Abstract
In the last decade, the study of human sperm anatomy, at molecular level, has revealed the presence of several nuclear protein receptors. In this work, we examined the expression profile and the ultrastructural localization of liver receptor homolog-1 (LRH-1) in human spermatozoa. We evidenced the presence of the receptor by Western blotting and real time-RT-PCR. Furthermore, we used immunogold electron microscopy to investigate the sperm anatomical regions containing LRH-1. The receptor was mainly located in the sperm head, whereas its expression was reduced in the neck and across the tail. Interestingly, we observed the presence of LRH-1 in different stages of testicular germ cell development by immunohistochemistry. In somatic cells, it has been suggested that the LRH-1 pathway is tightly linked with estrogen signaling and the important role of estradiol has been widely studied in sperm cells. To assess the significance of LRH-1 in male gametes and to deepen understanding of the role of estrogens in these cells, we investigated important sperm features such as motility, survival and capacitation. Spermatozoa were treated with 10 nm estradiol and the inhibition of LRH-1 reversed the estradiol stimulatory action. From our data, we discovered that human spermatozoa can be considered a new site of expression for LRH-1, evidencing its role in sperm motility, survival and cholesterol efflux. Furthermore, we may presume that in spermatozoa the LRH-1 effects are closely integrated with the estrogen signaling, supporting LRH-1 as a downstream effector of the estradiol pathway on some sperm functions.
Keywords: estrogen receptors, human sperm anatomy, human testis, liver receptor homolog-1, male genital tract, nuclear receptors
Introduction
Numerous studies have demonstrated the involvement of sex hormones in the regulation of reproductive system functions, in particular the role of estrogens in spermatogenesis and male fertility (O’Donnell et al. 2001; Carreau et al. 2002). The physiological responses to estrogens in target cells are known to be mediated by at least two distinct receptor subtypes, estrogen receptor (ER) α and β. Both receptors seem to be expressed in germ cells in various stages of development from spermatogonia to elongated spermatids and spermatozoa (Durkee et al. 1998; Aquila et al. 2004; Guido et al. 2011). When estradiol occupies its ligand-binding domain, the ERs can act through distinct ‘genomic’ and ‘non-genomic’ pathways. The occurrence of non-genomic effects is now established in different tissues and cell lines, including spermatozoa (Morley et al. 1992; Cato et al. 2002; Aquila et al. 2006, 2007). The ER non-genomic actions involve interactions with intracellular pathways, where the ligand–ER complex modulates the activity of several kinases and structural proteins, such as PI3K–Akt–GSK3 and MAPK. Previous data have demonstrated that several nuclear receptors, linked to rapid non-genomic actions, are expressed in human spermatozoa. Progesterone receptor is involved in the acrosomal reaction (Tesarik et al. 1993; De Amicis et al. 2011, 2012), whereas androgen receptor, ERα and ERβ evidenced their interaction with the phosphatidylinositol-3OH kinase (PI3K)/Akt pathway (Aquila et al. 2004, 2007). In addition, estradiol [estra-1,3,5,(10)-triene-3,17β-diol] (E2) significantly stimulates in vitro capacitation, acrosome reaction and fertilizing ability of mammalian spermatozoa (Adeoya-Osiguwa et al. 2003; Aquila et al. 2003, 2004).
The liver receptor homolog (LRH-1, also referred to as NR5A2, FTZ-F1, FTF or CPF), such as ER, is a member of the nuclear receptor superfamily; it is expressed in liver, intestine and exocrine pancreas where it plays vital roles in early development and cholesterol homeostasis (Annicotte et al. 2003). Recently, LRH-1 has been identified in many steroidogenic tissues such as ovary and testis in several species, suggesting that this receptor may play a critical role in development and differentiation of the endocrine and reproductive systems (Boerboom et al. 2000; Sirianni et al. 2002; Hinshelwood et al. 2003; Liu et al. 2003; Pezzi et al. 2004). LRH-1 has been reported to be expressed in some steps of spermatogenesis but in this context its role remains unknown. Several studies have showed LRH-1 expression in rat round spermatids and pachytene spermatocytes as well as an LRH-1 relationship with aromatase (Pezzi et al. 2004; Sierens et al. 2010). Aromatase is a critical enzyme, required for the conversion of androgens to estrogens, which was identified also in spermatozoa (Aquila et al. 2002, 2003). In breast cancer cells the expression of LRH is regulated by estrogens. In somatic cells, it was suggested that the LRH-1 signaling is tightly linked with the estrogen pathway (Annicotte et al. 2005). The structure of LRH-1 has been determined recently and much remains to be learned about this nuclear receptor.
In this work, we show, for the first time, the presence of LRH-1 in human ejaculated spermatozoa, providing evidence of its ultrastructural compartmentalization. Intriguingly, LRH-1 appears to mediate, as a downstream target of estrogenic signaling, the E2-induction of crucial sperm biological functions such as motility, survival and cholesterol efflux.
Material and methods
Chemicals
PMN Cell Isolation Medium was from BIOSPA (Milan, Italy). Total RNA Isolation System kit, enzymes, buffers, nucleotides 100-bp ladder used for RT-PCR were purchased from Promega (Milan, Italy). Moloney Murine Leukemia Virus (M-MLV) was from Gibco BRL-Life Technologies Italia (Milan Italy). Oligonucleotide primers were made by Invitrogen (Milan, Italy). Bovine serum albumin (BSA) protein standard, Laemmli sample buffer, prestained molecular weight markers, Percoll (colloidal PVP-coated silica for cell separation), hematoxylin, eosin, dimethylsulfoxide (DMSO), Earle’s balanced salt solution (EBSS), estradiol [estra-1,3,5,(10)-triene-3,17β-diol] (E2), 17alpha-estradiol (EA) and all other chemicals were purchased from Sigma-Aldrich (Milan, Italy). Acrylamide bisacrylamide was from Labtek Eurobio (Milan, Italy). Triton X-100 and Eosin Y were from Farmitalia Carlo Erba (Milan, Italy). Gel band purification kit, Enhanced chemiluminescence (ECL) Plus Western blotting detection system, Hybond™ ECL™, Hepes Sodium Salt were purchased from Amersham Pharmacia Biotech (Amersham, Buckinghamshire, UK). The cholesterol-oxidase (CHOD)-peroxidase (POD) enzymatic colorimetric kit was from Inter-Medical (Biogemina Sas, Catania, Italy). Monoclonal anti-human LRH-1/NR5A2 antibody (mouse anti-human LRH-1) was from R&D Systems (Minneapolis, MN, USA). Santa Cruz Biotechnology (Santa Cruz, CA, USA) provided LRH1/NR5A2 (N-16), an affinity purified goat polyclonal antibody raised against a peptide mapping at the N-terminus of NR5A2 of human origin (goat anti-human LRH-1), biotinylated anti-goat IgG, avidin-biotin-horseradish peroxidase (ABC) complex, normal goat serum, normal mouse serum, diaminobenzidine (DAB), goat polyclonal anti-actin Ab, anti-goat secondary Ab, anti-mouse secondary Ab, LRH-1 blocking peptide. A cell-permeable pyrazolylbiphenylethanone compound (specific antagonist of LRH-1) (LA) was from Calbiochem (Milan, Italy).
