Abstract
Recent research on the visual system has focused on investigating the relationship among eye (ocular), orbital, and visual cortical anatomy in humans. This issue is relevant in evolutionary and medical fields. In terms of evolution, only in modern humans and Neandertals are the orbits positioned beneath the frontal lobes, with consequent structural constraints. In terms of medicine, such constraints can be associated with minor deformation of the eye, vision defects, and patterns of integration among these features, and in association with the frontal lobes, are important to consider in reconstructive surgery. Further study is therefore necessary to establish how these variables are related, and to what extent ocular size is associated with orbital and cerebral cortical volumes. Relationships among these anatomical components were investigated using magnetic resonance images from a large sample of 83 individuals, which also included each subject’s body height, age, sex, and uncorrected visual acuity score. Occipital and frontal gyri volumes were calculated using two different cortical parcellation tools in order to provide a better understanding of how the eye and orbit vary in relation to visual cortical gyri, and frontal cortical gyri which are not directly related to visual processing. Results indicated that ocular and orbital volumes were weakly correlated, and that eye volume explains only a small proportion of the variance in orbital volume. Ocular and orbital volumes were also found to be equally and, in most cases, more highly correlated with five frontal lobe gyri than with occipital lobe gyri associated with V1, V2, and V3 of the visual cortex. Additionally, after accounting for age and sex variation, the relationship between ocular and total visual cortical volume was no longer statistically significant, but remained significantly related to total frontal lobe volume. The relationship between orbital and visual cortical volumes remained significant for a number of occipital lobe gyri even after accounting for these cofactors, but was again found to be more highly correlated with the frontal cortex than with the occipital cortex. These results indicate that eye volume explains only a small amount of variation in orbital and visual cortical volume, and that the eye and orbit are generally more structurally associated with the frontal lobes than they are functionally associated with the visual cortex of the occipital lobes. Results also demonstrate that these components of the visual system are highly complex and influenced by a multitude of factors in humans.
Keywords: eye, frontal lobe, occipital lobe, orbit, visual cortex
Introduction
The anatomical relationship among major components of the human visual system has been the subject of a number of recent inter-specific (Pearce et al. 2013; Meyer et al. 2014; Traynor et al. 2015), and intra-specific studies (Pearce & Dunbar, 2012; Pearce & Bridge, 2013). In human evolution, these topics are interesting when considering the species-specific cranial traits associated with hominid encephalization and facial morphology. Modern humans evolved large brains and small faces, which introduced spatial conflicts between these two anatomical blocks (Bruner et al. 2014). Eye morphogenesis is influenced by brain growth (Weale, 1982; Mak et al. 2006), and the orbit responds to facial growth (Waitzman et al. 1992), undergoing different (and opposite) evolutionary processes.
In Neandertals and modern humans, the two hominid species with larger brain sizes in absolute and relative terms, we observe an additional and specific constraint: where the facial block shifts under the neurocranial block, and the frontal lobes are consequently positioned directly above the orbital roof (Bruner & Holloway, 2010). Therefore, in these two species the orbits are partially constrained by a spatial relationship with the frontal cortex. Recently, further evolutionary correlations have been described between orbital and cerebral morphology, and tentatively interpreted with selective/adaptive processes integrating anatomy, vision, and geographical latitude (Pearce & Dunbar, 2012; Pearce et al. 2013).
In modern humans specifically, it has been stated that eye size dictates orbital and visual cortical size (Pearce & Bridge, 2013); and that increased ocular volume results in larger orbits and visual cortices (Pearce & Dunbar, 2012). However, further research on the anatomical relationship among the eye, orbit, and visual cortex is essential for elucidating the association among these traits, particularly given that eye size was shown to explain only 16.5% of variation in orbital size in an analysis of their anatomical relationship (Pearce & Bridge, 2013).
To propose a causal relationship between eye size, orbit size, and visual cortex size, a much stronger relationship must be shown to exist among the first two of these variables. This is particularly true when making inferences between orbit morphology, visual capacity, and environmental variables associated with differences in day length and light intensity across different latitudes. Realistically, to evaluate this functional network, it must first be shown that light levels dictate eye size, eye size determines orbital size, and orbital size determines adult volume of visual cortical areas of the occipital lobe.
A low R2 value of 16.5% does not fully support the idea that eye size dictates orbital morphology, which is a crucial element of the remaining logical pathway. Pearce and Bridge (2013) argue that the low R2 value, in their analysis of the relationship between ocular and orbital volume, may have been artificially reduced because it was not possible to include potentially confounding variables like body size. It was also argued that the R2 value was artificially low as a result of measurement error associated with having to reconstruct 49 of the 89 eye scans, and because visual acuity metrics were not available for individuals in their sample.
It is well established that myopic refractive error (nearsightedness) is associated with an overly large, axially elongated eye, increased vitreous depth, and increased focusing power of the cornea, which causes an image to be focused in front of the retina and results in blurred vision (Curtin, 1985; Working Group on Myopia Prevalence and Progression, 1989; Lam et al. 1999; Stone & Filtcroft, 2004; Dirani et al. 2006). Increased axial length (Ip et al. 2007; Mutti et al. 2007) and the overly large eye of myopes (Zadnik et al. 1994; Ip et al. 2007; Lam et al. 2008) have been shown to be among the biggest factors associated with this condition. However, this increased ocular growth has not been shown to have a direct influence on orbital size in humans (Schultz, 1940; Chau et al. 2004; Masters, 2012).
In fact, Chau et al. (2004) addressed this question specifically, investigating whether the larger eye of myopes corresponds with larger orbits in humans. This study was conducted because increased ocular growth had been shown to cause the orbit to grow larger in chickens (Wilson et al. 1997). However, in humans, this same relationship was not found to exist and, by contrast, orbital size was largely independent of eye size in this sample of Chinese adults, regardless of how large the myopic eye became (Chau et al. 2004).
A recent analysis of the relationship among the eye, orbit, and myopia using these same data (Chau et al. 2004), indicated that beyond simply the absolute size of the eye, the relative size of the eye within the orbit was associated with the prevalence and severity of myopia in this sample (Masters, 2012). Here it was shown that individuals with large eyes in small orbits had a higher rate of myopia and a greater degree of refractive error, whereas those with smaller eyes in relatively large orbits retained much more acute vision.
This further indicates that the eye does not directly influence orbital size in humans as it does in chickens (Wilson et al. 1997), but rather, that its excessive growth within the bony confines of an independently developing orbit may actually contribute to the development of juvenile-onset myopia. If the eye and orbit follow separate growth trajectories, which would be expected as a result of their distinct hard and soft tissue origins and anatomical associations, further enlargement of the eye may result in it becoming compressed against muscles, fat, and the orbit wall during ontogeny, resulting in the common ocular form of myopes, rather than a consequent increase in orbital size.
Schultz’s (1940) expansive research on the orbits of human and non-human primates also points to a lack of evidence for a direct causal relationship between eye and orbital size. In fact, he states that ‘In considering all the evidence produced it appears that the size of the orbit is dependent upon the size of the eyeball in only the most general way and that the two structures can vary in size independently to a surprising extent’ (Schultz, 1940, p. 408).
In the current study, we investigate the relationship among ocular, orbital, and visual cortical volumes with a large sample of magnetic resonance images (MRIs) to assess the cogency of using the orbit as a proxy for eye and visual cortex anatomy. This analysis also includes body height and visual acuity variables, which were not available in the Pearce & Bridge (2013) study, but were argued to have artificially reduced the R2 value in the relationship between eye and orbital volume by not being included in the analysis. Additionally, analyses investigating ocular anatomy in the current study included 83 individuals who possessed eyes that were fully visible in the MRI, and that were not moving during the scan, which can cause ripples to form in the image. As a result, no reconstruction was necessary to delineate accurately the borders of the eye, which reduces potential error associated with extrapolating the discernible ocular boundary.
Lastly, the relationship between the eye/orbit and visual cortex was assessed in the context of how ocular and orbital volumes vary in association with five frontal lobe gyri volumes generated using Brain Parser 56 ROI, and FreeSurfer 5.3.0. This was carried out to test whether the eye and orbit are more highly correlated with V1, V2, and V3 of the occipital lobe, or rather with frontal lobe gyri that are not directly related to visual processing, but that can be correlated with these features by means of structural spatial constraints.
In this way it is possible to evaluate the extent to which the eyes and orbits are associated with visual cortical size, by examining whether these hard and soft tissue components of the visual and craniofacial systems are more highly correlated with the visual areas of the occipital lobe, or with those of the frontal lobe that are not associated with vision. This is important because the eye is functionally associated with the occipital brain areas, but the orbit is structurally influenced by the frontal lobes, housed in the anterior cranial fossa and separated from the ocular space by only a thin bone forming the orbital roof.
