Abstract
Since their discovery in different human tissues by Zimmermann in 1898, primary cilia have been found in the vast majority of cell types in vertebrates. Primary cilia are considered to be cellular antennae that occupy an ideal cellular location for the interpretation of information both from the environment and from other cells. To date, in mammalian thyroid gland, primary cilia have been found in the thyrocytes of humans and dogs (fetuses and adults) and in rat embryos. The present study investigated whether the existence of this organelle in follicular cells is a general event in the postnatal thyroid gland of different mammals, using both immunolabeling by immunofluorescence and electron microscopy. Furthermore, we aimed to analyse the presence of primary cilia in various thyroid cell lines. According to our results, primary cilia are present in the adult thyroid gland of most mammal species we studied (human, pig, guinea pig and rabbit), usually as a single copy per follicular cell. Strikingly, they were not found in rat or mouse thyroid tissues. Similarly, cilia were also observed in all human thyroid cell lines tested, both normal and neoplastic follicular cells, but not in cultured thyrocytes of rat origin. We hypothesize that primary cilia could be involved in the regulation of normal thyroid function through specific signaling pathways. Nevertheless, further studies are needed to shed light on the permanence of these organelles in the thyroid gland of most species during postnatal life.
Keywords: follicular cells, mammals, primary cilia, thyroid gland
Introduction
Cilia are specialized dynamic organelles projecting from the cell surface that are composed of a basal body (the older centriole of the centrosome) and an emerging structure called axoneme, surrounded by the ciliary membrane, a specialized domain extension of the cell membrane. The axoneme contains nine doublet microtubules that originate at the triplet microtubules of the basal body and extend the length of the cilium and may exhibit a central microtubule pair (Satir & Christensen, 2007). On the basis of their structure and function, two main classes of cilia are considered: ‘secondary cilia’, with a 9 + 2 pattern, which are motile and present on multiciliated epithelial cells, and ‘primary cilia’, with a 9 + 0 pattern, which are usually solitary and non-motile, with the exception of motile primary cilia present on the node (Sulik et al. 1994; D’Angelo & Franco, 2009).
Since their discovery in different human tissues by Zimmermann in 1898, primary cilia have been found in the vast majority of cell types in vertebrates, such as renal tubules (Flood & Totland, 1977), bile ducts (De La Iglesia & Porta, 1967; Huang et al. 2006), pancreas (Aughsteen, 2001), neurons (Fuchs & Schwark, 2004; Whitfield, 2004), keratinocytes, endothelial cells and fibroblasts (Wandel et al. 1984). However, only in the last few years have investigations intensified about their possible functions (Wheatley, 2008). In addition to their well-established roles in sensory cells, as represented by photoreceptor cells (sight), vestibular cells (equilibrium), and olfactory cells (smell), evidence is accumulating that primary cilia in non-sensory cells may also function as sensory devices – mechanosensors or chemosensors – at a cellular level (Horst et al. 1987; Boekhoff et al. 1990; Praetorius & Spring, 2001; Singla & Reiter, 2006; Saqui-Salces et al. 2012).
In addition to these roles, the resorption and protrusion of primary cilia are regulated by cell cycle transitions (Pan et al. 2013). In cultured cells, primary cilia have been observed in serum-starved and confluent cultures during the stationary phase of the cell cycle (G0), and it is generally accepted that primary cilia are disassembled prior to mitosis so that the centrioles can function at the poles of the mitotic spindle (Tucker et al. 1979; Paridaen et al. 2013). Also, recent evidence suggests cancer cell lines and cells in primary tumors, which have deregulated cell growth, commonly lack cilia (Pan et al. 2013).
Since their discovery in humans (Zimmermann, 1898), in the thyroid gland, primary cilia have been described as emerging from the apex of follicular cells. Subsequently, ciliated follicular cells were reported in the thyroid gland of some embryos and adult vertebrate species, such as fish (Cowdry, 1921; Fujita & Machino, 1965), amphibians (Coleman et al. 1968; Larsen, 1968), birds (Fujita, 1963; Hilfer, 1979), reptiles (Rupik, 2013) and dogs (Nunez & Gershon, 1976). Moreover, several authors have observed primary cilia in human follicular cells of fetuses and adult thyroid glands using transmission or scanning electron microscopy (Klinck et al. 1970; Sobrinho-Simoes & Johannessen, 1981; Chan, 1983; Martin et al. 1988), reaching the conclusion that almost all follicular cells were endowed with between one and five of such organelles. To date, no other species of mammals have been reported to present primary cilia in thyroid follicular cells.
Therefore, the present study investigated whether the existence of primary cilia in follicular cells is a general event in the adult thyroid gland of different mammals. To accomplish that aim, we designed the immunolabeling of cilia by immunofluorescence, a method that facilitates the assessment of a larger number of cells and tissue area than electron microscopy, the previously used method for studies of thyroid primary cilia. However, we also used both scanning and transmission electron microscopy to study primary cilia morphology. Furthermore, we investigated the presence of primary cilia in the established cell-line models most widely used for the study of thyroid follicular cell function.
Materials and methods
Thyroid tissues
In this study, we analysed normal adult thyroid glands from several mammalian species: human (n = 5), guinea pig (n = 5), rat (n = 7), mouse (n = 4), rabbit (n = 3) and pig (n = 4). Human samples were obtained from patients undergoing thyroid surgery, diagnosed at the Department of Pathology of the University Hospital Virgen Macarena of Seville in the 1980s. Samples of guinea pig, rat and mouse were obtained from the Experimental Animal Service of the Medical School of Seville; pig samples were obtained from The Institute of Biomedicine of Seville (IBIS); and rabbit samples were obtained from The Production Center and Animal Experimentation, University of Seville (CPEA). All animals were sacrificed for several other purposes, and none of them had suffered from diseases related to the thyroid gland. Animals were anesthetized with intraperitoneal (i.p.) injection of ketamine/Diacepam in a specific dose (mg kg−1) depending on the species. Tissue samples were collected with approval from the Research Ethics Committee of the Virgen Macarena University Hospital (C.P.-C.I. 1921). All experiments were conducted in accordance with guidelines proposed in The Declaration of Helsinki (http://www.wma.net) involving the use of laboratory animals.
Thyroid glands were fixed in 10% neutral buffered formalin, embedded in paraffin using a standard procedure, sectioned at 4–5 μm thickness, and mounted on silane-coated glass slides. Consecutive tissue sections were stained with hematoxylin-eosin for histological diagnosis and to select thyroid tissue with a normal appearance to perform immunofluorescence (IF).
