Abstract
Background: Infants with lower respiratory tract infections (LRTIs) are at an increased risk of developing childhood wheezing illnesses (including asthma), but it is not currently possible to predict those at risk for these long-term outcomes. The current objective was to examine whether urine levels of club cell 16-kDa secretory protein (CC16) at the time of an infant LRTI are associated with the development of childhood wheezing illnesses.
Methods: Prospective study of 133 previously healthy infants enrolled during a healthcare visit for a LRTI and followed longitudinally for childhood wheezing illnesses. Urine levels of CC16 at the time of enrollment were measured after validating a commercially available enzyme-linked immunosorbent assay kit for serum. The outcome of interest was parental report of subsequent childhood wheeze (defined as ≥1 episode of wheezing following the initial LRTI) at the 1-year follow-up visit. Logistic regression was used for the main analysis.
Results: The median (interquartile range) urine levels of CC16 (ng/mg of creatinine) at the time of an infant LRTI were 11.1 (7.7–20.1) for infants with subsequent childhood wheeze and 13.4 (8.3–61.1) for those without (p = 0.11). In the main multivariate analysis using a logarithmic transformation of the urine levels of CC16, a twofold increase in urine levels of CC16 was associated with ∼30% decreased odds (OR = 0.74 [95% confidence interval (CI) 0.56–0.98], p = 0.04) of subsequent childhood wheeze after adjustment for potential confounders.
Conclusions: An inverse association was found between urine levels of CC16 at the time of an infant LRTI and the odds of subsequent childhood wheeze. Urine CC16 may be a useful biomarker of the development of childhood wheezing illnesses after LRTIs in infancy.
Introduction
Lower respiratory tract infections (LRTIs) are a major cause of morbidity and mortality in early childhood worldwide.1,2 Bronchiolitis (the most common LRTI) is the number one cause of infant hospitalizations in the United States, and its inpatient-related costs have been estimated at ∼$543 million per year.2,3 In addition to their acute effects, LRTIs in infancy have also been associated with long-term outcomes, such as the development of childhood wheezing illnesses (including asthma).4–7 This association is likely influenced by the specific infectious agent, the timing and/or severity of the infection, and certain host factors that may alter susceptibility to infection (e.g., family history of asthma),7–9 but the precise underlying mechanisms remain largely unknown. Furthermore, although between 30% and 50% of infants with LRTIs subsequently develop childhood wheezing illnesses,9–11 it is currently not possible to identify infants at risk for these long-term outcomes. Nor do we understand why some develop asthma and others do not.
Club cell 16-kDa secretory protein (CC16) (also known as CC10, uteroglobin, urine protein 1, or formerly as Clara cell secretory protein) is a candidate biomarker of lung injury produced mainly by the club cells (formerly known as Clara cells) of the distal bronchioles and, to a lesser extent, by the prostate, kidneys, and endometrium.12–14 CC16 is secreted in large amounts into the bronchial epithelial lining fluid, and it is thought to have anti-inflammatory, immunoregulatory, and immunosuppressive effects.12,13,15 In healthy subjects, small amounts of CC16 leak across the respiratory epithelium into the blood, likely through passive diffusion.14 It is hypothesized that different lung disease mechanisms may result in increments or declines in serum levels of CC16.13,14 For instance, respiratory disorders that increase the epithelial permeability (e.g., acute respiratory distress syndrome,16 exposure to pro-inflammatory agents,17 or severe chest trauma18) have been associated with higher serum levels of CC16. On the other hand, respiratory illnesses leading to small airway inflammation (e.g., asthma,19–24 exposure to tobacco smoke,25–27 or chronic obstructive pulmonary disease25,27–29) have been associated with lower serum levels of CC16.
Because CC16 in blood is entirely eliminated through glomerular filtration, serum and urine levels of CC16 are highly correlated.30 As a result, there has been a recent interest in the utility of urine CC16 as a potentially useful, cost-effective, and noninvasive biomarker for respiratory disorders.30,31
The authors have previously demonstrated a severity-dependent relationship between infant LRTIs and the risk of developing asthma.8 As CC16 may be a marker of both lung injury and immune response, the authors hypothesized that urine levels of CC16 at the time of an infant LRTI are associated with the development of childhood wheezing illnesses. To test this hypothesis, data obtained as part of the Tennessee Children's Respiratory Initiative (TCRI), a study of infants enrolled during an acute respiratory illness and followed longitudinally for the development of respiratory sequelae, were analyzed.
