Abstract
The medial nucleus of the amygdala (Me) is a component of the neural circuit for the interpretation of multimodal sensory stimuli and the elaboration of emotions and social behaviors in primates. We studied the presence, distribution, diverse shape, and connectivity of dendritic spines in the human Me of adult postmortem men. Data were obtained from the five types of multipolar neurons found in the Me using an adapted Golgi method and light microscopy, the carbocyanine DiI fluorescent dye and confocal microscopy, and transmission electron microscopy. Three-dimensional reconstruction of spines showed a continuum of shapes and sizes, with the spines either lying isolated or forming clusters. These dendritic spines were classified as stubby/wide, thin, mushroom-like, ramified or with an atypical morphology including intermediate shapes, double spines, and thorny excrescences. Pleomorphic spines were found from proximal to distal dendritic branches suggesting potential differences for synaptic processing, strength, and plasticity in the Me neurons. Furthermore, the human Me has large and thin spines with a gemmule appearance, spinules, and filopodium. The ultrastructural data showed dendritic spines forming monosynaptic or multisynaptic contacts at the spine head and neck, and with asymmetric or symmetric characteristics. Additional findings included en passant, reciprocal, and serial synapses in the Me. Complex long-necked thin spines were observed in this subcortical area. These new data reveal the diversity of the dendritic spines in the human Me likely involved with the integration and processing of local synaptic inputs and with functional implications in physiological and various neuropathological conditions.
Keywords: 3D reconstruction, DiI dye, extended amygdala, Golgi method, neuronal morphology, spine shape, ultrastructure
Introduction
The human amygdala is a group of nuclei with different cytoarchitectonical, connectional, and functional features in the subcortical part of the dorsomedial pole of the temporal lobe, rostral to the hippocampus, and close to the inferior margins of the claustrum, putamen, and globus pallidus (Brodal, 1981; Everitt, 1995; de Olmos, 2004; Yilmazer-Hanke, 2012; Dall’Oglio et al. 2013; Janak & Tye, 2015). The medial nucleus of the amygdala (Me) has a superficial position and an irregular shape in the rostrocaudal axis, located lateral to the optic tract and at the fundus of the endorhinal sulcus (Everitt, 1995; Gloor, 1997; de Olmos, 2004; Yilmazer-Hanke, 2012), and forming a continuum with part of the bed nucleus of the stria terminalis (BST) in monkeys and humans (Martin et al. 1991; Heimer et al. 2008). The definitive subdivision of the human Me has not as yet been established (Dall’Oglio et al. 2013 and references therein).
Connectional data indicate the participation of the primate Me in multimodal sensory input processing, in the intra-amygdaloid control of information flow, and in the display of social behaviors. That is, the monkey Me receives intra-amygdaloid connections from the basal and lateral nuclei and projects to the accessory basal, anterior cortical and central nuclei, the periamygdaloid cortex, and the amygdalohippocampal area (Everitt, 1995; Freese & Amaral, 2009). Major extra-amygdaloid afferences come directly from the brainstem peripeduncular nucleus, the hypothalamic ventromedial nucleus, the lateral area, the supramammillary region, and the rostral insular cortex. Output projections reach the medial areas of the BST, the medial preoptic area and the anterior medial hypothalamus, including the paraventricular and supraoptic nuclei as well as the hypothalamic ventromedial, the dorsal and ventral premammillary nuclei, and the midline thalamic nuclei (Everitt, 1995; Freese & Amaral, 2009; reviewed in Dall’Oglio et al. 2013).
The Me is one of the less studied nuclei in the human amygdaloid complex. Nevertheless, some lines of evidence indicate that the Me is part of a neural network for the elaboration of emotions (Blood & Zatorre, 2001; Liberzon et al. 2003; Gamer et al. 2010) and responds to aversive olfactory stimulation (Zald & Pardo, 1997), elaborates the gonadal hormone-mediated premenstrual increase in stress-induced negative affect (Ossewaarde et al. 2013), and the perception and processing of aversive stimulus caused by fearful faces or the positive valence of happy faces (Gamer et al. 2010). The Me has also been associated with the pathogenesis of various neurological and psychiatric disorders, such as epilepsy, autism, schizophrenia, Parkinson’s disease, Huntington’s disease, and Alzheimer’s disease (cf. Sorvari, 1997; Schumann & Amaral, 2005).
These data support the study of the dendritic spines and the synaptic organization of the human Me. Spines increase the packing density of synapses per tissue volume (Bourne & Harris, 2007) and are mainly involved with signaling excitatory synaptic inputs (Fortin et al. 2012; Harnett et al. 2012; Brusco et al. 2014). Spines have varied shapes, sizes, and functions (Holtmaat & Svoboda, 2009; García-López et al. 2010; Stewart et al. 2014). For example, they promote voltage-sensitive responses with variable electrical coupling with the adjacent dendrite (Spruston et al. 2013; Tonnesen et al. 2014), buffer local synaptically mediated calcium increases (Segal, 2010), initiate intracellular biochemical cascades for information storage (Boersma et al. 2011; Yuste, 2011; Fortin et al. 2012), and modulate the electrophysiological activity of the neuron on integrated neural networks (Gulledge et al. 2012; Lee et al. 2012; Rochefort & Konnerth, 2012; Yuste, 2013; Tonnesen et al. 2014). The shape and plastic properties of dendritic spines can vary according to the synaptic demands and the integrative capacities of neurons (González-Ramírez et al. 2014), among brain areas (Jacobs et al. 1997, 2001; Anderson et al. 2009; Spruston et al. 2013; Lepousez et al. 2014), and species (Benavides-Piccione et al. 2002), including differences between humans and great apes (Bianchi et al. 2013).
Here, we describe the presence, distribution, diverse shapes, complexity, and connectivity of the dendritic spines in the human Me using two-dimensional and three-dimensional (3D)-reconstructed images obtained with the Golgi method and light microscopy, with carbocyanine DiI fluorescent dye and confocal microscopy, and by transmission electron microscopy (TEM). We found spines of different classes along a continuum of shapes and sizes, but some complex spines in the human Me had no counterparts in the Me of other species (e.g. rat, Rasia-Filho et al. 2004; Cooke et al. 2007; Rasia-Filho et al. 2012; Brusco et al. 2014; mouse, Bian et al. 2008; Niimi et al. 2012; Bian, 2013; cat, Hall, 1972; monkey, Pitkänen & Amaral, 1994; McDonald et al. 1995). Ultrastructural data provided evidence of dendritic spines with monosynaptic and multisynaptic sites, asymmetric and symmetric contacts, spinules, en passant, reciprocal, and serial axo-axo-dendritic synapses in the human Me.
Materials and methods
Subjects
Five adult men were studied. Age, time postmortem, cause of death, and type of fixation are shown in Table1. The brains were obtained according to the ethical and legal procedures and international regulatory standards based on the Helsinki Declaration of 1964, following approval of the Ethic Committee of the Department of Forensic Medicine (Instituto Geral de Perícias, City of Porto Alegre, Brazil; process number 03/08), the Federal University of Health Sciences of Porto Alegre (UFCSPA, Brazil; process number 541/07 and 1734/12), the Federal University of Rio Grande do Sul (Brazil; process number 20080/09), and the Pathology Facility, Clinical Hospital of Ribeirão Preto, University of São Paulo (FMRP-USP, Brazil; process number 01/09). A next of kin at the morgue signed an informed consent form for the removal of part of the bilateral temporal lobes during autopsy. Three brain blocks were used for the Golgi technique; two of them were recovered from deceased donors at the Department of Forensic Medicine, and the perfused block was a donation from the Department of Pathology (UFCSPA). The last two brain blocks were used for the DiI and TEM studies and come from the Pathology Facility (FMRP-USP; Table1).
Table 1.
Characteristics of the human male brain samples
| Age of death (years) | PMI (hours) | Gender | Cause of death | Type of fixation | Technique |
|---|---|---|---|---|---|
| 68 | 8:30 | Male | Acute pulmonary edema | Immersion | Golgi* |
| 75 | 12:15 | Male | Bilateral pneumonia | Immersion | Golgi* |
| Unknown | 6:00 | Male | Unknown | Perfusion | Golgi* |
| 67 | 6:00 | Male | Urothelial carcinoma | Immersion | DiI + TEM |
| 50 | 6:00 | Male | Liver cirrhosis/pneumonia | Immersion | DiI + TEM |
PMI, post mortem interval.
These three subjects were chosen for study because they had the best slices impregnated by the adapted Golgi method. Partly reprinted with permission (license no. 3475570918187) from Dall’Oglio et al. (2013); Copyright © 2012 Wiley Periodicals, Inc.
The selection criteria were the same described in Dall’Oglio et al. (2013), and included adult men with no violent cause of death and no neurological infectious diseases or stroke. Clinical data and information regarding prior comorbidities were obtained by direct interviews with the family members or legal representatives. Subjects were reportedly healthy neurologically and psychiatrically, with no previous neurosurgical intervention. Each subject received a code to protect the individual’s identification. A tissue sample from each subject was analyzed histologically by a neurologist/neuropathologist (Dr. Arlete Hilbig, Department of Internal Medicine/Neurology, UFCSPA) to confirm the absence of the most frequent vascular and neurodegenerative lesions (data not shown).