Specimens for scanning (SEM) and transmission (TEM) electron microscopy were obtained from the normal-appearing thyroid parenchyma adjacent to three different thyroid lesions. The Ethical Committee of the University Virgen Macarena Hospital of Seville approved the study. Patients gave written informed consent (C.P.-C.I. 1921). For TEM studies, pieces of human, pig and rat thyroid glands of approximately 1 mm3 in size were fixed for 4 h in 2.5% glutaraldehyde in 0.1 m cacodylate buffer (pH 7.2–7.4) at 4 °C and post-fixed in 1% osmium tetroxide (1 h at 4 °C). Specimens were then dehydrated in a graded acetone series and embedded in Spurr. Semithin sections were used to select areas with intact follicles. Ultrathin sections were then prepared without contrast, and photographed with a Philips CM 10 transmission electron microscope.
Similarly, for SEM studies, the fixation of samples of human, pig and rat thyroid glands was performed in the same glutaraldehyde solution, but extended for several days at room temperature, followed by post-fixation in 1% osmium tetroxide (1 h at 4 °C). After dehydration in a graded acetone series, specimens were thoroughly dried with the critical point method using CO2 and a Balzer critical point drier (CPD030, Leica, Germany), then sputter-coated with vacuum-evaporated gold, and photographed with a JEOL 6460 LV scanning electron microscope (Tokyo, Japan).
Cell cultures
Human cell lines Nthy-ori 3-1 (normal follicular cells, ECACC 90011609, UK) and 8505C (anaplastic thyroid carcinoma, ECACC 94090184, UK) were cultured in RPMI 1640 medium (Biowest); FTC-133 (follicular thyroid carcinoma, ECACC 94060901) in Ham’s F12 medium (Biowest). Every culture medium was supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, Germany), 2 mm L-glutamine, 50 U mL−1 penicillin and 50 μg mL−1 streptomycin.
Rat cell line PC-Cl3 (follicular cells, generously provided by Dr. Massimo Santoro, Centro di Endocrinologia e Oncologia Sperimentale di C.N.R Naples, Naples, Italy) was grown in 6H medium consisting of Coon’s modified F-12 (Biochem, Berlin, Germany) medium supplemented with 5% FBS, 2 mM L-glutamine, 50 U mL−1 penicillin, 50 μg mL−1 streptomycin and a mixture of six hormones – bovine TSH (10 U L−1), insulin (10 mg L−1), hydrocortisone (0.4 mg L−1), human transferrin (5 mg L−1), glycyl-L-histidyl-L-lysine acetate (10 μg L−1) and somatostatin (10 μg L−1). FRT rat cell line (follicular cells, generously provided by Dr. Pilar Santisteban, Instituto de Investigaciones Biomédicas ‘Alberto Sols’, Spain) was maintained in the same medium without the hormone mixture.
For experiments, cells were seeded at approximately 70% of confluence in a slide chamber (Lab-Tek Chamber Slide System 154534, Thermo Fisher Scientific Inc., USA) and grown until confluence to allow ciliogenesis (Tucker et al. 1979).
Immunofluorescence study on thyroid sections
Sections were dewaxed in xylene and hydrated through graded alcohols. Afterwards, an antigen retrieval step using EDTA buffer, pH 9 (Dako, Denmark) was performed in a heating instrument, PTLink (Dako), at 96 °C for 20 min, according to the manufacturer’s instructions. The slides were immersed in a washing solution (Dako Wash Buffer) for 5 min, and nonspecific binding was blocked with 10% normal donkey serum for 15 min (Jackson ImmunoResearch Laboratories, UK). The primary antibody, a monoclonal anti-acetylated α-tubulin (1 : 20 000, Sigma-Aldrich, Germany), which labels the axoneme, was added for 1 h at room temperature in a humidified chamber. Slides were washed and incubated with Cy3-labeled donkey anti-mouse IgG secondary antibody (1 : 100, Jackson ImmunoResearch Laboratories) for 30 min at room temperature in a humidified chamber. When double IF was performed, the slides were then incubated with polyclonal rabbit anti-E-cadherin antibody (1 : 100, Santa Cruz Biotechnology, USA) and, subsequently, with Cy2-labeled donkey anti-rabbit IgG antibody (1 : 100, Jackson ImmunoResearch Laboratories) under the same conditions as before. After washing in PBS, DAPI (Sigma-Aldrich) was added for nuclei counterstaining, and the slides were coverslipped with Mowiol 4-88 (Sigma-Aldrich). Samples were visualized with a fluorescent microscope or with a laser scanning confocal microscope (Leica, TCS-SP2, Germany) and analysed with Leica Confocal Software. Primary cilia lengths were morphometrically assessed using Image PRO-PLUS 7.0 software (Media Cybernetics, Rockville, MD, USA). To avoid underestimation of the obliquely sectioned cilia length, we used confocal microscopy z–axis stacks in which primary cilia were fully included within the 5-μm paraffin sample (Supporting Information Videos S1 and S2).
Controls for specificity of IF were performed as follows: (i) omitting any essential step of the immunoreaction and (ii) replacing the primary antibody with an appropriate dilution of IgG mouse serum (Sigma-Aldrich), followed by the IF protocol as outlined above. As a positive control, we used rat trachea and human pancreas sections. All experiments were repeated at least twice to ensure the consistency of the results.
To evaluate the frequency of cilia in different thyroid sections, the number of primary cilia was semiquantitatively estimated by fluorescence microscopy at low and higher magnification by two independent observers in a blinded manner. First, the relative amount of ciliated vs. non-ciliated follicles was estimated by analysing a minimum of 50 follicles per sample for the presence of follicular cells displaying primary cilia. Afterwards, the frequency of ciliated vs. non-ciliated follicular cells was assessed by analysing the relative number of cilia protruding from the apical surface of the epithelium vs. the number of nuclei in adequately oriented sections of those thyroid follicles.