Methods and Materials
Study design and setting
The TCRI is a prospective cohort of term, predominantly non–low birth weight, previously healthy infants (n = 630) enrolled during a healthcare visit for an acute respiratory illness at a single academic institution. The rationale and methods for the TCRI have been reported previously.32 In brief, eligible infants were recruited at the time of an unscheduled clinic visit, emergency department visit, or hospitalization for an acute upper (n = 175) or lower (n = 455) respiratory tract infection during four winter respiratory viral seasons (from September to May of 2004 to 2008). Follow-up of participants to ascertain childhood wheezing illnesses through age 6 years is ongoing. Mothers provided consent for both their participation and their infants'. This study was approved by the Institutional Review Board of Vanderbilt University (Nashville, TN).
Study procedures
During study enrollment, research nurses administered an in-person research questionnaire to the mothers to collect information on the infant's past medical history and current respiratory health, sociodemographic characteristics, maternal history of asthma, and current exposure to second-hand smoking (SHS). They also obtained bag urine samples (mostly from hospitalized infants), which were maintained in repository for later analyses (see below). Through an initial structured medical chart review, information was gathered on each infant's acute illness visit (including current symptoms, physical examination, past medical history, and hospital course). Final diagnoses were obtained through a second medical chart review conducted after the visit discharge. A diagnosis of LRTI was based on both (1) a physician's diagnosis and (2) documentation of symptoms with duration ≤10 days (including any two of the following: cough, nasal congestion, rhinorrhea, wheezing, dyspnea, or fever) and requirement for an acute care clinic visit, an emergency department visit, or hospitalization (including 23-h stay). A panel of pediatricians reviewed cases that were not clearly identified to establish the final diagnosis. The severity of the LRTI was determined using the bronchiolitis severity score (BSS).33 The BSS is a slightly modified version from the one by Tal et al.34 It incorporates information obtained through the initial chart review, and ranges from 0 to 12, with higher scores indicating more severe illness (see Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/ped). Infants enrolled are followed up on a yearly basis up to the age of 6 years (either in person or by a phone interview) to determine childhood wheezing illnesses using the validated International Study of Asthma and Allergies in Children (ISAAC) questionnaire.35,36
Selection of participants
Urine levels of CC16 were measured in 133/455 infants with LRTIs, as described below. These 133 infants were selected among those infants with LRTIs with urine samples (n = 342) based on the quantity of available urine, and were included in analyses (Supplementary Fig. S1).
Validation and measurement of urine CC16
As a first step, a commercially available enzyme-linked immunosorbent assay kit for serum CC16 (BioVendor, Chandler, NC) in urine was validated. The validation experiments included spike and recovery, multiple freeze–thaw cycles, and intra-assay controls on urine samples. This same kit has been previously validated for urine in another study.30 Next, urine levels of CC16 (ng/mL) were measured according to the manufacturer's recommendations. All samples were tested in duplicate. The values below the instrument's limit of detection (LOD; 2 ng/mL) were assigned a value one-half the LOD (i.e., 1 ng/mL). Finally, urine creatinine (mg/mL) was determined by a chemical assay based on Jaffe's reaction (Enzo Life Sciences, Farmingdale, NY). Urine levels of CC16 (ng/mL) were corrected to account for urine concentration by dividing them by urine creatinine values (mg/mL; see Methods in the Supplementary Data).
Statistical analyses
The predictor of interest was the urine level of CC16 at the time of an infant LRTI, as measured with the assay described above. The outcome of interest was parental report of subsequent childhood wheeze (defined as ≥1 episode of wheezing following the initial LRTI) at the 1-year follow-up visit.
Descriptive statistics are presented as frequencies (percent) for categorical variables and median (interquartile range [IQR]) for continuous variables. Comparisons between groups were conducted with Pearson's chi-square or Mann–Whitney U-tests, as appropriate.
For the main analysis, logistic regression was used to assess the unadjusted and adjusted association between urine levels of CC16 and subsequent childhood wheeze. The variables to be included in the multivariable models (infant's age, sex, and current exposure to SHS) were selected a priori based on published literature.12,26,29,37 First-order interactions were tested for between urine levels of CC16 and each of the covariates mentioned above.
A sensitivity analysis was also conducted treating the values below the LOD as missing values and applying multiple imputation methods (see Methods in the Supplementary Data). These results were then compared to the main analysis that assigned values below the LOD a value of one-half the LOD.