With regard to the current study, the relationship among ocular, orbital, and visual cortical anatomy is addressed as three primary research questions:
Is ocular volume correlated with orbital volume, and to what extent does ocular volume explain variation in orbital volume?
Does the correlation between ocular and orbital volume strengthen when additional variables such as height, visual acuity, age, and sex are included in the analysis, as suggested by Pearce & Bridge (2013)?
Is ocular and orbital volume more highly correlated with V1, V2, and V3 of the visual cortex, or with frontal lobe gyri not directly related to visual processing?
Materials and methods
Sample
Numerous fields of anonymized clinical data, including information about age, sex, ancestry, height, weight, uncorrected visual acuity (vision before correction with lasik, glasses, or contacts), and numerous other demographic, physical, cognitive, and disease-related variables, were obtained for 189 adult individuals who had previously undergone MRI scanning as part of the International Consortium for Brain Mapping (Mazziotta et al. 2001). Other than showing variable levels of myopia and astigmatism, no other diseases or deformities of the eye or orbit were present across subjects. These MRIs and associated clinical data were provided by the Laboratory of Neuro Imaging at the University of Southern California for the purposes of this research. These whole-head MRIs were acquired using a Siemens Magnetom Sonata syngo MR 2004A, Sag MPRAGE 8 Channel with total scan time of 8 minutes, 8 seconds, and voxel size of 1.0 × 1.0 × 1.0 mm. Further details regarding the parameters and scanning protocol used to obtain these images can be found at www.loni.usc.edu/ICBM/About/icbm.pdf.
Of the 189 individuals in the dataset, orbital volume, cerebral volumes, body height, age, sex, and far-vision acuity scores derived from eye exams were available for 126 individuals (female = 66, male = 60, aged 19–80 years), and 83 individuals who possessed fully intact eyes (female = 41, male = 42, aged 19–80 years). This allowed for a large sample of individuals for whom no ocular reconstruction was necessary, thus reducing potential measurement error associated with an ill-defined anterior portion of the eye. Far-vision Snellen scores were used in the current study as a continuous variable after conversion to their LogMAR equivalent, which is the most appropriate way to investigate visual acuity in statistical analyses (Holladay, 1997).
Ocular and orbital volume
Amira 5.4 was used to render a 3D volume of the eye and internal orbital cavity for each subject in the sample (Fig.1). Ocular volume was calculated by selecting areas with similar intensity values on the targeted region of interest (ROI; left vitreous humor, lens, anterior chamber, etc.) and adding the selected pixels to that designated object file. These were further refined by filling holes, smoothing the labeled regions, and conducting a slice-by-slice evaluation of the accuracy of the ocular segmentation in the 2D coronal, sagittal, and axial planes, as well as in the 3D viewer.
Figure 1.
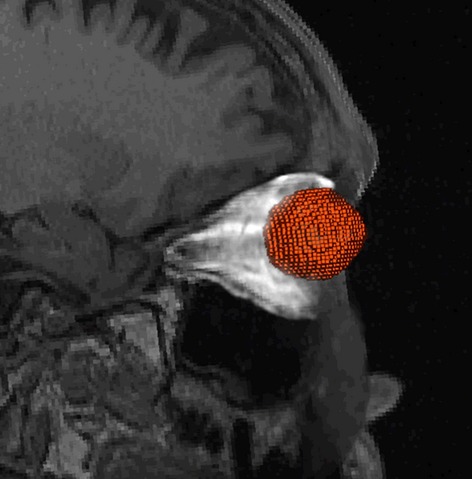
Lateral view of ocular and orbital segmentation for an individual in the sample, with eyes shown in red and orbital volume highlighted in white.
Orbital segmentation was carried out slice by slice in the sagittal view. Once the orbital border was delineated, each slice was examined and any errors were corrected in the coronal and axial planes, to ensure that the orbital boundaries were accurately represented. Additionally, to maintain consistency in defining the anterior margins of the orbits, prior to segmentation a vertical 3D line was drawn across the anterior orbital margins in the sagittal plane, and all slice-by-slice measurements were taken posterior to it. This was carried out to reduce measurement error associated with difficulty in defining the anterior margins in volumetric orbital measurements (Schultz, 1940). Because the image resolution of each MRI in the sample is 1 mm3, volume calculations for each 3D object file were easily quantified based on the count of voxels in each labeled object using the material statistics measurement protocol in amira.
Frontal and occipital gyri volume measurement
Both Brain Parser 56 ROI and FreeSurfer 5.3.0 cortical parcellation tools were used on the same sample of MRIs to ensure a more holistic and reliable result, as well as a better understanding of how the eye and orbit vary in relation to occipital and frontal cortical anatomy. This was also done as a result of a number of recently identified problems associated with the consistency of results using different versions of FreeSurfer, and when running the software on different operating systems (Gronenschild et al. 2012).
FreeSurfer is an open source software package developed by the Athinoula A. Martinos Center for Biomedical Imaging. Its analysis pipeline includes a surface-based stream and a volume-based stream. In the surface-based stream, boundaries between white matter, cortical gray matter, and the pial surface are constructed. The volume-based stream assigns subcortical tissue classification labels to volumes. Gronenschild et al. (2012) observed that the FreeSurfer version and workstation operating system can significantly affect the results of calculated volumes and cortical thicknesses, and advised authors using the program to provide system details. In light of these results, the current study was conducted using FreeSurfer 5.3.0, running on a High Performance Computing Cluster, which consists of 22 compute nodes, each containing two 8-core Intel Xeon 2.2 GHz processors (E5-2660). The cluster is a Linux system, which at the time of this study was running CentOS 6.5.
Gyri volumes for the frontal and occipital lobes were also calculated using the Laboratory of Neuro Imaging’s Brain Parser 56 ROI. This software can accurately and efficiently perform a whole brain segmentation to parse an MRI into 56 anatomical structures in < 30 min (Tu et al. 2008). Brain Parser identifies and segments these structures by overlaying an MRI atop a standard template derived from 40 subjects (Shattuck et al. 2008). The two images are aligned using topographically relevant anatomy, and the fit is refined using an AIR nonlinear warp, a 5th order polynomial. Segmentation and calculation of gyri volumes was carried out for all individuals in the available sample using the LONI Pipeline Brain Parser Workflow (Dinov et al. 2010).
Brain Parser designates the middle occipital gyrus as the Primary Visual Cortex V1, which corresponds to Brodmann’s Area 17 (Fig.2). The superior and inferior occipital gyri just beyond the banks of the calcarine fissure, which are part of V2 and V3 and correspond to Broadmann’s Areas 18 and 19, were also included in the analysis to investigate how these extrastriate visual association areas vary in relation to ocular and orbital volume. Lastly, cuneus and lingual gyrus volumes were included to provide a broader representation of overall striate and extrastriate cortical areas. The cuneus encompasses Brodmann area 17 at the inferior margins of the calcarine fissure, area 18 superior to that, and area 19 on the superoanterior margin at the parietooccipital fissure. The lingual gyrus also represents these same Brodmann areas on the inferior aspect of the occipital lobe, and together with the cuneus provides a broader anatomical representation of the visual cortex (Tong, 2003).
Figure 2.
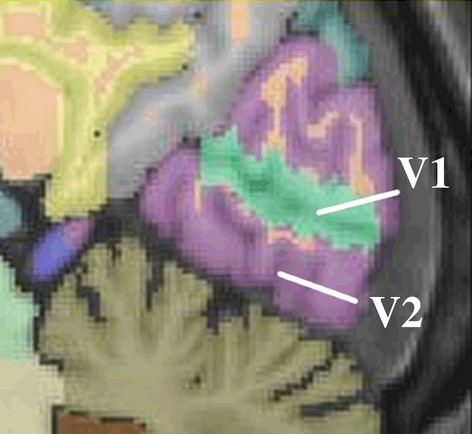
Sagittal view of V1, designating Brodmann area 17; labeled as the middle occipital gyrus on the upper and lower banks of the calcarine fissure, and V2, which encompasses Brodmann area 18 of the occipital lobe. From the ICBM (International Consortium for Brain Mapping) high-resolution single subject template used for frontal and occipital lobe gyri volume segmentation with Brain Parser 56 ROI in the LONI Pipeline Brain Parser Workflow.
