Abstract
Age-related neurological disorders are of growing concern among the elderly, and natural products with neuroprotective properties have been attracting increasing attention as candidates for the prevention or treatment of neurological disorders induced by oxidative stress. In an effort to explore natural resources, we collected some common marine seaweed from the Korean peninsula and Indonesia and screened them for neuroprotective activity against hypoxia/reoxygenation (H/R)-induced oxidative stress. Of the 23 seaweeds examined, the ethanol extract of Gracilariopsis chorda (GCE) provided maximum neuroprotection at an optimum concentration of 15 μg/mL, followed by Undaria pinnatifida. GCE increased cell viability after H/R, decreased the formation of reactive oxygen species (measured by 2′,7′-dichlorodihydrofluorescein diacetate [DCF-DA] staining), and inhibited the double-stranded DNA breaks (measured by H2AX immunocytochemistry), apoptosis (measured by Annexin V/propidium iodide staining), internucleosomal DNA fragmentation (measured by DNA laddering), and dissipation of mitochondrial membrane potential (measured by JC-1 staining). Using reverse-phase high-pressure liquid chromatography, we quantitated the arachidonic acid (AA) in GCE, which provides neuroprotection against H/R-induced oxidative stress. This neuroprotective effect of AA was comparable to that of GCE. These findings suggest that the neuroprotective effect of GCE against H/R-induced neuronal death is due, at least in part, to the AA content that suppresses neuronal apoptosis.
Key Words: : apoptosis, arachidonic acid, Gracilariopsis chorda, hippocampal neurons, hypoxia/reoxygenation, marine macroalgae, neuroprotection
Introduction
Increased longevity means that oxidative stress-related neurodegenerative disorders are more often encountered. Globally, the number of people aged 65 years or above is projected to increase dramatically, for example, it was predicted to increase from 420 million in 2000 to 973 million in 2030.1 Hypoxic brain injury represents a condition in which there is insufficient oxygen supply to the brain and has long been related to neuronal cell death associated with certain neurodegenerative events, such as Alzheimer's and Parkinson's diseases. The neuronal cell death observed in individuals after acute injury or in those with a chronic disease is frequently caused by oxidative stress.2 Hypoxia/reoxygenation (H/R) can cause oxidative stress in a process that involves the formation of reactive oxygen species (ROS), which include the superoxide anion (O2−), hydrogen peroxide (H2O2), and the hydroxyl radical (OH).3 Excessive ROS generation can induce apoptotic events that are characterized by nucleosomal fragmentation and the depolarization of mitochondrial membrane potential (MMP).4–7 Furthermore, since long-term exposure to drugs can cause side effects in patients, many researchers have sought to identify natural products capable of preventing free radical formation by H/R-induced oxidative stress for the treatment of neurological disorders.
Marine algae, also referred to as seaweeds, have been considered potential therapeutic agents for the treatment of oxidative stress-related neurodegenerative disorders.8 For example, the edible seaweed Eisenia bicyclis and its phlorotannin constituents have been shown to protect retinal ganglion cell death in the presence of oxidative stress.9 Fucoidan from the brown alga Laminaria japonica, effectively prevents the apoptosis of pheochromocytoma cells induced by H2O2.10 Recently, ethanol extracts of the marine seaweed Gelidium amansii and Sargassum fulvellum were shown to increase cell viability in normoxic hippoocampal cultures and to have neurotrophic effects.11–13 These results indicate that neurotrophic factors can have neuroprotective effects. Indeed, it has been reported that nerve growth factor attenuates injury in cultured hippocampal and cortical neurons exposed to various oxidative insults.14,15
However, no previous study has been conducted on the neuroprotective activity of marine seaweeds on central nervous system (CNS) neurons exposed to oxidative stress by H/R, a model system of in vitro oxidative stress. In this study, using a H/R rat hippocampal neuronal culture model, we screened the neuroprotective effects of different seaweeds collected in Korea and Indonesia.
