Abstract
Galago senegalensis is a habitual arboreal leaper that engages in rapid spinal extension during push-off. Large muscle excursions and high contraction velocities are important components of leaping, and experimental studies indicate that during leaping by G. senegalensis, peak power is facilitated by elastic storage of energy. To date, however, little is known about the functional relationship between epaxial muscle fiber architecture and locomotion in leaping primates. Here, fiber architecture of select epaxial muscles is compared between G. senegalensis (n = 4) and the slow arboreal quadruped, Nycticebus coucang (n = 4). The hypothesis is tested that G. senegalensis exhibits architectural features of the epaxial muscles that facilitate rapid and powerful spinal extension during the take-off phase of leaping. As predicted, G. senegalensis epaxial muscles have relatively longer, less pinnate fibers and higher ratios of tendon length-to-fiber length, indicating the capacity for generating relatively larger muscle excursions, higher whole-muscle contraction velocities, and a greater capacity for elastic energy storage. Thus, the relatively longer fibers and higher tendon length-to-fiber length ratios can be functionally linked to leaping performance in G. senegalensis. It is further predicted that G. senegalensis epaxial muscles have relatively smaller physiological cross-sectional areas (PCSAs) as a consequence of an architectural trade-off between fiber length (excursion) and PCSA (force). Contrary to this prediction, there are no species differences in relative PCSAs, but the smaller-bodied G. senegalensis trends towards relatively larger epaxial muscle mass. These findings suggest that relative increase in muscle mass in G. senegalensis is largely attributable to longer fibers. The relative increase in erector spinae muscle mass may facilitate sagittal flexibility during leaping. The similarity between species in relative PCSAs provides empirical support for previous work linking osteological features of the vertebral column in lorisids with axial stability and reduced muscular effort associated with slow, deliberate movements during anti-pronograde locomotion.
Keywords: elastic storage, epaxial muscle, fiber length, Galago, loris, physiological cross-sectional area, tendon
Introduction
The spine is the central element of the vertebrate skeleton, acting as a link between the head and limbs. The spine comprises bony elements that are both rigid enough to sustain compressive load and flexible enough to assist in locomotion. Variation in spinal morphology has been linked to differences in locomotor behavior in vertebrates, including primates (Keith, 1902, 1923; Schultz, 1938, 1961; Howell, 1944; Slijper, 1946; Washburn & Buettner-Janusch, 1952; Smith & Savage, 1956; Erikson, 1960, 1963; Ankel, 1965, 1972; Benton, 1967, 1974; Jenkins, 1970, 1974; Donisch, 1973; Clauser, 1975; Cartmill & Milton, 1977; English, 1980; Jungers, 1984; Hurov, 1987; Sanders, 1990, 1991; Pridmore, 1992; Shapiro, 1993, 1995, 2007; Ward, 1993; Johnson & Shapiro, 1998; Sargis, 2001; Shapiro et al. 2005; Schilling & Hackert, 2006; Granatosky et al. 2014a,b).
For example, primates with a more sagittally mobile spinal column (more dorsomobile vs. dorsostable) tend to have an elongated lumbar region, either because of a relative increase in the cranio-caudal length of the lumbar vertebral bodies, an increase in the number of lumbar vertebrae, or both (Erikson, 1963; Jungers, 1984; Shapiro, 1993, 2007; Ward, 1993; Sanders & Bodenbender, 1994; Johnson & Shapiro, 1998; Shapiro & Simons, 2002; Shapiro et al. 2005). It has been argued that enhanced flexibility in the lumbar vertebral column increases the range of sagittal plane vertebral movement (i.e. flexion–extension), which in turn results in an increase in stride and leap length (Howell, 1944; Slijper, 1946; Smith & Savage, 1956; English, 1980; Hurov, 1987; Shapiro, 1993, 1995, 2007; Sargis, 2001; Granatosky et al. 2014a). Enhanced lumbar flexibility, therefore, is advantageous for animals engaging in bounding, galloping and leaping behaviors. By contrast, features functionally linked with enhancing stability of the vertebral column have been associated with locomotor behaviors, such as vertical climbing, suspension and cantilevering/bridging (Jenkins, 1970, 1974; Cartmill & Milton, 1977; Shapiro, 1993, 1995, 2007; Ward, 1993; Johnson & Shapiro, 1998; Boyer & Bloch, 2008).
Differences in locomotor behavior between lesser galago (Galago senegalensis) and slow loris (Nycticebus coucang) are well documented. Galago senegalensis is a habitual arboreal leaper (Hall-Craggs, 1965a,b; Napier & Walker, 1967; Jenkins, 1974; Bennet-Clark, 1977; Aerts, 1998; Sargis, 2001; Off & Gebo, 2005; James et al. 2007), while N. coucang is a slow-moving arboreal quadruped that also engages in anti-pronograde suspension and cantilevering/bridging behaviors (Cartmill & Milton, 1977; Curtis, 1995; Shapiro & Demes, 1996; Shapiro et al. 2001; Nekaris & Bearder, 2011). These locomotor differences have been associated with differences in vertebral morphology between these two species. For example, compared with the slow loris and other lorisid primates, galagids exhibit features of the lumbar vertebrae that have been functionally linked to facilitating leaping, including relatively longer lumbar regions, dorsoventrally longer and more cranially oriented spinous processes resulting in longer extensor muscle lever arms, and more sagittally oriented prezygapophyses (vertebral articular processes), which promote sagittal plane motion while restricting motion in the coronal plane (Shapiro, 1993, 2007; Johnson & Shapiro, 1998; Shapiro & Simons, 2002).
By contrast, lorisids are characterized by relatively craniocaudally shorter and dorsoventrally higher lumbar vertebral bodies, relatively larger articular surface areas and more transversely oriented prezygapophyses, and dorsoventrally relatively shorter and more caudally oriented spinous processes (Shapiro, 2007). In addition, lorisids tend to demonstrate a relatively reduced lumbar region compared with galagids (Schultz & Straus, 1945; Cartmill & Milton, 1977; Granatosky et al. 2014a). Collectively, these characteristics have been argued to reduce intervertebral space, thereby passively restricting the range of vertebral column flexion and extension, and allow the erector spinae muscles to more effectively stabilize the trunk by resisting vertebral flexion (by aligning bony levers closer to the axis of extension; Jenkins, 1970; Cartmill & Milton, 1977; Gál, 1993a,b; Shapiro, 1993, 1995; Shapiro & Jungers, 1994; Curtis, 1995; Sargis, 2001; Shapiro et al. 2001; Boyer & Bloch, 2008; Granatosky et al. 2014a). The relatively greater transverse orientation of lorisid prezygapophyses is argued to be advantageous for lateral spinal movements such as bridging and climbing, for which lorisids are well known (Shapiro, 2007). An additional advantage of regulating vertebral movements by osseo-ligamentous structures such as articular surfaces is the reduction in muscular effort required to control spinal mobility, thereby reducing the overall costs of locomotion (Shapiro & Jungers, 1994). Indeed, lorisids share many of these osteological characteristics with atelines and hominoids – primates that are also characterized by enhanced thoraco-lumbar stability (Rose, 1975; Ward, 1993; Shapiro, 1995, 2007; Johnson & Shapiro, 1998; Shapiro et al. 2001, 2005; Shapiro & Simons, 2002).
The epaxial muscles are responsible for generating the spinal movements and forces associated with locomotion and trunk posture. Lorisids (N. coucang) reportedly use lateral flexion of the spine to increase hindlimb stride length. In N. coucang, lateral flexion becomes more prominent at higher velocities, resembling a pattern seen in amphibians (Shapiro et al. 2001). Epaxial muscles also play an important role during leaping by facilitating sagittal plane flexion and extension of the spine (Hall-Craggs, 1965a,b; Jenkins, 1974; Bennet-Clark, 1977; Fleagle, 1977; Aerts, 1998; Sargis, 2001; Walker, 2005; James et al. 2007). Sagittal plane flexion followed by extension allows for an increase in leap length by extending the spine from its flexure at the beginning of the push-off phase (Sargis, 2001). The combined movements of flexion and extension may also store (during flexion) and then release (during extension) strain energy in the tendons and aponeuroses. In the only kinetic study to date of leaping performance in G. senegalensis, Aerts (1998) used in vivo kinetic data and inverse dynamics to calculate power during push-off. His results indicate that elastic energy is stored in the vastus muscle–tendon unit during the crouching phase of leaping and is rapidly released to amplify power during knee extension at the tail end of push-off. Aerts (1998) also estimates comparatively modest power output by the back.
Studies of primate epaxial muscles have focused primarily on gross anatomical descriptions (Mivart, 1865; Howell & Straus, 1933; Donisch, 1973; Kumakura & Inokuchi, 1992; Curtis, 1995; Kumakura et al. 1996), muscle weights (Fleagle, 1977; Grand, 1977), metabolic profiles (Bagnall et al. 1983; Ford et al. 1986; Kojima & Okada, 1996; Neufuss et al. 2014) and muscle activity patterns (Shapiro & Jungers, 1988, 1994). Muscle weights, in particular, have been used by some investigators to draw inferences about muscle function and locomotor behavior (Haxton, 1947; Fleagle, 1977). However, muscle weights tend to be inconsistently applied among workers (Stern, 1971), and the relationship between muscle mass and a muscle’s maximum excursion or force-generating capacity is not straightforward (Gans, 1982; Powell et al. 1984; Lieber, 2010). Despite the potential importance of epaxial muscle fiber architecture for facilitating movements and forces associated with locomotion, to date there have been no studies of epaxial muscle fiber architecture in any primate.
In this study, we evaluated epaxial muscle fiber architecture in G. senegalensis (lesser galago), whose habitual mode of locomotion is vertical clinging-and-leaping (Napier & Walker, 1967; Gebo, 1987; Off & Gebo, 2005). We compared Galago senegalensis with N. coucang (slow loris), a cautious arboreal quadruped that engages in bridging, cantilevering and anti-pronograde suspension (Walker, 1969; Dykyj, 1980; Demes et al. 1990; Oxnard et al. 1990; Sellers, 1996; Fleagle, 2013; Shapiro et al. 2001; Nekaris & Bearder, 2011). We examined fiber architecture of the iliocostalis, longissimus and multifidus muscles was examined because experimental studies in quadrupedal primates and non-primate mammals have demonstrated that these muscles play an important role in spinal extension (Carlson et al. 1979; English, 1980; Shapiro & Jungers, 1988, 1994; Ritter et al. 2001; Schilling, 2009; Schilling & Carrier, 2009, 2010).
Muscle fiber architecture and function
Fiber architecture refers to the internal arrangement of muscle fibers in relation to the muscle’s force-generating axis (Gans & Bock, 1965; Gans, 1982). Different architectural configurations can have profound effects on the contractile ability and function of a whole muscle (Gans & Bock, 1965; Gans, 1982; Anapol & Jungers, 1986; Gans & de Vree, 1987; Anapol & Herring, 1989; Taylor & Vinyard, 2009). For example, muscle fibers may be arranged either in parallel with, or at an angle to (i.e. pinnate), the force-generating axis of the muscle (Gans & Bock, 1965; Gans, 1982). For a given muscle volume, parallel-fibered muscles will have longer fibers and therefore tend to facilitate large whole-muscle excursions and contraction velocities (Gans, 1982). All sarcomeres in a fiber are of similar length and are believed to contract more or less over the same distance simultaneously. Thus, the more sarcomeres in series, the greater the distance over which a whole muscle can shorten or lengthen. Fiber length is therefore proportional to maximum muscle excursion (Gans, 1982; Lieber, 2010). The velocity of whole-muscle contraction is a function of excursion over time. As a result, contraction velocity is also proportional to fiber length (Bodine et al. 1982).