These same five occipital gyri volumes obtained using Brain Parser were also selected from the FreeSurfer output aparc.a2009s (Destrieux et al. 2010). V1 and V2 gray matter volumes were also taken from the Brodmann Area output to investigate how ocular and orbital volume vary in relation to gyral-based cortical parcellation of these visual cortical areas, which are specifically designated as Brodmann areas 17 and 18 in FreeSurfer (Desikan et al. 2006; Hinds et al. 2008, 2009).
In general, V1 and V2 are among the best documented and most easily defined areas of the visual cortex in humans and non-human primates (Rosa & Tweedale, 2005). Additionally, automated, atlas-based delineation software has been shown to correspond well with other method of delineating V1 in humans (Hinds et al. 2008). Although it is not possible to use the stria of Gennari to define the striate cortex because this study uses MRIs, the atlas-based approach using LONI Brain Parser and FreeSurfer allows for delineation of these visual cortical areas across a large sample, and facilitates the examination of results in the context of previous research in this area.
As previously stated, to evaluate the importance of, and degree to which, the eye and orbit are related to visual cortical areas of the occipital lobe, a separate analysis was carried out to test the association between ocular/orbital volumes and five cerebral gyri of the frontal cortex. These include the middle frontal, superior frontal, and inferior frontal gyri, as well as the gyrus rectus and middle orbital frontal gyrus. These frontal ROIs are not directly related to vision, but can be structurally associated with upper facial morphology because of direct contact, spatial proximity, and reciprocal influence during morphogenesis. This additional analysis acts as a control of the relationship between ocular/orbital volume and occipital gyri, and helps frame the relevance of the eye and orbit in shaping adult human V1, V2, and V3 visual cortical volumes.
Results
Orbital and ocular volume
Analysis of the relationship between orbital and ocular volume reveals that these two variables are significantly correlated (t82 = 3.81, P < 0.01) (Fig.3A), although with a relatively low R2 value, where mean eye volume explains only 14.7% of the variance in mean orbital volume. The statistically significant correlations between ocular and orbital volumes observed in the above bivariate regression analyses, were found to be reduced below the level of significance at α = 0.05 when body height and LogMAR visual acuity were included in a generalized least-squares regression analysis (t80 = 1.53, P = 0.13). This change was primarily the result of including height in the analysis, which was found to be highly positively correlated with orbital volume (t125 = 8.58, P < 0.01, R2 = 0.37) and, to a lesser extent, with ocular volume (t82 = 3.08, P < 0.01, R2 = 0.10).
Figure 3.
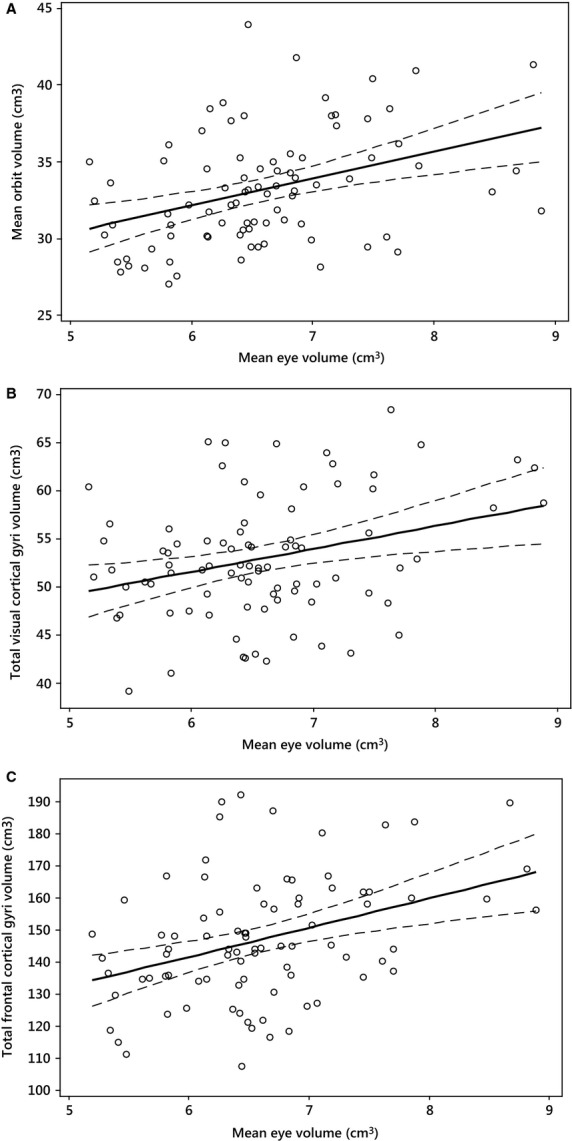
(A) Fitted line plots of mean eye volume vs. mean orbital volume, (B) mean eye volume vs. total of visual cortical gyri volumes generated using Brain Parser 56 ROI, and (C) mean eye volume vs. total of frontal cortical gyri volumes using Brain Parser 56 ROI.
Age (t78 = 4.56, P < 0.01) and sex (t78 = −2.71, P = 0.01) also contributed significantly to the least-squares regression model. Further examination of sex differences in orbital volume using a two-sample t-test, and after accounting for size differences between these two groups (orbital volume divided by body height for each individual), indicates that males possess larger orbits than females (t111 = −2.51, P = 0.01). By contrast, females had slightly larger eyes than males after accounting for body size, but this difference was not significant (t81 = 0.61, P = 0.54).
Individual regression analyses of age vs. orbital and ocular volume, show that age is significantly negatively associated with eye volume (t82 = −2.20, P = 0.031, R2 = 0.06) but positively correlated with orbital volume, although at slightly above α = 0.05 (t118 = 1.78, P = 0.078, R2 = 0.03). This indicates that orbital volume increases while eye volume decreases with advancing age, although the low R2 values in each regression indicate that age explains only a small percentage of the variance for both of these variables.
Additionally, after including age and sex in the least-squares regression analysis, the relationship between ocular and orbital volume returned to below the level of statistical significance (t78 = 2.82, P = 0.01). And together, ocular volume, body size, age, and sex explain approximately half of the variance in orbital volume (Adjusted R2 = 0.52), with age and body height contributing most to the model.
Eye, orbit, and occipital cortical volume
Bivariate regression analysis of ocular volume vs. mean middle occipital gyrus volume (V1) obtained using Brain Parser, shows that these two variables are positively significantly correlated (t82 = 2.87, P = 0.01); however, the relationship is again relatively weak, with eye volume explaining only 9% of the variance in V1 (Table1a). This result is nearly identical using the FreeSurfer generated volume, in which ocular volume explains 9.6% of the variance in middle occipital gyrus volume (Table1b). However, it can be seen that this relationship is weaker using the ROI specifically designated as V1 by FreeSurfer (t82 = 2.10, P = 0.04, R2 = 0.05).
Table 1.
Results of bivariate regression analyses of ocular and orbital volume vs. visual cortex gyri volumes obtained using (a) Brain Parser 56 ROI and (b) FreeSurfer 5.3.0-generated visual cortical gyri volumes
| Ocular volume | Orbital volume | |||||
|---|---|---|---|---|---|---|
| t | P | R 2 | t | P | R 2 | |
| (a) | ||||||
| Middle occipital gyrus – V1 | 2.87 | 0.00 | 0.09 | 4.91 | 0.00 | 0.16 |
| Superior occipital gyrus – V2/V3 | 3.38 | 0.00 | 0.12 | 5.07 | 0.00 | 0.17 |
| Inferior occipital gyrus – V2/V3 | 1.36 | 0.18 | 0.02 | 5.18 | 0.00 | 0.18 |
| Cuneus – V1-V3 | 2.76 | 0.01 | 0.08 | 4.50 | 0.00 | 0.14 |
| Lingual gyrus – V1-V3 | 2.10 | 0.04 | 0.05 | 4.41 | 0.00 | 0.13 |
| Total visual cortical gyri | 2.91 | 0.00 | 0.09 | 5.82 | 0.00 | 0.21 |
| (b) | ||||||
| Middle occipital gyrus – V1 | 2.97 | 0.00 | 0.10 | 2.91 | 0.00 | 0.06 |
| Superior occipital gyrus – V2/V3 | 2.35 | 0.02 | 0.06 | 2.33 | 0.02 | 0.04 |
| Inferior occipital gyrus – V2/V3 | 2.27 | 0.03 | 0.06 | 3.45 | 0.00 | 0.09 |
| Cuneus – V1-V3 | 1.13 | 0.26 | 0.01 | 1.97 | 0.05 | 0.03 |
| Lingual gyrus – V1-V3 | 4.22 | 0.00 | 0.18 | 3.67 | 0.00 | 0.10 |
| FreeSurfer designated V1 | 2.10 | 0.04 | 0.05 | 3.03 | 0.00 | 0.07 |
| FreeSurfer designated V2 | 2.80 | 0.01 | 0.09 | 3.33 | 0.00 | 0.08 |
| Total visual cortical gyri | 3.15 | 0.00 | 0.11 | 3.67 | 0.00 | 0.10 |
Of the visual cortical areas examined using Brain Parser, the relationship between eye volume and the total V1, V2, and V3 gyri volumes was statistically significant (t82 = 2.91, P < 0.01), with ocular volume explaining 9% of the total variance for these combined visual cortical areas (Fig.3B). This result was again consistent when ocular volume was regressed against the total of these visual cortical areas obtained using FreeSurfer (t82 = 3.15, P < 0.01, R2 = 0.11).