Semen samples and spermatozoa preparations
Human semen was collected, according to the World Health Organization (WHO, 2010) recommended procedure by masturbation from men undergoing semen analysis in our laboratory. This study included sperm samples with normal parameters of semen volume, sperm count, motility, vitality and morphology, according to the WHO Laboratory Manual (WHO, 2010). In each experiment three normal samples were pooled. The study was approved by the local medical-ethics committee and all participants gave their informed consent.
Processing of ejaculated sperm
After liquefaction, three normal semen samples were pooled and subjected to centrifugation (800 g) on a discontinuous Percoll density gradient (80 : 40% v/v) (WHO, 2010). The 80% Percoll fraction was examined using an optical microscope, equipped with a ×100 oil objective, to ensure that a pure sample of sperm was obtained. An independent observer, who observed several fields for each slide, inspected the cells. Percoll-purified sperm were washed with unsupplemented EBSS medium and incubated in the same medium (uncapacitating medium) for 30 min at 37 °C and 5% CO2, without (control) or with treatments (experimental). The treatments were as follows: 10 nm E2 or 5 μm LA or 10 nm EA or 10 nm E2 plus 5 μm LA. The LA exhibited no inhibitory effect against AR (androgen hormone receptor), ERα, or TRβ (thyroid hormone receptor β) at concentrations of up to 10 μm (Benod et al. 2013). When the cells were treated with the LA, a pretreatment of 15 min was performed and subsequently the spermatozoa were incubated with E2 for 30 min. E2 and LA were dissolved in dimethylsulfoxide (DMSO, 0.1% final concentration in culture); 17EA was dissolved in ethanol (EtOH, 0.02% final concentration in culture). Neither DMSO nor EtOH, when used as solvent controls, induce any positive results in any of the in vitro assays.
RNA extraction, cDNA synthesis and real time RT-PCR
Total RNA was isolated from purified human ejaculated spermatozoa as previously described (Aquila et al. 2002). RNA was treated with DNase, and the purity and integrity of the RNA was confirmed spectroscopically and by gel electrophoresis before use. DNase-treated RNA 2-μg samples were transcribed by 200 IU M-MLV reverse transcriptase in a reaction volume of 20 μL (0.4 μg oligo-dT, 0.5 mM deoxy-NTP and 24 IU RNasin) for 30 min at 37 °C, followed by heat denaturation for 5 min at 95 °C. PCRs were performed in the ABI Prism 7000 Sequence Detection System (Applied Biosystems) in a total volume of 30 μL reaction mixture, following the manufacturer’s recommendations, using the SYBR Green Universal PCR Master Mix 2× (Applied Biosystems) and 0.1 μm of each primer. Negative controls contained water instead of first-strand cDNA. Each sample was normalized on the basis of its 18S ribosomal RNA content. The relative LRH-1 gene expression levels were normalized to a calibrator that was chosen to be the sample with the highest threshold cycle (Ct). Final results, expressed as n-fold differences in orphan nuclear receptor gene expression relative to 18S rRNA and calibrator, were calculated following the ΔΔCt method as follows: n-fold = 2−(ΔCtsample−ΔCtcalibrator), where ΔCt values of the sample and calibrator are determined by subtracting the average Ct value of the nuclear receptor gene from the average Ct value of the 18S rRNA reference gene. Before using the ΔΔCt method for relative quantification, we performed validation experiments to demonstrate that efficiencies of target and reference were approximately equal, following Applied Biosystems instructions [http://docs.appliedbiosystems.com/pebiodocs/04303859.pdf (page 14)]. Primer pairs were as follows: LRH-1 (GenBank accession number NM_003822), Forward: 5′-TACCGACAAGTGGTACATGGAA-3′ (exon 6), Reverse: 5′-CGGCTTGTGATGCTATTATGGA-3′ (exon 7), giving an 89-bp fragment. TM3 mouse Leydig cells were used as positive control (Pezzi et al. 2004).
Western blot analysis of sperm proteins
Sperm samples, washed twice with EBSS (uncapacitating medium), were incubated without or with the indicated treatments, and then centrifuged for 5 min at 5000 g. The pellet was resuspended in lysis buffer as previously described (Aquila et al. 2007, 2008). Equal amounts of protein (70 μg) were boiled for 5 min, separated by 10% polyacrylamide gel electrophoresis, transferred to nitrocellulose sheets and probed with an appropriate dilution of the LRH-1 Ab. The bound of the secondary Ab was revealed with the ECL Plus Western blotting detection system according to the manufacturer’s instructions. As internal control, all membranes were subsequently stripped (glycine 0.2 m, pH 2.6 for 30 min at room temperature) of the first antibody and reprobed with anti-β-actin Ab.
Immunogold labeling of LRH-1
Immunogold labeling for LRH-1 was performed as previously reported (Aquila et al. 2010a). Briefly, spermatozoa fixed overnight in 4% paraformaldehyde were washed in phosphate-buffered saline (PBS) to remove excess fixative. Then they were dehydrated in a graded alcohol, infiltrated in LR white resin, polymerized in a vacuum oven at 45 °C for 48 h, and 60-nm ultrathin sections were cut and placed on coated nickel grids for post-embedding immunogold labeling for anti-human LRH-1 Ab. Potential non-specific labeling was blocked by incubating the sections in PBS containing 5% normal goat serum, 5% BSA, and 0.1% cold water fish gelatine at room temperature for 1 h. Sections were incubated overnight at 4 °C with anti-LRH-1 primary Ab (1 : 500) in PBS buffer and then incubated in 10 nm colloidal gold-conjugated anti-goat secondary Ab (1 : 50) for 2 h at room temperature. The sections were subsequently washed in PBS, fixed in glutaraldehyde, counterstained with uranyl acetate and lead acetate, and examined using a Zeiss EM 900 transmission electron microscope (TEM). To assess the specificity of the immunolabeling, a negative control was performed in sperm sections incubated with the colloidal gold-conjugated secondary Ab and with normal goat serum instead the primary Ab.