Tissue processing
The Golgi method and the image processing in light microscopy
We used the ‘single-section’ Golgi technique (Gabbott & Somogyi, 1984), modified by Izzo et al. (1987) and Bolam (1992), and adapted for the human nervous tissue by Dall’Oglio et al. (2010, 2013). Briefly, bilateral brain blocks of the anterior temporal lobe (cases 1 to 3, Table1) were sectioned to approximately 1.5 cm3 to contain the Me. Tissue blocks were maintained immersed in 10% unbuffered formaldehyde solution at room temperature (RT) for 1–42 months prior to the Golgi processing. Samples were submitted to a post-fixation procedure with 4% paraformaldehyde and 1.5% picric acid in phosphate buffer solution (PBS, 0.1 m, pH = 7.4) at RT from 5 to 90 days and then coronal sections were obtained (200 μm thick) along the rostrocaudal axis using a vibratome (1000S; Leica, Germany). After an additional 72 h immersed in the same post-fixation solution at RT, the sections were: (i) rinsed in PBS and transferred to a solution of 0.1% osmium tetroxide (Sigma Chemicals Co., USA) in PBS for 10–30 min; (ii) rinsed in PBS and immersed in 3% potassium dichromate (Merck, Germany) at 4 °C in the dark for 48 h; (iii) rinsed again in distilled water, ‘sandwiched’ between coverslips, and placed in a solution of 1.5% silver nitrate (Merck) at RT in the dark for 24 h; and (iv) washed in distilled water, placed on gelatin-coated histological slides, dried at RT, dehydrated in an ascending series of ethanol, cleared in xylene, and covered with synthetic balsam and coverslips.
The parahippocampal formation, the optic tract, and the endorhinal sulcus served as anatomical references to localize the Me (Fig.1; based on the images provided by DeArmond et al. 1989; Gloor, 1997; Sorvari, 1997; de Olmos, 2004; Mai et al. 2008). Well-impregnated neurons of both hemispheres must meet the following inclusion criteria: (i) have neuronal cell bodies located within the boundaries of the Me, (ii) be isolated from neighboring cells to avoid ‘tangled’ dendrites, (iii) have tapering dendrites with defined borders, and (iv) have dendritic spines clearly distinguishable from the background and clearly visible along the ‘z’ axis.
Figure 1.

(A) Photographs of a formalin fixed human brain and the respective (B) tissue block (coronal section) at the level of the medial amygdaloid nucleus (Me, pointed) in the ventral part of the temporal lobe. Courtesy of the Discipline of Anatomy, Department of Basic Sciences, Federal University of Health Sciences of Porto Alegre, Brazil. Bar = 1 cm.
Imaging of the general morphology of Me cells (Figs8) was performed at a final magnification of ×260 (using an ×20 Olympus UPlanSApo 0.6 NA air objective lens, magnification ×1.3) and a light microscope (Olympus BX-61 coupled to a CCD DP72 camera, Japan). Images were acquired with a resolution of 1360 × 1024 pixels, 24-bit color depth, and submitted to dynamic deconvolution using the Image Pro Plus 7.0 software (Media Cybernetics, USA). Dendritic spines were imaged at a final magnification of ×1300 using an × 100 Olympus UPlanSApo 1.4 NA oil immersion objective lens. Each image was acquired with a high resolution (2070 × 1548 pixels), 48-bit color depth, and submitted to auto-contrast and dynamic deconvolution using the same image analysis software mentioned above. Spines were sampled along different dendritic lengths, providing data from proximal to distal branches in all different neurons (Figs8).
Figure 8.

(A) Location of the medial amygdaloid nucleus (MeA; 9.3 mm posterior to the midpoint of the anterior commissure) in which a Golgi-impregnated large cell of the spiny rectangular neuron was observed. (B) Dendritic spines were classified as stubby (s), wide (w), thin (t), mushroom (m), and atypical (a). m* = spinule in a mushroom spine. Other details according to the legend of Fig.3.
The 3D-reconstruction of the Golgi-impregnated dendritic spines was performed with the ‘3D Constructor’ software module for Image Pro Plus 7.0. Each spine was fully imaged controlling the focus along the ‘z’ axis. The z-stacks were acquired with a 0.1-μm z-step and images were sequentially summed and aligned. We then checked and confirmed the shape of each reconstructed spine.
DiI dye immunofluorescence and image processing in confocal microscopy
We adapted the experimental protocol used by Brusco et al. (2010) and depicted previously by Rasia-Filho et al. (2010). Tissue fixation (cases 4 and 5, Table1) was performed with 4% paraformaldehyde in PBS for 1 day, and 1.5% paraformaldehyde in PBS in the following day. Blocks were sectioned coronally (200-μm-thick slices) in cold PBS using a vibratome. Both hemispheres were studied. The slices containing the Me were placed on glass slides, and finely powdered DiI (catalog code D-282; Molecular Probes, USA) was directly applied on the surface of the Me. The slices were covered with PBS and the DiI was allowed to diffuse along the tissue for 20 h at RT in the dark. An extra fixation was performed with 4% paraformaldehyde in PBS for 40 min. The slices were washed with PBS and the cell nuclei were stained with DAPI (Sigma, USA; 0.13 μL DAPI in 6.25 mL PBS) for 4 min. The slices were then washed again with PBS, mounted with an anti-fading solution (Fluoromount EMS, USA) under coverslips, and maintained at −4 °C. The samples were studied along 1 week following this procedure. The inclusion criteria of neurons and dendritic spines were the same as mentioned above.
Images were obtained using a confocal microscope (Leica TCS SP5 AOBS) with a Plan-Apochromat × 40, 1.25 NA oil-immersion lens. Spectral detectors were adjusted to capture emissions from helium/neon lasers at a wavelength of 550–700 nm. The ‘z’-stack acquisition was performed at a step size of 0.4 μm. The images were acquired at a resolution of 1024 × 1024 pixels per frame with 4× zoom, avoiding oversaturated or undersaturated pixels. All the dendritic spines were 3D reconstructed using the Leica Microsystems LAS AF Lite 2.2.1 software. We checked and confirmed the shape of each spine. Then, we compared these confocal results with the Golgi data to ascertain the spine shape and classification.
Spines were classified in accordance with the descriptions of Harris et al. (1992), Fiala & Harris (1999), Arellano et al. (2007a), Brusco et al. (2010), González-Burgos et al. (2012), Brusco et al. (2014), González-Ramírez et al. (2014), and Stewart et al. (2014). For both light and confocal microscopy studies, the criteria for assigning dendritic spines to different classes were based on the presence, length and diameter of a neck, number of protrusions from a single stalk, head diameter, and head shape. Spines were classified as: thin (a single protrusion with a long and narrow neck ending at a bulbous enlargement at the tip, the length of the spine greater than the diameter of the neck), stubby/wide (a protrusion from the cell membrane with no apparent neck, the length of the spine either similar or longer than its diameter), mushroom-like (a characteristically large-headed spine with the diameter of the head greater than the diameter of the neck), ramified (a single stalk giving rise to two or more heads) and ‘other’ types, which included spines that were morphologically atypical with more complex shapes, including double spines (‘a neck protruding from the parent dendrite ending in a bulb, from which a second neck protruded, in turn ending in another bulb’; González-Ramírez et al. 2014), spines with racemose appendages (with a lobed appearance and various bulbous enlargements and heads; Fiala & Harris, 1999), and thorny excrescences (densely packed outgrowths showing fairly large spines with various round heads grouped around the stems; Lauer & Senitz, 2006; Lu et al. 2013; Stewart et al. 2014). We also looked for the presence and shape of spinules (smaller protrusions usually extending from the head of a spine; Brusco et al. 2014; Stewart et al. 2014) and filopodium (a thin and elongated protrusion with no apparent head; García-López et al. 2010).
Transmission electron microscopy
We used the methodological procedure described in Dall’Oglio et al. (2013). Briefly, brain tissue blocks were cut to 2 mm3 to contain the left Me at approximately 8 mm posterior to the midpoint of the anterior commissure (Mai et al. 2008). The blocks were fixed in a solution containing 2% glutaraldehyde and 2% paraformaldehyde in cacodylate buffer (CB; 0.1 N, pH 7.4) for 48 h under gentle agitation, washed in CB, and immersed in a 1% osmium tetroxide solution on ice for 90 min. The samples were rinsed in CB and in a sodium acetate buffer (SABS; 0.1 m, pH 5.0) and stained en bloc with 0.5% uranyl acetate in SABS on ice for 1 day in the dark. The samples were then washed in SABS, dehydrated using a graded series of ethanol ending with 100% propylene oxide, infiltrated in EMbed 812 resin (Electron Microscopy Sciences, USA), and heated at 75 °C for 48 h to polymerize. The plastic blocks were trimmed and sectioned with a Leica Ultracut UCT ultramicrotome. Ultrathin sections (60–70 nm) were picked up on Pyoloform B Resin (SPI Supplies, USA) and carbon-coated single-slot grids, and contrasted with uranyl acetate and lead citrate.
Electron micrographs were obtained using a Zeiss 10 Transmission Electron Microscope (Germany). Approximately 850 electron micrographs were taken from the Me neuropil which was not intensely disrupted by postmortem autolysis. Data were obtained equally from both subjects studied here. It was not possible to obtain 150–300 ultra-thin serial sections to perform 3D reconstructions of TEM images from our samples as obtained for rats or mice (see Fiala & Harris, 1999; Brusco et al. 2014; Stewart et al. 2014).