Orbital volume was generally more strongly associated with these same visual cortical gyri using the Brain Parser-derived ROI volumes (t125 = 5.82 P < 0.01, R2 = 0.21) compared with the above analysis of their relationship to ocular volume (Table1a). Although the FreeSurfer-generated visual cortical gyri volumes were also positively correlated with orbital volume, the relationship was found to be substantially weaker (t125 = 3.67, P < 0.01, R2 = 0.10) across the seven ROIs considered (Table1b).
Despite being correlated with ocular and orbital volume in the above bivariate analyses, in the least-squares regression analysis, stepwise regression showed that body height did not significantly contribute to the statistical models, and it was therefore not included in the analysis of ocular/orbital volume and these visual cortical areas. Vision also was not an important contributor to these models and was not included in the least-squares regression analysis. However, age and sex were again found to be important factors, and were consistently highly correlated with each response variable.
After accounting for the variance explained by sex and age, the relationship between ocular and total visual cortical gyri volumes was no longer significant using Brain Parser (t80 = 1.59, P = 0.12, R2 = 0.32) or FreeSurfer (t80 = 1.60, P = 0.11, R2 = 0.45), and only the superior occipital gyrus and lingual gyrus remained significantly correlated with ocular volume using Brain Parser and FreeSurfer ROIs, respectively (Table2a,b).
Table 2.
Least-square regression analysis of the relationship between ocular and orbital volumes vs. occipital lobe gyri volumes generated using (a) Brain Parser 56 ROI, after accounting for age and sex, and (b) FreeSurfer 5.3.0-generated visual cortical gyri volumes
| Ocular volume | Orbital volume | |||||
|---|---|---|---|---|---|---|
| t | P | Adj. R2 for model | t | P | Adj. R2 for model | |
| (a) | ||||||
| Middle occipital gyrus – V1 | 1.67 | 0.10 | 0.25 | 4.01 | 0.00 | 0.29 |
| Superior occipital gyrus – V2/V3 | 2.58 | 0.01 | 0.22 | 2.99 | 0.00 | 0.25 |
| Inferior occipital gyrus – V2/V3 | −0.19 | 0.85 | 0.28 | 4.12 | 0.00 | 0.32 |
| Cuneus – V1-V3 | 1.63 | 0.11 | 0.23 | 3.22 | 0.00 | 0.28 |
| Lingual gyrus – V1-V3 | 0.85 | 0.40 | 0.22 | 3.04 | 0.00 | 0.22 |
| Total visual cortical gyri | 1.59 | 0.12 | 0.32 | 4.42 | 0.00 | 0.37 |
| (b) | ||||||
| Middle occipital gyrus – V1 | 1.58 | 0.12 | 0.42 | 1.63 | 0.11 | 0.39 |
| Superior occipital gyrus – V2/V3 | 0.72 | 0.47 | 0.36 | 1.57 | 0.12 | 0.33 |
| Inferior occipital gyrus – V2/V3 | 0.63 | 0.53 | 0.38 | 1.84 | 0.07 | 0.38 |
| Cuneus – V1-V3 | −0.24 | 0.81 | 0.20 | 1.15 | 0.25 | 0.20 |
| Lingual gyrus – V1-V3 | 3.15 | 0.00 | 0.29 | 2.85 | 0.01 | 0.22 |
| FreeSurfer designated V1 | 0.74 | 0.46 | 0.25 | 2.25 | 0.03 | 0.24 |
| FreeSurfer designated V2/V3 | 1.26 | 0.21 | 0.39 | 2.09 | 0.04 | 0.37 |
| Total visual cortical gyri | 1.60 | 0.11 | 0.45 | 2.57 | 0.01 | 0.43 |
The relationship between orbital volume and the five Brain Parser-derived visual cortical ROIs was also slightly reduced with the addition of these variables. However, this occurred to a lesser extent than with ocular volume, as these occipital gyri, and the total of the Brain Parser-generated visual cortical gyri, remained significantly correlated with orbital volume (t116 = 4.42, P < 0.01, R2 = 0.37) (Table2a). The relationship between orbital volume and the seven FreeSurfer-derived occipital gyri volumes was diminished to a greater extent with the addition of age and sex to the model, but as a whole, it remained significantly correlated with the total of these visual cortical gyri volumes (t116 = 2.57, P = 0.01, R2 = 0.43) (Table2b).
Eye, orbit, and frontal lobe volume
Individual bivariate regression analyses using Brain Parser-derived frontal lobe ROIs indicate that ocular volume is highly correlated with these five frontal gyri volumes (t82 = 5.28, P < 0.01, R2 = 0.14), and to a greater extent than those of the visual cortex (Table3a, Fig.3C). This was also supported by stronger correlations between ocular volume and frontal gyri volumes generated by FreeSurfer, which were again consistent with the results of this analysis using Brain Parser-generated frontal gyri volumes (Table3b).
Table 3.
Results of bivariate regression analyses of ocular and orbital volume vs. frontal lobe gyri volumes obtained using (a) Brain Parser 56 ROI and (b) FreeSurfer 5.3.0-generated frontal lobe gyri volumes. Total visual cortical gyri results from the above analysis are shown for comparison
| Ocular volume | Orbital volume | |||||
|---|---|---|---|---|---|---|
| t | P | R 2 | t | P | R 2 | |
| (a) | ||||||
| Middle frontal gyrus | 3.18 | 0.00 | 0.11 | 5.80 | 0.00 | 0.21 |
| Superior frontal gyrus | 3.25 | 0.00 | 0.11 | 4.80 | 0.00 | 0.16 |
| Inferior frontal gyrus | 3.41 | 0.00 | 0.12 | 4.54 | 0.00 | 0.14 |
| Middle orbitofrontal gyrus | 3.09 | 0.00 | 0.10 | 4.64 | 0.00 | 0.15 |
| Gyrus rectus | 3.24 | 0.00 | 0.11 | 4.57 | 0.00 | 0.14 |
| Total frontal gyri | 5.28 | 0.00 | 0.14 | 5.48 | 0.00 | 0.20 |
| Total visual cortical gyri | 2.91 | 0.00 | 0.09 | 5.82 | 0.00 | 0.21 |
| (b) | ||||||
| Middle frontal gyrus | 3.66 | 0.00 | 0.14 | 2.73 | 0.01 | 0.06 |
| Superior frontal gyrus | 3.01 | 0.00 | 0.10 | 1.64 | 0.10 | 0.02 |
| Inferior frontal gyrus | 1.99 | 0.05 | 0.05 | 1.48 | 0.14 | 0.02 |
| Middle orbitofrontal gyrus | 2.73 | 0.00 | 0.08 | 1.60 | 0.11 | 0.02 |
| Gyrus rectus | 3.99 | 0.00 | 0.16 | 3.99 | 0.00 | 0.11 |
| Total frontal gyri | 3.49 | 0.00 | 0.13 | 2.69 | 0.01 | 0.06 |
| Total visual cortical gyri | 3.15 | 0.00 | 0.11 | 3.67 | 0.00 | 0.10 |
Stepwise regression analysis showed that sex and age also significantly contributed to the model across each frontal gyri analysis, and again diminished the relationship between ocular and frontal volumes (Table4a,b). However, this occurred to a much smaller extent than in the above analysis of the relationship between ocular and occipital gyri volumes; in fact, ocular volume remained significantly correlated with total frontal gyri volumes using Brain Parser (t80 = 2.46, P = 0.02, R2 = 0.40) and FreeSurfer frontal gyri volumes (t80 = 2.17, P = 0.03, R2 = 0.57) even after accounting for the variance explained by these variables. This is in contrast to the above analysis of ocular volume vs. total occipital gyri volum es, which was reduced below the level of statistical significance with the addition of these variables (Table2a,b). Additionally, the explanatory power of the model is higher for the frontal lobe (Brain Parser Adjusted R2 = 0.40, FreeSurfer Adjusted R2 = 0.57), compared with the total of the visual cortical gyri in the above analysis (Brain Parser Adjusted R2 = 0.32, FreeSurfer Adjusted R2 = 0.45).