Immunohistochemistry
Paraffin-embedded human testis sections, 5 μm thick, were mounted on slides precoated with poly-lysine, and then de-paraffinized and dehydrated (seven to eight serial sections). Immunohistochemical experiments were performed after heat-mediated antigen retrieval in 0.01 m citrate buffer, pH 6. Hydrogen peroxide (3% in distillate water) was used for 30 min to inhibit endogenous peroxidase activity, whereas normal goat serum (10%) was used for 30 min to block the non-specific binding sites. Immunodetection was carried out using anti-LRH-1 (1 : 50) primary Ab at 4 °C overnight. A biotinylated anti-goat IgG was then applied (1 : 600) for 1 h at RT, followed by the avidin- biotin-horseradish peroxidase complex (ABC/HRP). Immunoreactivity was visualized using the diaminobenzidine chromogen (DAB). Testis sections were also counterstained with hematoxylin. The primary Ab was replaced by normal goat serum in negative control sections. Absorption controls utilized primary Ab preabsorbed with an excess (5 nmol mL−1) of the purified respective blocking peptides, at 4 °C for 48 h. Unaltered human testicular tissues were provided by the archives of the Anatomy-Pathological Unit of Annunziata Hospital, Cosenza, Italy. Three different testicular tissues were used in the experiments and each analysis was repeated three times. Six to seven serial sections were processed for each sample.
Evaluation of sperm motility and viability
Sperm motility and viability were assessed by means of light microscopy examining an aliquot of each sperm sample which had been incubated in the absence (NC) or in presence of 10 nm E2 or 5 μm LA or 10 nm EA or 10 nm E2 plus 5 μm LA. We decided to treat spermatozoa with E2 at 10 nm concentration since it was the most effective in our previous study (Guido et al. 2011). Sperm motility was expressed as percentage of total motile spermatozoa, including the rapid progressive (PR) plus the slow progressive (NP) cells (normal values: PR + NP > 40% as reported by WHO 2010). Viability was assessed by red-eosin exclusion test using Eosin Y to evaluate potential toxic effects of the treatments. An independent observer scored 200 cells for stain uptake (dead cells) or exclusion (live cells). Sperm viability was expressed as the percentage of total live sperm. Viability was evaluated before and after pooling the samples and there were no adverse effects among the different treatments on human sperm survival (Aquila et al. 2003; Cappello et al. 2012).
Measurement of cholesterol in the sperm culture medium
Cholesterol was measured in duplicate by a CHOD – POD enzymatic colorimetric method according to manufacturer’s instructions in the incubation medium from human spermatozoa as previously reported (Aquila et al. 2010b; Cappello et al. 2012). Sperm samples, washed twice with uncapacitating medium, were incubated in the same medium (control, NC) for 30 min at 37 °C and 5% CO2. Other samples were incubated in the presence of 10 nm E2 or 5 μm LA or 10 nm EA or 10 nm E2 plus 5 μm LA. At the end of the sperm incubation, the culture media were recovered by centrifugation, then lyophilized and dissolved in 1 mL of buffer reaction. The samples were incubated for 10 min at room temperature, and the cholesterol content was measured at 505 nm. Cholesterol standard used was 200 mg dL−1. Cholesterol results are presented as mg per 10 × 106 number of spermatozoa.
Statistical analysis
The experiments for real time RT-PCR, immunogold labeling and immunohistochemistry were repeated on at least three independent occasions, and Western blot analysis was performed in at least four independent experiments. The data obtained from motility, viability (six replicate experiments using duplicate determinations) and the CHOD – POD enzymatic colorimetric method (six experiments using duplicate determinations) were presented as the mean ± SEM. The differences in mean values were calculated by the one-way analysis of variance (anova). The Wilcoxon test was used after anova as a post-hoc test.
Results
LRH-1 protein is expressed in human spermatozoa
To address a possible role of LRH-1 in human spermatozoa, we investigated the presence of LRH-1 in normal human ejaculated spermatozoa by Western blot analysis. Two different antibodies detected a band of the expected size at about 64 kDa for LRH-1 protein (Fig.1), according to the results obtained in TM3 Leydig cells, used as positive control (Pezzi et al. 2004).
Figure 1.
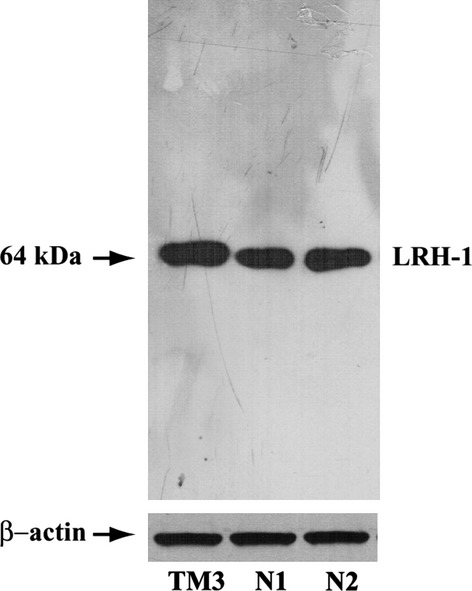
LRH-1 is present in human ejaculated spermatozoa. Western blot of LRH-1 protein in human sperm, expression in two samples of ejaculated spermatozoa from normal men (N1, N2). TM3 extract was used as positive control and β-actin as internal control. The experiments were repeated at least four times and the autoradiograph of the figure shows the results of one representative experiment.
LRH-1 mRNA is expressed by human spermatozoa
To quantify mRNA levels of LRH-1 in human spermatozoa, RNA was isolated from Percoll-separated spermatozoa of different normozoospermic samples (N1, N2) followed by quantitative real-time PCR. As shown in Fig.2, LRH-1 mRNA was expressed at appreciable levels in TM3 cells, as previously reported (Pezzi et al. 2004), and in ejaculated spermatozoa.