Immunolabeling of primary cilia in cell culture
For immunodetection of primary cilia, cells were serum-starved for 48 h before experiments. Cells were double-fixed, first with 4% paraformaldehyde for 10 min followed by cold methanol for 5 min. Slides were washed three times in phosphate buffered saline (PBS) after each fixation. Then, cells were incubated with the primary monoclonal antibody anti-acetylated α-tubulin (clon 6-11B-1, 1 : 10 000, Sigma-Aldrich) for 1 h at room temperature. Afterwards, slides were incubated for 40 min with anti-mouse secondary antibodies conjugated to Cy-3 (1 : 200, Jackson ImmunoResearch Laboratories, Newmarket, UK). After washing in PBS, cells were incubated for 1 h with polyclonal goat anti-γ-tubulin (1 : 50, Santa Cruz Biotechnology) at room temperature and washed in PBS. Slides were then incubated for 40 min with FITC-labeled anti-goat secondary antibodies (1 : 200, Jackson ImmunoResearch Laboratories). After washing in PBS, DAPI was added for nuclei counterstaining. Finally, slides were mounted with antifading mounting medium (Mowiol 4-88) and observed under a fluorescence microscope (Olympus BX50, Hamburg, Germany). Images were acquired using an ORCA-03G digital camera (Hamamatsu, Bridgewater, USA) and analysed using Image pro-plus 7.0 software (Media Cybernetics). Immunodetection was performed in seven independent experiments. Quantification of ciliated vs. non-ciliated cells was performed using fluorescence microscopy, counting a minimum of 400 nuclei from at least seven fields from at least two independent experiments for every thyroid cell line.
Results
Primary cilia detection by immunofluorescence in thyroid tissues
The presence of both secondary and primary cilia was detected using acetylated α-tubulin antibody in different paraffin-embedded tissue samples. Therefore, motile cilia observed in the tracheal epithelium (Figs1 and 2) and primary cilia in renal tubules and pancreatic ducts (in rats, mice and humans, data not shown) were used as positive controls. In thyroid glands, primary cilia were found in human, rabbit, pig and guinea-pig thyroid follicles; no cilia were observed in either rats or mice (Figs1 and 2). Generally, primary cilia emerged from the apical surface of follicular cells and entered the colloid perpendicularly.
Figure 1.
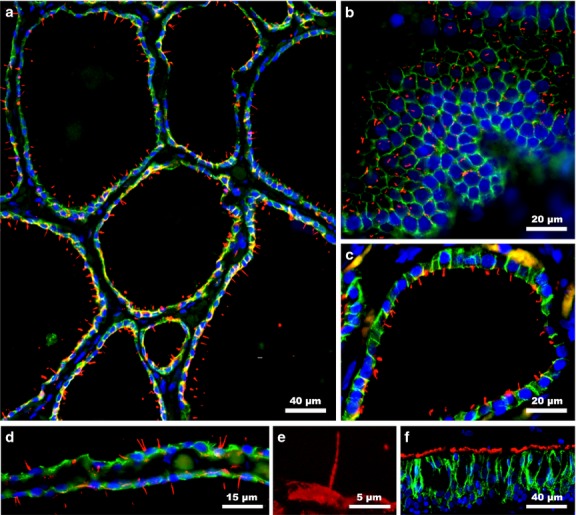
Primary cilia are present in human folli-cular cells. (a) Thyroid follicles exhibit numerous cilia oriented towards the colloid. (b) Primary cilia are located in the center of the apical cell surfaces (tangential section). Virtually every follicular cell displays at least one cilium (c). However, the presence of two primary cilia emerging in close proximity is not uncommon (d). Detail of the primary cilia (e). Secondary cilia of the tracheal epithelium are demonstrated as positive control (f). Double immunofluorescence staining: E-cadherin (green); acetylated α-tubulin (red); blue (nuclear counterstaining with DAPI).
Figure 2.
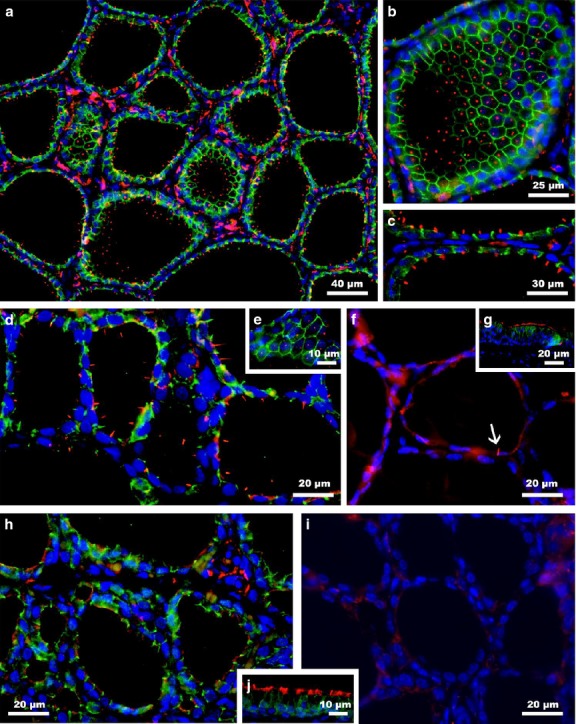
Primary cilia are observed in follicular cells of pigs (a-c), rabbits (d-e) and guinea pigs (f). Cilia are abundant in the follicular epithelium of pig and rabbit thyroid glands but are very scarce in guinea pigs (arrow). Normally, they emerged as single elements from the center of the cell apex, as observed in tangential sections of thyroid follicles (b,e). In contrast to those species, primary cilia were not detected in either rat (h) or mouse thyroid tissue (i). Ciliary tracheal epithelium from guinea pig (g) or rat (j) was used as positive control. Double immunofluorescence staining: E-cadherin (green); acetylated α-tubulin (red); blue (nuclear counterstaining with DAPI).
In human thyroid gland, all thyroid follicles exhibit ciliated cells. When the same material was analysed at higher magnification, nearly every follicular cell harbored a unique primary cilium, although there were some cells that showed two cilia or, even more rarely, a larger number of cilia (Fig.1). In those cases, cilia emerged usually close together and in a V- shape (Fig.1d). To confirm their location and frequency, E-cadherin labeling was performed to mark the epithelial cell perimeter. Thus, the cilium was emergent in tangential sections of the thyroid follicles mainly from the central area of the apical surface of each follicular cell (Fig.1b). Several thyroid follicles were then randomly chosen, and the mean length of primary cilia was confirmed to be 7.3 ± 1.2 μm (maximum 10.7 μm, minimum 5.0 μm).