The urine levels of CC16 were transformed to a logarithmic (log2) scale for regression modeling analyses, and results were backtransformed for ease of interpretation. All statistical analyses were performed using R v3.1.0.38 Statistical significance was defined as a p < 0.05.
Results
The baseline characteristics of the 133 infants with LRTIs included in the analyses are presented in Table 1. The majority of these infants were younger than 6 months of age, male, white, and enrolled at the time of a hospitalization. Most of them had a relatively high BSS, no history of maternal asthma or current exposure to SHS, and government health insurance for low-income families (Medicaid). There were no significant associations between the urine levels of CC16 at the time of an infant LRTI and any of the baseline characteristics, including BSS (p > 0.05).
Table 1.
Baseline Characteristics of Infants with LRTIs (N = 133)
| Age at enrollment (weeks) | 11.0 (6.0–17.0) |
| Female sex | 41 (31%) |
| Race/ethnicity | |
| Black | 23 (17%) |
| White | 85 (64%) |
| Hispanic | 17 (13%) |
| Other | 8 (6%) |
| Gestational age (weeks) | 39 (38–39) |
| Birth weight (g) | 3,345 (3,090–3,579) |
| Current exposure to SHS | 29 (22%) |
| Maternal asthma | 21 (16%) |
| Insurance type | |
| Private | 54 (41%) |
| Medicaid | 71 (53%) |
| None | 8 (6%) |
| BSS | 7.0 (4.5–9.0) |
| LRTI visit type | |
| Unscheduled outpatient visit | 3 (2%) |
| Emergency department visit | 21 (16%) |
| Hospitalization | 109 (82%) |
| Urine CC16 (ng/mL) | 1.0 (1.0–5.8) |
| Urine CC16 (ng/mg of creatinine) | 12.5 (7.7–35.7) |
Data are presented as the number (%) for binary variables or median (interquartile range) for continuous variables. Percentage calculated for children with complete data.
LRTI, lower respiratory tract infection; SHS, second-hand smoking; BSS, bronchiolitis severity score; CC16, club cell 16-kDa secretory protein.
Compared with infants with LRTIs included in analyses, those with LRTIs not included were more likely to be female and to have been enrolled at the time of an unscheduled outpatient visit. They were also more likely to have a history of current exposure to SHS, no insurance, and a lower BSS. There were no other significant differences between infants with LRTIs included and not included in analyses (see Supplementary Table S2).
Of the 133 infants with LRTIs, 109 (∼82%) had 1-year follow-up data (Supplementary Fig. S1). Of these, 61 (∼56%) had parental report of subsequent childhood wheeze (with 35 [∼57%] of them reporting ≥4 episodes of wheezing since the enrollment visit). The median (IQR) age for follow-up was 1.1 (1.0–1.9) years in those with subsequent childhood wheeze, and 1.1 (1.0–2.0) in those without (p = 0.6). The median (IQR) urine levels of CC16 (ng/mg of creatinine) at the time of an infant LRTI were 11.1 (7.7–20.1) for infants with subsequent childhood wheeze and 13.4 (8.3–61.1) for those without (p = 0.11; Supplementary Fig. S2). Infants with LRTIs without 2-year follow-up data were more likely to be Hispanic, to have a history of maternal asthma, and to have Medicaid insurance. There were no other significant differences between infants with LRTIs with and without 2-year follow-up data (see Supplementary Table S3).
In the main unadjusted analysis using the logarithmic transformation of the urine levels of CC16, a significant inverse association of the urine levels of CC16 at the time of an infant LRTI with the risk of subsequent childhood wheeze was found (unadjusted OR = 0.79 [95% CI 0.63–0.99], p = 0.0497). The adjustment for infant's age, sex, and current exposure to SHS did not weaken the association. In the main adjusted analysis, a twofold increase in urine levels of CC16 (ng/mg of creatinine) at the time of an infant LRTI was associated with ∼30% decreased odds (adjusted OR = 0.74 [95% CI 0.56–0.98], p = 0.04) of subsequent childhood wheeze (Fig. 1). No significant first-order interactions between urine levels of CC16 and the a priori selected covariates were detected (p > 0.05 for each interaction term). Consistent results were obtained when analyzing urine CC16 without the creatinine correction (ng/mL; Table 2).