Figure 2.
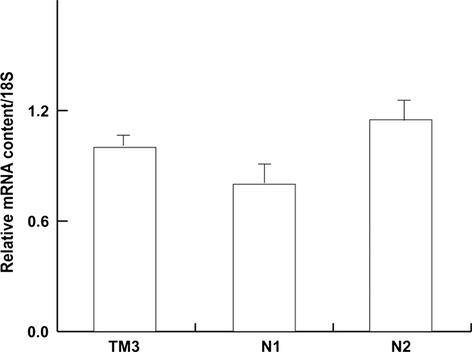
Real time RT-PCR analysis of human LRH-1 gene in human spermatozoa. Real time RT-PCR was used to quantify the level of LRH-1 mRNA obtained from ejaculates of two normozoospermic patients (N1, N2) and TM3 Leydig cells, used as positive control for LRH-1. Data represent the mean ± SEM of RNA samples obtained from TM3 cells, while the columns of the different ejaculates from normozoospermic patients were repeated at least three times, both expressed as relative difference from the calibrator.
Immunogold localization of LRH-1 in human sperm
Different findings on human sperm anatomy suggest the sperm molecules are compartmentalized in the regions where they are needed. Thus, sperm morphological features, at molecular levels, may be indicative of the function carried out. In the present study, post-embedding immunogold labeling was used to characterize the distribution of LRH-1 at the ultrastructural level. In the ultrathin sections of sperm from normozoospermic donors, LRH-1 immunolabeling was predominantly situated in the sperm head (Fig.3A-C), whereas only low labeling was observed in both the midpiece and the tail (Fig.4A-C). The high resolution and magnification of the TEM allowed us to identify the presence of gold particles not only in sperm nuclei but also around the sperm head. Negative controls have been also used to confirm the specificity of the method (primarily the specificity of the LRH-1 antibody) and to exclude the presence of nonspecific staining (Figs3D and 4D). To further examine the region-specific expression of LRH-1 across the sperm cell, we performed the quantification of gold particles. The results were expressed in terms of the number of gold particles in different regions of the sperm cell: the nucleus, the nuclear membrane, the midpiece and the tail (Supporting Information Fig. S1).
Figure 3.
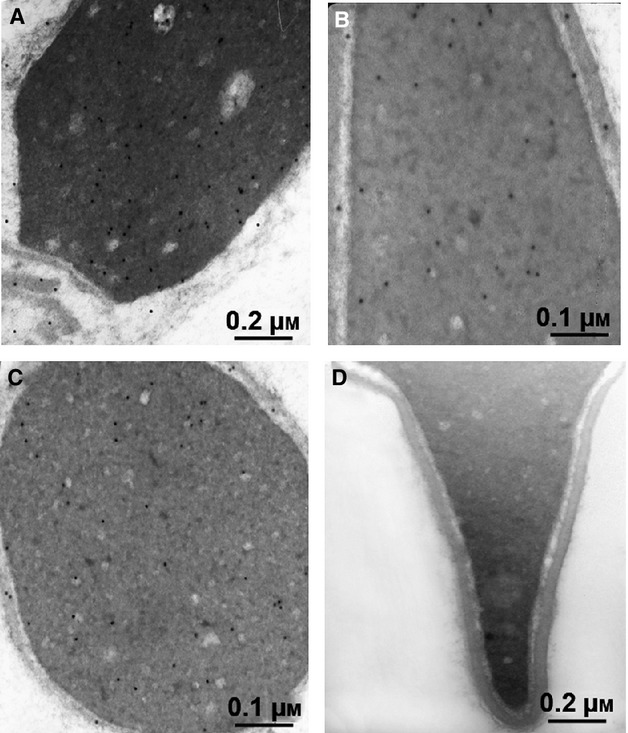
Immunoelectron microscopy demonstrated LRH-1 in the head of human ejaculated spermatozoa. Sperm from normozoospermic subjects was processed as reported in Material and methods. Electron micrographs of sperm allowed to react with Ab directed against LRH-1. (A,B) Longitudinal section of the head (×50 000 and ×85 000, respectively)/ (C) Transversal section of the head (×50 000). (D) Negative control performed as described in Material and methods (×50 000).
Figure 4.
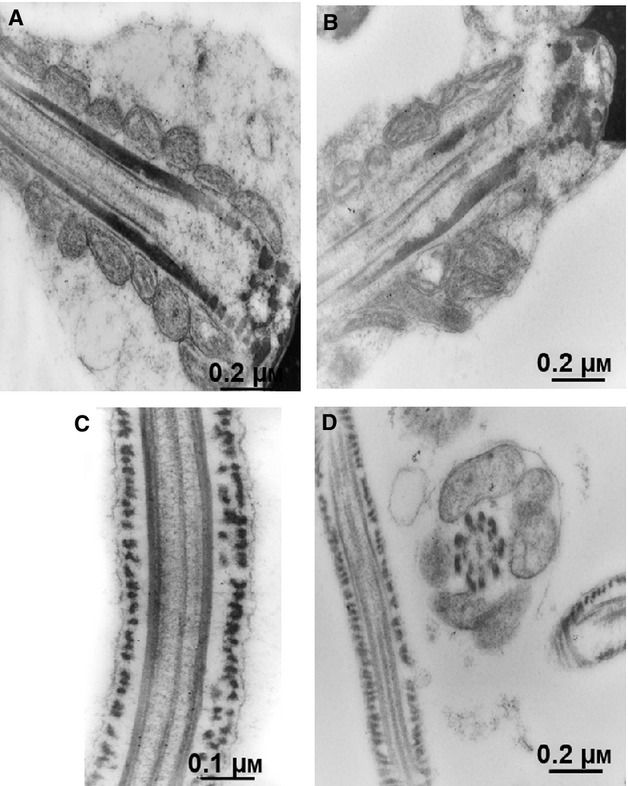
Immunoelectron microscopy did not show LRH-1 in the sperm tail. Sperm from normozoospermic subjects was processed as reported in Material and methods. Electron micrographs of sperm allowed to react with Ab directed against LRH-1. (A,B) Electron micrographs of sagittal section of the midpiece with its mitochondria (×40 000). (C) Longitudinal section of the tail (×50 000). (D) Negative control performed as described in Material and methods; longitudinal section of the end piece and a cross-section of the midpiece with mitochondria (×40 000).
