many factors may contribute to the high incidence of postoperative pulmonary complications (e.g. preoperative radiation therapy) but the inflammatory response is thought to be a pivotal factor (D’Journo et al. 2010; Eichenbaum & Neustein, 2010; Bosch et al. 2014). In their prospective study of 96 patients D’Journo et al. (2010) showed that the development of systemic inflammatory response syndrome (SIRS) within 48 h postoperatively was the sole independent predictor of the occurrence of later pulmonary complications (33 vs. 6%). These findings suggest that inflammation may be the final common pathway leading to postoperative pulmonary complications.
Vagotomy is an integral part of oesophagectomy due to the close topographical relationship between the oesophagus and the vagus nerve. The vagus nerve controls important pulmonary functions and characteristics such as the cough reflex, mucus production and bronchus diameter (Belvisi, 2002; Mazzone & Canning, 2013). Furthermore, the vagus nerve plays a crucial role in the regulation of systemic and local inflammatory responses (Luyer et al. 2005; Tracey, 2009; Lubbers et al. 2010a,b;). Interestingly, Pyere et al. studied a technique to spare the entire vagus nerve during transhiatal oesophagectomy for benign and pre-malignant conditions. In particular, the postoperative pneumonia and respiratory failure rates were reduced following vagus sparing surgery (4 and 0%, n = 49) compared with conventional transhiatal surgery (18 and 15%, n = 39). Furthermore, the median length of hospital stay was reduced from 16 to 12 days (Peyre et al. 2007). However, such a vagus sparing transhiatal technique would not be possible in the majority of patients with more advanced malignant disease because radicality would be compromised. For these patients, selective sparing of the pulmonary branches of the vagus nerve might be advantageous, and it has been hypothesized that sparing these branches might reduce postoperative pulmonary complications (Fujita et al. 1988).
The pulmonary branches of the vagus nerve are transected as part of the extensive mediastinal lymph node dissection during transthoracic oesophagectomy. Precise knowledge of the course and the extent of pulmonary innervation of these branches is a prerequisite for developing operative techniques to spare them. However, existing anatomical descriptions of the pulmonary branches of the vagus nerve are brief and insufficient. Therefore, we aimed to provide a detailed, surgically oriented description of the pulmonary nerve supply based on microscopic dissections.
Methods
Dissection
Six adult cadavers without signs of previous cervical or thoracic surgery that were fixed with 3% formaldehyde were included. The specimens were derived from bodies that entered the anatomy department through a donation programme. Written informed consent was obtained from these persons when they were alive, which allowed the use of their entire bodies for educational and research purposes. The anterolateral thoracic wall, including the parietal pleura, was removed, leaving the spine and both sympathetic trunks in situ. Both lungs were partially resected, leaving the pulmonary hilum behind.
At the level of the thyroid gland, the vagus nerve was identified between the common carotid artery and the internal jugular vein. From there, the nerve and its branches were exposed through careful microscopic dissection using a binocular surgical microscope (10582; Carl Zeiss, Oberkochen, Germany). Pulmonary vagus nerve branches were dissected as far as the lung lobe that they supplied. In the caudal direction, vagus nerve dissection was continued to the last of the pulmonary branches. Additionally, both sympathetic trunks were identified at the first rib, and the sympathetic nerves to the lungs were exposed through microscopic dissection. Finally, the entire mediastinum, including the oesophagus, the trachea, the aorta and the heart, was removed from the spine. This facilitated the dissection of all vagus and recurrent nerve branches towards the trachea. Photographs were taken at all stages of dissection.
Measurements
The nerve branches that supplied the lung were counted. In the final results, only those tissue strands that were histologically confirmed to contain neuronal tissue, were presented. Nerve branches were visually graded as large, i.e. easily identifiable without magnification, or small, i.e. identifiable only with the aid of a microscope. The cranial–caudal distance at which the posterior pulmonary branches left the vagus nerve in relation to the cranial and caudal edges of the main bronchus was recorded. The distance between the carina and the location where the vagus nerve crossed the inferior edge of the main bronchus was measured. This distance was regarded as an intra-operatively easily identifiable distance that might aid in finding the vagus nerve.