For two muscles of comparable volume, fibers angled relative to the muscle’s force-generating axis (pinnate fibers) tend to be shorter than parallel fibers (Gans, 1982). Because of the angular deviation from the force-generating axis, pinnation leads to some loss of force along the axis of the tendon of attachment (Gans, 1982). However, the geometry of pinnate-fibered muscles allows for more fibers to be packed adjacent to each other. Maximum isometric muscle force is governed by the number and diameter of fibers aligned in parallel to each other. Muscle physiological cross-sectional area (PCSA) represents the cross-sectional areas of all of the fibers within a muscle, accounting for pinnation angle, and is therefore proportional to a muscle’s maximum force-generating capacity (Gans & Bock, 1965; Gans, 1982; Powell et al. 1984). Theoretically (Gans & Bock, 1965; Gans, 1982) and empirically (Taylor et al. 2009), there is an architectural trade-off between maximizing muscle excursion vs. muscle force, when muscle mass is held constant.
Tendons and elastic energy storage
Elastic tissues such as tendons and aponeuroses are arranged in series with the contractile element of a muscle. Tendons serve a variety of functional requirements (Hildebrand, 1974). For example, tendons concentrate the forces of large muscles (or multiple synergistic muscles with different actions) into a relatively small area of attachment. Because tendons are compact compared with large muscle bellies, tendons permit forces to be transmitted across joints without sacrificing joint mobility. For example, the long digital flexor tendons allow the small size of the manual digits to be maintained with adequate strength by positioning their effecting muscles proximally in the forearm. Tendons also permit forces to be transmitted around ‘corners’, such as when tendons pass through ligamentous pulleys (retinacula) or bony protrusions (e.g. the sustenaculum tali).
During locomotion, elastic storage and release of energy in the tendon can contribute importantly to work and power (Alexander & Bennet-Clark, 1977; Bennet-Clark, 1977; Cavagna & Kaneko, 1977; Cavagna et al. 1980; Biewener et al. 1981; Biewener, 1998; Roberts & Azizi, 2010; Richards & Sawicki, 2012; Higham & Irschick, 2013), and to preventing damage to the contractile elements (Roberts & Azizi, 2010; Konow et al. 2011; Roberts & Konow, 2013). Experimental data indicate that in a manner similar to tendons, aponeuroses are capable of storing and releasing elastic energy (Alexander et al. 1985; Aerts, 1998; Azizi et al. 2009).
It has been suggested that small animals may take advantage of energy storage in tendons to generate large amounts of instantaneous power, which is critical in determining leaping distance (Bennet-Clark, 1977; Aerts, 1998; James et al. 2007). Energy storage may also provide a substantial reduction in metabolic work (Cavagna et al. 1964; Alexander & Vernon, 1975; Alexander & Bennet-Clark, 1977).
Hypotheses to be tested
We address three hypotheses relating the functional consequences of epaxial muscle fiber architecture to spinal movements and forces during locomotor behaviors in G. senegalensis and N. coucang.
Hypothesis 1: Relatively long fibers result in greater maximum whole-muscle excursion and contraction velocity. Relatively long tendons increase elastic strain storage. Together, these features are potentially important during leaping because they increase the range of sagittal plane vertebral movement. Elastic strain storage may provide power and reduce metabolic work during the push-off phase of leaping. Hypothesis 1 thus predicts that the leaper, G. senegalensis, will display relatively longer, less pinnate fibers and higher tendon length-to-fiber length ratios compared with N. coucang.
Hypothesis 2: Muscles comprised of shorter, more pinnate fibers tend to have larger PCSAs, given that PCSA is inversely proportional to fiber length (Gans & Bock, 1965; Gans, 1982). Thus, Hypothesis 2 predicts that G. senegalensis will have relatively smaller PCSAs compared with N. coucang. Larger PCSAs facilitate the generation of larger maximum muscle forces. There are little behavioral data to predict that N. coucang generates relatively greater muscle forces compared with G. senegalensis. Thus, a finding of relatively larger PCSAs in N. coucang would initially be interpreted as an architectural trade-off of their relatively shorter, more pinnate fibers compared with G. senegalensis.
Hypothesis 3: If G. senegalensis experiences an architectural trade-off between maximizing whole-muscle excursion and contraction velocity vs. muscle force, this trade-off can only be achieved by maintaining muscles of comparable volume to N. coucang. Therefore, Hypothesis 3 predicts that G. senegalensis and N. coucang will not differ in epaxial muscle weights.
Materials and methods
Samples
We took architectural measurements of the iliocostalis, longissimus and multifidus muscles and linear measurements of the vertebral column on captive adult specimens of G. senegalensis (four males) and N. coucang (one female and three males; Table1; 1).1 Sample sizes were limited by the rarity of these specimens. Adult status was determined by the eruption of the mandibular third molar (Fleagle, 2013; Ankel-Simons, 2007). All cadavers were previously fixed and stored in either alcohol or formalin.
Table 1.
Measurements included in the study
| Measurement | Abbreviation | Definition |
|---|---|---|
| Thoraco-lumbar spine length (mm) | TLS | The maximum distance between the tips of the spinous processes of T1 and the last lumbar vertebra |
| Proximal tendon length (mm) | TP | Measured as the maximum distance between the proximal tendon attachment to bone and the proximal tendon attachment to the myotendinous junction (Fig.1) |
| Distal tendon length (mm) | TD | Measured as the maximum distance between the distal tendon attachment to bone and the distal tendon attachment to the myotendinous junction (Fig.1) |
| Total tendon length | TL | Σ (TP + TD) |
| Tendon-to-fiber length ratio | – | TL/(NLf + TL) |
| Muscle belly length (mm) | Lb | Measured as the maximum length along the long axis of each muscle segment |
| Muscle weight (g) | MWt | Wet weights of each thoracic and lumbar segment of iliocostalis, longissimus and multifidus |
| Normalized fiber length (mm) | NLf | Average linear distance between the proximal and distal myotendinous junctions of a fasciculus, normalized by standard sarcomere length |
| Surface pinnation angle (°) | θ | Measured as the surface angle of a fiber relative to the muscle’s line of action, divided by NLf (Fig.2) |
| Physiological cross-sectional area (cm2) | PCSA | Muscle mass × cos (pinnation angle)/NLf × 1.0564 (gm/cm3) |
| Potential maximum whole-muscle excursion | h | NLf [cos θ – √(cos2 θ + n2 – 1)] |
Data collection
The skin and extrinsic back muscles overlying the left epaxial muscles were reflected, and the iliocostalis, longissimus and multifidus muscles were identified. Thoraco-lumbar spine length was measured as the maximum distance between the tips of the spinous processes of T1 and the last lumbar vertebra with a measuring tape accurate to the nearest 0.1 cm. Prior to carefully removing the muscles from their bony attachments, proximal (TP) and distal (TD) tendon lengths were measured in situ from their bony attachments to their proximal and distal myotendinous junctions, respectively (Fig.1). Proximal and distal tendon lengths were measured for a minimum of 10 fibers. The thoracic and lumbar segments of the left epaxial muscles then were dissected free from their bony attachments, and trimmed of excess fat, fascia and connective tissues. Thoracic and lumbar segments in these species were differentiated based on their caudal attachments, i.e. a slip of multifidus that was caudally attached to a thoracic vertebra was considered thoracic multifidus while a caudal attachment to a lumbar vertebra was considered lumbar multifidus. Whole-muscle belly length (Lb) was measured as the maximum length along the long axis of each muscle segment with digital calipers accurate to the nearest 0.01 mm (Anapol et al. 2004). Muscles then were blotted dry, and individual weights of each muscle segment (MWt) were measured to the nearest 0.0001 g (Table1).
Figure 1.
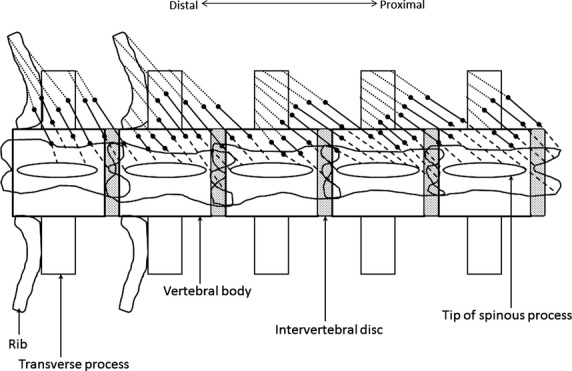
Schematic of tendon length measurements. Muscle fiber lengths (Lf) are represented by the solid black lines. The dashed lines represent proximal tendon lengths (TP), measured as the length of tendon from its proximal bony attachment to the proximal end of Lf (proximal myotendinous junction). The dotted lines represent distal tendon lengths (TD), measured as the length of tendon from its distal bony attachment to the distal end of Lf (distal myotendinous junction).
To estimate fiber length (Lf), each muscle segment was immersed in 30% nitric acid solution for chemical digestion (Loeb & Gans, 1986). When muscles were properly digested such that small fiber bundles (i.e. fasciculi) could be teased apart without breakage, they were placed under a dissecting microscope (either an Olympus-5000 or a Nikon SM-1500) for manual dissection. Fibers were mounted on slides and a minimum of 10 fibers was measured for each muscle segment using digital calipers accurate to the nearest 0.01 mm. Only straight fibers with rounded/squared ends were measured. Following fiber length measurements, slides were cover-slipped and air-dried.
Surface pinnation angle (θ′) was measured directly with a protractor as the angle the muscle fibers made relative to the muscle’s line of action (Fig.2). Unlike more complex muscles (e.g. masseter), the epaxial muscles do not have superficial and deep compartments. Because they are thin, surface pinnation provides a reasonable estimate for comparative purposes between species (Powell et al. 1984; Organ et al. 2009; Lieber, 2010; Mathewson et al. 2014). Depending on muscle size and fiber orientation, pinnation angle measurements were taken at one or more sites (e.g. cranial, middle and caudal) of the same muscle segment and the mean was used for data analysis.
Figure 2.
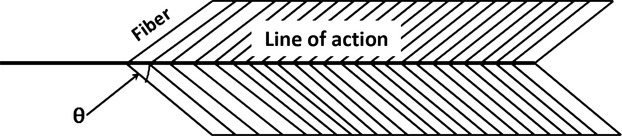
Schematic depicting measurement of surface pinnation angle on a bipinnate muscle. Pinnation angle is measured as the angle formed between the muscle fiber and the muscle’s line of action (modified from Organ et al. 2009).
Specimens used in this study were fixed with their trunks in a variety of postures such that some epaxial muscles were either shortened or stretched relative to their presumed resting lengths. To adjust for this postural variation in fiber length, raw fiber length (Lf) was normalized to a standardized sarcomere length (Anapol & Barry, 1996; Felder et al. 2005). Sarcomere length (Ls) measurements were obtained from the mounted fibers using laser diffraction (to the nearest 0.01 μm; Lieber et al. 1984; Felder et al. 2005; Taylor & Vinyard, 2009). Values for Lf normalized for Ls were calculated using the following equation:
where NLf is normalized fiber length, Lf is raw fiber length, Ls is measured sarcomere length in vitro, and 2.41 μm is the empirically determined optimal sarcomere length in Macaca mulatta limb muscles (Walker & Schrodt, 1974).
Normalized fiber length was used to standardize surface pinnation angle (θ) using the following equation (Anapol & Barry, 1996):
where θ = normalized pinnation angle, and θ′ = measured mean surface pinnation angle.