Immunohistochemistry for LRH-1 in human testis
We performed immunohistochemistry in human testis sections to determine the expression site of LRH-1 in the different stages of germ cell development. Our results showed a nuclear sub-cellular localization of LRH-1 in developing germ cells of human testes, whereas a cytoplasmic immunoreactivity was revealed predominately in somatic testicular cells (Sertoli and Leydig cells) together with nuclear staining (Fig.5). No immunoreactivity was observed in the negative (data not shown) and absorption controls (inserts).
Figure 5.
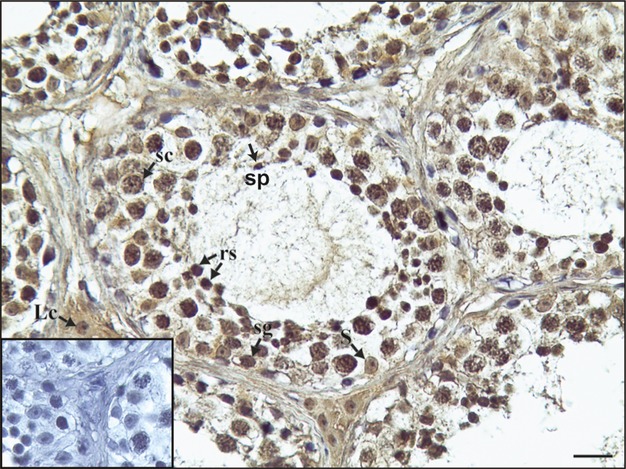
LRH-1 immunoreactivity in human testis. Sg, Spermatogonium; sc, spermatocyte; rs, round spermatid; sp, spermatozoon, Lc, Leydig cell; S, Sertoli cell. Scale bar: 12.5 μm. Insert: immunonegative absorption control.
LRH-1 inhibition abrogates the stimulatory effect of E2 on human sperm motility and survival
Assessment of the motile sperm fraction is perhaps the most widely used measure of semen quality, since motility describes the ability of sperm to move properly towards an egg. Therefore, we next investigated the effect of E2, as well as its combination with 5 μm LA, on this important sperm feature. As shown in Fig.6A, sperm motility was significantly enhanced after 10 nm E2 treatment, whereas the co-treatment with 5 μm LA reduced the effect. By treating sperm with 10 nm EA, an inactive isomer of E2, no effect was observed, as previously reported (Cheng & Boettcher, 1979). Another important parameter of human sperm quality consists in the capacity of sperm to survive as long as possible to have the chance to find and fertilize the oocyte. LRH-1 inhibition reduced the E2-effect, suggesting its role in the estrogen signaling (Fig.6B). Neither DMSO nor EtOH, when used as solvent controls, induced any positive results.
Figure 6.
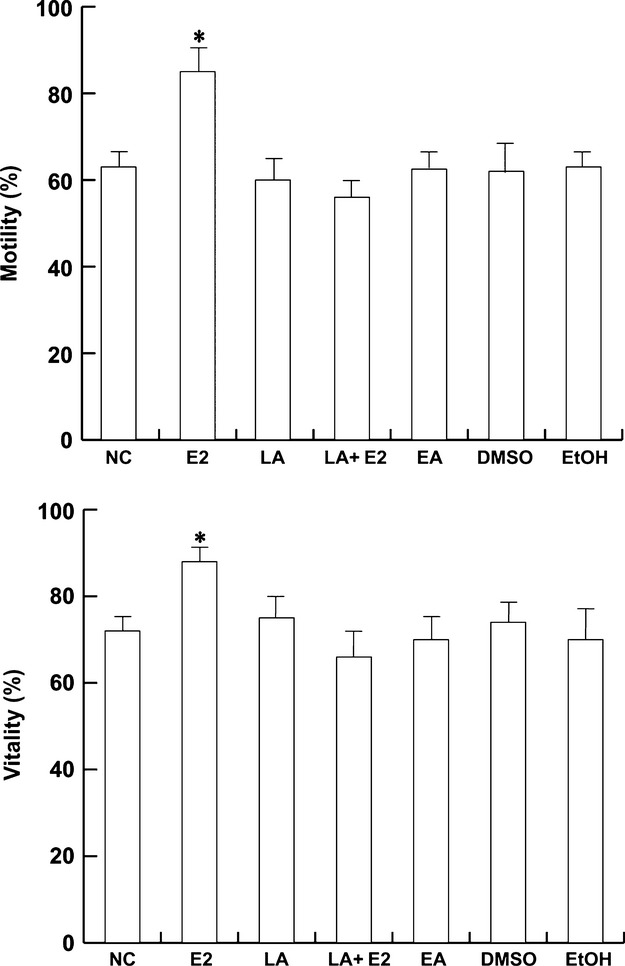
Estradiol effect on sperm motility and viability is mediated by LRH-1. Washed spermatozoa were incubated in the unsupplemented Earle’s medium for 30 min at 37 °C and 5% CO2, in the absence (NC) or in the presence of 10 nm E2 or 5 μm LA or 10 nm EA or 10 nm E2 plus 5 μm LA. Top panel: Sperm motility was expressed as the percentage of total motile sperm including the rapid progressive (PR) plus slow progressive (NP) sperm. Bottom panel: Sperm viability was assessed using Eosin Y as described in Material and methods. DMSO and EtOH were used as solvent controls. Columns are mean ± SEM of six independent experiments performed in duplicate. *P < 0.05 vs. control.
LRH-1 partly mediated E2-induced cholesterol efflux in human sperm
In vivo, the fertilization process is completed in the female reproductive tract, where spermatozoa undergo biochemical modifications, such as membrane cholesterol efflux. The latter is an important step in initiating transmembrane signaling events to complete the sperm maturation process and became able to fertilize (Travis & Kopf, 2002). LRH-1 has an important role in cholesterol homeostasis in somatic cells and therefore might be important in sperm. Pooled sperm from normal samples were treated as indicated in Material and methods and centrifuged. The upper phase of the sample was used to determine the cholesterol levels. As shown in Fig.7, the cholesterol efflux increased with 10 nm E2, in agreement with our previous studies, whereas LA partly reversed this effect, suggesting an involvement of LRH-1 in the estrogen-induction of sperm capacitation. EA 10 nm had no effect. Neither DMSO nor EtOH, when used as solvent controls, induced any positive results.