Immunohistochemistry
After the measurements, all nerve branches to the lungs were removed. This included all branches arising from the plexuses coursing towards the lung. From the right anterior pulmonary plexus next to the pulmonary branches, a few branches also coursed towards the heart; these cardiac branches were not included in the immunohistochemical analysis. The nerve branches were embedded in an optimum cutting temperature formulation of water-soluble glycols and resins (Tissue-Tek® O.C.T.™; Sakura, Alphen aan den Rijn, The Netherlands). Using a cryostat, 10-μm-thick sections were cut. Immunohistochemistry was performed using an automated procedure. Two sections of each pulmonary nerve branch were pre-treated for immunohistochemistry with a solution containing 2-methyl-3-isothiazolone, boric acid, ethylenediaminetetraacetic acid and Tris(hydroxymethyl)aminomethane (CC1®; Ventana Medical Systems, Inc., Tukson, Arizona). This Tris-based buffer disrupts the covalent tissue bonds formed by formaldehyde, which makes the proteins more accessible to antibodies. Subsequently, the tissue sections were exposed to antibodies that bind neurofilaments (Monosan mouse anti-human neurofilament clone 2F11; 1 : 800) for 32 min in the Ventana immunostainer® to confirm the presence of neuronal tissue. Optiview HRP Multimer® (Roche, Basel, Switzerland) was used as the secondary antibody, with an incubation time of 8 min. Neurofilament antibody binding was visualized by reaction with 3,3′-diaminobenzidine (DAB), with an incubation time of 8 min. A section of the upper cervical part of the vagus nerve was used as a positive control.
Computerized image analysis
One section of each pulmonary branch was selected for image analysis and was photographed. The selected section was characterized by the absence of artefacts and good neurofilament labelling. The images were analysed using ImageJ (Schneider et al. 2012). The images were split into red, green and blue channels, and the blue channel was selected for further analysis because it showed the best contrast. The best threshold for distinguishing neurofilament from other tissues was visually determined and applied, resulting in binary images. The nerve fibre area was measured and expressed in mm2. To correct for differences among the cadavers, the size of each nerve was presented as the percentage of the sum of the size of all nerve branches innervating the corresponding lung.
Statistical analysis
Individualized data are presented as absolute numbers and percentages for each cadaver dissected. Continuous data are summarized as the median (range) values.
Results
Course of the right vagus nerve
In all specimens, the right vagus nerve was identified in the neck between the common carotid artery and the internal jugular vein. Without exception, the recurrent nerve branched from the vagus nerve just inferior to the subclavian artery. From here, the vagus nerve coursed caudally lateral to the trachea and slightly dorsally to pass the main right bronchus. At the superior edge of this bronchus, the vagus nerve divided into two or three main divisions. These divisions formed the oesophageal plexus distal to the inferior edge of the right main bronchus. The median distance between the tracheal bifurcation and the location at which the vagus nerve crossed the inferior edge of the bronchus was 9 (5–15) mm.
Right pulmonary plexuses
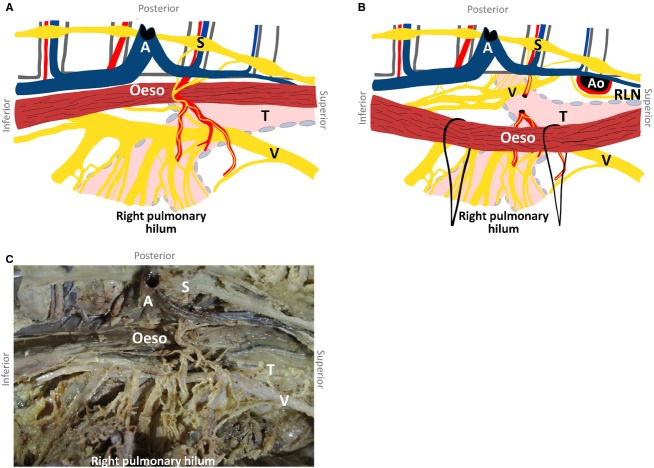
Several cardiopulmonary branches arose from the right vagus nerve between the subclavian artery and the main bronchus (Fig.1). These branches coursed caudally and medially over the trachea and were pretracheally embedded in loose connective tissue. Medially, the most superior branches coursed anterior to the trachea and posterior to the superior vena cava, leading to the heart. The most inferior branches formed an anterior pulmonary plexus, coursing in between or just dorsal to the pretracheal lymph nodes. The anterior pulmonary plexus was formed just superior to the right pulmonary artery. From this plexus, a median of three (two to three) nerve branches, containing a median of 23% (0–38%) of the total number of nerve fibres to the right lung, supplied any of the right lung lobes (Table1A). Other branches coursed medially to supply the heart. Figure2 shows an example of neurofilament staining. Nerve fibre area measurements of these sections determined the innervation percentages as presented in Table1.