Physiological cross-sectional area was computed using the following equation (Haxton, 1944; Schumacher, 1961; Lieber, 2010):
where 1.0564 g cm−3 is the specific density of the mammalian skeletal muscle (Murphy & Beardsley, 1974).
We estimated potential maximum whole-muscle excursion (h) (Benninghoff & Rollhäuser, 1952; Anapol & Barry, 1996) using the following equation: h (mm) = NLf [cos θ – √(cos2 θ + n2 – 1)], where n (coefficient of contraction) is estimated to be 0.769 (Gans & Bock, 1965; Muhl, 1982). This variable provides an estimate of whole-muscle maximum velocity (which is proportional to excursion/time).
Total tendon length (TL) for each muscle was computed as the sum of TP and TD and used to compute the ratio of TL/(TL + NLf). A higher ratio indicates a greater capacity for elastic storage involving tendons (Anapol & Barry, 1996).
All measurements were collected from muscle segments between the T1 and last lumbar vertebra, thereby excluding any tail musculature in the analysis. All architectural measurements were taken separately on the thoracic and lumbar segments of each muscle. The data from both segments then were averaged for each muscle and the average used for data analysis.
Data analysis
We used two-tailed Mann–Whitney U-tests to evaluate significant differences between G. senegalensis and N. coucang in thoraco-lumbar spine (TLS) length and absolute architectural measurements. To examine relative differences between species in architectural variables, we created dimensionless shape ratios. To assess relative differences in muscle weights, we divided MWt by body mass. To evaluate relative differences in fiber length and whole-muscle excursion, we divided NLf by TLS and h by normalized muscle belly length (NLb), reconstructed from whole-muscle length (Lb) at excision (Muhl, 1982; Anapol & Gray, 2003): NLb ≈ Lb + [1.2987 (NLf – Lf)]. We generated an estimate of weighted species mean body mass (Table2) using previously published body mass data (Smith & Jungers, 1997), and divided PCSA0.5 by body mass0.33 and by TLS length. We used one-tailed Mann–Whitney U-tests to address the hypothesis that G. senegalensis exhibits significantly relatively longer, less pinnate fibers, relatively higher maximum whole-muscle excursion/contraction velocity (h), and relatively greater tendon length-to-fiber length ratios compared with those of N. coucang. Similarly, the one-tailed Mann–Whitney U-test was used to address the hypothesis that G. senegalensis has epaxial muscles with significantly relatively smaller muscle PCSAs compared with N. coucang. We used two-tailed Mann–Whitney U-tests to address the hypothesis that G. senegalensis and N. coucang do not differ in relative muscle weights. We set an a priori α = 0.05 and employed the sequential Bonferroni adjustment to minimize the potential for Type I error (Rice, 1989; Sokal & Rohlf, 1995). Small sample sizes for each species limited the statistical power and thus the findings are regarded as preliminary. However, the rarity of these strepsirrhines and the limited data on epaxial muscle morphology make these fiber architecture data important for contributing to understanding of muscle mechanics during locomotion. Thus, in addition to reporting statistical results, we also describe trends in the data. All statistical analyses were performed using ms-excel 2010 (Microsoft) and spss v.16 (IBM-SPSS) software.
Table 2.
Body mass data, vertebral number, means (± SD) and tests of absolute differences in thoraco-lumbar spine length and muscle architectural variables.*,†,‡
| Measurement | Galago senegalensis | Nycticebus coucang | Two-tailed P-value |
|---|---|---|---|
| Mean (± SD) | Mean (± SD) | ||
| Body mass (g) | |||
| Males (n) | 315 (8) | 679 (56) | – |
| 227 (80) | 1100 (4) | ||
| Females (n) | 250 (9) | 626 (44) | – |
| 199 (67) | 1020 (2) | ||
| Weighted mean body mass (g) | |||
| Males | 235 | 707 | – |
| Females | 205 | 643 | – |
| Vertebral number (range) | |||
| Thoracic | 11–12 | 13–15 | – |
| Lumbar | 8–9 | 9 | – |
| TLS length (mm) | 100.53 (9.78) | 157.7 (5.97) | 0.021 |
| Iliocostalis | |||
| MWt (g) | 0.774 (0.422) | 0.899 (0.692) | 1.000 |
| NLb (mm) | 79.37 (8.89) | 106.30 (6.72) | 0.021 |
| NLf (mm) | 31.60 (5.75) | 18.31 (3.05) | 0.021 |
| h | 7.32 (1.34) | 4.27 (0.71) | 0.021 |
| TL (mm) | 62.55 (5.71) | 14.81 (1.57) | 0.029 |
| PCSA (cm2) | 0.246 (0.15) | 0.505 (0.48) | 0.375 |
| Longissimus | |||
| MWt (g) | 0.980 (0.352) | 2.147 (1.459) | 0.343 |
| NLb (mm) | 102.16 (9.40) | 145.38 (5.15) | 0.021 |
| NLf (mm) | 35.85 (3.49) | 18.04 (3.27) | 0.021 |
| h | 8.33 (0.83) | 4.22 (0.76) | 0.021 |
| TL (mm) | 74.83 (4.32) | 14.59 (2.02) | 0.029 |
| PCSA (cm2) | 0.262 (0.11) | 1.26 (1.08) | 0.029 |
| Multifidus | |||
| MWt (g) | 0.118 (0.048) | 0.298 (0.225) | 0.343 |
| NLb (mm) | 38.05 (2.14) | 60.83 (4.40) | 0.021 |
| NLf (mm) | 20.99 (1.43) | 9.71 (2.35) | 0.021 |
| h | 4.86 (0.34) | 2.26 (0.55) | 0.021 |
| TL (mm) | 22.65 (1.44) | 8.08 (1.99) | 0.029 |
| PCSA (cm2) | 0.053 (0.02) | 0.302 (0.23) | 0.149 |
TLS length, thoraco-lumbar spine length; MWt, muscle weight; NLb, normalized muscle belly length; NLf, normalized fiber length; h, maximum whole-muscle potential excursion; TL, tendon length; PCSA, physiological cross-sectional area.
Body mass data are from Smith & Jungers (1997: 539–540). The published data and sample sizes were used to compute a weighted mean body mass for the all-male sample of G. senegalensis (n = 4) and for the mixed-sex sample of N. coucang (n = 1 female, n = 3 males).
Lumbar vertebrae were defined by zygapophyseal orientation.
Results based on two-tailed Mann–Whitney U-tests. The sequential Bonferroni adjustment was applied separately for each muscle (α = 0.05/6) and no differences were significant following the sequential Bonferroni correction.
Results
Gross morphology of the muscles
In both lesser galago and slow loris, iliocostalis attaches caudally to the iliac crest and thoracolumbar aponeurosis, and cranially to ribs by tendons (Figs3 and 4). In lesser galago, longissimus attaches to the thoracolumbar aponeurosis, ribs and transverse processes of thoracic vertebrae, and to the transverse and accessory processes of lumbar vertebrae (Fig.3). The more caudally located fibers of longissimus then converge on a tendon over the sacrum; this tendon proceeds to the tail (Fig.3). In the slow loris, longissimus attaches caudally to lumbar transverse processes and to the thoracolumbar aponeurosis, and cranially to ribs and thoracic transverse processes. The more cranial thoracic portion of longissimus (at T1–T3/4 vertebral levels) does not have any costal attachment in the slow loris (Fig.4). In lesser galago, the thoracolumbar regions of multifidus attach caudally to a vertebral transverse process by a muscular slip spanning two–four segments, and attach by a short tendon to the spinous process of a more cranially located vertebra (Fig.5). The cranial and caudal attachments of thoracolumbar multifidus are similar for the slow loris, with the exception that slips of their multifidus span four–five vertebral segments (Figs5 and 6).
Figure 3.
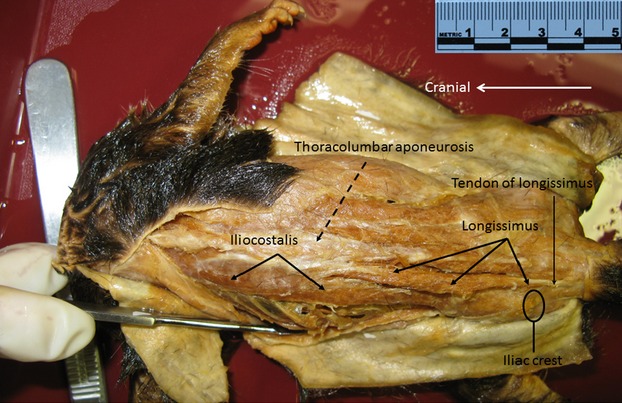
Photograph of G. senegalensis iliocostalis and longissimus muscles in situ. The fibers of longissimus are seen to be converging on a tendon. This tendon continues caudally to the tail.
Figure 4.
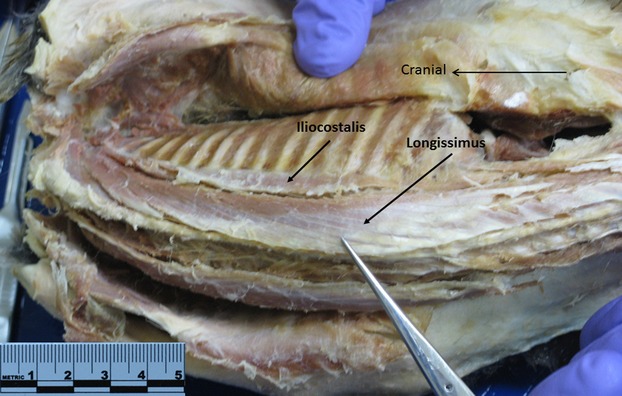
Nycticebus coucang iliocostalis and longissimus in situ.
Figure 5.
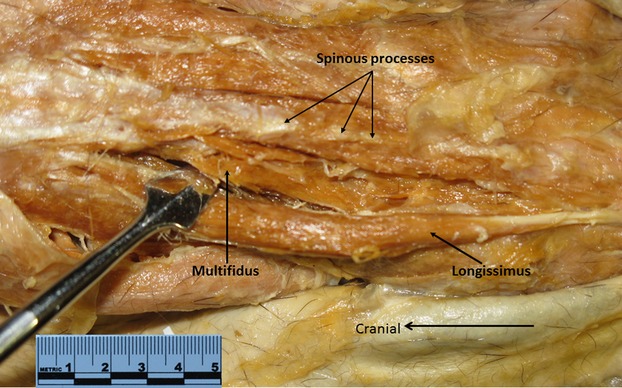
Photograph of G. senegalensis multifidus in situ (black arrow; tip of probe is separating longissimus from multifidus).
Figure 6.
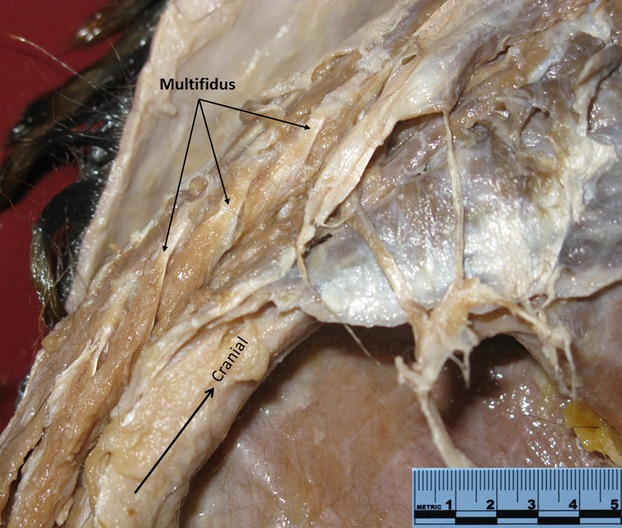
Slips of multifidus of N. coucang in situ (three black arrows).