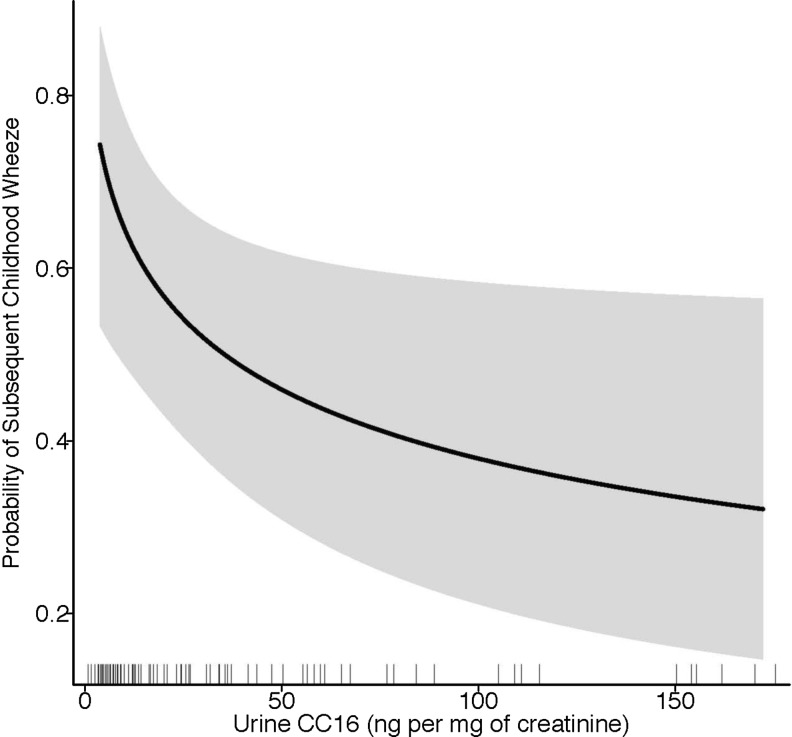
FIG. 1.
Predicted probability of subsequent childhood wheeze by urine CC16 at the time of an infant LRTI. The solid black line represents the predicted probability values, and the shaded gray bands represent the lower and upper 95% confidence intervals. These estimates were obtained from a logistic regression model adjusted for infant's age, sex, and current exposure to second-hand smoking. CC16, club cell 16-kDa secretory protein; LRTI, lower respiratory tract infection.
Table 2.
Analysis of Urine CC16 at the Time of an Infant LRTI and Subsequent Childhood Wheeze Without Creatinine Correction
| OR [95% CI]a | p-Value | |
|---|---|---|
| Unadjusted | ||
| Urine CC16 (ng/mL) | 0.85 [0.68–1.07] | 0.2 |
| Adjustedb | ||
| Urine CC16 (ng/mL) | 0.76 [0.58–0.99] | 0.04 |
OR [95% CI] for a twofold increase in urine levels of CC16.
Logistic regression model adjusted for infant's age, sex, and current exposure to SHS.
There were 75 (56%) samples with urine CC16 values below the LOD. A sensitivity analysis was conducted by treating the values below the LOD as missing data and applying multiple imputations methods, obtaining similar results when compared to the main analysis. In both the unadjusted and adjusted models using multiple imputations, the urine levels of CC16 were inversely associated with the risk of subsequent childhood wheeze (see Supplementary Table S4).
Discussion
Infants with LRTIs have substantially higher odds of subsequently developing childhood wheezing illnesses (including asthma) when compared with the general population,4–11 but it is not currently possible to identify those at risk for these long-term outcomes. This lack of prognostic data has led to substantial uncertainty among caregivers and healthcare providers as to which patients would benefit from preventive strategies, education, early intervention, or referral to a specialist. Thus, for both clinical and research purposes, noninvasive biomarkers of the development of childhood wheezing illnesses following LRTIs are needed.9 Urine biomarkers offer several advantages compared with serum samples, as they simplify collection, reduce costs, and may decrease participant attrition.30 In addition, biomarkers can shed light on the mechanisms of disease pathogenesis. In the present study, an inverse association was found between urine levels of CC16 at the time of an infant LRTI and the odds of subsequent childhood wheeze.
The precise function of CC16 is unknown, although it appears to protect the respiratory tract against epithelial injury, inflammation, and oxidative stress.12,14 CC16 modulates the expression and/or activity of inflammatory proteins such as interferon γ, tumor necrosis factor α, and phospholipase A2.12,39–41 Phospholipase A2 is involved in the arachidonic acid cascade, and indirectly controls the availability of prostaglandins and leukotrienes.42 CC16 may also attenuate mucous secretion and suppress Th2 T-cell differentiation.43,44 Following acute viral infections, transgenic mice deficient in CC16 develop a more severe lung inflammation with increased Th2 cytokine levels, airway reactivity, and mucous production, as well as prolonged viral persistence.45,46 Reconstitution of CC16 in mice is able to reverse these altered phenotypes.46 The CC16 gene maps to a genetic region (11q12–13) that has been linked to asthma and atopy in some studies.47,48 The A38G polymorphism in the promoter region of this gene decreases the amount of expressed CC16 and has been found to be associated with wheezing disorders in children and adults.19,21,22,49,50 Taken together, these findings support the concept that CC16 can have an important role in the pathogenesis of these diseases.