Table 4.
Least-square regression analysis of the relationship between ocular and orbital volumes vs. frontal lobe gyri volumes generated using (a) Brain Parser 56 ROI, after accounting for age and sex, and (b) FreeSurfer 5.3.0. Total visual cortical gyri results from the above analysis are shown for comparison
| Ocular volume | Orbital volume | |||||
|---|---|---|---|---|---|---|
| t | P | Adj. R2 for model | t | P | Adj. R2 for model | |
| (a) | ||||||
| Middle frontal gyrus | 2.04 | 0.04 | 0.35 | 4.29 | 0.00 | 0.41 |
| Superior frontal gyrus | 2.05 | 0.04 | 0.34 | 3.64 | 0.00 | 0.38 |
| Inferior frontal gyrus | 2.21 | 0.03 | 0.33 | 3.34 | 0.00 | 0.36 |
| Middle orbitofrontal gyrus | 1.64 | 0.11 | 0.39 | 3.09 | 0.00 | 0.43 |
| Gyrus rectus | 2.16 | 0.03 | 0.24 | 3.11 | 0.00 | 0.27 |
| Total frontal gyri | 2.46 | 0.02 | 0.40 | 4.28 | 0.00 | 0.45 |
| Total visual cortical gyri | 1.59 | 0.12 | 0.32 | 4.42 | 0.00 | 0.37 |
| (b) | ||||||
| Middle frontal gyrus | 2.38 | 0.02 | 0.44 | 3.10 | 0.00 | 0.46 |
| Superior frontal gyrus | 1.52 | 0.13 | 0.54 | 2.01 | 0.05 | 0.54 |
| Inferior frontal gyrus | 0.57 | 0.52 | 0.27 | 1.29 | 0.20 | 0.31 |
| Middle orbitofrontal gyrus | 1.12 | 0.27 | 0.47 | 1.24 | 0.22 | 0.47 |
| Gyrus rectus | 2.76 | 0.01 | 0.35 | 2.50 | 0.01 | 0.34 |
| Total frontal gyri | 2.17 | 0.03 | 0.57 | 2.80 | 0.01 | 0.59 |
| Total visual cortical gyri | 1.60 | 0.11 | 0.45 | 2.57 | 0.01 | 0.43 |
The relationship between orbital and frontal cortical gyri volumes is generally comparable to what was observed for the occipital lobes, where the orbit is again significantly correlated with the total of these frontal gyri volumes using Brain Parser (t125 = 5.48, P < 0.00, R2 = 0.20) and to a lesser extent with the FreeSurfer ROIs (t125 = 2.69, P = 0.01, R2 = 0.06) (Table3a,b). This similarity in the association between orbital volume and both the occipital and frontal lobe volumes also holds true when age and sex are included in the analysis, where orbital volume remains significantly correlated with the total of these frontal gyri using Brain Parser (t116 = 4.28, P < 0.00, R2 = 0.45) and FreeSurfer ROIs (t116 = 2.80, P = 0.01, R2 = 0.59) (Table4a,b).
Despite a similar test statistic between orbital volume and both the total occipital and frontal gyri volumes, the explanatory power of the model is again higher for the frontal lobe (Brain Parser Adjusted R2 = 0.45, FreeSurfer Adjusted R2 = 0.59) compared with the total of the visual cortical gyri in the above analysis (Brain Parser Adjusted R2 = 0.37, FreeSurfer Adjusted R2 = 0.43), indicating that the orbit is also generally more structurally integrated with the frontal lobes than it is functionally associated with the visual cortex of the occipital lobes.
Discussion
Eye and orbit
The above test of the relationship between ocular and orbital volumes is generally in line with that reported by Pearce & Bridge (2013), where these variables were shown to be significantly correlated, but with a low R2 value of 0.17. In the current study, ocular and orbital volumes were also correlated; however, the amount of variance in orbital volume explained by ocular volume was found to be slightly lower (R2 = 0.15). Both of these values from the two separate studies are quite low and are much less than what would be expected if ocular size directly dictates orbital size in humans. The general lack of influence that eye volume has on the development of orbital volume observed in this study also corroborates previous research by Chau et al. (2004), who found that orbit volume was poorly correlated with eyeball volume (r = 0.13, P > 0.005), and Schultz (1940), who showed that ocular size is only loosely associated with orbital size across numerous human and non-human primate groups.
The low R2 value of 0.17 reported by Pearce & Bridge (2013) was argued to be artificially reduced because it was not possible to include visual acuity data and actual body size in the analysis, as brain size was used as a proxy for body size. These data were available for the current study, and whereas visual acuity was generally not found to be an important explanatory variable, body size was highly correlated with both ocular and orbital volume in bivariate analyses. However, once body size was accounted for, contrary to the predicted increase in the explanatory power of eye on orbital volume, the relationship between these variables actually fell below the level of statistical significance. This is a particularly important result, considering that Pearce & Bridge (2013) stated that the eye and orbit scale independently of overall body size. However, here it was shown that height, a proxy for overall body size, was highly correlated with orbital volume, and to a lesser extent with ocular volume.
In fact, the difference between the eye and orbit regarding how they vary in association with body size, in which orbital volume increases to a greater extent than ocular volume as overall body size increases, also corroborates the results of Schultz (1940). His comprehensive study of ocular and orbital anatomy across a large sample of primates demonstrated a negative allometric relationship between the eye and orbit with respect to body size, meaning that orbital volume increased more rapidly than eyeball volume as size increased.
This relationship also held true for humans and was observable between the sexes, where larger-bodied males had eyes that occupied a relatively small percentage of the orbit, compared with females with smaller bodies, who possessed eyes that occupied a larger proportion of the orbital cavity. This same sex dichotomy was also consistent across all primate groups sampled, where eye size, relative to both orbital and overall body size, was always greater in females than in males of the same species (Schultz, 1940).
In the current study, sex was also shown to be an important variable for understanding the relationship between ocular and orbital volume, even after accounting for body size differences between the sexes. Additionally, after controlling for body height, the relationship between these variables was reduced to below the level of statistical significance. However, after also including sex and age along with ocular volume and body size in the least-squares regression analysis, the relationship between ocular and orbital volume returned to below the level of significance. Together, these variables explained approximately 52% of the variance in orbital volume, where age and height contributed most to the model, and to a much greater extent than ocular volume as a predictor of orbital volume.
The above results, and specifically the extent to which age and sex are correlated with both ocular and orbital volume, may also have implications for understanding the development of certain forms of myopic refractive error such as astigmatism and juvenile-onset myopia. For example, the observed sex difference in orbital volume and negative allometric relationship between the eye and orbit with respect to body size are of particular interest given that the relative size of the eye within the orbit has been shown to be associated with nearsightedness in a sample of Chinese adults (Masters, 2012). An overly large eye relative to orbital size has also been implicated in a case of high-myopia, in which the enlarged eye is clearly compressed and malformed against the walls of the orbit (Palmowski-Wolfe et al. 2009).
Among the sample investigated in the current study, eye size was generally the same between males and females (though slightly larger in females); however, males possessed larger orbits, even after accounting for body size differences between the sexes. In the context of an increased rate and severity of myopia as the eye grows larger relative to orbital size (Masters, 2012; Bruner et al. 2014), this sex dichotomy, in which females possess the same and even slightly larger eyes but in a smaller orbit, may help explain why women have a higher frequency of myopia than men, develop the condition earlier in life, and retain a higher degree of refractive error throughout ontogeny by comparison (Angle & Wissmann, 1980; Grosvenor & Goss, 1990; Parssinen & Lyyra, 1993; Lam et al. 1999; Ip et al. 2007, 2008).
The opposing relationship, with regard to age changes in ocular volume (negative correlation), and orbital volume (positive correlation), may also be important for understanding deviations in the pattern of myopic development and diminishment during ontogeny and senescence, respectively. More specifically, in the current study it was shown that with advancing age, orbital volume increased as eye volume decreased. This also corroborates the results of previous research demonstrating that orbital breadth increases with age (Weaver et al. 2010), and that a prominent pattern of curve distortion and widening of the superomedial upper orbit and inferolateral lower orbit is also observable with advanced age (Pessa and Chen, 2002).