We focused our ultrastructural study on local dendrites, spines, and axons with identifiable synapses. The general ultrastructural characteristics of these elements and the synaptic terminals, classified as either asymmetric or symmetric to the postsynaptic density (PSD), were based on previous descriptions (Peters et al. 1991; Pannese, 1994; Brusco et al. 2014; Stewart et al. 2014). Images had final fine adjustments of contrast and sharpness made in Photoshop CS3 (Adobe Systems, Inc., USA) without altering their content.
Results
We found five classes of Golgi-impregnated multipolar spiny neurons in the human Me (confirming Dall’Oglio et al. 2013). Spines were found at the cell body and along the different orders of the dendritic branches. Somatic spines were small stubby/wide spines or thin spines intermingled with few mushroom spines, and, less frequently, ramified spines (data not shown). Both the Golgi method and the DiI dye technique provided data for the 3D-reconstruction of dendritic spines. Although there were signs of tissue damage at the ultrastructural level, it was still possible to identify different neuronal components in the Me neuropil.
We have no evidence to conclude that there were differences in the Golgi data between subjects due to the different time of fixation and post-fixation. The same kind of Golgi-impregnated neurons were found in the subjects studied here. Some neurons and spines were observed more often than others and these findings were reproducible across the human subjects. The densely spiny round/ovoid neurons, the thorny excrescences, and the filopodium described below were observed less frequently in our samples.
The Golgi impregnation also provided the opportune identification of fibrous and protoplasmic astrocytes in the Me neuropil. Fibrous astrocytes showed a smooth surface (Fig.2B), whereas local protoplasmic astrocytes have thorny processes covered with small ‘spine-like’ structures along their processes (Fig.2C a–e).
Figure 2.

(A) Schematic diagram of a coronal section of the adult human brain showing the location of the medial amygdaloid nucleus (MeA; 5.4 mm posterior to the midpoint of the anterior commissure; adapted from Mai et al. 2008) in which representative examples of Golgi-impregnated fibrous (B) and protoplasmic (C) astrocytes were observed. (a–e) Small spine-like structures (arrows) were found in thorny processes radiating in all directions from a local protoplasmic astrocyte. (D) Schematic diagram of a coronal section of the adult human brain showing the location of the medial amygdaloid nucleus (MeA; 5.4 mm posterior to the midpoint of the anterior commissure; adapted from Mai et al. 2008) in which a Golgi-impregnated spiny fusiform neuron type was observed (marked with an asterisk). (E) Digitized microscopic image under light microscopy of a representative small fusiform neuron. Photoshop CS3 adjustments (Adobe Systems, Inc.). I, inferior; L, lateral; M, medial; S, superior.
Golgi-impregnated neurons and dendritic spines
The human Me neurons were classified according to the cell body shape, the number of primary dendrites, and the pattern of dendritic branching. They usually were (i) fusiform neurons with variable sizes, from relatively small cells (Fig.2E) to large cells (Fig.3). These neurons had two primary dendrites and both tapering branches near the cell body (Fig.2E) or long shafts extending in the Me neuropil (Fig.3). The other neuronal types showed: (ii) an angular soma with three primary dendrites with no evident definition of basal and apical dendrites, and tapering shafts with few branching points (Fig.4); (iii) a round to pear-shaped soma with two primary dendrites branching near the cell body (Fig.5); (iv) a small round to ovoid cell body with more than four primary dendrites, various branching points, short and thin dendritic shafts (Fig.6; in this case, an ovoid-shaped neuron with seven primary dendrites; see the scale bar for comparisons with the other neurons); and (v) a rectangular soma with variable sizes (small in Fig.7, large in Fig.8) and four symmetrically positioned primary dendrites with sparse ramification.
Figure 3.

(A) Schematic diagram of a coronal section of the adult human brain showing the location of the medial amygdaloid nucleus (MeA; 12 mm posterior to the midpoint of the anterior commissure; adapted from Mai et al. 2008) in which a Golgi-impregnated large cell of the spiny fusiform neuron was observed (marked with an asterisk). This procedure to describe the location of neurons is relevant to the subsequent Figs8. (B) Digitized microscopic image under light microscopy. Details about the presence, distribution, and shape of dendritic spines are shown in the corresponding inserts at higher magnification. Spines were classified as stubby (s), wide (w), thin (t), mushroom (m), ramified (r), or atypical (a) with double-spine (in c) or racemose appearance (in h). The irregular appearance of the dendritic surface represents spines in other focal planes. Photoshop CS3 adjustments (Adobe Systems, Inc.). I, inferior; L, lateral; M, medial; S, superior. Bars = 50 μm for the general view of the neuron and 2 μm for the inserts. This procedure to demonstrate the dendritic spines is also relevant to the subsequent Figures 8. Partly reprinted with permission (license 3407680263986) from Dall’Oglio et al. (2013); Copyright © 2012 Wiley Periodicals, Inc.
Figure 4.

(A) Location of the medial amygdaloid nucleus (MeA; 9.3 mm posterior to the midpoint of the anterior commissure) in which a Golgi-impregnated spiny neuron of the angular type was observed. (B) Dendritic spines were classified as stubby (s), wide (w), thin (t), mushroom (m), ramified (r), and atypical (a) with thorny excrescence aspect (in g). t* and w* = spinule in thin and wide spines, respectively. Other details according to the legend of Fig.3.
Figure 5.

(A) Location of the medial amygdaloid nucleus (MeA; 9.3 mm posterior to the midpoint of the anterior commissure) in which a Golgi-impregnated spiny neuron of the round to pear-shaped type was observed. (B) Dendritic spines were classified as stubby (s), wide (w), thin (t), mushroom (m), and atypical (a). Other details according to the legend of Fig.3.
Figure 6.

(A) Location of the medial amygdaloid nucleus (MeA; 10.7 mm posterior to the midpoint of the anterior commissure) in which a Golgi-impregnated highly dense spiny neuron of the small round to ovoid type was observed. (B) Dendritic spines were classified as stubby (s), wide (w), thin (t), mushroom (m), and atypical (a), including long spines with a gemmule aspect (in a). w* = spinule in a wide spine. Bars = 20 μm for the general view of the neuron and 2 μm for the inserts. Other details according to the legend of Fig.3.
Figure 7.

(A) Location of the medial amygdaloid nucleus (MeA; 5.4 mm posterior to the midpoint of the anterior commissure) in which a Golgi-impregnated small cell of the spiny rectangular neuron type was observed. (B) Dendritic spines were classified as stubby (s), wide (w), thin (t), and mushroom (m). Other details according to the legend of Fig.3.
Dendritic spines were observed from primary branches to more distal branches in all Golgi-impregnated neuronal types (Figs8). Low to high densities of spines were found along the dendritic branches in the same neuron (e.g. compare Fig.3a–c; Fig.6a,e). The density of dendritic spines also varied between neuronal types in the Me. The round/ovoid neuron showed a myriad of intermingled spines and the highest density of spines, irrespective of dendritic branching order or distance from the cell body (Fig.6).
Spines in the Me showed a continuum of sizes and shapes. This was observed both in bidimensional images (Figs8) and after 3D reconstructions (Fig.9). Thin spines were observed as small pedunculated protrusions (e.g. Fig.3B), with either thin or thick necks that ended at a large head (Fig.9A) or with a gemmule appearance of long necks ending at a bulbous head (Fig.6ag). Stubby and wide spines had round to ‘cone’ shapes (Figs7b,c and 9B) ranging from small (Figs3a and 7a) to large protrusions (Fig.5d). Mushroom spines had different head shapes and sizes. Some were small, with a short neck and a proportional large head (Fig.5d), others had large globous or flat heads (Figs5c and 9C). Ramified spines had a single stalk branching into two separate short processes resembling thin spines (Fig.4f). These spines also showed different diameters and lengths of the primary and secondary stalks, heads of irregular round to oval shapes (Figs3d and 9D first and second image), or some small protrusions, bigger than thin spinules, arising from the ending heads (Fig.9D, third image).
Figure 9.
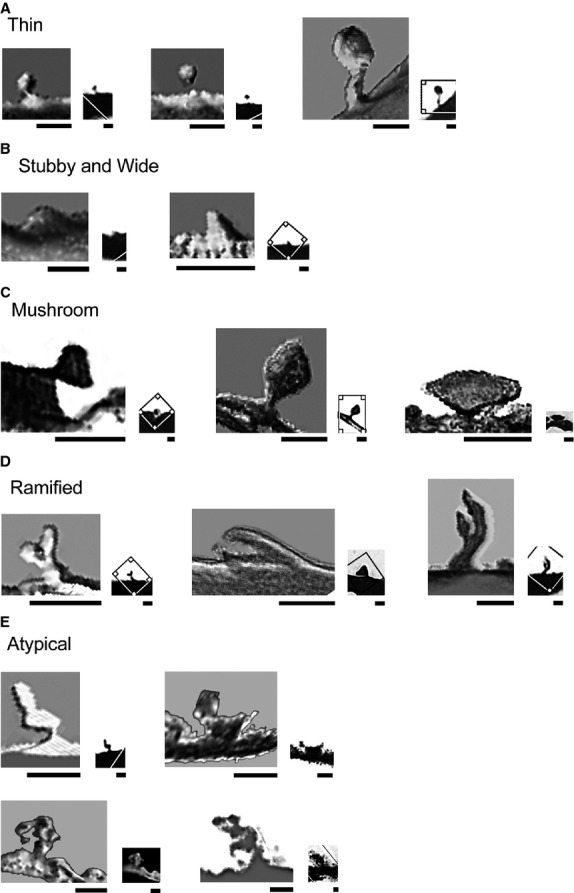
Three-dimensional reconstructed images of Golgi-impregnated dendritic spines from the adult human medial amygdaloid nucleus classified as (A) thin, (B) stubby/wide, (C) mushroom, (D) ramified, and (E) atypical. The corresponding original images are shown at lower magnification (right) as observed by light microscopy. Photoshop CS3 adjustments (Adobe Systems, Inc.). Bar = 1 μm, adapted to the bars in all images.