In the case of pig and rabbit thyroid follicles, a very similar distribution pattern of primary cilia to that described in human was observed because almost every follicular cell exhibited at least one primary cilium (Fig.2a–e). In particular, the mean length of the cilia in rabbits was 5.2 ± 2.4 μm (maximum 8.8 μm, minimum 2.9 μm), whereas in pigs, the cilia were somewhat shorter, with a mean length of 4.6 ± 0.7 μm (maximum 5.7 μm, minimum: 4.0 μm). Nevertheless, in guinea pig, one of 50 follicles were ciliated follicles and, among them, there were very few ciliated cells, less than one of 20 follicular cells (Fig.2f). On such occasions, the cilia looked very similar to those described above, with a mean length of 4.9 ± 1.4 μm (maximum 7.1 μm, minimum 3.2 μm). Finally, and surprisingly, in rat and mouse thyroid samples, no primary cilia were found, not even sporadically (Fig.2g,h).
Ultrastructure of primary cilia in thyroid tissue
TEM analysis of specimens of human and pig thyroid glands revealed a sporadic presence of primary cilia located on the apical surface of follicular cells protruding into the colloid (Fig.3). Usually, only one cilium per cell was present, although two cilia were occasionally observed together. Proximally, the cilium ended in a typical basal body, in whose proximity a centriole was frequently observed (Fig.3e), although not always at a right angle. Occasionally, ciliary rootlets were visible, extending from the vicinity of the basal body (Fig.3f). In longitudinal sections, a thinning at the distal end of the cilia was observed. When cross-sectioned, the typical 9 + 0 pattern of primary cilia was clearly recognizable at the base of the shaft.
Figure 3.
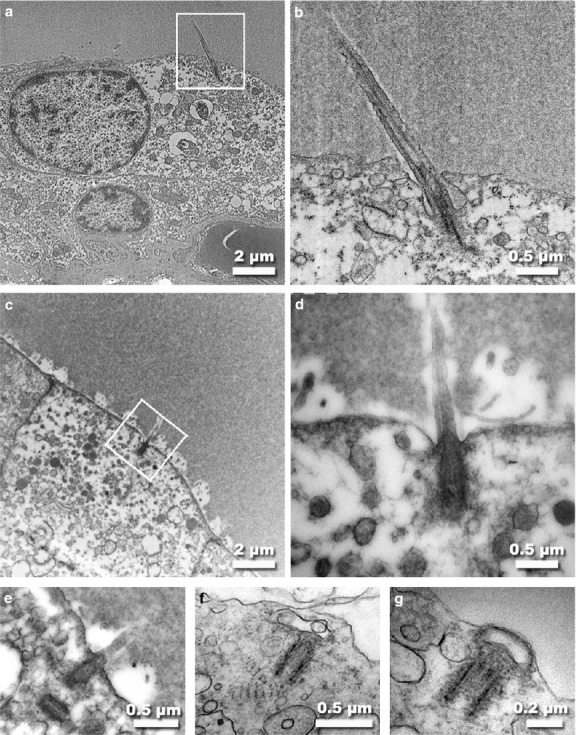
TEM micrographs of human (a,b) and pig (c-g) thyroid follicular cells. As observed in the apical surface of the human thyrocyte (a), a long primary cilium extends up into the colloid. At higher magnification (b), the basal body and microtubular doublets within the ciliary shaft are observed. In pig thyroid tissue, a much shorter primary cilium is found emerging from the apex into the lumen (c-e). The cilium ended in a typical basal body, in whose proximity a centriole was frequently observed (e). On some occasions, a pair of rootlets was found near the basal body (f) or the ciliary membrane was present in association with the centriole after cilium retraction (g).
SEM observation of thyroid samples from human (Fig.4) and pig (Fig.5) revealed that follicular cells had a polyhedral outline with four to seven sides. The cell surface was generally convex and presented numerous microvilli, and one primary cilia emerged among them from the geometric center of the cell protruding into the follicular lumen. Frequently in human thyroid cells, more than one cilium was observed (Fig.4c,d), often emerging in the characteristic V-shaped pattern (Fig.4e,f). Conversely, follicular cells from the rat thyroid gland always lacked primary cilia, although sharing the same polygonal morphology with other species (Fig.5d).
Figure 4.
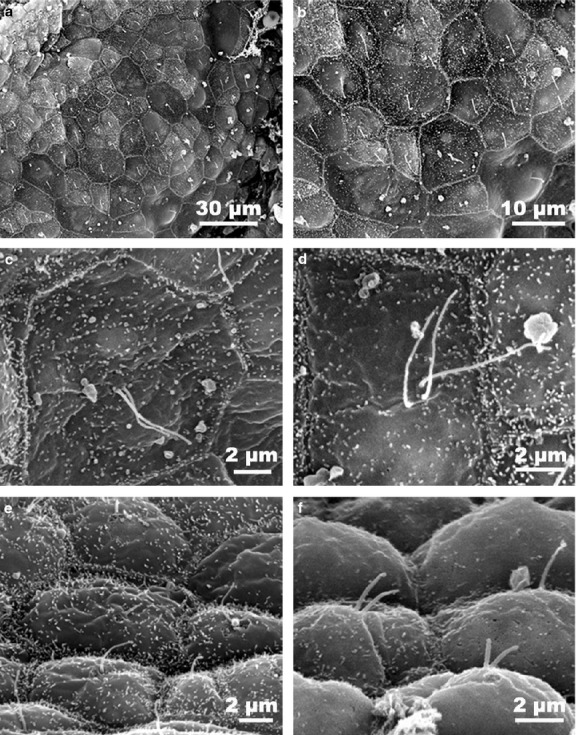
SEM micrographs of primary cilia in hu-man thyroid follicular cells. Cells with polyhedral outlines and protruding central cilia line the follicular lumen (a,b). At higher magnification, numerous microvilli can be observed on the apical cell surface with mostly one, two or even more considerably long cilia (c,d) emerging among them. When a lateral focus of the epithelium was obtained (e,f), it was possible to see the pronounced convexity of the cellular apex, in addition to the characteristic V-shaped pattern of the primary cilia.
Figure 5.
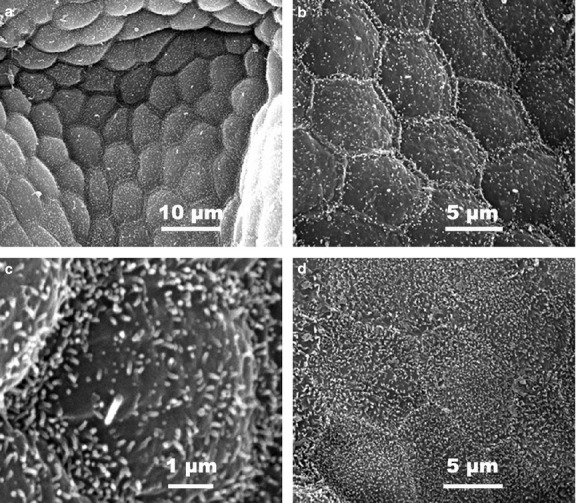
SEM micrographs of pig (a–c) and rat (d) thyroid follicular cells. In the pig thyroid gland, cell apexes are slightly raised with a moderate density of microvilli (a). At higher magnification, follicular cells display a very regular polyhedral outline together with a unique and short primary cilium emerging from the apical surface (b,c). Conversely, in rat follicular cells (d), no primary cilia are observed but the microvilli are rather more abundant.