The timing of maturation within the human skull is different for the different cranial areas; in general, the braincase matures earlier, followed by the cranial base, and lastly by the facial block (Bastir et al. 2006). Maturation involves changes in both shape and size of the elements implicated. Accordingly, during ontogeny the elements maturing earlier constrain the elements maturing later, influencing their spatial parameters and limiting their possible spatial adjustments. Beyond this chain of processes, minor adjustments can involve small secondary changes as well. For example, the anterior endocranial morphology (including frontal lobe geometry) can be subtly altered by late adjustments of the orbital areas (Neubauer et al. 2009). In apes, facial morphology is much more influential than in humans because of their larger muzzle dimensions and prolonged splanchnocranial development (Mitteroecker et al. 2004). Conversely, in humans the reduction of the facial block, in terms of size, timing, and variation, leads to a decreased capacity to regulate spatial adjustments. Taking into account this mismatch between facial and braincase maturation, if eye volume decreases while orbital size continues to increase, even after ontogeny ceases, this may help explain why juvenile-onset myopia commonly begins during early adolescence, becomes stable in most individuals in their 20s and 30s, and then typically moves toward hyperopia, or farsightedness, in later life (Atchison et al. 2008).
If the eye is provided more space within the orbit as it decreases in size by approximately 0.013 cm3 per year, while the orbit increases in volume by about 0.041 cm3 per year, as indicated by the above regression analyses, it could reduce pressure applied during ontogeny to the eye and surrounding ocular tissues, and gradually allow the globe to return to a more spherical refractive state. Previous research also supports this, as mean ocular protrusion, which is also highly correlated with myopia and is hypothesized to be associated with increased axial length and corneal curvature as the eye is forced anteriorly toward the narrowing rim of the orbit (Bruner et al. 2014), has been shown to decrease by approximately 0.06 mm per year in humans (Ahmadi et al. 2007).
If the results of the current study can be interpreted as a cohort effect, where younger individuals possess larger eyes and smaller orbits compared with older individuals in the sample, it may indicate that eyes are growing to occupy a larger percentage of the orbit among younger age groups. If this is the case, because the eye may become malformed against surrounding muscles and fat within the confines of the bony orbit during growth and development, it may help explain a more recent trend toward increased rates of myopia throughout much of the industrialized world. This is also supported by previous research indicating that an increase in the rate of myopia is occurring among numerous regional industrialized societies (Tay et al. 1992; Matsumura & Hirai, 1999; Rose et al. 2001). However, because the age range among individuals in the current study sample is only 61 years (minimum = 19 years, maximum = 80 years), some caution is warranted in interpreting this as a cohort effect rather than a volumetric change in these features as age increases. Nevertheless, future research in this area could seemingly benefit from the inclusion of neighboring structurally and functionally integrated soft and hard tissue anatomic components of the eye, orbit, viscerocranium, neurocranium, and frontal and temporal lobe morphology, particularly given that the current study strengthens the notion that eye size is co-regulated genetically with brain size (Todd et al. 1940; Weale, 1982; Miller, 1992; Weiss, 2002; Mak et al. 2006).
Eye, orbit, and brain
Analysis of the relationship between eye volume and V1, V2, and V3 showed that some association exists between the eye and these components of the visual system. However, each of the correlations were weak, and were generally consistent with those observed by Pearce & Bridge (2013), who also showed that total visual cortical volume was only weakly correlated with ocular and orbital volume. Additionally, after age and sex were accounted for, the relationship between ocular volume and the total of the visual cortical gyri volumes was reduced below the level of statistical significance. These results suggest that the eye should not be considered a strong predictor of visual cortical morphology, at least when dealing with intra-specific differences among adult individuals.
The relationship between orbital and visual cortical volumes was also reduced after the inclusion of these cofactors in the regression model, and reduced below the level of significance for four of the occipital gyri volumes generated using FreeSurfer, while remaining significantly correlated with the total of these, and each of the Brain Parser-generated gyri volumes. However, orbital volume was found to be equally or more strongly correlated with frontal lobe gyri than with occipital lobe gyri involved with vision. This indicates that the orbit is only a modest predictor of visual cortical anatomy in humans, and that both the eye and orbit are generally more structurally associated with the frontal lobes than they are functionally associated with the visual cortex of the occipital lobes.
Results obtained using Brain Parser and FreeSurfer were generally consistent in each analysis investigating the anatomic relationship between eye/orbit volumes and frontal and occipital gyri volumes. However, the association between orbital volume and both the frontal and occipital gyri generated with Brain Parser were slightly stronger. This was particularly apparent in the bivariate regression analyses between orbital and frontal gyri volumes, where these relationships were less consistent with those obtained using FreeSurfer ROIs. More specifically, although orbital volume was highly correlated with each frontal gyrus volume using Brain Parser, only two were found to be significantly associated using FreeSurfer.
Inconsistencies in results obtained using these different anatomical analysis tools may be partly attributable to the fact that this study focused exclusively on frontal and occipital regions, which in a study of landmark-based and automatic cortical registration techniques had been shown to generate larger differences, compared with language-related areas, which have less variability in sulcal anatomy among subjects (Pantazis et al. 2010). This increased frontal and occipital variability likely relates to left occipital–right frontal petalia torque asymmetry, which generally increases neural mass on the right frontal lobe and left occipital lobe in right-handed individuals. This type of cerebral asymmetry is common among modern humans, hominoids, and fossil hominids (Holloway & De La Costelareymondie, 1982; Balzeau et al. 2012; Gómez-Robles et al. 2013), and can be seen on endocasts as early as 1.8 million years ago in Homo rudolfensis, specimen KNM-ER 1470 (Holloway et al. 2009).
As a cautionary methodological note, it must be remembered that P statistics, here as in any inferential approach, concerns the probability of a non-random arrangement of the data, and not the evidence of the correctness of a specific hypothesis (Nuzzo, 2014). Significant results must be interpreted as support and agreement with a specific hypothesis, and not as its verification. Morphometric analyses must be intended as exploratory tools to investigate the spatial relationships among anatomical components, and research in embryology, histology, and physiology is then necessary to evaluate the actual structural interactions between tissues and functions.
Eye and human evolution
In general, these results indicate that a relationship is observable between orbits and the frontal and occipital brain areas of modern humans in bivariate analysis, which have also been described at an intra-specific level (Pearce & Bridge, 2013). Correlations between cranial and brain components can be extremely informative in paleoneurology, allowing for indirect inference about brain structures in fossils. It is worth noting that this information is relevant independent of the causal interpretation behind the correlation itself. In this sense, an effort must be made to separate the correlations (actual and useful numerical outputs) from the biological and evolutionary interpretation of the underlying processes. Hence, independently of hypotheses put forward to interpret a specific covariation of characters, correlation analysis can reveal underlying relationships that merit attention.
These relationships with orbital morphology are not exclusive to the visual cortical areas, as our analysis showed that orbital volume is just as highly correlated with frontal lobe volumes that are not related to vision. Additionally, the explanatory value of the model when the above cofactors were included along with orbital volume, was substantially higher for the frontal lobe than for the occipital lobe. This is interesting considering that the upper face and the frontal lobes are intimately integrated in terms of morphogenesis, and because of the physical contiguity between the anterior cranial fossa, housing the prefrontal cortex, and the orbital areas (Moss & Young, 1960; Enlow, 1990; Bruner, 2015).
Beyond this structural interaction between orbits and frontal lobes, modern humans also evolved relatively larger temporal lobes (Rilling, 2006), which are located in an even more anterior position than in non-modern human species (Bastir et al. 2008), approaching the orbital areas posteriorly. Finally, human cranial ontogeny is characterized by a progressive rotation of the facial block, which further approaches the posterior face to the endocranial base as the brain grows larger (Enlow, 1990).
All of these changes (contiguity between orbits and frontal lobes, reduced facial block, and enlarged temporal lobes) involve a spatial conflict between endocranial and orbital areas during growth and development, potentially leading to a deformation of the eye bulb and vision defects among more recent modern humans (Masters, 2012; Bruner et al. 2014). Such a situation is further stressed by an opposite genetic effect, considering that the eye is genetically associated with brain growth (increasing in Homo sapiens) and the orbit is genetically associated with facial growth (decreasing in H. sapiens).
Interestingly, the constraints between the frontal and orbital areas are probably more pronounced in modern humans and Neandertals, taking into account that in most extinct human species and living apes the orbits are in front of the anterior cranial fossa, and not under it (Bruner & Manzi, 2008). It is therefore interesting that in the two human species in which the eyes are positioned below the frontal lobes, namely modern humans and Neanderthals, we also observed a lateral widening of the prefrontal cortex, which is compatible with vertical limits associated with the underlying orbital constraints (Bruner & Holloway, 2010). The fact that both the occipital and frontal areas correlate with orbit morphology, provides evidence once again that both structural and functional factors contribute to the final phenotype. Hence, beyond the quantitative correlation results, it seems reasonable that interpretations of the evolutionary and morphogenetic processes should not rely on single and isolated factors, but rather on their interactions.