Absolute comparisons
Depending on body mass estimates, N. coucang males are between two and five times larger than male G. senegalensis; female N. coucang are between three and five times larger than female G. senegalensis (Table2). The weighted species mean body mass estimate similarly shows male N. coucang are three times larger compared with G. senegalensis males. Some absolute bony and muscle architectural variables track these differences in body mass (Table2). For example, the larger-bodied N. coucang has significantly longer TLS and, on average, longer NLb, larger MWt and larger PCSAs, though these fiber architecture differences are not significant (P > 0.05) following Bonferroni adjustment. The greater number of vertebral bodies (most consistently observed for the thoracic region) likely accounts, in part, for the significantly greater TLS length in N. coucang. Notably, the smaller-bodied G. senegalensis has, on average, absolutely longer NLf, greater muscle excursions (h) and longer TL, though these differences are not significant following Bonferroni adjustment.
Hypotheses and predictions
Hypothesis 1: We predicted that compared with N. coucang, the leaper G. senegalensis would have architectural features of the epaxial muscles that facilitate the generation of relatively greater muscle excursion, higher contraction velocity, and have the capacity for greater elastic storage. As predicted, G. senegalensis display epaxial muscles with significantly relatively longer fibers, relatively greater potential whole-muscle excursion (h/NLb), and relatively higher ratios of TL/(TL + NLf) compared with N. coucang (Table3; Figs7, 8 and 9). Galago senegalensis has epaxial muscles that are less pinnate compared with those of N. coucang (Table3; Fig.10). These differences are significant for iliocostalis, and trend towards significance for longissimus and multifidus.
Hypothesis 2: We predicted that compared with N. coucang, G. senegalensis would display epaxial muscles with relatively smaller PCSAs. However, while G. senegalensis longissimus and multifidus PCSAs are, on average, relatively smaller, neither of these differences is significant (Table3; Fig.1).2
Hypothesis 3: We predicted that G. senegalensis and N. coucang would not differ in relative epaxial muscle weights. This prediction was borne out by the current data. Galago senegalensis has, on average, larger muscle weights relative to body mass compared with N. coucang, but none of these differences is significant (Table3).
Table 3.
Means, standard deviations (SD) and tests of relative differences in muscle architectural variables between Galago senegalensis and Nycticebus coucang.*,†
| Measurement | Prediction | Galago senegalensis | Nycticebus coucang | Differs as predicted? | P-value |
|---|---|---|---|---|---|
| Mean (± SD) | Mean (± SD) | ||||
| Iliocostalis | |||||
| NLf/TLS length | G.s. > N.c. | 0.318 (0.076) | 0.116 (0.021) | Yes | 0.0105 |
| h/NLb | G.s. > N.c. | 0.093 (0.023) | 0.041 (0.008) | Yes | 0.0105 |
| TL/(TL + NLf) | G.s. > N.c. | 0.666 (0.023) | 0.449 (0.016) | Yes | 0.0105 |
| Pinnation angle (θ) | G.s. < N.c. | 3.66 (1.10) | 7.01 (0.8) | Yes | 0.0105 |
| PCSA0.5/body mass0.33 | G.s. < N.c. | 0.078 (0.03) | 0.077 (0.04) | No | 0.5000 |
| MWt/body mass | G.s. = N.c. | 0.0033 (0.002) | 0.0013 (0.001) | Yes | 0.1489 |
| Longissimus | |||||
| NLf/TLS length | G.s. > N.c. | 0.358 (0.031) | 0.115 (0.025) | Yes | 0.0105 |
| h/NLb | G.s. > N.c. | 0.082 (0.006) | 0.029 (0.006) | Yes | 0.0105 |
| TL/(TL + NLf) | G.s. > N.c. | 0.676 (0.015) | 0.449 (0.017) | Yes | 0.0105 |
| Pinnation angle (θ) | G.s. < N.c. | 5.33 (1.00) | 7.89 (2.25) | No | 0.0416 |
| PCSA0.5/body mass0.33 | G.s. < N.c. | 0.083 (0.017) | 0.122 (0.06) | No | 0.1241 |
| MWt/body mass | G.s. = N.c. | 0.0042 (0.002) | 0.0032 (0.002) | Yes | 0.5637 |
| Multifidus | |||||
| NLf/TLS length | G.s. > N.c. | 0.211 (0.033) | 0.062 (0.016) | Yes | 0.0105 |
| h/NLb | G.s. > N.c. | 0.128 (0.005) | 0.037 (0.009) | Yes | 0.0105 |
| TL/(TL + NLf) | G.s. > N.c. | 0.519 (0.023) | 0.453 (0.01) | Yes | 0.0105 |
| Pinnation angle (θ) | G.s. < N.c. | 3.78 (1.20) | 4.96 (0.75) | No | 0.0745 |
| PCSA0.5/body mass0.33 | G.s. < N.c. | 0.037 (0.009) | 0.058 (0.030) | No | 0.1241 |
| MWt/body mass | G.s. = N.c. | 0.0005 (0.001) | 0.0004 (0.001) | Yes | 0.7728 |
body mass, sex-specific weighted mean body mass; h, maximum whole-muscle potential excursion; MWt, muscle weight; NLb, normalized muscle belly length; NLf, normalized fiber length; PCSA, physiological cross-sectional area; TL, tendon length; TLS length, thoraco-lumbar spine length.
Results based on one-tailed Mann–Whitney U-tests with the exception of MWt/body mass, which is based on a two-tailed Mann–Whitney U-test. Bold P-values indicate a significant difference (α = 0.05) following the sequential Bonferroni correction. The sequential Bonferroni correction was applied for each hypothesis.
Results for PCSA0.5/TLS length were the same as for PCSA0.5/body mass0.33 and are thus not reported.
Figure 7.
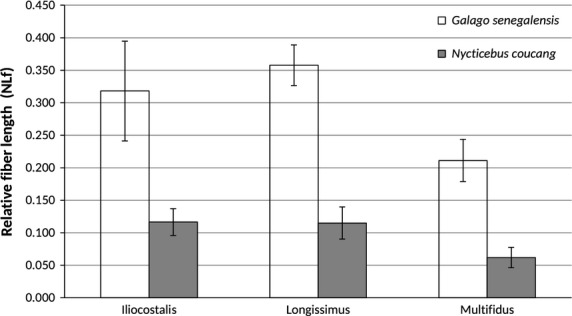
Bar graph of relative epaxial muscle fiber length (mean ± SD). In all cases, G. senegalensis has significantly relatively longer muscle fibers compared with N. coucang.
Figure 8.
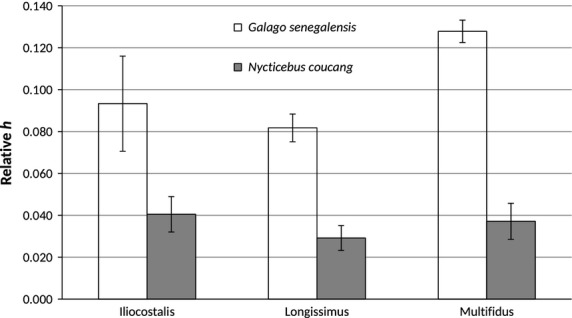
Bar graph of relative potential whole-muscle excursion (h/NLb) (mean ± SD). As predicted, G. senegalensis has significantly greater h/Lb (P = 0.0105) compared with N. coucang.
Figure 9.
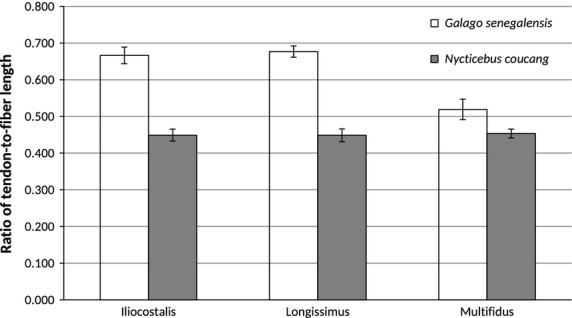
Bar graph of tendon length-to-fiber length ratios (mean ± SD). As predicted, G. senegalensis has significantly higher ratios compared with N. coucang, indicating enhanced capacity for elastic energy storage.
Figure 10.
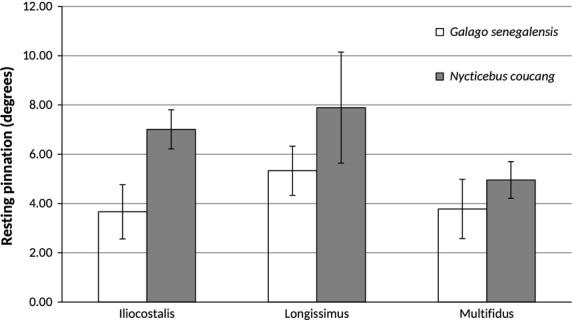
Bar graph of resting surface pinnation (mean ± SD). Nycticebus coucang epaxial muscles have, on average, larger pinnation angles, significantly so for iliocostalis (P = 0.0105) and trending towards significant for longissimus (P = 0.0416).
Figure 11.
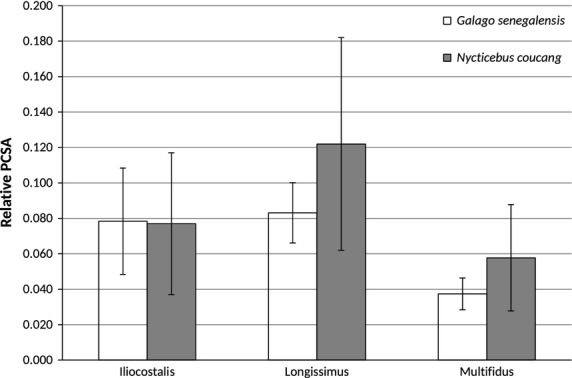
Bar graph of relative epaxial muscle physiological cross-sectional areas (PCSAs; mean ± SD). Contrary to predictions, G. senegalensis does not have relatively smaller PCSAs compared with N. coucang. Note, however, the high standard deviations for N. coucang, particularly for longissimus PCSA.
Discussion
Comparative assessment of epaxial fiber architecture in lesser galago and slow loris
Results from Hypothesis 1 indicate that G. senegalensis has relatively longer fibers, relatively higher maximum whole-muscle excursions, and relatively higher tendon length-to-fiber length ratios compared with N. coucang. Thus, relatively longer fibers and higher ratios of tendon length-to-fiber length should be included in the complex of vertebral column features in G. senegalensis that differentiate G. senegalensis from N. coucang. Galago senegalensis also has iliocostalis (and likely longissimus) fibers that are significantly less pinnate compared with N. coucang. However, contrary to our prediction for Hypothesis 2, G. senegalensis and N. coucang have similar relative epaxial muscle PCSAs. Thus, G. senegalensis does not display an architectural trade-off between relative NLf and relative PCSA. As predicted by Hypothesis 3, G. senegalensis and N. coucang do not differ in relative muscle weights. Given that the smaller-bodied G. senegalensis has, on average, relatively smaller muscle PCSAs but relatively larger muscle weights, it appears that G. senegalensis has added muscle mass by lengthening its muscle fibers (Table3), while N. coucang has increased muscle pinnation (Table2). By increasing relative muscle fiber length without significantly decreasing relative PCSA, G. senegalensis is effectively capable of generating relatively greater maximum epaxial muscle excursions, higher contraction velocities, and storing and releasing elastic strain energy, without compromising relative maximum force production.