Figure 1.
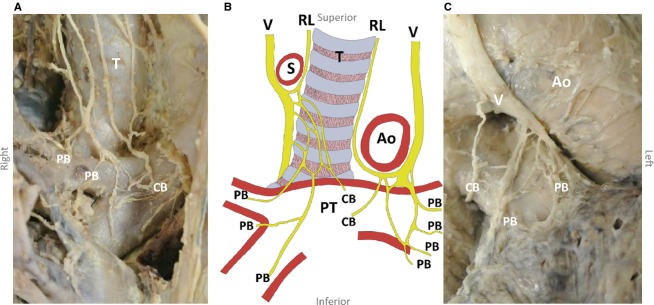
Schematic drawing of the right and left anterior pulmonary plexuses from a frontal view (B) with corresponding photographs of both plexuses (A,C). Ao, aorta; CB, cardiac branch; PB, pulmonary branch; PT, pulmonary trunk; RL, recurrent laryngeal nerve; T, trachea; S, subclavian artery; V, vagus nerve.
Table 1.
The number of branches of the pulmonary plexuses to the lungs and the percentages of nerve fibres in these branches
| Subject | Anterior branches | Posterior branches | |||
|---|---|---|---|---|---|
| n | Supply (%) | n | Supply (%) | ||
| 1A right lung | |||||
| 1 | Female | 2 | 21 | 14 | 79 |
| 2 | Male | 3 | 0 | 10 | 100 |
| 3 | Male | 3 | 32 | 18 | 68 |
| 4 | Female | 3 | 25 | 13 | 75 |
| 5 | Female | 3 | 38 | 12 | 62 |
| 6 | Male | 3 | 12 | 13 | 88 |
| 1B left lung | |||||
| 1 | Female | 3 | 16 | 11 | 84 |
| 2 | Male | 3 | 40 | 8 | 60 |
| 3 | Male | 3 | 36 | 9 | 64 |
| 4 | Female | 4 | 28 | 13 | 72 |
| 5 | Female | 2 | 17 | 12 | 83 |
| 6 | Male | 2 | 24 | 13 | 75 |
Figure 2.
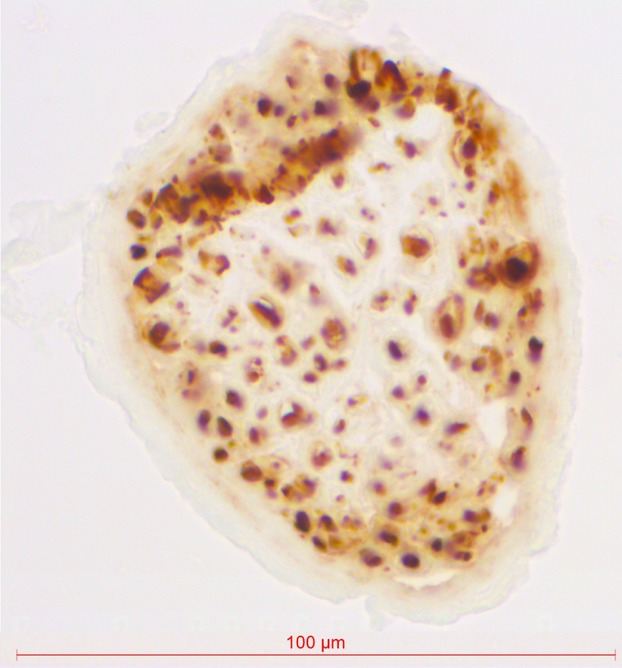
Section of a pulmonary vagus nerve branch labelled by neurofilament immunohistochemistry, magnification: 40×. The location of neurofilament antibody binding is stained dark brown by the DAB reaction.
Posterior to the right main bronchus, 13 (10–18) nerves to the lung arose from the right vagus nerve inferior to the superior edge of the right main bronchus (Fig.3). The most superior branches supplied the superior lobe, and the most inferior branches supplied the middle and inferior lobes. Differentiating between the nerve branches supplying the middle and the inferior lobes was not possible. All posterior branches together are termed the posterior pulmonary plexus. The last large posterior pulmonary branch was found 11 (5–26) mm caudal to the right main bronchus. Overall, the right posterior pulmonary plexus contained 77% (62–100%) of the total nerve supply of the right lung (Table1A).