It is, however, important to consider that intra-specific and inter-specific patterns and correlations can be based on very different principles and processes (Martin & Barbour, 1989). These two types of analyses are complementary, supplying information on different aspects, and most of all when considering size effects, evolutionary, ontogenetic, and static (adult) patterns of variation that can be based on distinct kinds of relationships (Cheverud, 1982). Therefore, evidence from intra-specific studies and from phylogenetic variability must be properly integrated, and not evaluated as alternatives to support or reject specific hypotheses.
Conclusions
Results of this study indicate that human ocular and orbital volumes are correlated, but that eye volume is not a major factor in determining adult volume of the orbit. Other variables, such as height, age and sex are more highly correlated with orbital volume, further indicating that size of the eye is only loosely associated with this feature. Additionally, although both ocular and orbital volumes are associated with V1, V2, and V3 visual cortical volumes, these relationships also diminish when sex and age variability are accounted for. Lastly, the relationship between the eye/orbit and cerebral gyri associated with visual function is slightly weaker in comparison with five frontal lobe gyri, indicating that important structural influences exist in the anterior skull that may be even more relevant than the functional relationships between the anterior and posterior aspects of the visual system.
It is worth noting as well that in terms of endocranial and cranial morphology, no strong patterns of integration have been observed across long anatomical distances, suggesting that local factors are categorically more important in shaping anatomical elements (Bruner & Ripani, 2008). In terms of structural relationships, local factors strictly depend on spatial proximity and maturation timing. In this study, we had two different and opposite lines of evidence in this sense. From one side, the correlation between orbits and frontal lobes specifies the importance of structural factors based on spatial contiguity. On the other hand, the lack of a strong correlation between the eye and orbit demonstrates that physical proximity does not always involve or require reciprocal influence among anatomical elements.
Consequently, the human cranium is further confirmed to be a system based on many independent factors, in which genetic, functional, and structural components must somehow integrate following distinct developmental patterns and constraints. These results are at the same time relevant to approach issues in human anatomy and evolutionary biology. Such approaches in functional craniology are essential in biomedical fields, in order to understand the actual network of variables involved in normal and pathological processes. Correlations between bone and brain features can be extremely important to put forward an ‘indirect paleoneurology’, which aids in investigating neuroanatomy in fossils by virtue of the association between hard and soft tissues.
Acknowledgments
This research was funded by Montana INBRE – National Institute of General Medical Sciences of the National Institutes of Health, award numbers 8 P20 GM103474-12 and P20GM103474-13. LONI Software Funding Citations for LONI Brain Parser – NIH-NCRR 9P41EB015922-15 and 2-P41-RR-013642-15NIH-NCRR U54 RR021813, and for the LONI Pipeline Processing Environment – NIH-NCRR 9P41EB015922-15 and 2-P41-RR-013642-15, NIH-NCRR U54 RR021813, NIH-NIMH R01 MH071940. The authors do not receive funding from any company or organization that would benefit from this article, and have no relevant affiliations or conflicts of interest.
References
- Ahmadi H, Shams PN, Davies NP, et al. Age-related changes in the normal sagittal relationship between globe and orbit. J Plast Reconstr Aesthet Surg. 2007;60:246–250. doi: 10.1016/j.bjps.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Angle J, Wissmann D. The epidemiology of myopia. Am J Epidemiol. 1980;11:220–228. doi: 10.1093/oxfordjournals.aje.a112889. [DOI] [PubMed] [Google Scholar]
- Atchison DA, Markwell EL, Kasthurirangan S, et al. Age-related changes in optical and biometric characteristics of emmetropic eyes. J Vis. 2008;8:29. doi: 10.1167/8.4.29. [DOI] [PubMed] [Google Scholar]
- Balzeau A, Gilissen E, Grimaud-Hervé D. Shared pattern of endocranial shape asymmetries among great apes, anatomically modern humans, and fossil hominins. PLoS ONE. 2012;7:e29581. doi: 10.1371/journal.pone.0029581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastir M, Rosas A, O’Higgins P. Craniofacial levels and the morphological maturation of the human skull. J Anat. 2006;209:637–654. doi: 10.1111/j.1469-7580.2006.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastir M, Rosas A, Lieberman D, et al. Middle cranial fossa anatomy and the origin of modern humans. Anat Rec. 2008;291:130–140. doi: 10.1002/ar.20636. [DOI] [PubMed] [Google Scholar]
- Bruner E. Functional craniology and brain evolution. In: Bruner E, editor. Human Paleoneurology. Switzerland: Springer International Publishing; 2015. pp. 57–94. ) [Google Scholar]
- Bruner E, Holloway RL. A bivariate approach to the widening of the frontal lobes in the genus Homo. J Hum Evol. 2010;58:138–146. doi: 10.1016/j.jhevol.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Bruner E, Manzi G. Paleoneurology of an early Neanderthal: endocranial size, shape, and features of Saccopastore 1. J Hum Evol. 2008;54:729–742. doi: 10.1016/j.jhevol.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Bruner E, Ripani M. A quantitative and descriptive approach to morphological variation of the endocranial base in modern humans. Am J Phys Anthropol. 2008;137:30–40. doi: 10.1002/ajpa.20837. [DOI] [PubMed] [Google Scholar]
- Bruner E, de la Cuétara JM, Masters M, et al. Functional craniology and brain evolution: from paleontology to biomedicine. Front Neuroanat. 2014;8:1–15. doi: 10.3389/fnana.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau A, Fung K, Pak K, et al. Is eye size related to orbit size in human subjects? Ophthalmic Physiol Opt. 2004;24:35–40. doi: 10.1046/j.1475-1313.2003.00159.x. [DOI] [PubMed] [Google Scholar]
- Cheverud JM. Relationships among ontogenetic, static, and evolutionary allometry. Am J Phys Anthropol. 1982;59:139–149. doi: 10.1002/ajpa.1330590204. [DOI] [PubMed] [Google Scholar]
- Curtin BJ. The Myopias. Basic Science and Clinical Management. Philadelphia: Harper and Row; 1985. [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, et al. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 2010;53:1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinov ID, Lozev K, Petrosyan P, et al. Neuroimaging study designs, computational analyses and data provenance using the LONI pipeline. PLoS ONE. 2010;5:e13070. doi: 10.1371/journal.pone.0013070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirani M, Chamberlain M, Shekar SN, et al. Heritability of refractive error and ocular biometrics: the Genes in Myopia (GEM) twin study. Invest Ophthalmol Vis Sci. 2006;47:4756–4761. doi: 10.1167/iovs.06-0270. [DOI] [PubMed] [Google Scholar]
- Enlow DH. Facial Growth. Philadelphia: Saunders; 1990. [Google Scholar]
- Gómez-Robles A, Hopkins WD, Sherwood CC. Increased morphological asymmetry, evolvability and plasticity in human brain evolution. Proc Biol Sci. 2013;280:20130575. doi: 10.1098/rspb.2013.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronenschild EH, Habets P, Jacobs HI, et al. The effects of FreeSurfer version, workstation type, and Macintosh operating system version on anatomical volume and cortical thickness measurements. PLoS ONE. 2012;7:e38234. doi: 10.1371/journal.pone.0038234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosvenor TP, Goss DA. Clinical Management of Myopia. Boston: Butterworth-Heinemann; 1990. [Google Scholar]
- Hinds OP, Rajendran N, Polimeni JR, et al. Accurate prediction of V1 location from cortical folds in a surface coordinate system. NeuroImage. 2008;39:1585–1599. doi: 10.1016/j.neuroimage.2007.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds O, Polimeni JR, Rajendran N, et al. Locating the functional and anatomical boundaries of human primary visual cortex. NeuroImage. 2009;46:915–922. doi: 10.1016/j.neuroimage.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. 1997;13:388–391. doi: 10.3928/1081-597X-19970701-16. [DOI] [PubMed] [Google Scholar]