Figure 7.
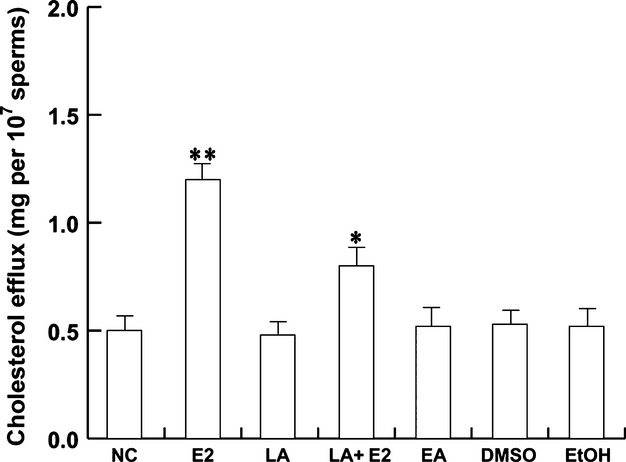
LRH-1 is involved in the estradiol-induced cholesterol efflux. Washed spermatozoa were incubated in unsupplemented Earle’s medium for 30 min at 37 °C and 5% CO2, in the absence (NC) or presence of 10 nm E2 or 5 μm LA or 10 nm EA or 10 nm E2 plus 5 μm LA. Cholesterol in culture medium from human ejaculated spermatozoa was measured by enzymatic colorimetric assay. DMSO and EtOH were used as solvent controls. Columns are mean ± SEM of six independent experiments performed in duplicate. Data are expressed in mg/107 sperm. *P < 0.05 vs. control, **P < 0.01 vs. control.
Discussion
The role of estrogen in spermatogenesis and male reproduction is now shown taking into account the simultaneous presence of a biologically active aromatase and the widespread distribution of estrogen receptors in germ cells (Carreau et al. 2012). The presence of several nuclear receptors, including ERs, has been widely demonstrated in human spermatozoa (Tesarik et al. 1993; Aquila et al. 2004, 2007). In this study we have evaluated the presence of LRH-1 in human ejaculated spermatozoa, evidencing its new role in the regulation of sperm function. Interestingly, LRH-1 appears to be able to mediate the responsiveness to E2 of human male gamete on motility, survival and cholesterol efflux.
We showed the presence of LRH-1 in human male gamete by Western blotting and real time-RT-PCR. Besides, by immunogold analysis, we demonstrated that LRH-1 is abundantly localized in the sperm head, both on the membrane and in the nucleus. The gold particles were present but reduced in the midpiece and the tail. Immunohistochemical analysis of the human testis has shown that the LRH-1 is expressed in seminiferous tubules and in the interstitium. The presence of LRH-1 led us to investigate whether it may be involved in sperm biology.
In somatic cells, it was suggested that the LRH-1 pathway is tightly associated with estrogen signaling (Annicotte et al. 2003; Lee et al. 2006). So, to investigate a functional role for LRH-1 in spermatozoa, we hypothesized a possible link between the E2/ER pathway and LRH-1 in male gametes. We therefore studied some sperm representative events such as motility and survival, by treating the cells with estradiol and inhibiting LRH-1 signaling. Our data confirmed the stimulatory estrogen action on motility and viability (Aquila et al. 2003, 2004) and demonstrated that LRH-1 is part of estrogen signaling, since its inhibition reduced the E2-induced effects. Even if the receptor is mainly located in the sperm head, on the basis of our results, we can speculate that LRH-1 influences the sperm motility through a link with the ERβ shown on the sperm tail (Aquila et al. 2004; Guido et al. 2011), although the molecular mechanism needs to be defined.
Because of the critical roles of this receptor in human physiology and pathophysiology, identification of specific regulatory ligands, modulators of LRH-1 activity, is extremely important. Recently, a synthetic antagonist of LRH-1 has been described (Benod et al. 2013). This cell-permeable pyrazolylbiphenylethanone compound targets the LRH-1/NR5A2 ligand binding domain via direct affinity interaction, preventing LRH-1 from assuming an active conformation. Estrogenic signaling in spermatozoa has been shown to have a number of rapid effects, including the activation of phosphatidylinositol 3-kinase (PI3K) and the mitogen-activated protein kinase (MAPK) pathways (Aquila et al. 2003, 2004). It was shown that LRH-1 activity can be also modulated by posttranslational events and it was reported that ERK enhances human LRH-1 activation (Lee et al. 2006). Nonetheless, the stimulation of MAPK and PI3K signaling pathways by estrogen coincides with mechanisms proposed to activate NR5A nuclear receptors, including LRH-1, in different cell types (Lin et al. 2009). LRH-1 has also been shown to be a downstream effector of estrogen-mediated cell proliferation (Annicotte et al. 2005). From the above observations we assume that the effects of LRH-1 are closely integrated with the estrogen signaling pathway in spermatozoa, too.
Morphologically mature spermatozoa leaving the testis undergo several functional maturation steps. They acquire the potential for motility in the epididymis, and complete fertilization capacity in vivo during their migration through the female reproductive tract (Austin, 1951; Chang, 1951). The biological processes that allow the ejaculated spermatozoa to be able to penetrate and fertilize an egg have been termed capacitation (Yanagimachi, 1994; Visconti & Kopf, 1998).
Undoubtedly, the membrane cholesterol efflux is important in starting transmembrane signaling events and successive intracellular signal transduction, leading to the acquisition of fertilizing capacity (De Lamirande et al. 1997; Travis & Kopf, 2002). LRH-1 plays important roles in metabolism, being involved in the regulation of cholesterol homeostasis and steroidogenesis (Fayard et al. 2004), so the evidence of new regulators of cholesterol efflux in spermatozoa is of great interest. On the basis of our data, we discovered in human spermatozoa a new expression site for LRH-1 which appears partly to mediate E2 action on cholesterol efflux. Therefore, in spermatozoa, estradiol may modulate the activity of LRH-1, which can be considered a downstream target of the estrogenic stimuli.