Materials and Methods
Collection and processing of seaweed samples
Samples of 23 seaweed types were collected, epiphytes cleaned off, salts removed by mechanical washing with fresh water, and dried in the shade at room temperature for 1 week. Dried samples were then pulverized using a grinder (HMF-340; Hanil Co., Seoul, Korea) and stored in sealed plastic bags in the dark at −20°C until required. Botanical names were authenticated using local or common names.16 Voucher specimens were deposited in the laboratory of Dr. Y. K. Hong (Pukyong National University, Busan, Korea). Seaweed samples were given random codes before screening.
Extraction of seaweeds
Ethanol (95%) was poured into a conical flask containing 2 g of a seaweed powder at a ratio of 0.02 g/mL. The mixture was placed on an orbital shaker at 200 rpm (VS-202D; Vision Scientific Co. Ltd., Seoul, Korea) at room temperature for 24 h in the dark, the slurry obtained was centrifuged at 17,888 g (Sorvall T6000D benchtop centrifuge; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and the resulting supernatant was filtered through sterile cotton. The filtrate was concentrated in vacuo and dried completely under a stream of nitrogen gas. The dried ethanol extract was weighed and extract yield (w/w%) was calculated (data not shown). The yield of the ethanol extract of Gracilariopsis chorda (GCE) was 0.22% (w/w). Extracts were dissolved in dimethyl sulfoxide (DMSO) at a concentration of 8 mg/mL and stored in foil-wrapped vials at −20°C for further experiments.
Reverse-phase high-pressure liquid chromatography analysis of arachidonic acid in GCE
The arachidonic acid (AA) standard sample (>99% purity; Sigma-Aldrich, St. Louis, MO, USA) was dissolved in methanol with concentrations ranging from 0.82 to 3.28 mM. A standard calibration curve was made for quantification. GCE was dissolved in methanol to make a final concentration of 10 mg/mL, and 100 μL was injected into a semi-prep C18 reverse-phase high-pressure liquid chromatography (RP-HPLC column, Ultrasphere; Beckman Coulter, Fullerton, CA, USA). The HPLC mobile phase was a two solvent system: acetonitrile with 0.1% trifluoroacetic acid and water with 0.1% trifluoroacetic acid. The UV detector was monitored at 213 nm. The amount of AA in G. chorda sample was quantitated by comparing the peak area of the algal sample to the standard curve of pure AA.
Primary culture of hippocampal neurons and treatment
All reagents used for primary cell cultures were purchased from Invitrogen (Carlsbad, CA, USA), unless otherwise stated. Pregnant rats (Sprague-Dawley) were ordered on the 13th day of pregnancy and housed in a controlled environment with ad lib access to food and water. Experiments were approved beforehand by the Institutional Animal Care and Use Committee of the College of Medicine, Dongguk University. On the 19th day of pregnancy, rats were euthanized with isofluorane and fetuses were collected. The fetal hippocampi were excised from brains, and hippocampal neuronal cultures were prepared as described previously.17 Briefly, hippocampi were collected in Hank's balanced salt solution (HBSS), tissues were dissociated by digestion with 0.25% trypsin in HBSS for 12 min at 37°C with gently shaking at 4 min intervals and triturated through fire-polished graded Pasteur pipettes. The dissociated cells were counted using a hemocytometer and plated at a density of 3.0×104. For DNA extraction, cells were plated at a density of 40.0×104 cells/cm2 onto poly-DL-lysine (PDL) (Sigma-Aldrich)-coated Ø12-mm glass coverslips in preincubated serum-free neurobasal medium supplemented with B27 (Invitrogen) (24-well culture plates with 800 μL of medium/well) and incubated at 37°C under 5% CO2 and 95% air. Sample extracts, AA, or control (DMSO, final concentration <1.0%) were added to media before cell plating.