Figure 3.
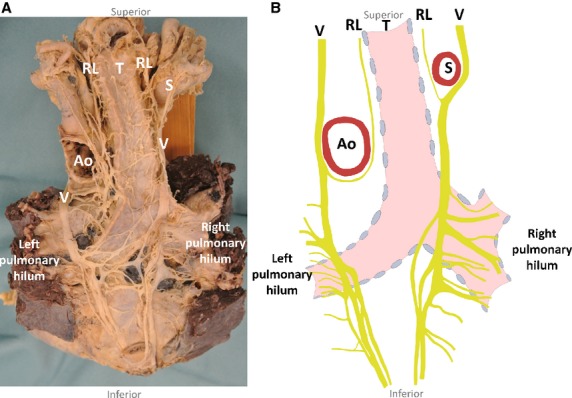
Schematic drawing of the right and left posterior pulmonary plexuses from a posterior view (B) with the corresponding photograph (A). Ao, aorta; RL, recurrent laryngeal nerve; S, subclavian artery; T, trachea; V, vagus nerve.
Course of the left vagus nerve
The left vagus nerve was identified between the left common carotid artery and the internal jugular vein in all specimens. The nerve coursed caudally and passed the aortic arch ventrally, where the recurrent laryngeal nerve left the vagus nerve and entered the aortopulmonary window in all cases. The vagus nerve then coursed dorsally to the left pulmonary hilum to join the oesophagus. Between the level of the left pulmonary artery and the left pulmonary vein, the vagus nerve divided into two to four main divisions. These joined the divisions of the right vagus nerve inferior to the main bronchi to form the oesophageal plexus. The median distance between the tracheal bifurcation and the location where the left vagus nerve crossed the inferior edge of the principal bronchus was 32 (24–36) mm.
Left pulmonary plexuses
The left anterior pulmonary plexus was formed by three (two to four) vagus nerve branches on the anterior side of the left pulmonary artery (Table1B, Fig.1). These nerve branches coursed in between or immediately dorsal to lymph nodes lying antero-superior to the left pulmonary artery. On the anterior side of the left pulmonary artery, a small nerve plexus from which all branches ran to the superior lung lobe was formed. The anterior pulmonary plexus contained 26% (16–40%) of the total number of nerve fibres to the left lung,
The left posterior pulmonary plexus comprised a median of 12 (8–13) vagus nerve branches, arising from the vagus nerve between the superior edge of the pulmonary artery and directly below the left pulmonary vein (Fig.2). These branches coursed laterally to supply the superior and inferior pulmonary lobes; the most superior branches innervated the superior lobe, and the most inferior branches innervated the inferior lobe. The caudalmost large left posterior pulmonary branch was found 13 (−1 to 37) mm under the left main bronchus. The nerves forming the left posterior pulmonary plexus contained 74% (60–84%) of the left pulmonary vagus nerve fibres.
Tracheal branches
Many branches from both the vagus and the recurrent laryngeal nerves coursed medially to the trachea and the oesophagus. A median of 12 (6–20) tracheal branches were found on the right side; four (one to nine) branches were from the right recurrent laryngeal nerve, and nine (3–13) branches were from the right vagus nerve. A median of 16 (14–23) branches innervated the trachea from the left side; 12 (8–17) branches were from the left recurrent laryngeal nerve, and six (one to nine) were from the left vagus nerve. These branches coursed medially to form a nerve plexus on the membranous wall of the trachea. Divisions of many of these vagus nerve branches also coursed towards the oesophagus.
Sympathetic nerves
On the right and the left sides, several thin pulmonary splanchnic nerves arose from the highest thoracic sympathetic trunk ganglia. On the right side, these nerves coursed medially and caudally to join the right bronchial artery on its way to the right main bronchus, directly medially to the azygos vein (Figs4 and 5). Even if the right bronchial artery was absent (n = 1), the nerves joined directly medially to the azygos vein and coursed ventrally to the right main bronchus to supply the lung. The course of the pulmonary splanchnic nerves from the left sympathetic trunk ganglia to the left lung was similar to that on the right side, except that they passed the aortic arch dorsally or ventrally to join the bronchial arteries coursing to the left lung.
Figure 4.