Most19–24 but not all51 studies in children or adults have found an inverse association between serum levels of CC16 and prevalent asthma or its related phenotypes. In a recent cross-sectional study of 203 adult elite athletes, low serum levels of CC16 were also associated with a history of frequent upper respiratory tract infections.52 To date, only a few pediatric studies have been conducted in this topic, all of which used serum levels of CC16 and had a cross-sectional design. In a study of 100 Australian children aged 0–18 years, asthmatics had lower serum levels of CC16 when compared with nonasthmatics.19 Similar results were obtained in a study of 51 children aged 0–14 years in Greece.20 Two other larger studies in Chinese children found lower serum levels of CC16 in children with childhood wheezing illnesses, although these findings were mostly present in children who were homozygous for the A38G polymorphism.21,22 In a recent large prospective study of children in three different countries, low serum levels of CC16 at 4–6 years of age were associated with decreased lung function by 16 years of age, although the outcome of asthma was not assessed.29 To the authors' knowledge, this is the first study to examine the relationship between urine CC16 and the development of childhood wheezing illnesses.
The mechanisms behind an inverse association between serum or urine levels of CC16 and childhood wheezing illnesses found in this and other studies can only be speculated on. In both LRTIs and childhood wheezing illnesses (including asthma), there can be substantial small airway inflammation with resultant damage of the club cells of the distal airways. This can lead to a decrease production of CC16 and, thus, lower amounts of this biomarker leaking across the respiratory epithelium into the blood as long as the epithelial permeability remains intact.27 In other respiratory diseases where the epithelial permeability is severely affected (such as acute respiratory distress syndrome), a positive correlation between serum or urine levels of CC16 and these conditions may occur, as has been previously shown.16
The present study has considerable strengths, including a prospective design, the use of a commercially available test in urine samples, and an analytical approach accounting for potential confounders. Several limitations to the findings are also recognized. First, there were significant differences in certain baseline characteristics between infants included and not included in analyses and those with and without follow-up data, which may suggest a selection bias. However, some of these differences can be explained by the study design, as urine samples were more likely available for hospitalized infants. Second, as is the case for any observational study, there could be residual confounding by measured or unmeasured variables. The variables to be included in the multivariable models (age, sex, and current exposure to SHS) were selected a priori based on the published literature.12,26,37 Due to the relatively small sample size, more variables in the same multivariable models could not be included, but additional analysis replacing current exposure to SHS with other potential confounders (such as maternal asthma or BSS) did not change the results (data not shown). Urine levels of CC16 could be also affected by several other factors (e.g., post-renal excretion of CC16, diurnal variation, and physical activity).13,30,53 However, it is unknown if these factors are truly important in children, as the limited data available are in adults.13,14 Furthermore, urine levels of CC16 have been highly correlated to serum levels (which are less likely to be affected by some of these factors) in other studies using a similar instrument.14,29,30 In the present study, similar results were obtained when analyzing urine CC16 with and without the creatinine correction, but there could still be residual confounding by the renal function and/or hydration status at the time of the urine sample. Third, there were a large number of values below the LOD. It was not possible to evaluate if this differs from other studies examining urine levels of CC16, as there are only a limited number of publications on this field and this information is rarely included in the results. To overcome this limitation, a sensitivity analysis was conducted using multiple imputation methods, obtaining similar results to those of the main analysis (which further supports the conclusions). Fourth, due to limited power, sensitivity or specificity analyses could not be conducted to evaluate the utility of urine CC16 for risk prediction assessment. Fifth, urine levels of CC16 could be a predictor of asthma independent of LRTIs. Sixth, longitudinal measurements of urine CC16 were not available. Lastly, although the results were significant, the overall strength of the association was relatively weak. Thus, it is unlikely that urine CC16 can be used as a standalone biomarker. Nonetheless, the data raise the possibility of using urine CC16 in combination with other clinical risk factors to select research participants at high risk of developing childhood wheezing illnesses following LRTIs and/or to understand better the mechanisms of disease development.