There is little published work on epaxial muscle fiber architecture in strepsirrhine primates specifically, or in primates more generally, limiting our ability to evaluate and compare these findings for G. senegalensis with other vertical clingers and leapers. Early comparative work on the bony vertebral column in ricochetal and generalized quadrupedal rodents (Hatt, 1932) led to the suggestion that morphological differences in vertebral transverse and spinous processes were reflective of larger epaxial muscles in both ricochetal rodents and primate leapers (e.g. Saimiri; Johnson & Shapiro, 1998). Fleagle (1977) did observe that the leaper Presbytis melalophos exhibits significantly larger lumbar (but not thoracic) trunk dry muscle mass relative to fresh body mass compared with the quadrupedal P. obscura.
While no significant differences between species in epaxial muscle weights relative to body mass (Table3) or TLS length (P > 0.05) were observed, it is notable that the smaller-bodied galago has, on average, relatively larger muscle weights compared with the loris. When we generated a combined estimate of muscle weight, we further confirmed that G. senegalensis has, on average, larger epaxial muscle mass relative to body mass (G. senegalensis
 = 0.0080 vs. N. coucang
= 0.0080 vs. N. coucang
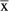 = 0.0050), but significantly larger epaxial muscle mass relative to TLS length (G. senegalensis
= 0.0050), but significantly larger epaxial muscle mass relative to TLS length (G. senegalensis
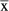 = 0.1223 vs. N. coucang
= 0.1223 vs. N. coucang
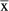 = 0.0903; Mann–Whitney U-test statistic = 16.00; P = 0.0209; Chi-square approximation = 5.333). It has been theoretically argued (Johnson & Shapiro, 1998) that an increased erector spinae muscle mass should serve to facilitate sagittal flexibility required by leaping. The current findings can thus be interpreted as providing some preliminary evidence in support of this hypothesis.
= 0.0903; Mann–Whitney U-test statistic = 16.00; P = 0.0209; Chi-square approximation = 5.333). It has been theoretically argued (Johnson & Shapiro, 1998) that an increased erector spinae muscle mass should serve to facilitate sagittal flexibility required by leaping. The current findings can thus be interpreted as providing some preliminary evidence in support of this hypothesis.
We note that the standard deviations for N. coucang muscle weights (and PCSAs) are high, both relative to mean values and in comparison to those of G. senegalensis (Table3). In particular, one specimen of N. coucang has markedly larger muscle masses in comparison to the other three individuals in the sample (1). Because muscle mass increases to the 0.33 power of linear dimensions, within-species variability in muscle mass contributes disproportionately to variation in estimates of muscle PCSA. Notable within-species variation in muscle mass has been observed in previous studies (Taylor & Vinyard, 2013; Terhune et al. 2015), and may be attributed to a variety of factors, including differences in sample preservation, individual differences in size and age, and muscle plasticity. This within-species variation, coupled with the current small sample sizes and limited statistical power, emphasize the need for continued evaluation of epaxial muscle fiber architecture in habitual leapers. Additionally, more work is needed to better understand the influence of factors such as age and health status on studies of locomotor mechanics in both living and fossil taxa.
While there are relatively little empirical data to inform predictions regarding epaxial muscle fiber architecture in the slow loris and performance during antipronograde suspension, bridging or cantilevering, lorisids do have morphological features of the vertebral column that suggest a reduction in the muscular effort required to control spinal mobility (Shapiro, 2007). For example, compared with galagids, the relatively reduced spinous processes and attendant shorter lever arms for the epaxial muscles have been functionally linked to the frequent use by lorisids of anti-pronograde suspension, during which the trunk is in a flexed position and active back extension does not appear to be involved (Walker, 1974; Jouffroy, 1989; Jouffroy & Petter, 1990). Lorisids also have a relatively reduced lumbar region (Schultz & Straus, 1945; Cartmill & Milton, 1977; Shapiro, 2007; Granatosky et al. 2014a), relatively wider vertebral laminae and more transversely oriented prezygapophyses (Shapiro, 2007), all of which have been argued to play an important role in governing vertebral column movements and promoting lumbar stability. Based on gross anatomical description, slow loris also displays a markedly smaller spinalis muscle, and has longissimus and semispinalis muscles in the thoracic region and at the thoraco-lumbar junction that are much better differentiated from each other compared with the leaper Saimiri (Curtis, 1995). These gross morphological differences have been functionally linked to the importance of unilateral contraction of longissimus to facilitate lateral flexion of the spine (vs. contraction of semispinalis for rotation) during bridging and cantilevering. We can speculate that the more pinnate-fibered epaxial muscles in the slow loris are linked to their elongated thoracic region and may function to promote lateral flexion (vs. sagittal plane flexion/extension). Thus, compared with lesser galago, our current findings of relatively smaller muscle masses and comparable PCSAs in slow loris support previous work, suggesting adequate axial stability is achieved through osseous (and perhaps ligamentous) structures, and that powerful back extension is not required for slow, cautious movements observed in antipronograde species (Granatosky et al. 2014a).
Functional implications of a relatively longer muscle–tendon complex in G. senegalensis
By increasing whole-muscle excursion and contraction velocity, we speculate that the relatively longer fibers in G. senegalensis are functionally linked to the rapid spinal extension and acceleration that is an important component of leaping (Bennet-Clark, 1977). Previous research (Shapiro & Simons, 2002, Shapiro, 2007) has shown that G. senegalensis has relatively craniocaudally longer lumbar vertebral bodies, sagittally oriented lumbar zygapophyses that resist rotation, and improved leverage for spinal extension compared with slow loris. These features have been functionally linked to facilitating spinal extension and to enhanced propulsive effort during leaping (Erikson, 1963). We suggest that relative increases in epaxial fiber length can be added to the suite of vertebral column features in G. senegalensis that may be functionally linked to their biological role of leaping (Bock & Von Wahlert, 1965).
Galago senegalensis does not exhibit the predicted architectural trade-off between relative NLf and relative PCSA as they have increased relative NLf primarily by decreasing pinnation but without reducing their epaxial muscle mass. By increasing relative NLf without markedly decreasing relative PCSA, lesser galago is capable of increasing relative muscle excursion and contraction velocity without necessarily compromising epaxial muscle force. A similar lack of an architectural trade-off in fiber architecture has been observed in the tail musculature in prehensile-tailed monkeys (Organ et al. 2009), as well as the jaw-closing muscles of Cebus apella (Taylor & Vinyard, 2009) and male Macaca fascicularis (Terhune et al. 2015). Collectively, these results suggest that the benefits of adding (or maintaining) muscle mass outweigh the metabolic costs. As muscle performance is influenced by additional mechanical and physiological factors (e.g. fiber type), future work should be aimed at integrating fiber architecture with other components of the locomotor system.
The higher tendon length-to-fiber length ratios in G. senegalensis suggest that the galago back has enhanced capacity to store and release elastic strain energy. There are several potential benefits to increased elastic energy savings during leaping. First, elastic energy storage may provide a necessary component of the power required during the propulsive phase of the leap by releasing energy rapidly through elastic recoil at push-off (Alexander & Bennet-Clark, 1977; Biewener et al. 1981; Alexander, 1988). Second, elastic storage may decrease metabolic work by reducing muscle work during take-off (Alexander & Bennet-Clark, 1977). This is because only the contractile elements (i.e. the actin and myosin filaments) consume metabolic energy by hydrolyzing ATP during activation. Thus, a greater tendon length-to-fiber length ratio reduces the expense of contraction without compromising tension (Hill, 1938; Wilkie, 1968; Paul, 1983; Anapol et al. 2004). A third potential benefit of elastic energy storage might occur during the landing phase of the leap if the tendons act as mechanical buffers by rapidly absorbing energy and releasing it more slowly, thereby limiting power input to lengthening muscle fibers and protecting fibers from the damaging effects of eccentric contractions (Roberts & Azizi, 2010; Konow & Roberts, 2015).
High peak power requirements for jumping by G. senegalensis have been theoretically supported (Bennet-Clark, 1977) and empirically demonstrated (Günther, 1985; Aerts, 1998). In vivo experimental data indicate that the push-off stance of the lesser galago is a crouch, where the spine is in flexion (Hall-Craggs, 1965a; Aerts, 1998). While Aerts’s (1998) analysis suggested the majority of power derives from the vasti muscle–tendon complex, the current results are consistent with his finding of power output in the back during push-off and suggest that elastic storage in the epaxial muscles of G. senegalensis may reduce muscle work and/or provide power to improve leaping performance. Future work should be aimed at experimentally testing precisely when and how elastic storage energy in the epaxial muscles is released during leaping in G. senegalensis and other primates.
Limitations of the study
The most obvious limitations of this work are small sample sizes and the restriction of the comparison to only two species (Garland & Adolph, 1994). Given the rarity of these strepsirrhines and the lack of data on epaxial fiber architecture in any primate, we view the data and results as important both for preliminary testing of current functional hypotheses as well as for raising future hypotheses and predictions that can be tested on additional primate species. The absence of data on body mass and absolute age of individual specimens is also a potential limitation. Age-related loss of skeletal muscle mass, or sarcopenia, and a corresponding decrease in muscle force has been observed in mammals, including humans (Lowe et al. 2001; Faulkner et al. 2007, 2008). While the current specimens were dentally adult, some of the observed variation in muscle mass and PCSA may be attributable to differential age at death and opportunistic sampling. Importantly, age-related muscle loss is unlikely to account for the observed differences in fiber length and tendon length-to-fiber length ratios (Burkholder, 2001).
Conclusion
We investigated the functional relationship between epaxial muscle fiber architecture and locomotion in the leaping primate G. senegalensis. Compared with N. coucang, a slow arboreal quadruped, G. senegalensis epaxial muscles display relatively longer, less pinnate fibers and higher ratios of tendon length-to-fiber length. These architectural features indicate that G. senegalensis epaxial muscles have the capacity to generate relatively larger muscle excursions and greater contraction velocities, and have greater elastic storage capacity. We hypothesize that these architectural features facilitate spinal extension and enhanced propulsive effort during leaping. Additional comparative data on epaxial musculature are needed to better understand the functional roles of these muscles during various locomotor and positional behaviors.
Acknowledgments
Galago senegalensis specimens were provided courtesy of Dr William Jungers (Dept. of Anatomical Sciences, Stony Brook University, Stony Brook, NY, USA); specimens of N. coucang were provided courtesy of Dr Jandy Hanna (West Virginia School of Osteopathic Medicine, Lewisburg. WV, USA), the Duke Lemur Center (Durham, NC, USA) and the US National Museum of Natural History (Washington, DC, USA). The authors thank Sarah Zehr and Erin Ehmke (Duke Lemur Center, Durham, NC, USA), and Linda Gordon (US National Museum of Natural History, Washington, DC, USA) for providing access to cadavers. Drs John Fleagle, Jack Stern, Jr and William Jungers provided valuable comments on the original research and earlier versions of this manuscript. Two anonymous reviewers provided very helpful comments and suggestions that have substantially improved the quality of this work. The research was supported by the Stony Brook University, the L.S B. Leakey Foundation, the Turkana Basin Institute, the National Institutes of Health (R24 HD050837-01 to Andrea B. Taylor) and the National Science Foundation (BCS-0452160 to Andrea B. Taylor and BCS-0094522 to Christine E. Wall). This is Duke Lemur Center Publication no. 1293.