Other branched and atypical spines with complex shapes included: (i) double spine with a thin stalk and two continuous bulbous protrusions (Fig.9E, first image, see also Fig.3c a); (ii) large stalk protrusion giving rise to both a thin thorny projection and a bulbous head resembling a mushroom-shaped spine (Fig.9E, second image); (iii) racemose appearance with a small stalk giving rise to various bulbous enlargements and round heads (Fig.9E, second row); and (iv) thorny excrescence (Fig.4g a). Some spines showed intermediate/transition shapes with overlaps between thin and mushroom spines or between wide and mushroom spines. Finally, spinules were found in various classes of spines (e.g. in a thin spine in Fig.4e, in a wide spine in Figs4i and 6c, and in a mushroom spine in Fig.8d).
Dendritic spines imaged by confocal microscopy
The 3D-reconstruction of confocal microscopy data confirmed the Golgi results and provided additional images for the variety and complexity of spine shapes in the human Me. We observed dendrites with low to high densities of spines (Fig.10A). Thin spines (Fig.10B) and stubby/wide spines (Fig.10C) varied from small to relatively large shapes. Mushroom spines showed various sizes and both large heads with a flat (Fig.10D, first to sixth images) or a ‘perforated’ appearance (Fig.10D, last image). Ramified spines had a single large stalk branching into two heads (Fig.10E, first and second images) or a single stalk giving rise to a branch with no evident head (Fig.10E, last image).
Figure 10.

(A) Three-dimensional reconstructed fluorescent image of dendritic spines from the human medial amygdaloid nucleus (Me) using confocal microscopy. (A) Dendrites showed low to high density of pleomorphic spines. Spines were classified as (B) thin, (C) stubby/wide, (D) mushroom, (E) ramified, and (F) atypical, also shown in A and B (‘a’). (G) A filopodium with elongated neck and no evident head. Photoshop CS3 adjustments (Adobe Systems, Inc.). Bar = 2 μm. The scale shown in the first image of A applies to all other images, except those with their own corresponding bar.
Atypical spines from confocal microscopy images included: (i) double spine (Fig.10B, fifth image a); (ii) spines with a single, large, elongated, and thick protrusion (Fig.10F, first and second images in the first row); (iii) spines with an enlarged finger-like neck and a fusiform head (Fig.10F, third image in the first row); (iv) spines with a convoluted shape (Fig.10F, fourth and fifth images in the first row); (v) branched spine with various bulbous protrusions (Fig.10A, second image a); (vi) spines with a racemose appearance (Fig.10F, last image in the first row); (vii) spines with intermediate shapes (Fig.10F, first to third images in the second row); (viii) spines with a thin, elongated neck resembling a gemmule aspect with an atypical head (Fig.10F, fourth image in the second row, left); (ix) spines with a single bulbous protrusion giving rise, from one side, to a spinule and, from the other side, to a transition-like spine with thick neck and head (Fig.10F, fifth image in the second row); and (x) spines with an enlarged, bulbous base ending at a single thick head protrusion (Fig.10F, last image in the second row). We also found a filopodium (Fig.10G).
Ultrastructure of dendritic spines and synapses
Synapses were found directly on the dendritic shafts and on differently shaped spines in the Me (Figs1 and 2). We did not observe spines as presynaptic elements in any of our samples. Dendritic shaft synapses showed both asymmetric and symmetric contacts. In this sense, axon terminals formed isolated (Fig.1A) or multiple asymmetric contacts (Fig.1B). The presynaptic terminals of the asymmetric synapses showed round, small, electron-lucent vesicles (e.g. Fig.1A,C,D). In the symmetric presynaptic terminals, the vesicles were usually small and pleomorphic or intermingled with large, dense-core vesicles (e.g. Fig.1E,G, top). Axo-dendritic synapses occurred close to axo-spine ones (Fig.1E).
Figure 11.
Ultrastructure of dendrites (slightly highlighted in green), spines (blue), and synapses in the human medial amygdaloid nucleus. (A) Isolated or (B) multiple, adjacent axon terminals making asymmetric synapses on dendritic shafts. Dendritic spines were classified as (C, D) stubby, (E) wide, (F) thin with small or (G) long necks, (H) with transition aspect (spinule indicated by an arrow), (I–K) mushroom with small to (L) big head (spinule indicated by an arrow) and macular postsynaptic densities. (M) Spine with a transitional aspect between mushroom and ramified shapes. Synapses showed asymmetric (e.g. C, D, F, J, K) or symmetric contacts (E, I). Multisynaptic spine showed two asymmetric contacts (G, right at the spine head and at the neck) and a symmetric contact (G, top of the spine head). Synapses were found at the spine base (C), neck (G), and head (I). Spines in G and I partly reprinted with permission (license no. 3475570918187) from Dall’Oglio et al. (2013); Copyright © 2012 Wiley Periodicals, Inc. Photoshop CS3 adjustments (Adobe Systems, Inc.). Bar = 0.2 μm.
Figure 12.
Ultrastructure of dendrites (slightly highlighted in green), spines (blue), axons (yellow), and synapses in the human medial amygdaloid nucleus. (A) Mushroom spine with a typical large head and a perforated postsynaptic density contacting an axonal bouton. (B) Spine with atypical shape with ramification and protrusions or (C) with an elongated and wide shape and a spinule (arrow). (D) Complex synaptic arrangements showing an axo-dendritic asymmetric contact and axonal enveloping of the spine head, (E, arrows) axo-axonal reciprocal synapse (arrows close synaptic vesicles) and axo-spiny asymmetric contact (arrowhead) in an axonal spine (asterisk) with an atypical ‘spinule-like’ double-membrane evagination (right), (F) serial axo-axo-dendritic synapse (arrows) and an en passant axo-spiny synapse (asterisk), and (G) close appositions of membranes of a gap-like junction (arrow; at high magnification in the insert G′). Photoshop CS3 adjustments (Adobe Systems, Inc.). Bar = 0.2 μm, except for the inserts where bar = 20 nm.
Dendritic spines had various shapes and sizes and showed axo-spinous monosynaptic or multisynaptic sites at the spine head and neck (Fig.1F–M). These synapses were usually of the asymmetric type (e.g. Fig.1F,H), but we also observed axo-spinous symmetric contacts (e.g. Fig.1E). Synaptic terminals contacted different locations of stubby/wide spines (base, Fig.1C; top, Fig.1D; or lateral, Fig.1E). Thin spines were found varying from small protrusions (Fig.1F) to long spines (Fig.1G). In this case, there were coexisting asymmetric and symmetric contacts at opposite sites on the spine head, and an asymmetric contact at the spine neck (Fig.1G). Spines with a transitional shape between thin and mushroom aspects showed a protruding spinule with no evident PSD (Fig.1H). Mushroom spines showed variable sizes with small to large spine heads (Figs1I–M and 2A). These spines also established both symmetric and asymmetric macular synapses (Fig.1I) or usually asymmetric contacts at the spine head (Fig.1J,K). We also observed mushroom spines with a large head and perforated PSD (Fig.2A). Spinules were also found in multisynaptic mushroom spines (Fig.1L). Other spines had a transitional shape between a mushroom aspect to a ramified one (Fig.1M). Atypical shapes are shown in Fig.2B,C.
Other synaptic arrangements were noteworthy: (i) an axonal envelopment close to the symmetric synapse and involving the remaining perimeter of the spine head (Fig.2D); (ii) reciprocal synapses, with vesicles in adjacent parts of pre-/postsynaptic structures (Fig.2E, top); a multisynaptic spine protruding from an axon showing an unusual spinule-like double-membrane evagination (Fig.2E, bottom and right); (iii) a serial axo-axo-dendritic synapse (Fig.2F, bottom); (iv) an en passant axo-spiny synapse (Fig.2F, top); and (v) the close apposition between cellular membranes suggestive of a gap-like junction (Fig.2G and insert).
Discussion
Our data describe the diversity of the spine shapes and address relevant possibilities for the synaptic organization of the human Me. The implications for the local cellular functioning and the information processing are discussed below.
Methodological considerations
There are some inherent methodological limitations in studies involving postmortem human tissue. For example, the causes of death, the time between death and histological processing [postmortem intervals vary in the literature from 1 to 2 h (Jacobs et al. 2001; Lu et al. 2013; respectively) to 48 h (Sorvari et al. 1996a,b) or as long as 120 h (Garey et al. 2014)], the dynamic changes in the spine shapes over time (Kuwajima et al. 2013; Tonnesen et al. 2014; van der Zee, 2015), and the existence of previous non-reported or undiagnosed neurological and psychiatric disturbances (e.g. see data in Kirov et al. 1999; Fiala et al. 2002). Our study involved samples coming from adult men, but brains from women and from younger subjects would be desirable for comparison (see Goldstein et al. 2001; Kilpatrick et al. 2006; Anderson et al. 2009; Uematsu et al. 2012).