In summary, it was found that urine levels of CC16 at the time of an infant LRTI are associated with subsequent childhood wheeze. The results of the present study suggest that urine CC16 may be a useful, readily accepted, noninvasive biomarker of the development of childhood wheezing illnesses following infant LRTIs. Larger studies are needed to confirm the utility of urine CC16 as a predictive tool of childhood wheezing illnesses following LRTIs, establish standard values, and validate these findings in other populations.
Supplementary Material
Acknowledgments
T.V.H. received support from NIH grants U19 AI095227, K24 AI77930, and U54 RR24975, the Thrasher Research Fund, and MedImmune. K.N.C. received support from NIH grant K01 AI070808. E.K.M. received support from NIH grants K12 RR24977, K23 AI 091691, and R03 AI 101629, the Vanderbilt Institute for Clinical and Translational Research (VICTR), March of Dimes, and the Thrasher Research Fund. Part of this article was presented as a poster at the American Thoracic Society International Conference 2014 (San Diego, CA).
Author Disclosure Statement
T.V.H. has provided consultation services for the Merck Research Advisory Board on a topic unrelated to this manuscript. E.K.M. has provided consultation services for Ameda on a topic unrelated to this manuscript.
No competing financial interests exist for the remaining authors.
References
- 1.Guthrie R. Community-acquired lower respiratory tract infections: etiology and treatment. Chest 2001;120:2021–2034 [DOI] [PubMed] [Google Scholar]
- 2.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA 1999;282:1440–1446 [DOI] [PubMed] [Google Scholar]
- 3.Pelletier AJ, Mansbach JM, Camargo CA., Jr. Direct medical costs of bronchiolitis hospitalizations in the United States. Pediatrics 2006;118:2418–2423 [DOI] [PubMed] [Google Scholar]
- 4.Perez-Yarza EG, Moreno A, Lazaro P, Mejias A, Ramilo O. The association between respiratory syncytial virus infection and the development of childhood asthma: a systematic review of the literature. Pediatr Infect Dis J 2007;26:733–739 [DOI] [PubMed] [Google Scholar]
- 5.Ramsey CD, Gold DR, Litonjua AA, Sredl DL, Ryan L, Celedon JC. Respiratory illnesses in early life and asthma and atopy in childhood. J Allergy Clin Immunol 2007;119:150–156 [DOI] [PubMed] [Google Scholar]
- 6.Nafstad P, Magnus P, Jaakkola JJ. Early respiratory infections and childhood asthma. Pediatrics 2000;106:E38. [DOI] [PubMed] [Google Scholar]
- 7.Busse WW, Lemanske RF, Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 2010;376:826–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll KN, Wu P, Gebretsadik T, et al. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol 2009;123:1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bacharier LB, Cohen R, Schweiger T, et al. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol 2012;130:91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000;161:1501–1507 [DOI] [PubMed] [Google Scholar]
- 11.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med 2008;178:667–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broeckaert F, Bernard A. Clara cell secretory protein (CC16): characteristics and perspectives as lung peripheral biomarker. Clin Exp Allergy 2000;30:469–475 [DOI] [PubMed] [Google Scholar]
- 13.Lakind JS, Holgate ST, Ownby DR, et al. A critical review of the use of Clara cell secretory protein (CC16) as a biomarker of acute or chronic pulmonary effects. Biomarkers 2007;12:445–467 [DOI] [PubMed] [Google Scholar]
- 14.Hermans C, Bernard A. Lung epithelium-specific proteins: characteristics and potential applications as markers. Am J Respir Crit Care Med 1999;159:646–678 [DOI] [PubMed] [Google Scholar]
- 15.Seys SF, Hox V, Van Gerven L, et al. Damage-associated molecular pattern and innate cytokine release in the airways of competitive swimmers. Allergy 2015;70:187–194 [DOI] [PubMed] [Google Scholar]
- 16.Lesur O, Langevin S, Berthiaume Y, et al. Outcome value of Clara cell protein in serum of patients with acute respiratory distress syndrome. Intensive Care Med 2006;32:1167–1174 [DOI] [PubMed] [Google Scholar]