Appendix A1
Architectural measurements for each specimen*
| Specimen | Sex | TLS length (mm) | Iliocostalis | Longissimus | Multifidus | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MWt (g) | NLb (mm) | NLf (mm) | Pinnation Angle (θ) | PCSA (cm2) | TL (mm) | MWt (g) | NLb (mm) | NLf (mm) | Pinnation Angle (θ) | PCSA (cm2) | TL (mm) | MWt (g) | NLb (mm) | NLf (mm) | Pinnation Angle (θ) | PCSA (cm2) | TL (mm) | |||
| G. senegalensis | M | 89.1 | 0.2938 | 69.07 | 37.59 | 3.36 | 0.0739 | 67.91 | 1.283 | 91.89 | 35.20 | 4.53 | 0.3440 | 72.67 | 0.0488 | 37.47 | 21.87 | 5.36 | 0.0210 | 22.94 |
| W. Jungers collection | ||||||||||||||||||||
| G. senegalensis | M | 112 | 1.1786 | 90.55 | 34.71 | 4.79 | 0.3203 | 64.54 | 0.7065 | 113.43 | 40.96 | 6.69 | 0.1622 | 79.10 | 0.122 | 35.28 | 18.85 | 2.73 | 0.0612 | 23.39 |
| W. Jungers collection | ||||||||||||||||||||
| G. senegalensis | M | 97 | 0.55 | 80.64 | 29.51 | 4.23 | 0.1759 | 63.24 | 0.6438 | 97.66 | 33.98 | 5.47 | 0.1785 | 77.71 | 0.1516 | 39.55 | 21.65 | 4.07 | 0.0661 | 20.55 |
| W. Jungers collection | ||||||||||||||||||||
| G. senegalensis | M | 104 | 1.0728 | 77.21 | 24.57 | 2.27 | 0.4129 | 54.51 | 1.285 | 105.68 | 33.28 | 4.63 | 0.3643 | 69.85 | 0.1496 | 39.90 | 21.61 | 2.96 | 0.0655 | 23.73 |
| W. Jungers collection | ||||||||||||||||||||
| N. coucang | F | 151.8 | 0.3341 | 99.07 | 18.44 | 7.5 | 0.1700 | 15.15 | 0.8666 | 140.35 | 20.05 | 8.16 | 0.4050 | 16.00 | 0.2326 | 60.37 | 10.13 | 5.92 | 0.2163 | 8.80 |
| NMNH 297828 | ||||||||||||||||||||
| N. coucang | M | 165 | 1.8444 | 112.77 | 14.36 | 7.12 | 1.2063 | 12.76 | 3.9425 | 152.41 | 13.79 | 6.3 | 2.6896 | 12.43 | 0.3832 | 62.60 | 6.62 | 4.18 | 0.5465 | 5.23 |
| NMNH 502559 | ||||||||||||||||||||
| N. coucang | M | 154 | 0.4326 | 111.22 | 18.63 | 5.85 | 0.2186 | 14.75 | 1.0568 | 145.57 | 21.09 | 6.13 | 0.4716 | 16.58 | 0.0238 | 54.99 | 9.79 | 4.61 | 0.0229 | 8.43 |
| J. Hanna collection | ||||||||||||||||||||
| N. coucang | M | 160 | 0.9856 | 102.16 | 21.79 | 7.56 | 0.4244 | 16.57 | 2.7221 | 143.20 | 17.22 | 10.98 | 1.4692 | 13.33 | 0.552 | 65.37 | 12.32 | 5.11 | 0.4225 | 9.85 |
| DLC 1906 m | ||||||||||||||||||||
NMNH, National Museum of Natural History, Washington, DC; DLC, Duke Lemur Center.
See Table1 for variable definitions.
Notes
Age-at-death was available for only one male N. coucang specimen (22 years old).
Results are similar when muscle weights0.333 and PCSAs0.5 are divided by TLS length.
Author contributions
E.H. conceived the original project. E.H., C.E.W. and A.B.T. were involved in data collection and analysis. E.H. wrote the initial draft. E.H., C.E.W. and A.B.T. interpreted the data, and contributed to the writing and editing of the manuscript.
References
- Aerts P. Vertical jumping in Galago senegalensis: the quest for an obligate mechanical power amplifier. Phil Trans R Soc Lond B. 1998;353:1607–1620. [Google Scholar]
- Alexander RM. Elastic Mechanisms in Animal Movement. Cambridge: Cambridge University Press; 1988. [Google Scholar]
- Alexander RM, Bennet-Clark HC. Storage of elastic strain energy in muscle and other tissue. Nature. 1977;265:114–117. doi: 10.1038/265114a0. [DOI] [PubMed] [Google Scholar]
- Alexander RM, Vernon A. The mechanics of hopping by kangaroos (Macropodidae) J Zool. 1975;177:265–303. [Google Scholar]
- Alexander RM, Dimery NJ, Ker RF. Elastic structures in the back and their role in galloping in some mammals. J Zool. 1985;207:467–482. [Google Scholar]
- Anapol FC, Barry K. Fiber architecture of the extensors of the hindlimb in semiterrestrial and arboreal guenons. Am J Phys Anthropol. 1996;99:429–447. doi: 10.1002/(SICI)1096-8644(199603)99:3<429::AID-AJPA5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Anapol FC, Gray JP. Fiber architecture of the intrinsic muscles of the shoulder and arm in semiterrestrial and arboreal guenons. Am J Phys Anthropol. 2003;122:51–65. doi: 10.1002/ajpa.10269. [DOI] [PubMed] [Google Scholar]
- Anapol FC, Herring SW. Length-tension relationships of masseter and digastric muscles of miniature swine during ontogeny. J Exp Biol. 1989;143:1–16. doi: 10.1242/jeb.143.1.1. [DOI] [PubMed] [Google Scholar]
- Anapol FC, Jungers WL. Architectural and histochemical diversity within the quadriceps femoris of the brown lemur (Lemur fulvus. Am J Phys Anthropol. 1986;69:355–375. doi: 10.1002/ajpa.1330690308. [DOI] [PubMed] [Google Scholar]
- Anapol F, Shahnoor N, Gray JP. Fiber architecture, muscle function, and behavior: gluteal and hamstring muscles of semiterrestrial and arboreal guenons. In: Anapol F, German RZ, Jablonski NG, editors. Shaping Primate Evolution. Cambridge: Cambridge University Press; 2004. pp. 99–133. [Google Scholar]
- Ankel F. Der Canalis sacralis als Indikator für die Länge der Caudalregion der Primaten. Folia Primatol. 1965;3:263–276. doi: 10.1159/000155038. [DOI] [PubMed] [Google Scholar]
- Ankel F. Vertebral morphology of fossil and extant primates. In: Tuttle RH, editor. The Functional and Evolutionary Biology of Primates. Chicago: Aldine-Atherton; 1972. pp. 223–240. [Google Scholar]
- Ankel-Simons F. Primate Anatomy. 3rd edn. Burlington: Elsevier, Inc; 2007. [Google Scholar]
- Azizi E, Halenda GM, Roberts TJ. Mechanical properties of the gastrocnemius aponeurosis in wild turkeys. Integr Comp Biol. 2009;49:51–58. doi: 10.1093/icb/icp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnall KM, Ford DM, McFadden KD, et al. A comparison of vertebral muscle fiber characteristics between human and monkey tissue. Acta Anat. 1983;117:51–57. doi: 10.1159/000145770. [DOI] [PubMed] [Google Scholar]
- Bennet-Clark HC. Scale effects in jumping animals. In: Pedley T, editor. Scale Effects in Animal Locomotion. London: Academic Press; 1977. pp. 185–201. [Google Scholar]
- Benninghoff A, Rollhäuser H. Zur inneren Mechanik des gefiederten Muskels. Pflügers Arch ges Physiol. 1952;254:527–548. doi: 10.1007/BF00362785. [DOI] [PubMed] [Google Scholar]
- Benton RS. Morphological evidence for adaptations within the epaxial region of the primates. In: Vagtborg H, editor. The Baboon in Medical Research. Vol. 2. Austin: University of Texas Press; 1967. pp. 201–216. ). In:, Vol. [Google Scholar]
- Benton RS. Structural patterns in the pongidae and cercopithecidae. Yearb Phys Anthropol. 1974;18:65–88. [Google Scholar]
- Biewener A. Muscle function in vivo: a comparison of muscles used for elastic energy savings versus muscles used to generate mechanical power. Am Zool. 1998;38:703–717. [Google Scholar]
- Biewener A, Alexander McN, Heglund NC. Elastic energy storage in the hopping of kangaroo rats (Dipodomys spectabilis. J Zool. 1981;195:369–383. [Google Scholar]
- Bock WJ, Von Wahlert G. Adaptation and the form-function complex. Evolution. 1965;19:269–299. [Google Scholar]
- Bodine SC, Roy RR, Meadows DA, et al. Architectural, histochemical, and contractile characteristics of a unique biarticular muscle: the cat semitendinosus. J Neurophysiol. 1982;48:192–201. doi: 10.1152/jn.1982.48.1.192. [DOI] [PubMed] [Google Scholar]
- Boyer DM, Bloch JI. Evaluating the mitten-gliding hypothesis for Paromomyidae and Micromomyidae (Mammalia, “Plesiadapiformes”) using comparative functional morphology of new Paleogene skeletons. In: Sargis EJ, Dagosto MJ, editors. Mammalian Evolutionary Morphology. A Tribute to Frederick S. Szalay. New York: Springer; 2008. pp. 233–284. [Google Scholar]
- Burkholder TJ. Age does not influence muscle fiber length adaptation to increased excursion. J Appl Physiol. 2001;91:2466–2470. doi: 10.1152/jappl.2001.91.6.2466. [DOI] [PubMed] [Google Scholar]
- Carlson H, Halbertsma J, Zomlefer M. Control of the trunk during walking in the cat. Acta Physiol Scand. 1979;105:251–253. doi: 10.1111/j.1748-1716.1979.tb06338.x. [DOI] [PubMed] [Google Scholar]
- Cartmill M, Milton K. The lorisiform wrist joint and the evolution of “brachiating” adaptations in the Hominoidea. Am J Phys Anthropol. 1977;47:249–272. doi: 10.1002/ajpa.1330470206. [DOI] [PubMed] [Google Scholar]
- Cavagna GA, Kaneko M. Mechanical work and efficiency in level walking and running. J Physiol. 1977;268:467–481. doi: 10.1113/jphysiol.1977.sp011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavagna GA, Saibene FP, Margaria R. Mechanical work in running. J Appl Physiol. 1964;19:249–256. doi: 10.1152/jappl.1964.19.2.249. [DOI] [PubMed] [Google Scholar]
- Cavagna GA, Citterio G, Jacini P. Elastic storage: role of tendons and muscles. In: Schmidt-Nielsen K, Bolis L, Taylor CR, editors. Comparative Physiology: Primitive Mammals. Cambridge: Cambridge University Press; 1980. pp. 231–242. [Google Scholar]
- Clauser DA. The numbers of vertebrae in three African cercopithecine species. Folia Primatol. 1975;23:308–319. doi: 10.1159/000155679. [DOI] [PubMed] [Google Scholar]
- Curtis DJ. Functional anatomy of the trunk musculature in the slow loris (Nycticebus coucang. Am J Phys Anthropol. 1995;97:367–379. doi: 10.1002/ajpa.1330970404. [DOI] [PubMed] [Google Scholar]
- Demes B, Jungers WL, Nieschalk U. Size- and speed-related aspects of quadrupedal walking in slender and slow lorises. In: Jouffroy FK, Stack MH, Niemitz C, editors. Gravity, Posture and Locomotion in Primates. Florence: Il Sedicesimo; 1990. pp. 175–197. [Google Scholar]
- Donisch E. A comparative study of the back muscles of gibbon and man. In: Rumbaugh DM, editor. Gibbon and Siamang. Vol. 2. Basel: Karger; 1973. pp. 96–120. : Anatomy, Dentition, Taxonomy, Molecular Evolution and Behavior. (ed. ). In:, Vol. [Google Scholar]
- Dykyj D. Locomotion of a slow loris in a designed substrate context. Am J Phys Anthropol. 1980;52:577–586. [Google Scholar]
- English AW. The functions of the lumbar spine during stepping in cat. J Morphol. 1980;165:55–66. doi: 10.1002/jmor.1051650106. [DOI] [PubMed] [Google Scholar]
- Erikson GE. The vertebral column of New World primates. Anat Rec. 1960;138:346–347. [Google Scholar]
- Erikson GE. Brachiation in New World monkeys and in anthropoid apes. Symp Zool Soc Lond. 1963;10:135–164. [Google Scholar]