Previous studies have evidenced LRH-1 expression in human and rat testis (Sirianni et al. 2002; Pezzi et al. 2004) as well as in rat round spermatids and pachytene spermatocytes (Pezzi et al. 2004; Sierens et al. 2010). In spermatozoa, estrogens activate the ERs and consequently initiate several intracellular signaling enzymes such as receptor tyrosine kinase, phosphatidylinositol 3-kinase, Akt, Src, MAPK, protein kinase C, phospholipase D and phospholipase C. From our data, it appears that estrogens also are able to activate LRH-1 signaling in male gamete.
The identification of signaling pathways that modulate the LRH-1 activity as well as further functional studies are of great importance to investigate LRH-1 biology. Our study may represent a good starting point for further exploration that will help identify the role of this nuclear receptor in health and disease, validating LRH-1 as a novel therapeutic target for the treatment of male fertility disorders.
Acknowledgments
Our special thanks to Dr. Vincenzo Cunsolo (Biogemina Italia Srl, Catania, Italy) for technical and scientific assistance. We would like also to thank Enrico Perrotta for excellent technical and scientific assistance and Maria Clelia Gervasi for the English language review of the manuscript.
Author contributions
Daniela Montanaro: acquisition and critical evaluation of Western blotting data; Marta Santoro: culturing of the cells, performing experimental conditions, survival and motility analysis, interpretation of data and critical revision for intellectual content; Amalia Carpino: acquisition, interpretation and critical revision of immunohistochemical data; Ida Perrotta: acquisition and interpretation and critical revision of immunoelectron microscopy data; Francesca De Amicis: interpretation of data; Rosa Sirianni: acquisition and critical interpretation of RT-PCR data; Vittoria Rago: acquisition of immunohistochemical data; Serena Gervasi: interpretation of data and revision of English language; Aquila Saveria: conception, design, analysis, interpretation of data, critical revision for important intellectual content, final approval of the submitted version of the article.
Disclosure of interests
The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
Supporting Information
Fig. S1. The histograms show quantification of gold particles associated with the different regions of the sperm cell. Data are presented as mean ± SEM of the gold particles counted in 100 cells from six fields. *P < 0.01 vs. nucleus.
References
- Adeoya-Osiguwa SA, Markoulaki S, Pocock V, et al. 17beta-Estradiol and environmental estrogens significantly affect mammalian sperm function. Hum Reprod. 2003;18:100–107. doi: 10.1093/humrep/deg037. [DOI] [PubMed] [Google Scholar]
- Annicotte JS, Fayard E, Swift GH, et al. Pancreatic-duodenal homeobox 1 regulates expression of liver receptor homolog 1 during pancreas development. Mol Cell Biol. 2003;23:6713–6724. doi: 10.1128/MCB.23.19.6713-6724.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annicotte JS, Chavey C, Servant N, et al. The nuclear receptor liver receptor homolog-1 is an estrogen receptor target gene. Oncogene. 2005;24:8167–8175. doi: 10.1038/sj.onc.1208950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquila S, Sisci D, Gentile ME, et al. Human ejaculated spermatozoa contain active P450 aromatase. J Clin Endocrinol Metab. 2002;87:3385–3390. doi: 10.1210/jcem.87.7.8633. [DOI] [PubMed] [Google Scholar]
- Aquila S, Sisci D, Gentile M, et al. Towards a physiological role for cytochrome P450 aromatase in ejaculated human sperm. Hum Reprod. 2003;18:1650–1659. doi: 10.1093/humrep/deg340. [DOI] [PubMed] [Google Scholar]
- Aquila S, Sisci D, Gentile ME, et al. Estrogen receptor (ER) α and ER β are both expressed in human ejaculated spermatozoa: evidence of their direct interaction with phosphatidylinositol-3-OH kinase/Akt pathway. J Clin Endocrinol Metab. 2004;89:1443–1451. doi: 10.1210/jc.2003-031681. [DOI] [PubMed] [Google Scholar]
- Aquila S, Bonofiglio D, Gentile M, et al. Peroxisome proliferator-activated receptor (PPAR)gamma is expressed by human spermatozoa: its potential role on the sperm physiology. J Cell Physiol. 2006;209:977–986. doi: 10.1002/jcp.20807. [DOI] [PubMed] [Google Scholar]
- Aquila S, Middea E, Catalano S, et al. Human sperm express a functional androgen receptor: effects on PI3K/AKT pathway. Hum Reprod. 2007;22:2594–2605. doi: 10.1093/humrep/dem243. [DOI] [PubMed] [Google Scholar]
- Aquila S, Guido C, Perrotta I, et al. Human sperm anatomy: ultrastructural localization of 1alpha,25-dihydroxyvitamin D receptor and its possible role in the human male gamete. J Anat. 2008;213:555–564. doi: 10.1111/j.1469-7580.2008.00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquila S, Guido C, Santoro A, et al. Human sperm anatomy: ultrastructural localization of the cannabinoid1 receptor and a potential role of anandamide in sperm survival and acrosome reaction. Anat Rec. 2010a;293:298–309. doi: 10.1002/ar.21042. [DOI] [PubMed] [Google Scholar]
- Aquila S, Guido C, Santoro A, et al. Rimonabant (SR141716) induces metabolism and acquisition of fertilizing ability in human sperm. Br J Pharmacol. 2010b;159:831–841. doi: 10.1111/j.1476-5381.2009.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin CR. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B. 1951;4:581–596. doi: 10.1071/bi9510581. [DOI] [PubMed] [Google Scholar]
- Benod C, Carlsson J, Uthayaruban R, et al. Structure-based discovery of antagonists of nuclear receptor LRH-1. J Biol Chem. 2013;288:19830–19844. doi: 10.1074/jbc.M112.411686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerboom D, Pilon N, Behdjani R, et al. Expression and regulation of transcripts encoding two members of the NR5A nuclear receptor subfamily of orphan nuclear receptors, steroidogenic factor-1 and NR5A2, in equine ovarian cells during the ovulatory process. Endocrinology. 2000;141:4647–4656. doi: 10.1210/endo.141.12.7808. [DOI] [PubMed] [Google Scholar]