Cell feeding and H/R
Hippocampal cells were fed every 4 days during culture by replacing 1/4 the media with fresh prewarmed serum-free neurobasal media supplemented with B27, with or without sample extract. H/R of rat hippocampal neurons was carried out as described previously,18,19 with some modification. Briefly, after the indicated times, culture plates were transferred into a modular hypoxia chamber (Modular Incubator Chamber MIC-101; Billups-Rothenberg, Inc., Del Mar, CA, USA) in 94% N2, 5% CO2, and 1% O2 atmosphere, and incubated at 37°C. To reoxygenate hypoxic cells, culture plates were placed in a normoxic incubator (95% air, 5% CO2) and incubated at different times for further experiments.
Propidium iodide cell viability assay
At indicated times, cells were stained with propidium iodide (PI) at a final concentration of 1.0 μM for 12 min at room temperature and washed with Dulbecco's phosphate-buffered saline (D-PBS; Invitrogen). Phase-contrast and red-fluorescence photographs were then taken. PI-stained neurons, which fluoresced red, were considered dead cells. Results were normalized versus PI-stained no H/R controls.
Lactate dehydrogenase cytotoxicity assay
Cytotoxicities were evaluated by measuring lactate dehydrogenase (LDH) released into culture media by hippocampal cells treated with control (No H/R or H/R) or extracts at different concentrations. After exposure to H/R, LDH assays were performed using the CytoTox96 Nonradioactive Assay Kit (Promega, Madison, WI, USA); quantification was performed at a wavelength of 490 nm. LDH activity is the percentage of the ratio of experimental LDH release to the maximum LDH release. Results were normalized versus the amount of LDH released from the no H/R control cells (100%).
Assessment of intracellular ROS
ROS production in cells was determined using the green fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA) (Molecular Probes, Eugene, OR, USA). DCF-DA was added to culture media at 10 μM for 20 min in the dark. After washing with prewarmed PBS, phase-contrast and fluorescence images were obtained, and numbers of ROS-positive cells were counted.
Immunocytochemistry using anti-phospho-H2AX antibody
At indicated times, neurons on coverslips were rinsed briefly with D-PBS (Invitrogen) and fixed using a sequential paraformaldehyde/methanol fixation procedure.20 For immunostaining, coverslips were incubated with a rabbit polyclonal anti-phospho-H2AX antibody (1:500; Upstate Biotechnology, Inc., now Millipore, Billerica, MA, USA), followed by secondary antibodies (Alexa Fluor 568-conjugated donkey anti-rabbit IgG [1:1000]; Molecular Probes) and mounted on slides as previously described.20
Detection of apoptotic cells by Annexin V/PI staining
Cells were washed with D-PBS and stained with Alexa Fluor® 488 Annexin V (AV; Invitrogen) and PI (Sigma-Aldrich) for 20 min in the dark at room temperature. Cells were then washed twice with the AV binding buffer. Phase-contrast and fluorescence images were obtained using green or red channels. Apoptotic cells were counted, and results were normalized versus no H/R control cells (100%).
DNA fragmentation by agarose gel electrophoresis
DNA was extracted from dissociated hippocampal cells (5×106) as previously described.21 DNA was separated on 1.5% agarose gel containing 0.5 μg/mL ethidium bromide and visualized by ultraviolet transillumination.
Measurement of MMP by JC-1 staining
Cells were washed with fresh media and stained with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) (1 μg/mL; Molecular Probes) for 20 min in a CO2 incubator and then washed twice with fresh media. Fluorescence images were obtained using green or red channels and the intensity ratio of the red/green fluorescence of each neuron was assessed using ImageJ (version 1.45).
Trypan blue exclusion assay
Neuronal viability was assessed using Trypan blue exclusion assay at the indicated time of cultures. The cultures were stained with 0.4% Trypan blue for 15 min at room temperature. After washing with D-PBS, the cultures were then fixed with 4% paraformaldehyde. The viable cells will exclude Trypan blue dye, whereas dead cells will not, thus appearing dark blue in phase-contrast image. Results were normalized versus Trypan blue-stained no H/R controls.