The postmortem human brain tissue is susceptible to anoxic and autolytic disturbances. Focal dendritic swelling and the expansion of varicosities in the edematous neuropil might alter the number of stubby spines (Fiala et al. 2002; García-López et al. 2007) and spinules (Tarrant & Routtenberg, 1977). As much as technically possible, we gathered images from tissue that maintained a relative integrity. The apparent spine length and the diameters of the spine head and neck would be estimated from confocal microscopy images. However, due to postmortem conditions and tissue processing, these quantifications might not represent actual values. It was also not possible to have tissue under controlled conditions to combine with immunocytochemical techniques.
Having accepted the above general limitations for the available material (Nimchinsky et al. 1999; Anderson et al. 2009), we used three complementary methodologies for the study of Me spines: (i) the Golgi method as a classic technique for ascertaining the types and morphological details of local neurons (Peters & Jones, 1984; Jacobs et al. 1997; García-López et al. 2010; Bianchi et al. 2013; Vargas-Barroso & Larriva-Sahd, 2013; and see Mancuso et al. 2012), (ii) the fluorescent dye DiI for the image reconstruction of spines using confocal microscopy (Kim et al. 2007; Rasia-Filho et al. 2010), and (iii) TEM to reveal ultrastructural details on the connectivity and synaptic features involving dendritic shafts and spines (Stewart et al. 2014).
Cellular types and functional implications for the human Me
As a parallel finding, the present Golgi method allowed the identification of different astrocytes in the neuropil of the adult human Me. Based on a stereological counting procedure, we previously found more glial cells (∼ 72%) than neurons in the adult human Me (Dall’Oglio et al. 2013). Fibrous astrocytes have a smooth surface, whereas protoplasmic astrocytes have a thorny surface with simple, small, and thin processes that appear as spine-like structures (Pannese, 1980). Both types of astrocytes were observed in the Me neuropil. The same occurs in the anterior cingulate cortex, where protoplasmic astrocytes also have thorny branches with spine-like structures (Torres-Platas et al. 2011). The function of the human Me astrocytes is not currently known, but they might establish interactions between neuronal microdomains and the extracellular matrix to modulate spine shape and synaptic function (Araque et al. 1999; Lippman & Dunaevsky, 2005; Dityatev & Rusakov, 2011; Giaume & Liu, 2012; Kuwajima et al. 2013; Medvedev et al. 2014). This opens an interesting research line.
The human Me is composed of multipolar neurons of five types classified according to their general aspect (Dall’Oglio et al. 2013). Pyramidal-shaped cells were previously described in the human Me by Sims & Williams (1990). From our data, only the angular neuron would resemble a pyramidal-like structural feature (shown in Fig.4). However, typical pyramidal neurons have a triangular soma, single and characteristic apical dendrite extending to a recognized surface; basal dendrites radiate out from the base of the soma and ramify close to it, and dendritic spines cover the most distal portions of primary, secondary, and tertiary branches (Feldman, 1984; Peters & Jones, 1984). This is not exactly the case for any Me neuron described here (Figs8). Therefore, these Me neurons can be considered nonpyramidal cells in this subcortical area.
There are no current studies that characterized the human Me neurons electrophysiologically. Nevertheless, the length and branching pattern of the dendrites of the different Me multipolar neurons would impose differences for the local synaptic processing (Dall’Oglio et al. 2008; Spruston et al. 2013). Dendrites are involved in the direction of information flow and their extent and branching complexity affect the neuronal voltage (Spruston et al. 2013; Sun et al. 2014). Long dendrites offer a large surface for multiple axonal connections throughout the Me neuropil. Thus, it is highly likely that the human Me neurons are gathering synaptic information with different spatial organization of the incoming connections. They could compartmentalize the synaptic processing or integrate various temporospatially synchronized synaptic activity. For example, the more extensive dendritic array of large fusiform neuron could receive a great sampling of afferent information if various axonal inputs contact these dendrites along their extensions. This would increase the neuronal integrative capacity and processing of information within a network (Jacobs et al. 1997). Otherwise, the Me round/ovoid-shaped neurons have a more restricted, circumjacent receptive field, and a high packed density of synapses represented by the great density of dendritic spines. These might be interneurons with a distinct expression of calcium-binding proteins in the human Me (Sorvari, 1997).
These findings also have to be linked with the afferents to the Me neurons. Available connectional data have come mostly from non-human primates and other mammals. The insula, the cortical-like amygdaloid nuclei, and the intercalated nuclei innervate the Me (Yilmazer-Hanke, 2012). Various classical neurotransmitters and neuropeptides can modulate the local excitatory and inhibitory synaptic transmission (reviewed in Martin et al. 1991; Benzing et al. 1992; Gloor, 1997; de Olmos, 2004; Yilmazer-Hanke, 2012). Indeed, the human Me has synapses with asymmetric and symmetric aspects contacting both dendritic shafts and spines. Dendritic shaft synapses can promote prompt responses and induce larger synaptic currents recorded at the soma when compared with the spine synapses, with the exception of contacts made on stubby spines (Segal, 2010). Dendritic spines with variable sizes, shapes, packing density, and complexity can differently modulate the incoming synaptic activity. We found dendritic spines either innervated by a single axon terminal or contacted by an en passant axon in the Me. That is, these axonal features support connectional possibilities ranging from a direct contact on a specific end-target element to a more diffuse synaptic relationship with various cells. The human Me neuropil has coarse and finely beaded axons, indicating the presence of en passage synapses, and collateral axonal branches forming terminal boutons ranging from simple appendages to multiple bulbous forms (Dall’Oglio et al. 2013). This latter forms a glomerular-like synaptic organization, that is, a bulbous axonal ending making asymmetric contacts with various dendrites at the same time (Dall’Oglio et al. 2013). The Me connectional organization can now be completed by the inclusion of reciprocal (axo-axonal connection) and serial (axo-axo-dendritic connection) synapses. In conjunction, it is suggested that the incoming synaptic activity in the Me promotes a divergence in the information processing. This could rapidly modulate the activity of various neurons in parallel and promote orchestrated responses for the interpretation of emotions and the prompt display of social behaviors.
Spine shapes and functional implications for the human Me
Dendritic spine density varies from 1.5 to 5.2 spines μm−1 in Golgi-impregnated neurons in the human Me (Dall’Oglio et al. 2013). Here, we found the presence of pleomorphic spines along different dendrites, from proximal to distal branches, either isolated or forming clusters. Similar ‘multiplex’ spines were found in other human brain areas, such as the cingulate (Yuste, 2013) and the visual cerebral cortex (García-López et al. 2010). The structure–function coupling of spines and the activity-driven changes related to synaptic demand, stability, and plasticity are under intensive investigation (Kasai et al. 2010; Segal, 2010; Chen et al. 2011; Fortin et al. 2012; Yuste, 2013; Morales et al. 2014; Oikonomou et al. 2014; Stewart et al. 2014; Tonnesen et al. 2014). Notably, the impact of the geometry of each spine on the postsynaptic signaling can be adjusted in a region- and neuron-specific manner (Bourne & Harris, 2008; Kasai et al. 2010; Mishchenko et al. 2010; Segal, 2010; Chen et al. 2011; Chen & Sabatini, 2012; Gulledge et al. 2012; Lee et al. 2012; Spruston et al. 2013).
It is conceivable that spines of different shapes and sizes can differ in the membrane surface available for receptor trafficking and fine-tuned synaptic processing (Bourne & Harris, 2008). Large heads in spines can increase biochemical compartmentalization, whereas shorter and wider necks may disperse chemical second messengers into the dendrite faster. Faster signaling may result in the ability to sustain stronger synaptic currents even during large or repeated synaptic conductance increases (Tonnesen et al. 2014) and to generate larger somatic potentials (Yuste, 2013). Moreover, “the possibility that excitatory postsynaptic potentials are filtered in the spine neck as they arrive into the dendritic shaft has interesting implications for spines that have longer necks because those spines appear to generate no significant depolarization at the soma… Are long spines electrically silent, as reserve connections? Perhaps their necks become shorter and wider during synaptic plasticity protocols…, ‘plugging in’ the presynaptic neuron they represent into the circuit and enabling fast circuit switching. Interestingly, human neurons have not only higher spine densities but also spines with characteristically long necks… This could reflect a higher degree of synaptic connectivity and plasticity by our brains” (Yuste, 2013 and references therein). Our present ultrastructural data provide evidence for this type of thin spine with long neck and complex multisynaptic connections in the Me.
Mushroom spines in the human Me are morphologically heterogeneous, with macular and perforated PSDs. These latter spines can increase their signaling strength by enhancing synaptic AMPA receptor expression in proportion to the distance of the synapse from the soma (van der Zee, 2015 and references therein). Mushroom spines can also standardize local postsynaptic potentials throughout the dendritic tree, reducing the location-dependent variability of local excitatory properties (Gulledge et al. 2012). That is, the synaptic strength on spines may be progressively larger at distal dendritic locations (Harnett et al. 2012). Also, synaptic amplification involving adjacent dendritic spines can enhance input cooperativity among coactive inputs (Harnett et al. 2012). Interestingly, mushroom spines intermingled among other clustered spines are found in distal branches of Me neurons.