- Faulkner JA, Larkin LM, Claflin DR, et al. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol. 2007;34:1091–1096. doi: 10.1111/j.1440-1681.2007.04752.x. [DOI] [PubMed] [Google Scholar]
- Faulkner JA, Davis CS, Mendias CL, et al. The aging of elite male athletes: age-related changes in performance and skeletal muscle structure and function. Clin J Sport Med. 2008;18:501–507. doi: 10.1097/JSM.0b013e3181845f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder A, Ward SR, Lieber RL. Sarcomere length measurement permits high resolution normalization of muscle fiber length in architectural studies. J Exp Biol. 2005;208:3275–3279. doi: 10.1242/jeb.01763. [DOI] [PubMed] [Google Scholar]
- Fleagle JG. Locomotor behavior and muscular anatomy of sympatric Malaysian leaf-monkeys (Presbytis obscura and Presbytis melalophos. Am J Phys Anthropol. 1977;46:297–308. doi: 10.1002/ajpa.1330460211. [DOI] [PubMed] [Google Scholar]
- Fleagle JG. Primate Adaptation and Evolution. 3rd edn. San Diego, CA: Academic Press; 2013. [Google Scholar]
- Ford DM, Bagnall KM, McFadden KD, et al. A comparison of muscle fiber characteristics at different levels of the vertebral column in the rhesus monkey. Acta Anat. 1986;126:163–166. doi: 10.1159/000146208. [DOI] [PubMed] [Google Scholar]
- Gál JM. Mammalian spinal biomechanics. I. Static and dynamic mechanical properties of intact intervertebral joints. J Exp Biol. 1993a;174:247–280. doi: 10.1242/jeb.174.1.247. [DOI] [PubMed] [Google Scholar]
- Gál JM. Mammalian spinal biomechanics. II. Intervertebral lesion experiments and mechanisms of bending resistance. J Exp Biol. 1993b;174:281–297. doi: 10.1242/jeb.174.1.281. [DOI] [PubMed] [Google Scholar]
- Gans C. Fiber architecture and muscle function. Exerc Sport Sci Rev. 1982;10:160–207. [PubMed] [Google Scholar]
- Gans C, Bock WJ. The functional significance of muscle architecture – a theoretical analysis. Ergeb Anat Entwicklungsgesch. 1965;38:115–142. [PubMed] [Google Scholar]
- Gans C, de Vree F. Functional bases of fiber length and angulation in muscle. J Morphol. 1987;192:63–85. doi: 10.1002/jmor.1051920106. [DOI] [PubMed] [Google Scholar]
- Garland T, Adolph SC. Why not to do two-species comparative studies: limitations on inferring adaptation. Physiol Zool. 1994;67:797–828. [Google Scholar]
- Gebo DL. Locomotor diversity in prosimian primates. Am J Primatol. 1987;13:271–281. doi: 10.1002/ajp.1350130305. [DOI] [PubMed] [Google Scholar]
- Granatosky MC, Lemelin P, Chester SGB, et al. Functional and evolutionary aspects of axial stability in euarchontans and other mammals. J Morphol. 2014a;275:313–327. doi: 10.1002/jmor.20216. [DOI] [PubMed] [Google Scholar]
- Granatosky MC, Miller CE, Boyer DM, et al. Lumbar vertebral morphology of flying, gliding, and suspensory mammals: Implications for the locomotor behavior of the subfossil lemurs Palaeopropithecus and Babakotia. J Hum Evol. 2014b;75:40–52. doi: 10.1016/j.jhevol.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Grand TI. Body weight: its relation to tissue composition, segment distribution, and motor function. I. Interspecific comparisons. Am J Phys Anthropol. 1977;47:211–240. doi: 10.1002/ajpa.1330470204. [DOI] [PubMed] [Google Scholar]
- Günther MM. Biomechanische Voraussetzungen beim Absprung des Senegalgalagos. Z Morph Anthrop. 1985;75:287–306. [PubMed] [Google Scholar]
- Hall-Craggs ECB. An analysis of the jump of the Lesser Galago (Galago senegalensis. J Zool. 1965a;147:20–29. [Google Scholar]
- Hall-Craggs ECB. An osteometric study of the hind limb of the Galagidae. J Anat. 1965b;99:119–126. [PMC free article] [PubMed] [Google Scholar]
- Hatt RT. The vertebral column of richochetal rodents. Bull Am Mus Nat Hist. 1932;63:599–738. [Google Scholar]
- Haxton HA. Absolute muscle force in the ankle flexors of man. J Physiol. 1944;103:267–273. doi: 10.1113/jphysiol.1944.sp004075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxton HA. Muscles of the pelvic limb: a study of the differences between bipeds and quadrupeds. Anat Rec. 1947;98:337–346. doi: 10.1002/ar.1090980304. [DOI] [PubMed] [Google Scholar]
- Higham TE, Irschick DJ. Springs, steroids, and slingshots: the roles of enhancers and constraints in animal movement. J Comp Physiol B. 2013;183:583–595. doi: 10.1007/s00360-012-0734-z. [DOI] [PubMed] [Google Scholar]
- Hildebrand M. Analysis of Vertebrate Structure. New York: John Wiley and Sons; 1974. [Google Scholar]
- Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond B Biol Sci. 1938;126:136–195. doi: 10.1098/rspb.1949.0019. [DOI] [PubMed] [Google Scholar]
- Howell AB. Speed in Animals: Their Specializations for Running and Leaping. Chicago: University of Chicago Press; 1944. [Google Scholar]
- Howell AB, Straus WL. The muscular system. In: Hartman CG, Straus WL, editors. The Anatomy of the Rhesus Monkey. New York: Hafner; 1933. pp. 89–175. [Google Scholar]
- Hurov JR. Terrestrial locomotion and back anatomy in vervets (Cercopithecus aethiops) and patas monkeys (Erythrocebus patas. Am J Primatol. 1987;13:297–311. doi: 10.1002/ajp.1350130307. [DOI] [PubMed] [Google Scholar]
- James RS, Navas CA, Herrel A. How important are skeletal muscle mechanics in setting limits on jumping performance? J Exp Biol. 2007;210:923–933. doi: 10.1242/jeb.02731. [DOI] [PubMed] [Google Scholar]
- Jenkins FA. Anatomy and function of expanded ribs in certain edentates and primates. J Mammal. 1970;51:288–301. [PubMed] [Google Scholar]
- Jenkins FA. Tree shrew locomotion and the origins of primate arborealism. In: Jenkins FA, editor. Primate Locomotion. New York: Academic Press; 1974. pp. 85–115. [Google Scholar]
- Johnson SE, Shapiro LJ. Positional behavior and vertebral morphology in atelines and cebines. Am J Phys Anthropol. 1998;105:333–354. doi: 10.1002/(SICI)1096-8644(199803)105:3<333::AID-AJPA4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Jouffroy FK. Quantitative and experimental approaches to primate locomotion: a review of recent advances. In: Seth P, Seth S, editors. Perspectives in Primate Biology. New Delhi: Today’s and Tomorrow’s Printers and Publishers; 1989. pp. 47–108. [Google Scholar]
- Jouffroy FK, Petter A. Gravity-related kinematic changes in lorisine horizontal locomotion in relation to position of the body. In: Jouffroy FK, Stack MH, Niemitz C, editors. Gravity, Posture and Locomotion in Primates. Florence: Il Sedicesimo; 1990. pp. 199–208. [Google Scholar]
- Jungers WL. Scaling of the hominoid locomotor skeleton with special reference to lesser apes. In: Preuschoft H, Chivers D, Brockelman W, Creel N, editors. The Lesser Apes: Evolutionary and Behavioral Biology. Edinburgh: Edinburgh University Press; 1984. pp. 146–169. [Google Scholar]
- Keith A. The extent to which the posterior segments of the body have been transmuted and suppressed in the evolution of man and allied primates. J Anat Physiol. 1902;37:18–40. [PMC free article] [PubMed] [Google Scholar]
- Keith A. Man’s posture: its evolution and disorders. II. The evolution of the orthograde spine. Br Med J. 1923;1:499–502. doi: 10.1136/bmj.1.3247.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima R, Okada M. Distribution of muscle fibre types in the thoracic and lumbar epaxial muscles of Japanese macaques (Macaca fuscata. Folia Primatol. 1996;66:38–43. doi: 10.1159/000157183. [DOI] [PubMed] [Google Scholar]
- Konow N, Roberts TJ. The series elastic shock absorber: tendon elasticity modulates energy dissipation by muscle during burst deceleration. Proc R Soc Lond B Biol Sci. 2015;282:20142800. doi: 10.1098/rspb.2014.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konow N, Azizi E, Roberts TJ. Muscle power attenuation by tendon during energy dissipation. Proc R Soc Lond B Biol Sci. 2011 doi: 10.1098/rspb.2011.1435. rspb20111435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumakura H, Inokuchi S. Morphological diversity of the lorisoid epaxial muscles. In: Matano S, Tuttle RH, Ishida H, Goodman M, editors. Topics in Primatology 3: Evolutionary Biology, Reproductive Endocrinology, and Virology. Tokyo: University of Tokyo Press; 1992. pp. 83–92. [Google Scholar]
- Kumakura H, Hirasaki E, Nakano Y. Organization of epaxial muscles in terrestrial and arboreal primates. Folia Primatol. 1996;66:25–37. doi: 10.1159/000157182. [DOI] [PubMed] [Google Scholar]
- Lieber RL. Skeletal Muscle Structure, Function, and Plasticity. Baltimore: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- Lieber RL, Yeh Y, Baskin RJ. Sarcomere length determination using laser diffraction: effect of beam and fiber diameter. Biophys J. 1984;45:1007–1016. doi: 10.1016/S0006-3495(84)84246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb GE, Gans C. Electromyography for Experimentalists. Chicago: The University of Chicago Press; 1986. [Google Scholar]
- Lowe DA, Surek JT, Thomas DD, et al. Electron paramagnetic resonance reveals age-related myosin structural changes in rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2001;280:C540–C547. doi: 10.1152/ajpcell.2001.280.3.C540. [DOI] [PubMed] [Google Scholar]
- Mathewson MA, Kwan A, Eng CM, et al. Comparison of rotator cuff muscle architecture between humans and selected vertebrate species. J Exp Biol. 2014;217:261–273. doi: 10.1242/jeb.083923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mivart St G. Contributions toward a more complete knowledge of the axial skeleton in the primates. Proc Zool Soc Lond. 1865;33:545–592. [Google Scholar]
- Muhl ZF. Active length-tension relation and the effect of muscle pinnation on fiber lengthening. J Morphol. 1982;173:285–292. doi: 10.1002/jmor.1051730305. [DOI] [PubMed] [Google Scholar]
- Murphy RA, Beardsley AC. Mechanical properties of the cat soleus muscle in situ. Am J Physiol. 1974;227:1008–1013. doi: 10.1152/ajplegacy.1974.227.5.1008. [DOI] [PubMed] [Google Scholar]