Identification of primary cilia in cell cultures by immunofluorescence
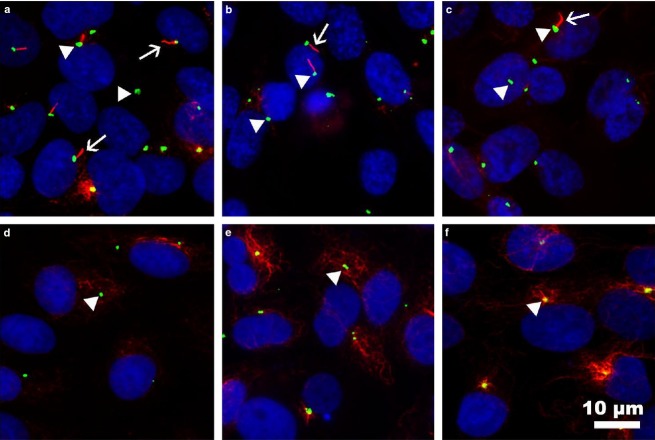
To confirm the formation of primary cilia in cultures of thyroid cells, we performed double IF analysis using an anti-acetylated α-tubulin antibody in addition to an anti-γ-tubulin antibody, which specifically labels the basal body. Cells were grown in media without serum for 48 h to induce cell cycle arrest and promote primary cilia assembly. As shown in Fig.6, primary cilia were observed in all human thyroid cell lines tested, both normal and cancerous, although clearly with a lower frequency in cancer cell lines. Specifically, the approximate percentages of ciliated cells were 43% for Nthy-ori 3-1, 21% for FTC-133, and 7% for 8505C cell line. Strikingly, primary cilia were not found in FRT and PC-Cl3 rat thyroid cells tested. As expected, primary cilia were absent in human thyroid cells grown in the presence of serum (Fig.6).
Figure 6.
Immunofluorescence staining of primary cilia in human and rat follicular cell lines. Primary cilia were studied in human Nthy-ori 3-1 (a,f), FTC-133 (b), and 8505C (c) and rat PC-Cl3 (d), and FRT (e) cell lines by staining the axonemes with anti-acetylated α-tubulin (red) and the basal bodies with anti-γ-tubulin (green). Nuclei were labeled with DAPI. Primary cilia were present in approximately 43% of normal human follicular cells (Nthy-ori 3-1), in 21% of neoplastic human FTC-133 cells and in 7% of anaplastic 8505C cells, but were absent in all the rat cell lines tested (PC-Cl3, FRT) and non-starved human control Nthy-ori 3-1 cells (f).
Discussion
In the present study, we show that primary cilia are present in the adult thyroid glands of human, pig, guinea pig and rabbit, usually as a single copy per follicular cell. We also observed primary cilia in all human thyroid cell lines tested, both in normal (Nthy-ori 3-1) and neoplastic follicular cells (FTC-133 and 8505C), although at a lower frequency in the latter. Strikingly, they were not found in mouse and rat postnatal thyroid tissues and, accordingly, cilia were not identified in cultured thyrocytes of rat origin (FRT and PC-Cl3).
Since their discovery by Zimmermann in 1898 in the human thyroid gland, as well as in various other tissues, primary cilia have been reported in almost every vertebrate cell (Wheatley et al. 1996). Specifically at the thyroid level, primary cilia have been described in follicular cells of various embryos and adult vertebrate species, such as fish (Cowdry, 1921; Fujita & Machino, 1965), amphibians (Coleman et al. 1968), reptiles (Rupik, 2013), birds (Fujita, 1963) and mammals. Thus far, in mammals, cilia have been found in the thyrocytes of humans (fetuses and adults) (Klinck et al. 1970; Johannessen et al. 1980; Sobrinho-Simoes & Johannessen, 1981; Chan, 1983; Martin et al. 1988), dogs (fetuses and adults) (Nunez & Gershon, 1976) and also in rat embryos (Calvert & Pusterla, 1973; Remy et al. 1980). Apart from thyroid follicular cells, the only type of apicobasal-polarized endocrine epithelium in mammals, primary cilia have been described in other endocrine tissues, such as adenohypophysis (Barnes, 1961), parathyroid (Munger & Roth, 1963), pancreatic islands (Aughsteen, 2001) and adrenal glands (Wheatley, 1967).
As shown before, we confirm that primary cilia in thyroid cell cultures were only observed when cells were serum-starved for 2 days to induce cell cycle arrest. Several authors have described the post-mitotic character of primary cilium (Tucker et al. 1979; Irigoin & Badano, 2011; Pan et al. 2013; Paridaen et al. 2013). Specifically, Irigoin & Badano (2011), studying various ciliary proteins by immunofluorescence in cultured fibroblasts during different stages of the cell cycle, demonstrated that primary cilia were only present during G0/G1 and at the beginning of S phase and disassembled at late S phase or the beginning of G2, when centrioles such as the one functioning as a basal body were needed to organize the mitotic spindle (Irigoin & Badano, 2011). Therefore, primary cilium is considered an organelle of cells in a quiescent or differentiated state (Pugacheva et al. 2007; D’Angelo & Franco, 2009).
Our studies using electron microscopy have clearly demonstrated that cilia of thyroid follicular cells of different mammals are structurally typical primary cilia, similar to those found in the follicular cells of other vertebrates, as well as in practically all organs during ontogenesis and postnatal life (Martin et al. 1988; Wheatley et al. 1996). Cilia were usually located in a central position on the thyrocyte surface, even when there were more than one, and were perpendicular to the apex. They exhibited a non-motile 9 + 0 axonemal configuration and had a rather uniform diameter, although they varied greatly in length among different samples and species (2.4–10.7 μm). The presence of a single basal body, accompanied or not by a centriole at various angles, was observed, in addition to some appendages that accompanied centriolar basal bodies such as the ciliary rootlets. These findings coincide with those described by Martin et al. (1988) in human thyroid gland and by Nunez and Gershon (1976) in fetuses and adult dogs.