- 17.Michel O, Murdoch R, Bernard A. Inhaled LPS induces blood release of Clara cell specific protein (CC16) in human beings. J Allergy Clin Immunol 2005;115:1143–1147 [DOI] [PubMed] [Google Scholar]
- 18.Wutzler S, Backhaus L, Henrich D, et al. Clara cell protein 16: A biomarker for detecting secondary respiratory complications in patients with multiple injuries. J Trauma Acute Care Surg 2012;73:838–842 [DOI] [PubMed] [Google Scholar]
- 19.Laing IA, Hermans C, Bernard A, Burton PR, Goldblatt J, Le Souef PN. Association between plasma CC16 levels, the A38G polymorphism, and asthma. Am J Respir Crit Care Med 2000;161:124–127 [DOI] [PubMed] [Google Scholar]
- 20.Gioldassi XM, Papadimitriou H, Mikraki V, Karamanos NK. Clara cell secretory protein: determination of serum levels by an enzyme immunoassay and its importance as an indicator of bronchial asthma in children. J Pharm Biomed Anal 2004;34:823–826 [DOI] [PubMed] [Google Scholar]
- 21.Ku MS, Sun HL, Lu KH, et al. The CC16 A38G polymorphism is associated with the development of asthma in children with allergic rhinitis. Clin Exp Allergy 2011;41:794–800 [DOI] [PubMed] [Google Scholar]
- 22.Yang KD, Ou CY, Chang JC, et al. Infant frequent wheezing correlated to Clara cell protein 10 (CC10) polymorphism and concentration, but not allergy sensitization, in a perinatal cohort study. J Allergy Clin Immunol 2007;120:842–848 [DOI] [PubMed] [Google Scholar]
- 23.Ye Q, Fujita M, Ouchi H, et al. Serum CC-10 in inflammatory lung diseases. Respiration 2004;71:505–510 [DOI] [PubMed] [Google Scholar]
- 24.Shijubo N, Itoh Y, Yamaguchi T, et al. Serum levels of Clara cell 10-kDa protein are decreased in patients with asthma. Lung 1999;177:45–52 [DOI] [PubMed] [Google Scholar]
- 25.Bernard A, Marchandise FX, Depelchin S, Lauwerys R, Sibille Y. Clara cell protein in serum and bronchoalveolar lavage. Eur Respir J 1992;5:1231–1238 [PubMed] [Google Scholar]
- 26.Robin M, Dong P, Hermans C, Bernard A, Bersten AD, Doyle IR. Serum levels of CC16, SP-A and SP-B reflect tobacco-smoke exposure in asymptomatic subjects. Eur Respir J 2002;20:1152–1161 [DOI] [PubMed] [Google Scholar]
- 27.Zhu L, Di PY, Wu R, Pinkerton KE, Chen Y. Repression of CC16 by cigarette smoke (CS) exposure. PloS One 2015;10:e0116159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lomas DA, Silverman EK, Edwards LD, et al. Evaluation of serum CC-16 as a biomarker for COPD in the ECLIPSE cohort. Thorax 2008;63:1058–1063 [DOI] [PubMed] [Google Scholar]
- 29.Guerra S, Halonen M, Vasquez MM, et al. Relation between circulating CC16 concentrations, lung function, and development of chronic obstructive pulmonary disease across the lifespan: a prospective study. Lancet Respir Med 2015;3:613–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersson L, Lundberg PA, Barregard L. Methodological aspects on measurement of Clara cell protein in urine as a biomarker for airway toxicity, compared with serum levels. J Appl Toxicol 2007;27:60–66 [DOI] [PubMed] [Google Scholar]
- 31.St Helen G, Holland NT, Balmes JR, et al. Utility of urinary Clara cell protein (CC16) to demonstrate increased lung epithelial permeability in non-smokers exposed to outdoor secondhand smoke. J Expo Sci Environ Epidemiol 2013;23:183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartert TV, Carroll K, Gebretsadik T, et al. The Tennessee Children's Respiratory Initiative: objectives, design and recruitment results of a prospective cohort study investigating infant viral respiratory illness and the development of asthma and allergic diseases. Respirology 2010;15:691–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carroll KN, Gebretsadik T, Minton P, et al. Influence of maternal asthma on the cause and severity of infant acute respiratory tract infections. J Allergy Clin Immunol 2012;129:1236–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tal A, Bavilski C, Yohai D, Bearman JE, Gorodischer R, Moses SW. Dexamethasone and salbutamol in the treatment of acute wheezing in infants. Pediatrics 1983;71:13–18 [PubMed] [Google Scholar]