Image acquisition, optimization, and quantification
A Leica Research Microscope DM IRE2 equipped with I3 S, N2.1 S, and Y5 filter systems (Leica Microsystems AG, Wetzlar, Germany) was used for phase-contrast and epifluorescence microscopy. Images (1388×1039 pixels) were acquired using a high-resolution CoolSNAP™ CCD camera (Photometrics, Inc., Tucson, AZ, USA) under the control of a computer running Leica FW4000 software. The quantifications of cells or puncta were performed using ImageJ (version 1.45) software with the cell counter plugin (National Institute of Health, Bethesda, MA, USA). Gel imaging was processed using the AlphaImager® HP system (www.alphainnotech.com).
Statistical analysis
Results are presented as means±standard errors of means. Data were analyzed using Student's t-test and one-way analysis of variance with Duncan's post hoc multiple comparison. Statistical significance was accepted for P-values <.05, and SPSS software (version 16.0; SPSS, Inc., Chicago, IL, USA) was used throughout.
Results
Optimization of the effect of H/R on neuronal viability
Schematic representation of the hippocampal culture and assays were outlined in Figure 1. On days in vitro (DIV) 9, hippocampal cultures were exposed to hypoxia (94% N2/5% CO2/1% O2 gas mixture) for 3 h, and then reoxygenated (i.e., returned to normoxic culture conditions) for different times ranging from zero (DIV 9) to 7 days (DIV 16). Neuronal viabilities were assessed by PI staining (Fig. 2). Results indicated that H/R affected neuronal viability in a time-dependent manner. As compared with no reoxygenation control, highly significant (P<.001) decreases were observed after 4 days (96 h) of reoxygenation (77.6%±1.51%). Therefore, we chose 3 h of hypoxia and 4 days (96 h) of reoxygenation for subsequent screening experiments, unless otherwise mentioned.
FIG. 1.
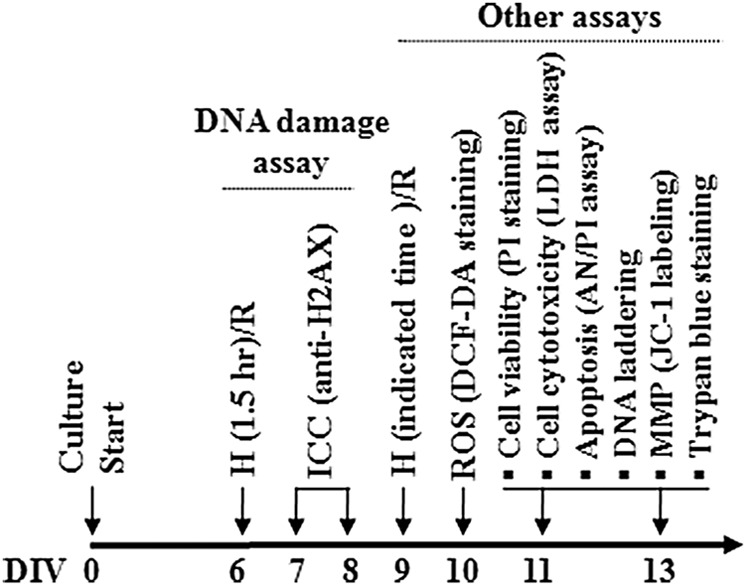
Schematic representation of hippocampal culture and assay schedules. For the assessment of DNA damage, hypoxia/reoxygenation (H/R) shock was given in early maturing stage of culture, on days in vitro (DIV) 6–8, when the integrity of DNA is expected to be best under normal condition. For reactive oxygen species (ROS) measurement, H/R shock was given in the later maturing stages of culture (DIV 9) and the effect of extracts was assessed in the early reoxygenation time (DIV 10). For other assessments, H/R shock was given on DIV 9 and the various effects were assayed in the delayed neuronal death periods (DIV 11–13).
FIG. 2.