Other types of spines were found in the human Me. The occurrence of thorny excrescences, large-headed spines, and thin spines with a gemmule appearance in the Me indicate that these spine classes are not unique features of the CA3 pyramidal neurons in humans (Lauer & Senitz, 2006; Lu et al. 2013). These Me spines provide the rationale for further evaluation of the role of these and other complex spines in the human amygdala in cases of temporal epilepsy (Drakew et al. 1996) and Down syndrome (see further discussion in Kuwajima et al. 2013). Also, the human Me showed varied ramified spines. The importance of them is based on a theoretical model for branching dendritic spines in which ‘… spine head morphology affects the level of local activity, whether the spines are modeled with passive membrane properties, or excitable membrane using Hodgkin–Huxley kinetics. The results indicate that merely separating the postsynaptic receptors on the surface of the spine may add to the diversity of circuitry, but does not change the efficacy of the synapse. However, when the surface area of the spine is a dynamic variable, efficacy of the synapse may vary continuously over time’ (Verzi & Noris, 2009). Compartmentalization in the ramified spine could confer differential temporal and spatial specificity and signaling microdomains for synapses (Chen & Sabatini, 2012) and the process of information in the Me neurons.
Our data also revealed additional synaptic plasticity in the adult Me. That is, the presence of filopodium is usually regarded as a sign of dynamic spine formation or elimination (García-López et al. 2010). The spines classified as atypical (the term used by Arellano et al. 2007a) can represent transient forms at varying stages of development or retraction (as ‘protospines’ and ‘multispines’ in García-López et al. 2010). Spinules can be involved in the transfer of large molecules and the electrical coupling between pre- and postsynaptic elements (Tarrant & Routtenberg, 1977; Spacek & Harris, 2004; Stewart et al. 2014). Spinules can also occur during the growth and remodeling of synaptic membranes (Matus, 2000; Stewart et al. 2014), be indicative of sustained synaptic activity (Tao-Cheng et al. 2009) as after the occurrence of LTP (Stewart et al. 2014), or serve to increase the motility of spines (Matus, 2000; Stewart et al. 2014).
We propose that the presence of different shapes of spines in the human Me ‘… aligns well with emerging theoretical models of synaptic learning that demonstrate that synapses exhibiting a gradation of states, each bridged by distinct metaplastic transitions, bestow neural networks with enhanced information storage capacity…’ (Lee et al. 2012). Given the possibility of functional subcompartments in the same spine (Chen & Sabatini, 2012), the synaptic processing can particularly be more complex in those Me double spines with two interconnected heads, and in bulbous and beaded racemose spines. Since we found neighboring spines with varying shapes and sizes in the same dendritic shafts of the human Me neurons, we also assume that ‘the morphological heterogeneity of spines, even for a local small portion of the dendrite, is consistent with the idea that synaptic strength is regulated locally, at the level of a single spine’ (Arellano et al. 2007a,b; see also Frick & Johnston, 2005; Chen et al. 2011; Lee et al. 2012). This is an important proposition for the human Me because primary changes in the structural organization of the amygdaloid occurred phylogenetically toward the primates, mainly in our species (Gloor, 1997), and can reflect either a more complex subcortical synaptic processing for sensory and emotional processing or an adaptation for species-specific social behaviors (Dall’Oglio et al. 2013; see a parallel discussion in Nimchinsky et al. 1999).
Finally, our ultrastructural data revealed three additional intriguing results: (i) the presence of an axonal enveloping the head of a spine adjacent to the synaptic site, which could serve to maintain a strong and selective connection, to restrict the neurotransmitter diffusion and/or receptors trafficking, or to isolate these elements from neighboring neurons or glial cells. (ii) An axonal spine as a postsynaptic element with asymmetric synapses. This spine would provide an additional site to increase axonal excitability and firing output. Interestingly, this axonal spine is connected with a presynaptic axon making a reciprocal synapse. Thus, the activity in this kind of network could generate synchronized, reverberated or self-limited activity upon the axonal spine, depending on the functional properties of the pre- and postsynaptic elements. (iii) The close apposition of cellular membranes that resembles a gap-like junction. The space between these apposed membranes is not obliterated and shows no evidence of a ‘zonulae occludentes’ (Peters et al. 1991) or for a ‘puncta adherentia’ with thick and electron-dense plaques at both sides of the membranes (Sätzler et al. 2002). This gap-like junction in the human Me is similar to the images of classic gap junctions described in the fish brain (fig.8E in Meek et al. 2004) and in the anterior pituitary gland of rats (fig.4B in Horiguchi et al. 2011).
Concluding remarks
The present work provides new data regarding the dendritic spine diversity and the synaptic ultrastructure of the adult human Me. Some spine shapes have not been reported in the Me of other species. These data are important when considering that the human Me neurons integrate subcortical circuits for the processing of ongoing multimodal stimuli and their emotional salience. Our findings also address likely functional implications for pleomorphic spines in the synaptic organization and plasticity of the Me, which can occur in normal conditions and in neurological and psychiatric disorders involving the human amygdala (cf. Sorvari, 1997; Schumann & Amaral, 2005; Heimer et al. 2008; Ossewaarde et al. 2013).
Acknowledgments
The authors are thankful to the relatives of the donors of the tissue samples studied here. We also acknowledge the skillful technical support provided by Mr. Hernan Mendoza (Olympus, Argentina). We are thankful to Dr. Arlete Hilbig (Department of Clinics/Neurology, UFCSPA, Brazil) and to Dr. Simone Marcuzzo (Department of Morphological Sciences, Laboratory of Comparative Morphophysiology, UFRGS, Brazil) for the use of equipment at a federal facility. A.A.R.F. is a CNPq researcher and A.D.O. is a grant recipient from CNPq (PDJ no. 150049/2014-5).
Conflict of interest
All authors state they have no actual or potential conflicts of interest.
Author contributions
Study concept and design: A.D.O., A.C.L.D., J.E.M., A.A.R.F. Acquisition of data: A.D.O., A.C.L.D., A.A.R.F. Interpretation of data: A.D.O., A.C.L.D., J.E.M., A.A.R.F. Critical revision of the manuscript: A.D.O., A.C.L.D., J.E.M., A.A.R.F. Administrative, technical, and material support: J.E.M., A.A.R.F.
References
- Anderson K, Bones B, Robinson B, et al. The morphology of supragranular pyramidal neurons in the human insular cortex: a quantitative Golgi study. Cereb Cortex. 2009;19:2131–2144. doi: 10.1093/cercor/bhn234. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, et al. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Arellano JI, Benavides-Piccione R, DeFelipe J, et al. Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front Neurosci. 2007a;1:131–143. doi: 10.3389/neuro.01.1.1.010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano JI, Espinosa A, Fairén A, et al. Non-synaptic dendritic spines in neocortex. Neuroscience. 2007b;145:464–469. doi: 10.1016/j.neuroscience.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Benavides-Piccione R, Ballesteros-Yáñes I, DeFelipe J, et al. Cortical area and species differences in dendritic spine morphology. J Neurocytol. 2002;31:337–346. doi: 10.1023/a:1024134312173. [DOI] [PubMed] [Google Scholar]
- Benzing WC, Mufson EJ, Jennes L, et al. Distribution of neurotensin immunoreactivity within the human amygdaloid complex: a comparison with acetylcholinesterase- and Nissl-stained tissue sections. J Comp Neurol. 1992;317:283–297. doi: 10.1002/cne.903170306. [DOI] [PubMed] [Google Scholar]
- Bian X. Physiological and morphological characterization of GABAergic neurons in the medial amygdala. Brain Res. 2013;1509:8–19. doi: 10.1016/j.brainres.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Bian X, Yanagawa Y, Chen WR, et al. Cortical-like functional organization of the pherormone-processing circuits in the medial amygdala. J Neurophysiol. 2008;99:77–86. doi: 10.1152/jn.00902.2007. [DOI] [PubMed] [Google Scholar]
- Bianchi S, Stimpson CD, Bauernfeind AL, et al. Dendritic morphology of pyramidal neurons in the chimpanzee neocortex: regional specializations and comparison to humans. Cereb Cortex. 2013;23:2429–2436. doi: 10.1093/cercor/bhs239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci USA. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma MCH, Dresselhaus EC, De Biase LM, et al. A requirement for nuclear factor-kB in developmental and plasticity-associated synaptogenesis. J Neurosci. 2011;31:5414–5425. doi: 10.1523/JNEUROSCI.2456-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP. Experimental Neuroanatomy: A Practical Approach. New York: Oxford University Press; 1992. [Google Scholar]
- Bourne JN, Harris KM. eLS. Chichester: John Wiley & Sons Ltd; 2007. Dendritic spines. In: http://www.els.net. doi: 10.1002/9780470015902.a0000093.pub2. [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodal A. Neurological Anatomy. New York: Oxford University Press; 1981. [Google Scholar]