- Napier JR, Walker AC. Vertical clinging and leaping–a newly recognized category of locomotor behaviour of primates. Folia Primatol. 1967;6:204–219. doi: 10.1159/000155079. [DOI] [PubMed] [Google Scholar]
- Nekaris KAI, Bearder SK. The lorisiform primates of Asia and mainland Africa: diversity shrouded in darkness. In: Campbell CJ, Fuentes A, MacKinnon KC, Bearder SK, Stumpf RM, editors. Primates in Perspective. 2nd edn. New York: Oxford University Press; 2011. pp. 34–54. [Google Scholar]
- Neufuss J, Hesse B, Thorpe SKS, et al. Fibre type composition in the lumbar perivertebral muscles of primates: implications for the evolution of orthogrady in hominoids. J Anat. 2014;224:113–131. doi: 10.1111/joa.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Off EC, Gebo DL. Galago locomotion in Kibale National Park, Uganda. Am J Primatol. 2005;66:189–195. doi: 10.1002/ajp.20137. [DOI] [PubMed] [Google Scholar]
- Organ JM, Teaford MF, Taylor AB. Functional correlates of fiber architecture of the lateral caudal musculature in prehensile and nonprehensile tails of the Platyrrhini (Primates) and Procyonidae (Carnivora) Anat Rec. 2009;292:827–841. doi: 10.1002/ar.20886. [DOI] [PubMed] [Google Scholar]
- Oxnard CE, Crompton RH, Lieberman SS. Animal Lifestyles and Anatomies: The Case of the Prosimian Primates. Seattle: University of Washington Press; 1990. [Google Scholar]
- Paul RJ. Physical and biochemical energy balance during an isometric tetanus and steady state recovery in frog sartorius at 0oC. J Gen Physiol. 1983;81:337–354. doi: 10.1085/jgp.81.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell PL, Roy RR, Kanim P, et al. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J Appl Physiol. 1984;57:1715–1721. doi: 10.1152/jappl.1984.57.6.1715. [DOI] [PubMed] [Google Scholar]
- Pridmore PA. Trunk movements during locomotion in the marsupial Monodelphis domestica (Didelphidae) J Morphol. 1992;211:137–146. doi: 10.1002/jmor.1052110203. [DOI] [PubMed] [Google Scholar]
- Rice WR. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Richards CT, Sawicki GS. Elastic recoil can either amplify or attenuate muscle-tendon power, depending on inertial vs. fluid dynamic loading. J Theor Biol. 2012;313:68–78. doi: 10.1016/j.jtbi.2012.07.033. [DOI] [PubMed] [Google Scholar]
- Ritter DA, Nassar PN, Fife M, et al. Epaxial muscle function in trotting dogs. J Exp Biol. 2001;204:3053–3064. doi: 10.1242/jeb.204.17.3053. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Azizi E. The series-elastic shock absorber: tendons attenuate muscle power during eccentric actions. J Appl Physiol. 2010;109:396–404. doi: 10.1152/japplphysiol.01272.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TJ, Konow N. How tendons buffer energy dissipitation by muscle. Exerc Sport Sci Rev. 2013;41:186–193. doi: 10.1097/JES.0b013e3182a4e6d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MD. Functional proportions of primate lumbar vertebral bodies. J Hum Evol. 1975;4:21–38. [Google Scholar]
- Sanders WJ. Weight transmission through the lumbar vertebrae and sacrum in australopithecines. Am J Phys Anthropol Suppl. 1990;81:289. [Google Scholar]
- Sanders WJ. Comparative study of hominoid lumbar neural canal dimensions. Am J Phys Anthropol. 1991;S12:157. [Google Scholar]
- Sanders WJ, Bodenbender BE. Morphometric analysis of lumbar vertebra UMP 67-28: implications for spinal function and phylogeny of the Miocene Moroto hominoid. J Hum Evol. 1994;26:203–237. [Google Scholar]
- Sargis EJ. A preliminary qualitative analysis of the axial skeleton of tupaiids (Mammalia, Scandentia): functional morphology and phylogenetic implications. J Zool. 2001;253:473–483. [Google Scholar]
- Schilling N. Metabolic profile of the perivertebral muscles in small therian mammals: implications for the evolution of the mammalian trunk musculature. Zoology. 2009;112:279–304. doi: 10.1016/j.zool.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Schilling N, Carrier DR. Function of the epaxial muscles during trotting. J Exp Biol. 2009;212:1053–1063. doi: 10.1242/jeb.020248. [DOI] [PubMed] [Google Scholar]
- Schilling N, Carrier DR. Function of the epaxial muscles in walking, trotting, and galloping dogs: implications for the evolution of epaxial muscle function in tetrapods. J Exp Biol. 2010;213:1490–1502. doi: 10.1242/jeb.039487. [DOI] [PubMed] [Google Scholar]
- Schilling N, Hackert R. Sagittal spinal movements of small therian mammals during asymmetrical gaits. J Exp Biol. 2006;209:3925–3939. doi: 10.1242/jeb.02400. [DOI] [PubMed] [Google Scholar]
- Schultz AH. The relative length of the regions of the spinal column in Old World primates. Am J Phys Anthropol. 1938;24:1–22. [Google Scholar]
- Schultz AH. Vertebral column and thorax. In: Hofer H, Schultz AH, Stark D, editors. Primatologia. Vol. 4. Basel: Karger; 1961. pp. 1–66. [Google Scholar]
- Schultz AH, Straus WL. The numbers of vertebrae in primates. Proc Am Phil Soc. 1945;89:601–626. [PubMed] [Google Scholar]
- Schumacher GH. Funktionelle Morphologie der Kaumuskulatur. Jena: Gustav Fischer; 1961. [Google Scholar]
- Sellers WI. A biomechanical investigation into the absence of leaping in the locomotor repertoire of the slender loris (Loris tardigradus. Folia Primatol. 1996;67:1–14. doi: 10.1159/000157202. [DOI] [PubMed] [Google Scholar]
- Shapiro LJ. Functional morphology of the vertebral column in primates. In: Gebo DL, editor. Postcranial Adaptation in Nonhuman Primates. DeKalb: Northern Illinois University Press; 1993. pp. 121–149. [Google Scholar]
- Shapiro LJ. Functional morphology of Indrid lumbar vertebrae. Am J Phys Anthropol. 1995;98:323–342. doi: 10.1002/ajpa.1330980306. [DOI] [PubMed] [Google Scholar]
- Shapiro LJ. Morphological and functional differentiation in the lumbar spine of lorisids and galagids. Am J Primatol. 2007;69:86–102. doi: 10.1002/ajp.20329. [DOI] [PubMed] [Google Scholar]
- Shapiro LJ, Demes B. Spinal kinematics in lorids and cheirogaleids. Am J Phys Anthropol Suppl. 1996;22:213. [Google Scholar]
- Shapiro LJ, Jungers WL. Back muscle function during bipedal walking in chimpanzee and gibbon: implications for the evolution of human locomotion. Am J Phys Anthropol. 1988;77:201–212. doi: 10.1002/ajpa.1330770208. [DOI] [PubMed] [Google Scholar]
- Shapiro LJ, Jungers WL. Electromyography of back muscles during quadrupedal and bipedal walking in primates. Am J Phys Anthropol. 1994;93:491–504. doi: 10.1002/ajpa.1330930408. [DOI] [PubMed] [Google Scholar]
- Shapiro LJ, Simons CVM. Functional aspects of strepsirrhine lumbar vertebral bodies and spinous processes. J Hum Evol. 2002;42:753–783. doi: 10.1006/jhev.2002.0560. [DOI] [PubMed] [Google Scholar]
- Shapiro LJ, Demes B, Cooper J. Lateral bending of the lumbar spine during quadrupedalism in strepsirhines. J Hum Evol. 2001;40:231–259. doi: 10.1006/jhev.2000.0454. [DOI] [PubMed] [Google Scholar]
- Shapiro LJ, Seiffert CVM, Godfrey LR, et al. Morphometric analysis of lumbar vertebrae in extinct Malagasy strepsirrhines. Am J Phys Anthropol. 2005;128:823–839. doi: 10.1002/ajpa.20122. [DOI] [PubMed] [Google Scholar]
- Slijper EJ. Comparative biologic-anatomical investigations on the vertebral column and spinal musculature of mammals. Verh K Ned Akad Wet. 1946;42:1–128. [Google Scholar]
- Smith RJ, Jungers WL. Body mass in comparative primatology. J Hum Evol. 1997;32:523–559. doi: 10.1006/jhev.1996.0122. [DOI] [PubMed] [Google Scholar]
- Smith JM, Savage RJG. Some locomotory adaptations in mammals. J Linn Soc Lond Zool. 1956;42:603–622. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. 3rd edn. New York: W. H. Freeman; 1995. [Google Scholar]
- Stern JT. Bibl Primatologica. Vol. 14. Basel: S. Karger; 1971. Functional myology of the hip and thigh of cebid monkeys and its implications for the evolution of erect posture. [Google Scholar]
- Taylor AB, Vinyard CJ. Jaw muscle fiber architecture in tufted capuchins favors generating relatively large muscle forces without compromising jaw gape. J Hum Evol. 2009;57:710–720. doi: 10.1016/j.jhevol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AB, Vinyard CJ. The relationships among jaw-muscle fiber architecture, jaw morphology, and feeding behavior in extant apes and modern humans. Am J Phys Anthropol. 2013;151:120–134. doi: 10.1002/ajpa.22260. [DOI] [PubMed] [Google Scholar]
- Taylor AB, Eng CM, Anapol FC, et al. The functional correlates of jaw-muscle fiber architecture in tree-gouging and non-gouging callitrichid monkeys. Am J Phys Anthropol. 2009;139:353–367. doi: 10.1002/ajpa.20991. [DOI] [PubMed] [Google Scholar]
- Terhune CE, Hylander WL, Vinyard CJ, et al. Jaw-muscle architecture and mandibular morphology influence relative maximum jaw gapes in the sexually dimorphic Macaca fascicularis. J Hum Evol. 2015;82:145–158. doi: 10.1016/j.jhevol.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Walker A. The locomotion of the lorises, with special reference to the Potto. Afr J Ecol. 1969;7:1–5. [Google Scholar]
- Walker A. Locomotor adaptations in past and present prosimian primates. In: Jenkins FA, editor. Primate Locomotion. New York: Academic Press; 1974. pp. 349–381. [Google Scholar]
- Walker SE. Leaping behavior of Pithecia pithecia and Chiropotes satanas in eastern Venezuela. Am J Primatol. 2005;66:369–387. doi: 10.1002/ajp.20162. [DOI] [PubMed] [Google Scholar]
- Walker SM, Schrodt GR. I segment lengths and thin filament periods in skeletal muscle fibers of the Rhesus monkey and the human. Anat Rec. 1974;178:63–81. doi: 10.1002/ar.1091780107. [DOI] [PubMed] [Google Scholar]
- Ward CV. Torso morphology and locomotion in Proconsul nyanzae. Am J Phys Anthropol. 1993;92:291–328. doi: 10.1002/ajpa.1330920306. [DOI] [PubMed] [Google Scholar]
- Washburn SL, Buettner-Janusch J. The definition of thoracic and lumbar vertebrae. Am J Phys Anthropol. 1952;10:251–252. [Google Scholar]
- Wilkie DR. Heat work and phosphorylcreatine break-down in muscle. J Physiol. 1968;195:157–183. doi: 10.1113/jphysiol.1968.sp008453. [DOI] [PMC free article] [PubMed] [Google Scholar]