Time course of neuronal viability after H/R. Embryonic (E19) rat hippocampal cells were cultured on poly-DL-lysine-coated coverslips in serum-free neurobasal media with B27 supplements. On DIV 9, cultures were exposed to 3 h hypoxia (5% CO2, 94% N2, and 1% O2) and subsequently to normoxia (95% air and 5% CO2) for different times ranging from no reoxygenation (day 0; DIV 9) to 7 days (day 7; DIV 16). Cell viabilities were determined by propidium iodide (PI) staining. Viabilities, which are expressed as percentages of PI-negative cells (live neurons), were normalized versus no reoxygenation (day 0). Bars represent the means±standard errors of means (SEMs; three independent experiments). Comparisons were made versus no reoxygenation control, day 0: *P<.05, **P<.01, and ***P<.001 (analysis of variance [ANOVA]).
Screening of seaweeds for neuroprotective activity against H/R
We collected 23 edible seaweed types from Korean and Indonesian coasts (Table 1). To screen for marine seaweeds with potent neuroprotective activity, rat hippocampal neurons (DIV 9) were cultured in the presence of ethanol extracts and cultures were exposed to H/R (3 h/96 h). Cultures were treated with three different concentrations of seaweed extract: 5, 15, and 30 μg/mL. About one third of the tested seaweeds exhibited neuroprotective activity (Table 2). Cell viability and cell cytotoxicity testing revealed that the ethanol GCE (code SW 23) afforded the most neuroprotection at a concentration of 15 μg/mL, at which GCE significantly increased cell viability (PI staining) to 119.0%±3.2% (P<.01), and decreased cell death (LDH assay) to 80.5%±10.3% (P<.01) of [GCE(−) no H/R controls] (Table 2). Undaria pinnatifida had almost the same level of neuroprotection as GCE, and others like S. fulvellum, Sargassum nigrifolium, Porphyra yezoensis, Gracilaria coronopifolia, Gracilaria tenuistipitata, Eisenia bicyclis, and Carpopeltis cornea also exhibited moderate neuroprotective effects (Table 2).
Table 1.
Classification of Seaweeds Used in This Study
| Division | Code | Botanical name | Harvested country |
|---|---|---|---|
| Chlorophyta (green algae) | SW 01 | Codium fragile subsp. fragile (Suringar) Hariot | Kor |
| SW 06 | Chaetomorpha antennina (Bory de Saint-Vincent) Kützing | Indo | |
| SW 07 | Enteromorpha linza (Linnaeus) J.Agardh (South korea) | Kor | |
| SW 18 | Capsosiphon fulvescens (C. Agardh) Setchell & N. L. Gardner | Kor | |
| SW 19 | Enteromorpha compressa (Linnaeus) Nees | Kor | |
| Phaeophyta (brown algae) | SW 05 | Sargassum polycystum C.Agardh | Indo |
| SW 10 | Padina australis Hauck | Indo | |
| SW 11 | Turbinaria conoides (J.Agardh) Kützing | Indo | |
| SW 13 | Sargassum fulvellum (Turner) C. Agardh | Kor | |
| SW 14 | Eisenia bicyclis (Kjellman) Setchell | Kor | |
| SW 15 | Undaria pinnatifida (Harvey) Suringar | Kor | |
| SW 16 | Sargassum nigrifolium Yendo | Kor | |
| SW 20 | Sargassum horneri (Turner) C. Agardh | Kor | |
| SW 21 | Hizikia fusiformis (Harvey) Okamura | Kor | |
| SW 22 | Laminaria japonica Areschoug | Kor | |
| Rhodophyta (red algae) | SW 02 | Corallina pilulifera Postels & Ruprecht | Kor |
| SW 03 | Pachymeniopsis elliptica (Holmes) Yamada | Kor | |
| SW 04 | Porphyra yezoensis f. kinositae Y.Yamada & T.Tanaka | Kor | |
| SW 08 | Gracilaria coronopifolia J.Agardh | Indo | |
| SW 09 | Gracilaria arcuata Zanardini | Indo | |
| SW 12 | Gracilaria tenuistipitata C. F. Chang & B. M. Xia | Kor | |
| SW 17 | Carpopeltis cornea (Okamura) Okamura | Kor | |
| SW 23 | Gracilariopsis chorda (Holmes) Ohmi | Kor |
SW, seaweed.