- Brusco J, Dall’Oglio A, Rocha LB. Descriptive findings on the morphology of dendritic spines in the rat medial amygdala. Neurosci Lett. 2010;483:152–156. doi: 10.1016/j.neulet.2010.07.083. et al. ( [DOI] [PubMed] [Google Scholar]
- Brusco J, Merlo S, Ikeda ÉT, et al. Inhibitory and multisynaptic spines, and hemispherical synaptic specialization in the posterodorsal medial amygdala of male and female rats. J Comp Neurol. 2014;522:2075–2088. doi: 10.1002/cne.23518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Sabatini BL. Signaling in dendritic spines and spine microdomains. Curr Opin Neurobiol. 2012;22:389–396. doi: 10.1016/j.conb.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Leischner U, Rochefort NL, et al. Functional mapping of single spines in cortical neurons in vivo. Nature. 2011;475:501–507. doi: 10.1038/nature10193. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Stokas MR, Woolley CS. Morphological sex differences and laterality in the prepubertal medial amygdala. J Comp Neurol. 2007;501:904–915. doi: 10.1002/cne.21281. [DOI] [PubMed] [Google Scholar]
- Dall’Oglio A, Gehlen G, Achaval M, et al. Dendritic branching features of posterodorsal medial amygdala neurons of adult male and female rats: further data based on the Golgi method. Neurosci Lett. 2008;430:151–156. doi: 10.1016/j.neulet.2007.10.051. [DOI] [PubMed] [Google Scholar]
- Dall’Oglio A, Ferme D, Brusco J, et al. The ‘single-section’ Golgi method adapted for formalin-fixed human brain and light microscopy. J Neurosci Methods. 2010;189:51–55. doi: 10.1016/j.jneumeth.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Dall’Oglio A, Xavier LL, Hilbig A, et al. Cellular components of the human medial amygdaloid nucleus. J Comp Neurol. 2013;521:589–611. doi: 10.1002/cne.23192. [DOI] [PubMed] [Google Scholar]
- DeArmond SJ, Fusco MM, Dewey MM. Structure of the Human Brain. New York: Oxford University Press; 1989. [Google Scholar]
- Dityatev A, Rusakov DA. Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol. 2011;21:1–7. doi: 10.1016/j.conb.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakew A, Müller M, Gähwiler BH, et al. Spine loss in experimental epilepsy: quantitative light and electron microscopic analysis of intracellularly stained CA3 pyramidal cells in hippocampal slice cultures. Neuroscience. 1996;70:31–45. doi: 10.1016/0306-4522(95)00379-w. [DOI] [PubMed] [Google Scholar]
- Everitt B. Limbic lobe and olfactory pathways. In: Berry MM, Bannister LH, Standring SM, editors. Gray’s Anatomy. London: Churchill Livingstone; 1995. pp. 1115–1141. [Google Scholar]
- Feldman ML. Morphology of the neocortical pyramidal neuron. In: Jones EG, Peters A, editors. Cerebral Cortex. Vol. 2. New York: Plenum Press; 1984. pp. 107–121. [Google Scholar]
- Fiala JC, Harris KM. Dendrite structure. In: Stuart G, Sprutson N, Häusser M, editors. Dendrites. New York: Oxford University Press; 1999. pp. 1–34. [Google Scholar]
- Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Rev. 2002;39:29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Fortin DA, Srivastava T, Soderling TRS. Structural modulation of dendritic spines during synaptic plasticity. Neuroscientist. 2012;18:326–341. doi: 10.1177/1073858411407206. [DOI] [PubMed] [Google Scholar]
- Freese JL, Amaral DG. Neuroanatomy of the primate amygdala. In: Whalen PJ, Phelps EA, editors. The Human Amygdala. New York: The Guilford Press; 2009. pp. 3–42. [Google Scholar]
- Frick A, Johnston D. Plasticity of dendritic excitability. J Neurobiol. 2005;64:100–115. doi: 10.1002/neu.20148. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Somogyi J. The ‘single’ section Golgi-impregnation procedure: methodological description. J Neurosci Methods. 1984;11:221–230. doi: 10.1016/0165-0270(84)90084-0. [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Büchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc Natl Acad Sci USA. 2010;107:9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-López P, García-Marín V, Freire M. The discovery of dendritic spines by Cajal in 1888 and its relevance in the present neuroscience. Prog Neurobiol. 2007;83:110–130. doi: 10.1016/j.pneurobio.2007.06.002. [DOI] [PubMed] [Google Scholar]
- García-López P, García-Marín V, Freire M. Dendritic spines and development: towards a unifying model of spinogenesis – a present day review of Cajal’s histological slides and drawings. Neural Plast. 2010;769207 doi: 10.1155/2010/769207. doi: 10.1155/2010/769207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, et al. Reduced dendritic spine density on cerebral cortical neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 2014;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Liu X. From a glial syncytium to a more restricted and specific glial networking. J Physiol. 2012;106:34–39. doi: 10.1016/j.jphysparis.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Gloor P. The Temporal Lobe and Limbic System. New York: Oxford University Press; 1997. [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- González-Burgos I, Rivera-Cervantes MCR, Velázquez-Zamora DA. Selective estrogen receptor modulators regulate dendritic spine plasticity in the hippocampus of male rats. Neural Plast. 2012;309494 doi: 10.1155/2012/309494. et al. ( 2012:. doi: 10.1155/2012/309494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Ramírez MM, Velázquez-Zamora DA, Olvera-Cortés ME, et al. Changes in the plastic properties of hippocampal dendritic spines underlie the attenuation of place learning in healthy aged rats. Neurobiol Learn Mem. 2014;109:94–103. doi: 10.1016/j.nlm.2013.11.017. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Carnevale NT, Stuart GJ. Electrical advantages of dendritic spines. PLoS One. 2012;7:e36007. doi: 10.1371/journal.pone.0036007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall EA. The amygdala of the cat: a Golgi study. Z Zellforsch Mikrosk Anat. 1972;134:439–458. doi: 10.1007/BF00307668. [DOI] [PubMed] [Google Scholar]
- Harnett MT, Makara JK, Spruston N, et al. Synaptic amplification by dendritic spines enhances input cooperativity. Nature. 2012;491:599–605. doi: 10.1038/nature11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW, Trimble M, et al. Anatomy of Neuropsychiatry – The New Anatomy of the Basal Forebrain and Its Implications for Neuropsychiatric Illness. San Diego, CA: Academic Press; 2008. [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Horiguchi K, Kouki T, Fujiwara K, et al. The extracellular matrix component laminin promotes gap junction formation in the rat anterior pituitary gland. J Endocrinol. 2011;208:225–232. doi: 10.1677/JOE-10-0297. [DOI] [PubMed] [Google Scholar]
- Izzo PN, Graybiel AM, Bolam JP. Characterization of substance P- and [Met]enkephalin-immunoreactive neurons in the caudate nucleus of cat and ferret by a single section Golgi procedure. Neuroscience. 1987;20:577–587. doi: 10.1016/0306-4522(87)90111-4. [DOI] [PubMed] [Google Scholar]
- Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J Comp Neurol. 1997;386:661–680. [PubMed] [Google Scholar]
- Jacobs B, Schall M, Prather M, et al. Regional dendritic and spine variation in human cerebral cortex: a quantitative Golgi study. Cereb Cortex. 2001;11:558–571. doi: 10.1093/cercor/11.6.558. [DOI] [PubMed] [Google Scholar]
- Janak PH, Tye KM. From circuits to behavior in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Fukuda M, Watanabe S, et al. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–129. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, et al. Sex-related differences in amygdala functional connectivity during resting conditions. NeuroImage. 2006;30:452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Kim BG, Dai H-N, McAtee M, et al. Labeling of dendritic spines with the carbocyanine dye DiI for confocal microscopic imaging in lightly fixed cortical slices. J Neurosci Methods. 2007;162:237–243. doi: 10.1016/j.jneumeth.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov SA, Sorra KE, Harris KM. Slices have more synapses than perfusion-fixed hippocampus from young and mature rats. J Neurosci. 1999;19:2876–2886. doi: 10.1523/JNEUROSCI.19-08-02876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima M, Spacek J, Harris KM. Beyond counts and shapes: studying pathology of dendritic spines in the context of the surrounding neuropil through serial section electron microscopy. Neuroscience. 2013;251:75–89. doi: 10.1016/j.neuroscience.2012.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer M, Senitz D. Dendritic excrescences seem to characterize hippocampal CA3 pyramidal neurons in humans. J Neural Transm. 2006;113:1469–1475. doi: 10.1007/s00702-005-0428-8. [DOI] [PubMed] [Google Scholar]
- Lee KFH, Soares C, Béïque JC. Examining form and function of dendritic spines. Neural Plast. 2012 doi: 10.1155/2012/704103. 2012:704103. doi: 10.1155/2012/704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepousez G, Nissant A, Bryant AK, et al. Olfactory learning promotes input-specific synaptic plasticity in adult-born neurons. Proc Natl Acad Sci USA. 2014;111:13984–13989. doi: 10.1073/pnas.1404991111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Phan KL, Decker LR, et al. Extended amygdala and emotional salience: a PET activation study of positive and negative affect. Neurospsychopharmacology. 2003;28:726–733. doi: 10.1038/sj.npp.1300113. [DOI] [PubMed] [Google Scholar]
- Lippman J, Dunaevsky A. Dendritic spine morphogenesis and plasticity. J Neurobiol. 2005;64:47–57. doi: 10.1002/neu.20149. [DOI] [PubMed] [Google Scholar]