In most models, primary cilia are considered to be cellular antennae that occupy an ideal cellular location for the interpretation of information both from the environment and from other cells. Depending on the cell type or tissue considered, cilia could be involved in various different biological processes. In addition to their well-established roles in sight, smell and mechanosensation, primary cilia are key participants in intracellular signaling related to cellular growth, differentiation and secretion. Specifically, in the inner ear (Singla & Reiter, 2006), retina (Horst et al. 1987) and olfactory mucosa (Boekhoff et al. 1990), they are sensory organelles; in renal tubules, they are mechanoreceptors that detect and respond to fluid flow through the tubule lumen (Praetorius & Spring, 2001); in pancreatic endocrine cells (B cells) and adenohypophysis (GH cells), they express SSTR3 and sense the inhibitory signal of somatostatin (Iwanaga et al. 2011); in gastric endocrine cells, primary cilia sense the components of food that modulate gastrin secretion and gastric acidity (Saqui-Salces et al. 2012); finally, in the central nervous system, they are involved in extra-synaptic signaling through different G protein-coupled receptors (Domire & Mykytyn, 2009).
The demonstration of primary cilia in almost every differentiated follicular cell supports the assumption that they may also play a functional role in the thyroid gland. Their hypothetical function as a contributor to mixing newly synthesized thyroglobulin in the colloid, proposed by Sobrinho-Simoes et al. in 1981, was later rejected because their character as a non-motile cilium was clearly demonstrated by Martin et al. (1988). Thereafter, a function related to the secretory process of thyroglobulin was also proposed based either on the structural relationship of primary cilia with the Golgi apparatus (Martin et al. 1988) or on their appearance precisely coinciding with the onset of colloidal secretion (Rupik, 2013).
According to different authors, primary cilium forms a separate cell compartment, providing a platform for several extracellular signals such as sonic hedgehog (Shh), planar cell-polarity (PCP) and Wnt signaling pathways, important regulators of proliferation and embryonic patterning (Singla & Reiter, 2006). Specifically, Wnt proteins that activate several distinct signaling pathways are classified as either β-catenin-dependent (the so-called canonical pathway) or β-catenin-independent (the noncanonical pathway) (Singla & Reiter, 2006). Corbit et al. (2008) have shown that the primary cilium restrains canonical Wnt/beta-catenin signaling in mouse embryos, primary fibroblasts, and embryonic cells. Although a direct relationship between a specific signaling pathway and primary cilium has not yet been demonstrated in thyrocytes, recent findings support that the Wnt/beta-signalling pathway is probably involved in the normal development of thyroid gland, provoking its deregulation with the appearance of several types of cancers, such as papillary thyroid carcinomas, which are the most common thyroid tumors (Garcia-Jimenez & Santisteban, 2007). Kim et al. (2007) have also confirmed that Wnt signaling contributes to the ability of TSH to simultaneously increase cell growth and functional, thyroid-specific, gene expression. Similarly, Chen et al. (2010) reported that the Wnt/beta-catenin pathway plays a significant role in the regulation of the proliferation of primary human thyrocytes; finally, Gilbert-Sirieix et al. (2011) have also demonstrated that this same pathway is a direct and forward driver of TTF-1 expression. Taking all these findings together, we suggest that primary cilia in follicular cells could also be involved in the normal development of the thyroid gland, as well as the regulation of thyroid function, through a specific Wnt-signaling pathway.
The thyroid gland is a unique endocrine organ composed of follicles, with the thyrocytes surrounding a lumen, in which their secretory product thyroglobulin is stored extracellularly and in large quantities (Colin et al. 2013). To synthesize thyroid hormones, follicular cells actively transport iodide from their basolateral domain and concentrate it in the follicle lumen. Iodide is then oxidated and bound to tyrosine residues to form iodothyroglobulin, which stores thyroid hormones. Thyrocytes constantly produce moderate amounts of H2O2 and other reactive oxygen species (ROS) that are physiologically required for thyroid hormone synthesis and, if not finely regulated, may become toxic (Denef et al. 1996; Poncin et al. 2010). The enzymatic machinery for thyroglobulin iodination is located and restricted at the apical plasma membrane of thyrocytes (Song et al. 2007; Ohye & Sugawara, 2010). Therefore, primary cilia might act in these cells as mechanosensors for the refilling of the follicular lumen with thyroglobulin to form the colloid. Moreover, taking advantage of its ideal localization and extending from the follicular cell apex into the colloidal lumen, ciliary membrane might possess specific receptors that could sense, in some specific way, either the level of iodinated thyroglobulin stored in the colloid or the thyroid oxidative charge associated to the outer surface of the plasma membrane, such as the presence of iodinated or peroxidized phospholipids that are presumed to be toxic (Song et al. 2007). These sensory activities would be coupled in coordination with the biosynthetic process through specific intracellular downstream signalling pathways.
In summary, we have shown that the presence of primary cilia is almost a constant event in adult mammalian thyroid follicular cells. However, in contrast to human and other mammal species, primary cilia are not present in either mouse or rat thyroid tissues, or in two of the most commonly used cell-culture models (FRT and PC-Cl-3) for the study of thyrocyte function. If primary cilia are present and can truly develop some of those theoretical functions in follicular cells of most vertebrates, we cannot explain why they are only present in mice and rats during thyroid development and disappear after birth. Perhaps the only explicable difference among species could be related to the average follicular size. As previously demonstrated (Saadeh & Babikian, 1978), follicular size tends to increase with increasing body size, whereas epithelium percentage decreases. Accordingly, in mouse and rat, where no primary cilia are found, follicular size ranged between 45.7 and 59.7 μm, respectively. In guinea pig, where only scattered cilia are observed, follicular size average reached 73.2 μm. Finally, in those species where cilia are almost a constant presence in every follicular cell, such as rabbit, pig and human, follicular size ranged from 81.6 to 179.6 μm and 198.1 μm, respectively. Nevertheless, further studies are needed to shed light on the permanence of these organelles in the thyroid gland of most species during the postnatal life and the functional role, if any, that primary cilium may play in thyrocytes. Moreover, on the basis of our findings, such a relevant dissimilarity among species should be taken into account when using rat or mouse models for the study of normal thyroid activity and thyroid cancer biology.