Schematic drawing of the right posterior (A) and left posterior (B) pulmonary plexuses from a right lateral view, as encountered during transthoracic oesophagectomy, with a corresponding photograph of the right posterior pulmonary plexus (C). The right bronchial artery with pulmonary splanchnic nerves is also shown. The azygos vein, aorta and bronchial artery are severed and partially resected. A, azygos vein; Ao, aorta; Oeso, oesophagus; RLN, left recurrent laryngeal nerve; S, sympathetic trunk; T, trachea; V, vagus nerve.
Figure 5.
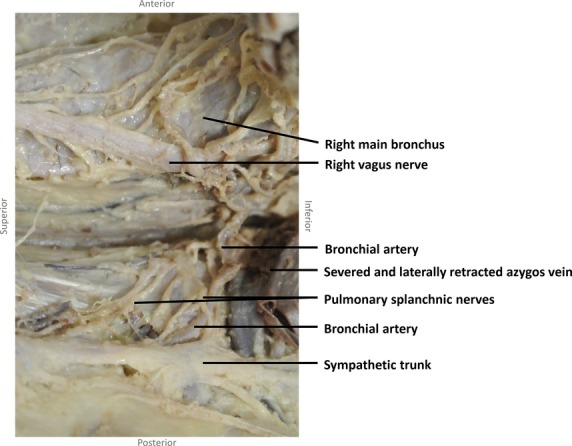
Photograph from a right lateral view showing that the pulmonary splanchnic nerves merge at the beginning of the right bronchial artery to follow the bronchial artery to the lung.
Landmarks for surgery
Figure4 shows the right vagus nerve from a right lateral view, which fits the most common approach for transthoracic oesophagectomy. The superior and inferior edges of the right main bronchus and the caudalmost large pulmonary branch of the right vagus nerve were easily identifiable. Table2 shows the proportion of pulmonary vagus nerve branches that are transected when the vagus nerve is cut at these landmarks. If the vagus nerve was transected at the superior edge of the main bronchus, 68% to approximately 100% of the total nerve supply of the right lung would be lost, and 86–100% of the inferior left lung lobe nerve supply. Severing the vagus nerve at the inferior margin of the main bronchus may save the nerve supply to the superior right lung lobe. From 0 to 8% and 0 to 13% of the nerve branches to the right middle and inferior lobe and left inferior lobe nerves, respectively, would be lost if the vagus nerve was transected distally to the caudalmost large pulmonary branch. The right and left caudalmost large pulmonary branches are encountered 5–26 mm and −1 to 37 mm below the inferior margin of the right and left main bronchi.
Table 2.
The proportion of pulmonary nerves severed at different levels of vagotomy
| Right lung | Left lung | |||||
|---|---|---|---|---|---|---|
| Lung (%) | Superior lobe (%) | Middle and inferior lobes (%) | Lung | Superior lobe (%) | Inferior lobe (%) | |
| Superior margin main bronchus | 68–100 | 55–98 | 64–100 | 48–72 | 0–6 | 86–100 |
| Inferior margin main bronchus | 16–64 | 0–0 | 20–78 | 2–45 | 0–0 | 5–76 |
| Inferior to last large pulmonary branch | 0–5 | 0–0 | 0–8 | 0–9 | 0–0 | 0–13 |
Discussion
The vagus nerves supply the lungs via a small nerve plexus anterior to the main bronchus and an extensive nerve plexus posterior to the main bronchus. Pulmonary splanchnic nerves reach the lung along the bronchial arteries. Oesophagectomy with extensive mediastinal lymphadenectomy results in a near complete vagotomy and sympathectomy of the right lung and a complete vagotomy of the inferior left lung lobe. Saving the vagus nerve supply of all lung lobes may be an important step in reducing postoperative pulmonary complications. This can be achieved by performing the vagotomy below the level of the caudalmost large and easily identifiable pulmonary branch.
The course of the pulmonary branches of the vagus nerve has been described previously but not in as much detail as in the current study (Hollinshead, 1982). Our study confirms that the vagus nerve branches reach the lungs through plexuses that are anterior and posterior to the main bronchus. New findings involve the quantitative sizes of the lung plexuses, the lobar distribution of the nerve branches, the significant proportion of pulmonary nerves that leave the vagus nerve below the level of the main bronchus and the course of the sympathetic pulmonary splanchnic nerves. The result of such findings is a highly detailed description of the regional anatomy. To the best of our knowledge, we demonstrate for the first time that mediastinal lymphadenectomy, as performed during transthoracic oesophagectomy, denervates the lungs to a great extent. This provides an incentive for further research on methods to spare the pulmonary vagus nerve branches and on the effects of pulmonary vagotomy. A limitation of this study is the relatively low number of specimens dissected. However, because the main findings were consistently present in all cadavers, this number appears to be sufficient to meet the descriptive purpose of this study.