- Cappello AR, Guido C, Santoro A, et al. The mitochondrial citrate carrier (CIC) is present and regulates insulin secretion by human male gamete. Endocrinology. 2012;153:1743–1754. doi: 10.1210/en.2011-1562. [DOI] [PubMed] [Google Scholar]
- Carreau S, Bourguiba S, Lambard S, et al. Reproductive system: aromatase and estrogens. Mol Cell Endocrinol. 2002;193:137–143. doi: 10.1016/s0303-7207(02)00107-7. [DOI] [PubMed] [Google Scholar]
- Carreau S, Bouraima-Lelong H, Delalande C. Role of estrogens in spermatogenesis. Front Biosci. 2012;4:1–11. doi: 10.2741/e356. [DOI] [PubMed] [Google Scholar]
- Cato AC, Nestl A, Mink S. Rapid actions of steroid receptors in cellular signaling pathways. Sci STKE. 2002;138:re9. doi: 10.1126/stke.2002.138.re9. [DOI] [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697–698. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Boettcher B. The effect of steroids on the in vitro migration of washed human spermatozoa in modified Tyrode’s solution or in fasting human blood serum. Fertil Steril. 1979;32:566–570. doi: 10.1016/s0015-0282(16)44361-x. [DOI] [PubMed] [Google Scholar]
- De Amicis F, Guido C, Perrotta I, et al. Conventional progesterone receptors (PR) B and PRA are expressed in human spermatozoa and may be involved in the pathophysiology of varicocoele: a role for progesterone in metabolism. Int J Androl. 2011;34:430–445. doi: 10.1111/j.1365-2605.2010.01111.x. [DOI] [PubMed] [Google Scholar]
- De Amicis F, Santoro M, Guido C, et al. Progesterone through progesterone receptors affects survival and metabolism of pig sperm. Anim Reprod Sci. 2012;135:75–84. doi: 10.1016/j.anireprosci.2012.09.004. [DOI] [PubMed] [Google Scholar]
- De Lamirande E, Leclerc P, Gagnon C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol Hum Reprod. 1997;3:175–194. doi: 10.1093/molehr/3.3.175. [DOI] [PubMed] [Google Scholar]
- Durkee TJ, Mueller M, Zinaman M. Identification of estrogen receptor protein and messenger ribonucleic acid in human spermatozoa. Am J Obstet Gynecol. 1998;178:1288–1297. doi: 10.1016/s0002-9378(98)70335-7. [DOI] [PubMed] [Google Scholar]
- Fayard E, Auwerx J, Schoonjans K. LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol. 2004;14:250–260. doi: 10.1016/j.tcb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Guido C, Perrotta I, Panza S, et al. Human sperm physiology: estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) influence sperm metabolism and may be involved in the pathophysiology of varicocele-associated male infertility. J Cell Physiol. 2011;226:3403–3412. doi: 10.1002/jcp.22703. [DOI] [PubMed] [Google Scholar]
- Hinshelwood MM, Repa JJ, Shelton JM, et al. Expression of LRH-1 and SF-1 in the mouse ovary: localization in different cell types correlates with differing function. Mol Cell Endocrinol. 2003;207:39–45. doi: 10.1016/s0303-7207(03)00257-0. [DOI] [PubMed] [Google Scholar]
- Lee YK, Choi YH, Chua S, et al. Phosphorylation of the hinge domain of the nuclear hormone receptor LRH-1 stimulates transactivation. J Biol Chem. 2006;281:7850–7855. doi: 10.1074/jbc.M509115200. [DOI] [PubMed] [Google Scholar]
- Lin BC, Suzawa M, Blind RD, et al. Stimulating the GPR30 estrogen receptor with a novel tamoxifen analogue activates SF-1 and promotes endometrial cell proliferation. Cancer Res. 2009;69:5415–5423. doi: 10.1158/0008-5472.CAN-08-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DL, Liu WZ, Li QL, et al. Expression and functional analysis of liver receptor homologue 1 as a potential steroidogenic factor in rat ovary. Biol Reprod. 2003;69:508–517. doi: 10.1095/biolreprod.102.011767. [DOI] [PubMed] [Google Scholar]
- Morley P, Whitfield JF, Vanderhyden BC, et al. A new, nongenomic estrogen action: the rapid release of intracellular calcium. Endocrinology. 1992;131:1305–1312. doi: 10.1210/endo.131.3.1505465. [DOI] [PubMed] [Google Scholar]
- O’Donnell L, Robertson KM, Jones ME, et al. Estrogen and spermatogenesis. Endocr Rev. 2001;22:289–318. doi: 10.1210/edrv.22.3.0431. [DOI] [PubMed] [Google Scholar]
- Pezzi V, Sirianni R, Chimento A, et al. Differential expression of steroidogenic factor-1/adrenal 4 binding protein and liver receptor homolog-1 (LRH-1)/fetoprotein transcription factor in the rat testis: LRH-1 as a potential regulator of testicular aromatase expression. Endocrinology. 2004;145:2186–2196. doi: 10.1210/en.2003-1366. [DOI] [PubMed] [Google Scholar]
- Sierens J, Jakody I, Poobalan Y, et al. Localization and regulation of aromatase liver receptor homologue-1 in the developing rat testis. Mol Cell Endocrinol. 2010;323:307–313. doi: 10.1016/j.mce.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Sirianni R, Seely JB, Attia G, et al. Liver receptor homologue-1 is expressed in human steroidogenic tissues and activates transcription of genes encoding steroidogenic enzymes. J Endocrinol. 2002;174:R13–R17. doi: 10.1677/joe.0.174r013. [DOI] [PubMed] [Google Scholar]
- Tesarik J, Carreras A, Mendoza C. Differential sensitivity of progesterone- and zona pellucida-induced acrosome reactions to pertussis toxin. Mol Reprod Dev. 1993;34:183–189. doi: 10.1002/mrd.1080340210. [DOI] [PubMed] [Google Scholar]
- Travis AJ, Kopf GS. The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J Clin Invest. 2002;110:731–736. doi: 10.1172/JCI16392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti PE, Kopf GS. Regulation of protein phosphorylation during sperm capacitation. Biol Reprod. 1998;59:1–6. doi: 10.1095/biolreprod59.1.1. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Laboratory Manual for the Examination and Processing of Human Semen. 5th edn. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill J, editors. Physiology of Reproduction. New York: Raven Press; 1994. pp. 189–317. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. The histograms show quantification of gold particles associated with the different regions of the sperm cell. Data are presented as mean ± SEM of the gold particles counted in 100 cells from six fields. *P < 0.01 vs. nucleus.