Acknowledgments
This work was supported by grants from the Consejería de Innovación, Ciencia y Empresa (refs. CTS-439/2011 and CTS-229/2011), and from the Consejería de Salud (ref. PI-0051-2013), Junta de Andalucía, Spain. The authors thank Mr. John Brown for correction of the English language, and the Microscopy Service of CITIUS (General Research Services, Seville University, Spain) for technical assistance with the electron microscopy study.
Supporting Information
Video S1. Animation of the Z-stack of confocal immunofluorescence optical sections used to measure cilia length on human thyroid tissue.
Video S2. Animation of the Z-stack of confocal immunofluorescence optical sections used to measure cilia length on pig thyroid tissue.
References
- Aughsteen AA. The ultrastructure of primary cilia in the endocrine and excretory duct cells of the pancreas of mice and rats. Eur J Morphol. 2001;39:277–283. doi: 10.1076/ejom.39.5.277.7380. [DOI] [PubMed] [Google Scholar]
- Barnes BG. Ciliated secretory cells in the pars distalis of the mouse hypophysis. J Ultrastruct Res. 1961;5:453–467. doi: 10.1016/s0022-5320(61)80019-1. [DOI] [PubMed] [Google Scholar]
- Boekhoff I, Tareilus E, Strotmann J, et al. Rapid activation of alternative second messenger pathways in olfactory cilia from rats by different odorants. EMBO J. 1990;9:2453–2458. doi: 10.1002/j.1460-2075.1990.tb07422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert R, Pusterla A. Formation of thyroid follicular lumina in rat embryos studied with serial fine sections. Gen Comp Endocrinol. 1973;20:584–587. doi: 10.1016/0016-6480(73)90091-9. [DOI] [PubMed] [Google Scholar]
- Chan AS. Ultrastructural observations on the formation of follicles in the human fetal thyroid. Cell Tissue Res. 1983;233:693–698. doi: 10.1007/BF00212236. [DOI] [PubMed] [Google Scholar]
- Chen G, Jiang Q, You Z, et al. Regulation of GSK-3 beta in the proliferation and apoptosis of human thyrocytes investigated using a GSK-3 beta-targeting RNAi adenovirus expression vector: involvement the Wnt/beta-catenin pathway. Mol Biol Rep. 2010;37:2773–2779. doi: 10.1007/s11033-009-9819-5. [DOI] [PubMed] [Google Scholar]
- Coleman R, Evennett PJ, Dodd JM. Ultrastructural observations on the thyroid gland of Xenopus laevis Daudin throughout metamorphosis. Gen Comp Endocrinol. 1968;10:34–46. doi: 10.1016/0016-6480(68)90006-3. [DOI] [PubMed] [Google Scholar]
- Colin IM, Denef JF, Lengele B, et al. Recent insights into the cell biology of thyroid angiofollicular units. Endocr Rev. 2013;34:209–238. doi: 10.1210/er.2012-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, et al. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- Cowdry EV. Flagellated thyroid cells in the dogfish (Mustelus canis. Anat Rec. 1921;22:289–299. [Google Scholar]
- D’Angelo A, Franco B. The dynamic cilium in human diseases. Pathogenetics. 2009;2:3. doi: 10.1186/1755-8417-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Iglesia FA, Porta EA. Ciliated biliary epithelial cells in the livers of non-human primates. Experientia. 1967;23:49–51. doi: 10.1007/BF02142263. [DOI] [PubMed] [Google Scholar]
- Denef JF, Many MC, van den Hove MF. Iodine-induced thyroid inhibition and cell necrosis: two consequences of the same free-radical mediated mechanism? Mol Cell Endocrinol. 1996;121:101–103. doi: 10.1016/0303-7207(96)03848-8. [DOI] [PubMed] [Google Scholar]
- Domire JS, Mykytyn K. Markers for neuronal cilia. Methods Cell Biol. 2009;91:111–121. doi: 10.1016/S0091-679X(08)91006-2. [DOI] [PubMed] [Google Scholar]
- Flood PR, Totland GK. Substructure of solitary cilia in mouse kidney. Cell Tissue Res. 1977;183:281–290. doi: 10.1007/BF00226625. [DOI] [PubMed] [Google Scholar]
- Fuchs JL, Schwark HD. Neuronal primary cilia: a review. Cell Biol Int. 2004;28:111–118. doi: 10.1016/j.cellbi.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Fujita H. Electron microscopic studies on the thyroid gland of domestic fowl, with special reference to the mode of secretion and the occurrence of a central flagellum in the follicular cell. Z Zellforsch Mikrosk Anat. 1963;60:615–632. doi: 10.1007/BF00331187. [DOI] [PubMed] [Google Scholar]
- Fujita H, Machino M. Electron microscopic studies on the thyroid gland of a teleost, Seriola Quinqueradiata. Anat Rec. 1965;152:81–97. doi: 10.1002/ar.1091520109. [DOI] [PubMed] [Google Scholar]
- Garcia-Jimenez C, Santisteban P. TSH signalling and cancer. Arq Bras Endocrinol Metabol. 2007;51:654–671. doi: 10.1590/s0004-27302007000500003. [DOI] [PubMed] [Google Scholar]
- Gilbert-Sirieix M, Makoukji J, Kimura S, et al. Wnt/beta-catenin signaling pathway is a direct enhancer of thyroid transcription factor-1 in human papillary thyroid carcinoma cells. PLoS ONE. 2011;6:e22280. doi: 10.1371/journal.pone.0022280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfer SR. Follicle formation in the embryonic chick thyroid. III. Initiation of follicle formation. Tissue Cell. 1979;11:727–740. doi: 10.1016/0040-8166(79)90027-2. [DOI] [PubMed] [Google Scholar]
- Horst CJ, Forestner DM, Besharse JC. Cytoskeletal-membrane interactions: a stable interaction between cell surface glycoconjugates and doublet microtubules of the photoreceptor connecting cilium. J Cell Biol. 1987;105:2973–2987. doi: 10.1083/jcb.105.6.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang BQ, Masyuk TV, Muff MA, et al. Isolation and characterization of cholangiocyte primary cilia. Am J Physiol Gastrointest Liver Physiol. 2006;291:G500–G509. doi: 10.1152/ajpgi.00064.2006. [DOI] [PubMed] [Google Scholar]