Pulmonary vagotomy may be a pivotal factor in the development of pulmonary complications (in general pneumonia, acute lung injury, acute respiratory distress syndrome, atelectasis) following transthoracic oesophagectomy. Many important pulmonary functions are controlled by the vagus nerve (Belvisi, 2002). Vagus nerve stimulation causes bronchoconstriction, mucus secretion and bronchial vasodilation. Important reflexes that are mediated via the vagus nerve are the cough reflex and the Hering-Breuer reflex, which prevents overinflation (Belvisi, 2002; Mazzone & Canning, 2013). Furthermore, the vagus nerve plays an important regulatory role in inflammation activated by mechanical or chemical stimulation or via nutrition (Luyer et al. 2005; Tracey, 2009; Lubbers et al. 2010a,b). Acetylcholine, released in target organs, binds to the α7-nACh receptor on inflammatory cells (Tracey 2009). This results in significantly decreased levels of pro-inflammatory cytokines (e.g. a significant reduction in tumour necrosis factor (TNF)-α from 83.5 to 17.9 ± 10.4 pg mL−1) and increased levels of anti-inflammatory cytokines (Luyer et al. 2005; Lubbers et al. 2010a,b). Ventilator- and lipopolysaccharide-induced lung injury was reduced in mice by electrical cervical vagus nerve stimulation (Bregeon et al. 2011; dos Santos et al. 2011) or by administration of the α7-nACh receptor agonists nicotine and GTS-2 (Kox et al. 2011; Mabley et al. 2011). Among other cytokines, lung IL-6 levels decreased from 67 to 2 ng g−1 following electrical cervical vagus nerve stimulation (Bregeon et al. 2011), and GTS-21 pretreatment significantly decreased the arterial-alveolar gradient from 156 to 140 mmHg (Kox et al. 2011) in rats with ventilator-induced lung injury. In addition, cervical transection of the vagus nerve significantly reduced survival in mice with Escherichia coli-induced pneumonia (87 vs. 66%) and significantly increased lung injury (Su et al. 2010). When activated, the sparse sympathetic nerve supply of the lungs causes pulmonary and bronchial vessel constriction and airway dilation (Mazzone & Canning, 2013).
The important regulatory functions of the vagus nerve are an incentive to spare the nerve during oesophagectomy. Previously, Akiyama described a technique to spare the entire vagus nerve during oesophagectomy (Akiyama et al. 1994). The technique involved stripping the oesophagus from the mediastinum with a vein stripper that was passed through the oesophagus, inverting the oesophagus along its length. This technique was validated in a cadaveric study in which the vagus nerves were preserved in all 20 cadavers subjected to vagus nerve sparing transhiatal oesophagectomy (Herbella et al. 2006). The technique was adopted by Banki and DeMeester et al., who showed that it preserved gastric function, measured as postoperative gastric emptying, in 73% (n = 15) of cases (Banki et al. 2002). Furthermore, significant reductions in the length of hospital stay (12 vs. 16 days) and the occurrences of pneumonia (4 vs. 18%) and respiratory failure (0 vs. 15%) were observed with vagus-sparing transhiatal oesophagectomy (n = 49) compared with conventional transhiatal oesophagectomy (n = 39) (Peyre et al. 2007). However, no lymphadenectomy was performed. Therefore, this procedure is not suitable for the majority of patients, including those with malignant disease extending into the submucosa or beyond and therefore those who are at risk for lymph node metastasis (DeMeester, 2010).
Fujita et al. (1988) described a technique to spare the pulmonary vagus nerve branches and bronchial artery during mediastinal lymphadenectomy in 22 patients. During this procedure, the pulmonary branches of the vagus nerve were retracted to prevent injury while the carinal and hilar lymph nodes were resected. Vagotomy was performed distal to the pulmonary branches of the vagus nerve. The rate of severe respiratory dysfunction, as measured based on the pulmonary shunt ratio, was 0% with this modified technique (n = 8) compared with 22% (n = 2) with conventional transthoracic oesophagectomy (n = 9). In-hospital mortality was 0% for the pulmonary vagus nerve branches and bronchial artery sparing technique vs. 11% (n = 1) for the conventional technique (Fujita et al. 1988). However, limitations were the small sample size, the comparison with a historical cohort, and the lack of description of the eligibility criteria and baseline patient characteristics. This technique was not validated and it remained unclear which (and how many) pulmonary vagus nerve branches were spared and how mediastinal lymphadenectomy was effected.