- Holloway RL, De La Costelareymondie MC. Brain endocast asymmetry in pongids and hominids: some preliminary findings on the paleontology of cerebral dominance. Am J Phys Anthropol. 1982;58:101–110. doi: 10.1002/ajpa.1330580111. [DOI] [PubMed] [Google Scholar]
- Holloway RL, Sherwood CC, Hof PR. Evolution of the brain in humans – Paleoneurology. In: Binder MD, Hirokawa N, editors. Encyclopedia of Neuroscience. Berlin, Heidelberg: Springer; 2009. pp. 1326–1334. ), et al. ( [Google Scholar]
- Ip JM, Huynh SC, Kifley A, et al. Variation of the contribution from axial length and other oculometric parameters to refraction by age and ethnicity. Invest Ophthalmol Vis Sci. 2007;48:4846–4853. doi: 10.1167/iovs.07-0101. [DOI] [PubMed] [Google Scholar]
- Ip J, Huynh S, Rogaei D, et al. Ethnic differences in refraction and ocular biometry in a population-based sample of 11–15-year-old Australian children. Eye. 2008;22:649–656. doi: 10.1038/sj.eye.6702701. [DOI] [PubMed] [Google Scholar]
- Lam C, Edwards M, Millodot M, et al. A 2-year longitudinal study of myopia progression and optical component changes among Hong Kong school children. Optom Vis Sci. 1999;76:370–379. doi: 10.1097/00006324-199906000-00016. [DOI] [PubMed] [Google Scholar]
- Lam DS, Fan DS, Lam RF, et al. The effect of parental history of myopia on children’s eye size and growth: results of a longitudinal study. Invest Ophthalmol Vis Sci. 2008;49:873–876. doi: 10.1167/iovs.06-1097. [DOI] [PubMed] [Google Scholar]
- Mak M, Kwan T, Cheng K, et al. Myopia as a latent phenotype of a pleiotropic gene positively selected for facilitating neurocognitive development, and the effects of environmental factors in its expression. Med Hypotheses. 2006;66:1209–1215. doi: 10.1016/j.mehy.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Martin R, Barbour AD. Aspects of line-fitting in bivariate allometric analyses. Folia Primatol. 1989;53:65–81. doi: 10.1159/000156409. [DOI] [PubMed] [Google Scholar]
- Masters MP. Relative size of the eye and orbit: an evolutionary and craniofacial constraint model for examining the etiology and disparate incidence of juvenile-onset myopia in humans. Med Hypotheses. 2012;78:649–656. doi: 10.1016/j.mehy.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Matsumura H, Hirai H. Prevalence of myopia and refractive changes in students from 3 to 17 years of age. Surv Ophthalmol. 1999;44:S109–S115. doi: 10.1016/s0039-6257(99)00094-6. [DOI] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos Trans R Soc Lond B Biol Sci. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Lewis JE, Fong MR, et al. Global patterns of human orbit size: implications for Neandertals. Am J Phys Anthropol. 2014;153:184–185. [Google Scholar]
- Miller EM. On the correlation of myopia and intelligence. Genet Soc Gen Psychol Monogr. 1992;118:361–383. [PubMed] [Google Scholar]
- Mitteroecker P, Gunz P, Bernhard M, et al. Comparison of cranial ontogenetic trajectories among great apes and humans. J Hum Evol. 2004;46:679–697. doi: 10.1016/j.jhevol.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Moss ML, Young RW. A functional approach to craniology. Am J Phys Anthropol. 1960;18:281–292. doi: 10.1002/ajpa.1330180406. [DOI] [PubMed] [Google Scholar]
- Mutti D, Hayes J, Mitchell G, et al. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2007;48:2510–2519. doi: 10.1167/iovs.06-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer S, Gunz P, Hublin JJ. The pattern of endocranial ontogenetic shape changes in humans. J Anat. 2009;215:240–255. doi: 10.1111/j.1469-7580.2009.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuzzo R. Statistical errors. Nature. 2014;506:150–152. doi: 10.1038/506150a. [DOI] [PubMed] [Google Scholar]
- Palmowski-Wolfe AM, Kober C, Berg I, et al. Globe restriction in a severely myopic patient visualized through oculodynamic magnetic resonance imaging (od-MRI) J Pediatr Ophthalmol Strabismus. 2009;13:322–324. doi: 10.1016/j.jaapos.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Pantazis D, Joshi A, Jiang J, et al. Comparison of landmark-based and automatic methods for cortical surface registration. NeuroImage. 2010;49:2479–2493. doi: 10.1016/j.neuroimage.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parssinen O, Lyyra A. Myopia and myopic progression among schoolchildren: a three-year follow-up study. Invest Ophthalmol Vis Sci. 1993;34:2794–2802. [PubMed] [Google Scholar]
- Pearce E, Bridge H. Is orbital volume associated with eyeball and visual cortex volume in humans? Ann Hum Biol. 2013;40:531–540. doi: 10.3109/03014460.2013.815272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E, Dunbar R. Latitudinal variation in light levels drives human visual system size. Biol Lett. 2012;8:90–93. doi: 10.1098/rsbl.2011.0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E, Stringer C, Dunbar RIM. New insights into differences in brain organization between Neanderthals and anatomically modern humans. Proc Biol Sci. 2013;280:20130168. doi: 10.1098/rspb.2013.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessa JE, Chen Y. Curve analysis of the aging orbital aperture. Plast Reconstr Surg. 2002;109:751–755. doi: 10.1097/00006534-200202000-00051. [DOI] [PubMed] [Google Scholar]
- Rilling JK. Human and nonhuman primate brains: are they allometrically scaled versions of the same design? Evol Anthropol. 2006;15:65–77. [Google Scholar]
- Rosa MGP, Tweedale R. Brain maps, great and small: lessons from comparative studies of primate visual cortical organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:665–691. doi: 10.1098/rstb.2005.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K, Smith W, Morgan I, et al. The increasing prevalence of myopia: implications for Australia. J Clin Exp Ophthalmol. 2001;29:116–120. doi: 10.1046/j.1442-9071.2001.00389.x. [DOI] [PubMed] [Google Scholar]
- Schultz AH. The size of the orbit and of the eye in primates. Am J Phys Anthropol. 1940;26:389–408. [Google Scholar]
- Shattuck DW, Mirza M, Adisetiyo V, et al. Construction of a 3D probabilistic atlas of human cortical structures. NeuroImage. 2008;39:1064–1080. doi: 10.1016/j.neuroimage.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone R, Filtcroft D. Ocular shape and myopia. Ann Acad Med Singapore. 2004;33:7–15. [PubMed] [Google Scholar]
- Tay MT, Au EK, Ng CY, et al. Myopia and educational attainment in 421,116 young Singaporean males. Ann Acad Med Singapore. 1992;21:785–791. [PubMed] [Google Scholar]
- Todd T, Beecher H, Williams G, et al. The weight and growth of the human eyeball. Hum Biol. 1940;12:1–20. [Google Scholar]
- Tong F. Primary visual cortex and visual awareness. Nat Rev Neurosci. 2003;4:219–229. doi: 10.1038/nrn1055. [DOI] [PubMed] [Google Scholar]
- Traynor S, Gurtov AN, Senjem JH, et al. Assessing eye orbits as predictors of Neandertal group size. Am J Phys Anthropol. 2015;157:680–683. doi: 10.1002/ajpa.22747. [DOI] [PubMed] [Google Scholar]
- Tu Z, Narr KL, Dollár P, et al. Brain anatomical structure segmentation by hybrid discriminative/generative models. EEE Trans Med Imaging. 2008;27:495–508. doi: 10.1109/TMI.2007.908121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitzman AA, Posnick JC, Armstrong DC, et al. Craniofacial skeletal measurements based on computed tomography: part II. Normal values and growth trends. Cleft Palate Craniofac J. 1992;29:118–128. doi: 10.1597/1545-1569_1992_029_0118_csmboc_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Weale R. A Biography of the Eye: Development, Growth, Age. London: H. K. Lewis & Co; 1982. [Google Scholar]
- Weaver AA, Loftis KL, Tan JC, et al. CT based three-dimensional measurement of orbit and eye anthropometry. Invest Ophthalmol Vis Sci. 2010;51:4892–4897. doi: 10.1167/iovs.10-5503. [DOI] [PubMed] [Google Scholar]
- Weiss K. How the eye got its brain. Evol Anthropol. 2002;11:215–219. [Google Scholar]
- Wilson KT, Sivak JG, Callender MG. Induced refractive anomalies affect chick orbital bone structure. Exp Eye Res. 1997;64:675–682. doi: 10.1006/exer.1996.0236. [DOI] [PubMed] [Google Scholar]
- Working Group on Myopia Prevalence and Progression. Myopia: Prevalence and Progression. Washington, DC: National Academy Press; 1989. [Google Scholar]
- Zadnik Z, Satariano WA, Mutti DO, et al. The effect of parental history of myopia on children’s eye size. J Am Med Assoc. 1994;271:1323–1327. [PubMed] [Google Scholar]