- Lu D, He L, Xiang W, et al. Somal and dendritic development of human CA3 pyramidal neurons from midgestation to middle childhood: a quantitative Golgi study. Anat Rec. 2013;296:123–132. doi: 10.1002/ar.22616. [DOI] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Voss T. Atlas of the Human Brain. New York: Academic Press; 2008. [Google Scholar]
- Mancuso JJ, Chen Y, Li X, et al. Methods of dendritic spine detection: from Golgi to high-resolution optical imaging. Neuroscience. 2012;251:129–140. doi: 10.1016/j.neuroscience.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Powers RE, Dellovade TL, et al. The bed nucleus-amygdala continuum in human and monkey. J Comp Neurol. 1991;309:445–485. doi: 10.1002/cne.903090404. [DOI] [PubMed] [Google Scholar]
- Matus A. Actin-based plasticity in dendritic spines. Science. 2000;290:754–758. doi: 10.1126/science.290.5492.754. [DOI] [PubMed] [Google Scholar]
- McDonald J, Mascagni F, Augustine JR. Neuropeptide Y and somatostatin-like immunoreactivity in neurons of the monkey amygdala. Neuroscience. 1995;66:959–982. doi: 10.1016/0306-4522(94)00629-j. [DOI] [PubMed] [Google Scholar]
- Medvedev N, Popov V, Henneberger C, et al. Glia selectively approach synapses on thin dendritic spines. Philos Trans R Soc Lond B Biol Sci. 2014;369:20140047. doi: 10.1098/rstb.2014.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek J, Kirchberg G, Grant K, et al. Dye coupling without gap junctions suggests excitatory connections of γ-aminobutiric acidergic neurons. J Comp Neurol. 2004;468:151–164. doi: 10.1002/cne.10951. [DOI] [PubMed] [Google Scholar]
- Mishchenko Y, Hu T, Spacek J, et al. Ultrastructural analysis of hippocampal neuropil from the connectomics perspective. PLoS One. 2010;67:1009–1020. doi: 10.1016/j.neuron.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales J, Benavides-Piccione R, Dar M, et al. Random positions of dendritic spines in human cerebral cortex. J Neurosci. 2014;34:10078–10084. doi: 10.1523/JNEUROSCI.1085-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi K, Horie S, Yokosuka M, et al. Heterogeneous electrophysiological and morphological properties of neurons in the mouse medial amygdala in vitro. Brain Res. 2012;1480:41–52. doi: 10.1016/j.brainres.2012.08.050. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Gilissen E, Allman JM, et al. A neuronal morphologic type unique to humans and great apes. Proc Natl Acad Sci USA. 1999;96:5268–5273. doi: 10.1073/pnas.96.9.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikonomou KD, Singh MB, Sterjanaj EV, et al. Spiny neurons of amygdala, striatum, and cortex use dendritic plateau potentials to detect network UP states. Front Neurosci. 2014;8:292. doi: 10.3389/fncel.2014.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Olmos JS. Amygdala. In: Paxinos G, Mai J, editors. The Human Nervous System. San Diego, CA: Elsevier; 2004. pp. 739–868. [Google Scholar]
- Ossewaarde L, van Wingen GA, Rijpkema M, et al. Menstrual cycle-related changes in amygdala morphology are associated with changes in stress sensitivity. Hum Brain Mapp. 2013;34:1187–1193. doi: 10.1002/hbm.21502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannese E. Caratteri strutturali utili per l’identificazione delle cellule gliali del sistema nervoso central. Istocitopatologia. 1980;2:129–136. [Google Scholar]
- Pannese E. Neurocitology: Fine Structure of Neurons, Nerve Processes, and Neuroglial Cells. New York: Thieme; 1994. [Google Scholar]
- Peters A, Jones EG. Classification of cortical neurons. In: Jones EG, Peters A, editors. Cerebral Cortex. Functional Properties of Cortical Cells. Vol. 2. New York: Plenum Press; 1984. pp. 107–121. [Google Scholar]
- Peters A, Palay SL, Webster H. The Fine Structure of the Nervous System. New York: Oxford University Press; 1991. [Google Scholar]
- Pitkänen A, Amaral DG. The distribution of GABAergic cells, fibers, and terminals in the monkey amygdaloid complex: an immunohistochemical and in situ hybridization study. J Neurosci. 1994;74:2200–2224. doi: 10.1523/JNEUROSCI.14-04-02200.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasia-Filho AA, Fabian C, Rigoti K. Influence of sex, estrous cycle and motherhood in dendritic spine density in the rat medial amygdala revealed by the Golgi method. Neuroscience. 2004;126:839–847. doi: 10.1016/j.neuroscience.2004.04.009. et al. ( [DOI] [PubMed] [Google Scholar]
- Rasia-Filho AA, Brusco J, Rocha LB. Dendritic spines observed by extracellular DiI dye and immunolabeling under confocal microscopy. Nat Protoc/Protoc Exch. 2010 et al. ( http://www.nature.com/protocolexchange/protocols/1890. [Google Scholar]
- Rasia-Filho AA, Dalpian F, Menezes IC, et al. Dendritic spines of the medial amygdala: plasticity, density, shape, and subcellular modulation by sex steroids. Histol Histopathol. 2012;27:985–1011. doi: 10.14670/HH-27.985. [DOI] [PubMed] [Google Scholar]
- Rochefort NL, Konnerth A. Dendritic spines: from structure to in vivo function. EMBO Rep. 2012;13:699–708. doi: 10.1038/embor.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sätzler K, Söhl LF, Bollmann JH, et al. Three-dimensional reconstruction of a Calyx of Held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. J Neurosci. 2002;22:10567–10579. doi: 10.1523/JNEUROSCI.22-24-10567.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological estimation of the number of neurons in the human amygdaloid complex. J Comp Neurol. 2005;491:320–329. doi: 10.1002/cne.20704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. Dendritic spines, synaptic plasticity and neuronal survival: activity shapes dendritic spines to enhance neuronal viability. Eur J Neurosci. 2010;31:2178–2184. doi: 10.1111/j.1460-9568.2010.07270.x. [DOI] [PubMed] [Google Scholar]
- Sims KS, Williams RS. The human amygdaloid complex: a cytologic and histochemical atlas using Nissl, myelin, acetylcholinesterase and nicotinamide adenine dinucleotide phosphate diaphorase staining. Neuroscience. 1990;2:449–472. doi: 10.1016/0306-4522(90)90440-f. [DOI] [PubMed] [Google Scholar]
- Sorvari H. 1997. Finland University of Kuopio Neurons containing calcium-binding proteins in the human amygdaloid complex. Ph.D. Thesis,
- Sorvari H, Soininen H, Pitkänen A. Calretinin-immunoreactive cells and fibers in the human amygdaloid complex. J Comp Neurol. 1996a;369:188–208. doi: 10.1002/(SICI)1096-9861(19960527)369:2<188::AID-CNE2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Sorvari H, Soininen H, Pitkänen A. Calbindin-D28K-immunoreactive cells and fibres in the human amygdaloid complex. Neuroscience. 1996b;75:421–443. doi: 10.1016/0306-4522(96)00296-5. [DOI] [PubMed] [Google Scholar]
- Spacek J, Harris KM. Trans-endocytosis via spinules in adult rat hippocampus. J Neurosci. 2004;24:4233–4241. doi: 10.1523/JNEUROSCI.0287-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N, Häusser M, Stuart G. Information processing in dendrites and spines. In: Squire LR, Berg D, Bloom FE, editors. Fundamental Neuroscience. Waltham: Elsevier; 2013. pp. 231–260. et al.). In: [Google Scholar]
- Stewart MG, Popov VI, Kraev IV. Structure and complexity of the synapse and dendritic spine. In: Pickel V, Segal M, editors. The Synapse. Kidlington: Academic Press; 2014. pp. 1–20. ), et al. (. In: [Google Scholar]
- Sun Q, Srinivas KV, Sotayo A, et al. Dendritic Na+ spikes enable cortical input to drive action potential output from hippocampal CA2 pyramidal neurons. eLIFE. 2014;3:e04551. doi: 10.7554/eLife.04551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng J-H, Dosemeci A, Gallant PE, et al. Rapid turnover of spinules at synaptic terminals. Neuroscience. 2009;160:42–50. doi: 10.1016/j.neuroscience.2009.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrant SB, Routtenberg A. The synaptic spinule in the dendritic spine: electron microscopic study of the hippocampal dentate gyrus. Tissue Cell. 1977;9:461–473. doi: 10.1016/0040-8166(77)90006-4. [DOI] [PubMed] [Google Scholar]
- Tonnesen J, Katona G, Rózsa B, et al. Spine neck plasticity regulates compartmentalization of synapses. Nat Neurosci. 2014;17:678–685. doi: 10.1038/nn.3682. [DOI] [PubMed] [Google Scholar]
- Torres-Platas SG, Hercher C, Davoli MA, et al. Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology. 2011;36:2650–2658. doi: 10.1038/npp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A, Matsui M, Tanaka C, et al. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One. 2012;7:e46970. doi: 10.1371/journal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Barroso V, Larriva-Sahd J. A cytological and experimental study of the neuropil and primary olfactory afferences to the piriform cortex. Anat Rec. 2013;296:1297–1316. doi: 10.1002/ar.22753. [DOI] [PubMed] [Google Scholar]
- Verzi DW, Noris OY. A compartmental model for activity-dependent dendritic spine branching. Bull Math Biol. 2009;71:1048–1072. doi: 10.1007/s11538-009-9393-y. [DOI] [PubMed] [Google Scholar]
- Yilmazer-Hanke DM. Amygdala. In: Paxinos G, Mai J, editors. The Human Nervous System. San Diego, CA: Elsevier; 2012. pp. 759–834. [Google Scholar]
- Yuste R. Dendritic spine and distributed circuits. Neuron. 2011;71:772–781. doi: 10.1016/j.neuron.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R. Electrical compartmentalization in dendritic spines. Annu Rev Neurosci. 2013;36:429–449. doi: 10.1146/annurev-neuro-062111-150455. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci USA. 1997;94:4119–4124. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zee EA. Synapses, spines and kinases in mammalian learning and memory, and the impact of aging. Neurosci Biobehav Rev. 2015;50:77–85. doi: 10.1016/j.neubiorev.2014.06.012. [DOI] [PubMed] [Google Scholar]