The transthoracic approach for oesophagectomy is performed mainly because it allows for better mediastinal dissection, including meticulous mediastinal lymphadenectomy. In a propensity-matched cohort study (n = 468), this approach significantly improved radicality (86 vs. 73%), lymph node yield (median of 27 vs. 17) and 5-year survival (squamous cell carcinoma, 25 vs. 9%; adenocarcinoma, 35 vs. 19%) compared with transhiatal oesophagectomy for locally advanced oesophageal cancer (Kutup et al. 2014). During transthoracic oesophagectomy, the pulmonary branches of the vagus nerve are found coursing over and sometimes between the lymph nodes that must be resected. The present study provides a detailed description of the anatomy of the pulmonary vagus nerve branches that may be useful for the further development of vagus sparing techniques. These future studies should determine the expected effects of the selective sparing of pulmonary vagus nerve branches and the impact of this technique on mediastinal lymphadenectomy.
Conclusions
Lungs have an extensive nerve supply that is composed of branches of the vagus nerve. Extensive mediastinal lymphadenectomy during oesophagectomy denervates the lungs to a great extent. Further research should determine the effects of the selective sparing of pulmonary vagus nerve branches. Furthermore, the feasibility of sparing pulmonary vagus nerve branches without compromising lymphadenectomy should be investigated.
Acknowledgments
We acknowledge the valuable contributions of Simon Plomp and Fiona van Zoomeren in organizing all the logistics regarding the cadavers used. We thank Suzanne Verlinde-Schellekens for creating the tissue sections and Domenico Castiglio for performing the neurofilament immunohistochemistry procedures.
Disclosure of interest
The authors declare that there are no conflicts of interest.
Author contributions
Teus Weijs performed the dissections and measurements and wrote the manuscript.
Jelle Ruurda, Misha Luyer, Grard Nieuwenhuijzen and Richard van Hillegersberg, who are surgeons specializing in oesophageal surgery, participated in the study design and critically revised the manuscript and figures. Ronald Bleys, an anatomist, supervised the project, participated in the study design, provided the materials and critically revised the manuscript and the figures.
References
- Akiyama H, Tsurumaru M, Ono Y, et al. Esophagectomy without thoracotomy with vagal preservation. J Am Coll Surg. 1994;178:83–85. [PubMed] [Google Scholar]
- Banki F, Mason RJ, DeMeester SR, et al. Vagal-sparing esophagectomy: a more physiologic alternative. Ann Surg. 2002;236:324–335. doi: 10.1097/00000658-200209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvisi MG. Overview of the innervation of the lung. Curr Opin Pharmacol. 2002;2:211–215. doi: 10.1016/s1471-4892(02)00145-5. [DOI] [PubMed] [Google Scholar]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379:1887–1892. doi: 10.1016/S0140-6736(12)60516-9. [DOI] [PubMed] [Google Scholar]
- Bosch DJ, Muijs CT, Mul VE, et al. Impact of neoadjuvant chemoradiotherapy on postoperative course after curative-intent transthoracic esophagectomy in esophageal cancer patients. Ann Surg Oncol. 2014;21:605–611. doi: 10.1245/s10434-013-3316-8. [DOI] [PubMed] [Google Scholar]
- Bregeon F, Xeridat F, Andreotti N, et al. Activation of nicotinic cholinergic receptors prevents ventilator-induced lung injury in rats. PLoS ONE. 2011;6:e22386. doi: 10.1371/journal.pone.0022386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMeester SR. Vagal-sparing esophagectomy: is it a useful addition? Ann Thorac Surg. 2010;89:S2156–S2158. doi: 10.1016/j.athoracsur.2010.03.039. [DOI] [PubMed] [Google Scholar]
- D’Journo XB, Michelet P, Marin V, et al. An early inflammatory response to oesophagectomy predicts the occurrence of pulmonary complications. Eur J Cardiothorac Surg. 2010;37:1144–1151. doi: 10.1016/j.ejcts.2009.11.033. [DOI] [PubMed] [Google Scholar]