- 35.Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995;8:483–491 [DOI] [PubMed] [Google Scholar]
- 36.Sole D, Vanna AT, Yamada E, Rizzo MC, Naspitz CK. International Study of Asthma and Allergies in Childhood (ISAAC) written questionnaire: validation of the asthma component among Brazilian children. J Investig Allergol Clin Immunol 1998;8:376–382 [PubMed] [Google Scholar]
- 37.Shijubo N, Itoh Y, Yamaguchi T, et al. Serum and BAL Clara cell 10 kDa protein (CC10) levels and CC10-positive bronchiolar cells are decreased in smokers. Eur Respir J 1997;10:1108–1114 [DOI] [PubMed] [Google Scholar]
- 38.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2006 [Google Scholar]
- 39.Levin SW, Butler JD, Schumacher UK, Wightman PD, Mukherjee AB. Uteroglobin inhibits phospholipase A2 activity. Life Sci 1986;38:1813–1819 [DOI] [PubMed] [Google Scholar]
- 40.Dierynck I, Bernard A, Roels H, De Ley M. Potent inhibition of both human interferon-gamma production and biologic activity by the Clara cell protein CC16. Am J Respir Cell Mol Biol 1995;12:205–210 [DOI] [PubMed] [Google Scholar]
- 41.Yao XL, Levine SJ, Cowan MJ, Logun C, Shelhamer JH. Tumor necrosis factor-alpha stimulates human Clara cell secretory protein production by human airway epithelial cells. Am J Respir Cell Mol Biol 1998;19:629–635 [DOI] [PubMed] [Google Scholar]
- 42.Miele L, Cordella-Miele E, Mukherjee AB. Uteroglobin: structure, molecular biology, and new perspectives on its function as a phospholipase A2 inhibitor. Endocr Rev 1987;8:474–490 [DOI] [PubMed] [Google Scholar]
- 43.Tokita E, Tanabe T, Asano K, Suzaki H, Rubin BK. Club cell 10-kDa protein attenuates airway mucus hypersecretion and inflammation. Eur Respir J 2014;44:1002–1010 [DOI] [PubMed] [Google Scholar]
- 44.Johansson S, Wennergren G, Aberg N, Rudin A. Clara cell 16-kd protein downregulates T(H)2 differentiation of human naive neonatal T cells. J Allergy Clin Immunol 2007;120:308–314 [DOI] [PubMed] [Google Scholar]
- 45.Harrod KS, Mounday AD, Stripp BR, Whitsett JA. Clara cell secretory protein decreases lung inflammation after acute virus infection. Am J Physiol 1998;275:L924–930 [DOI] [PubMed] [Google Scholar]
- 46.Wang SZ, Rosenberger CL, Bao YX, Stark JM, Harrod KS. Clara cell secretory protein modulates lung inflammatory and immune responses to respiratory syncytial virus infection. J Immunol 2003;171:1051–1060 [DOI] [PubMed] [Google Scholar]
- 47.Cookson WO, Sharp PA, Faux JA, Hopkin JM. Linkage between immunoglobulin E responses underlying asthma and rhinitis and chromosome 11q. Lancet 1989;1:1292–1295 [DOI] [PubMed] [Google Scholar]
- 48.Daniels SE, Bhattacharrya S, James A, et al. A genome-wide search for quantitative trait loci underlying asthma. Nature 1996;383:247–250 [DOI] [PubMed] [Google Scholar]
- 49.Laing IA, de Klerk NH, Turner SW, et al. Cross-sectional and longitudinal association of the secretoglobin 1A1 gene A38G polymorphism with asthma phenotype in the Perth Infant Asthma Follow-up cohort. Clin Exp Allergy 2009;39:62–71 [DOI] [PubMed] [Google Scholar]
- 50.Nie W, Xue C, Chen J, Xiu Q. Secretoglobin 1A member 1 (SCGB1A1) +38A/G polymorphism is associated with asthma risk: a meta-analysis. Gene 2013;528:304–308 [DOI] [PubMed] [Google Scholar]
- 51.Rava M, Tares L, Lavi I, et al. Serum levels of Clara cell secretory protein, asthma, and lung function in the adult general population. J Allergy Clin Immunol 2013;132:230–232 [DOI] [PubMed] [Google Scholar]
- 52.Kurowski M, Jurczyk J, Jarzebska M, et al. Association of serum Clara cell protein CC16 with respiratory infections and immune response to respiratory pathogens in elite athletes. Respir Res 2014;15:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helleday R, Segerstedt B, Forsberg B, et al. Exploring the time dependence of serum clara cell protein as a biomarker of pulmonary injury in humans. Chest 2006;130:672–675 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.