- Irigoin F, Badano JL. Keeping the balance between proliferation and differentiation: the primary cilium. Curr Genoms. 2011;12:285–297. doi: 10.2174/138920211795860134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga T, Miki T, Takahashi-Iwanaga H. Restricted expression of somatostatin receptor 3 to primary cilia in the pancreatic islets and adenohypophysis of mice. Biomed Res. 2011;32:73–81. doi: 10.2220/biomedres.32.73. [DOI] [PubMed] [Google Scholar]
- Johannessen JV, Sobrinho-Simoes M, Finseth I, et al. Scanning electron microscopy of the human thyroid gland. A simple method to remove colloid. J Submicrosc Cytol. 1980;12:301–305. [Google Scholar]
- Kim WB, Lewis CJ, McCall KD, et al. Overexpression of Wnt-1 in thyrocytes enhances cellular growth but suppresses transcription of the thyroperoxidase gene via different signaling mechanisms. J Endocrinol. 2007;193:93–106. doi: 10.1677/JOE-06-0025. [DOI] [PubMed] [Google Scholar]
- Klinck GH, Oertel JE, Winship T. Ultrastructure of normal human thyroid. Lab Invest. 1970;22:2–22. [PubMed] [Google Scholar]
- Larsen JH., Jr Ultrastructure of thyroid follicle cells of three salamanders (Ambystoma, Amphiuma, and Necturus) exhibiting varying degrees of neoteny. J Ultrastruct Res. 1968;24:190–209. doi: 10.1016/s0022-5320(68)90058-0. [DOI] [PubMed] [Google Scholar]
- Martin A, Hedinger C, Haberlin-Jakob M, et al. Structure and motility of primary cilia in the follicular epithelium of the human thyroid. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;55:159–166. doi: 10.1007/BF02896572. [DOI] [PubMed] [Google Scholar]
- Munger BL, Roth SI. The cytology of the normal parathyroid glands of man and Virginia deer; a light and electron microscopic study with morphologic evidence of secretory activity. J Cell Biol. 1963;16:379–400. doi: 10.1083/jcb.16.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez EA, Gershon MD. Appearance and disappearance of multiply ciliated follicular cells during development of the dog’s thyroid gland. Anat Rec. 1976;184:133–145. doi: 10.1002/ar.1091840202. [DOI] [PubMed] [Google Scholar]
- Ohye H, Sugawara M. Dual oxidase, hydrogen peroxide and thyroid diseases. Exp Biol Med (Maywood) 2010;235:424–433. doi: 10.1258/ebm.2009.009241. [DOI] [PubMed] [Google Scholar]
- Pan J, Seeger-Nukpezah T, Golemis EA. The role of the cilium in normal and abnormal cell cycles: emphasis on renal cystic pathologies. Cell Mol Life Sci. 2013;70:1849–1874. doi: 10.1007/s00018-012-1052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paridaen JT, Wilsch-Brauninger M, Huttner WB. Asymmetric inheritance of centrosome-associated primary cilium membrane directs ciliogenesis after cell division. Cell. 2013;155:333–344. doi: 10.1016/j.cell.2013.08.060. [DOI] [PubMed] [Google Scholar]
- Poncin S, Van Eeckoudt S, Humblet K, et al. Oxidative stress: a required condition for thyroid cell proliferation. Am J Pathol. 2010;176:1355–1363. doi: 10.2353/ajpath.2010.090682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- Pugacheva EN, Jablonski SA, Hartman TR, et al. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy L, Michel-Bechet M, Athouel-Haon AM, et al. Development of the thyroid gland in the rat fetus in vivo. An ultrastructural and radioautographic study. Arch Anat Microsc Morphol Exp. 1980;69:91–108. [PubMed] [Google Scholar]
- Rupik W. Ultrastructural studies of cilia formation during thyroid gland differentiation in grass snake embryos. Micron. 2013;44:228–237. doi: 10.1016/j.micron.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Saadeh FA, Babikian LG. A comparative histologic study of thyroid follicular size and epithelium percentage in certain mammals. Anat Anz. 1978;143:96–99. [PubMed] [Google Scholar]
- Saqui-Salces M, Dowdle WE, Reiter JF, et al. A high-fat diet regulates gastrin and acid secretion through primary cilia. FASEB J. 2012;26:3127–3139. doi: 10.1096/fj.11-197426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Sobrinho-Simoes M, Johannessen JV. Scanning electron microscopy of the normal human thyroid. J Submicrosc Cytol. 1981;13:209–222. [PubMed] [Google Scholar]
- Song Y, Driessens N, Costa M, et al. Roles of hydrogen peroxide in thyroid physiology and disease. J Clin Endocrinol Metab. 2007;92:3764–3773. doi: 10.1210/jc.2007-0660. [DOI] [PubMed] [Google Scholar]
- Sulik K, Dehart DB, Iangaki T, et al. Morphogenesis of the murine node and notochordal plate. Dev Dyn. 1994;201:260–278. doi: 10.1002/aja.1002010309. [DOI] [PubMed] [Google Scholar]
- Tucker RW, Pardee AB, Fujiwara K. Centriole ciliation is related to quiescence and DNA synthesis in 3T3 cells. Cell. 1979;17:527–535. doi: 10.1016/0092-8674(79)90261-7. [DOI] [PubMed] [Google Scholar]
- Wandel A, Steigleder GK, Bodeux E. Primary cilia in cells of the epidermis and dermis. Z Hautkr. 1984;59:389–392. 382, [PubMed] [Google Scholar]
- Wheatley DN. Cilia and centrioles of the rat adrenal cortex. J Anat. 1967;101:223–237. [PMC free article] [PubMed] [Google Scholar]
- Wheatley DN. Nanobiology of the primary cilium – paradigm of a multifunctional nanomachine complex. Methods Cell Biol. 2008;90:139–156. doi: 10.1016/S0091-679X(08)00807-8. [DOI] [PubMed] [Google Scholar]
- Wheatley DN, Wang AM, Strugnell GE. Expression of primary cilia in mammalian cells. Cell Biol Int. 1996;20:73–81. doi: 10.1006/cbir.1996.0011. [DOI] [PubMed] [Google Scholar]
- Whitfield JF. The neuronal primary cilium – an extrasynaptic signaling device. Cell Signal. 2004;16:763–767. doi: 10.1016/j.cellsig.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Zimmermann KW. Beiträge zur Kenntniss einiger Drüsen und Epithelien. Arch Mikrosk Anat. 1898;52:552–706. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Animation of the Z-stack of confocal immunofluorescence optical sections used to measure cilia length on human thyroid tissue.
Video S2. Animation of the Z-stack of confocal immunofluorescence optical sections used to measure cilia length on pig thyroid tissue.