- Eichenbaum KD, Neustein SM. Acute lung injury after thoracic surgery. J Cardiothorac Vasc Anesth. 2010;24:681–690. doi: 10.1053/j.jvca.2009.10.032. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Fujita H, Hawahara H, Yamana H, et al. Mediastinal lymphnode dissection procedure during esophageal cancer operation – carefully considered for preserving respiratory function. Jpn J Surg. 1988;18:31–34. doi: 10.1007/BF02470843. [DOI] [PubMed] [Google Scholar]
- Herbella FA, Regatieri CV, Moreno DG, et al. Vagal integrity in vagal-sparing esophagectomy: a cadaveric study. Dis Esophagus. 2006;19:406–409. doi: 10.1111/j.1442-2050.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- Hollinshead W. Anatomy for Surgeons. 3rd edn. Philadelphia: Harper & Row; 1982. pp. 122–125. [Google Scholar]
- Kox M, Pompe JC, Peters E, et al. α7 nicotinic acetylcholine receptor agonist GTS-21 attenuates ventilator-induced tumour necrosis factor-α production and lung injury. Br J Anaesth. 2011;107:559–566. doi: 10.1093/bja/aer202. [DOI] [PubMed] [Google Scholar]
- Kutup A, Nentwich MF, Bollschweiler E, et al. What should be the gold standard for the surgical component in the treatment of locally advanced esophageal cancer: transthoracic versus transhiatal esophagectomy. Ann Surg. 2014;260:1016–1022. doi: 10.1097/SLA.0000000000000335. [DOI] [PubMed] [Google Scholar]
- Lubbers T, De Haan JJ, Hadfoune M, et al. Lipid-enriched enteral nutrition controls the inflammatory response in murine gram-negative sepsis. Crit Care Med. 2010a;38:1996–2002. doi: 10.1097/CCM.0b013e3181eb90d7. [DOI] [PubMed] [Google Scholar]
- Lubbers T, de Haan JJ, Luyer MD, et al. Cholecystokinin/Cholecystokinin-1 receptor-mediated peripheral activation of the afferent vagus by enteral nutrients attenuates inflammation in rats. Ann Surg. 2010b;252:376–382. doi: 10.1097/SLA.0b013e3181dae411. [DOI] [PubMed] [Google Scholar]
- Luyer MD, Greve JW, Hadfoune M, et al. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med. 2005;17:1023–1029. doi: 10.1084/jem.20042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabley J, Gordon S, Pacher P. Nicotine exerts an anti-inflammatory effect in a murine model of acute lung injury. Inflammation. 2011;34:231–237. doi: 10.1007/s10753-010-9228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markar SR, Karthikesalingam A, Thrumurthy S, et al. Volume-outcome relationship in surgery for esophageal malignancy: systematic review and meta-analysis 2000–2011. J Gastrointest Surg. 2012;16:1055–1063. doi: 10.1007/s11605-011-1731-3. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Canning BJ. Autonomic neural control of the airways. Handb Clin Neurol. 2013;117:215–228. doi: 10.1016/B978-0-444-53491-0.00018-3. [DOI] [PubMed] [Google Scholar]
- Peyre CG, DeMeester SR, Rizzetto C, et al. Vagal-sparing esophagectomy: the ideal operation for intramucosal adenocarcinoma and Barrett with high-grade dysplasia. Ann Surg. 2007;246:665–671. doi: 10.1097/SLA.0b013e318155a7a1. [DOI] [PubMed] [Google Scholar]
- van de Poll-Franse LV, Lemmens VE, Roukema JA, et al. Impact of concentration of oesophageal and gastric cardia cancer surgery on long-term population-based survival. Br J Surg. 2011;98:956–963. doi: 10.1002/bjs.7493. [DOI] [PubMed] [Google Scholar]
- dos Santos CC, Shan Y, Akram A, et al. Neuroimmune regulation of ventilator-induced lung injury. Am J Respir Crit Care Med. 2011;183:471–482. doi: 10.1164/rccm.201002-0314OC. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluis PC, Verjage RJJ, van der Horst S. A new clinical scoring system to define pneumonia following esophagectomy for cancer. Dig Surg. 2014;31:108–116. doi: 10.1159/000357350. [DOI] [PubMed] [Google Scholar]
- Su X, Matthay MA, Malik AB. Requisite role of the cholinergic α7 nicotinic acetylcholine receptor pathway in suppressing gram-negative sepsis-induced acute lung inflammatory injury. J Immunol. 2010;184:401–410. doi: 10.4049/jimmunol.0901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]