Table 2.
Neuroprotective Activities of Seaweeds After Hypoxia/Reoxygenation
| Viability (% of no H/R) | Cytotoxicity (% of no H/R) | |||||||
|---|---|---|---|---|---|---|---|---|
| Code | 0 μg/mL | 5 μg/mL | 15 μg/mL | 30 μg/mL | 0 μg/mL | 5 μg/mL | 15 μg/mL | 30 μg/mL |
| SW(−) No H/R | 100±5.1 | 100±9.5 | ||||||
| SW(−) H/R | 90.6±2.7# | 116.7±8.2# | ||||||
| SW 01 | 97.5±4.8 | 100.5±10.2 | 101.6±13.8 | 109.6±13.0 | 118.5±12.3 | 103.2±4.8 | ||
| SW 02 | 103.0±14.3 | 84.9±10.2 | 72.4±4.5 | 100.6±12.3 | 124.4±12.8 | 139±7.0 | ||
| SW 03 | 98.9±5.1 | 103.9±17.5 | 93.9±14.4 | 107.2±14.3 | 99.4±2.0 | 112.7±7.8 | ||
| SW 04 | 103.6±9.9 | 108.1±7.0* | 92.7±6.8 | 102±22.2 | 93.8±5.9* | 115.5±8.9 | ||
| SW 05 | 75.4±35.8 | 69.5±14.3 | 57.8±7.0 | 136.3±8.5 | 167.8±28.6 | 158.9±4.9 | ||
| SW 06 | 99.8±2.9 | 96.4±6.7 | 95.0±3.5 | 103.8±11.7 | 110.7±12.3 | 110.3±8.2 | ||
| SW 07 | 92.5±10.1 | 73.3±17.3 | 45.7±6.0 | 118.1±11.5 | 138.7±17.0 | 175.8±33.1 | ||
| SW 08 | 100.1±2.2* | 103.6±5.5 | 93.8±5.4 | 92.4±10.7* | 101.2±9.5 | 104.8±10.3 | ||
| SW 09 | 94.2±13.7 | 91.9±1.1 | 86.2±10.3 | 110.0±8.2 | 114.7±3.3 | 121.9±8.1 | ||
| SW 10 | 94.7±8.2 | 86.0±0.6 | 42.2±2.9 | 112.1±5.5 | 124.5±25.5 | 178.6±5.0 | ||
| SW 11 | 101.3±2.4 | 94.6±15.6 | 75.4±0.8 | 103.2±2.5 | 112.3±12.4 | 136.7±5.9 | ||
| SW 12 | 104.9±6.1 | 106.6±5.9* | 109.4±10.1* | 98.3±4.3 | 96.1±11.4 | 92.5±17.2** | ||
| SW 13 | 108.1±9.6 | 109.8±6.4* | 112.5±8.5* | 94.3±12.2 | 92.0±7.9 | 88.5±10.1** | ||
| SW 14 | 103.0±8.7 | 110.5±12.6 | 110.3±17.9* | 100.8±12.5 | 99.3±16.3 | 91.4±5.6* | ||
| SW 15 | 112.7±2.7* | 116.0±4.4** | 105.1±14.1 | 85.1±8.3** | 84.3±21.2** | 97.3±1.2 | ||
| SW 16 | 108.8±8.5* | 113.5±9.8* | 84.0±22.2 | 96.1±26.0 | 86.5±31.3** | 125.3±23.8 | ||
| SW 17 | 105.3±20.4 | 105.3±4.1 | 111.7±5.5* | 98.2±5.3 | 97.8±16.2 | 89.5±12.5* | ||
| SW 18 | 106.7±7.9 | 109.9±1.9* | 108.3±13.9 | 96.1±18.3 | 91.9±6.2 | 94.0±23.4 | ||
| SW 19 | 103.2±6.8 | 104.5±10.9 | 107.4±8.0 | 102.9±0.8 | 98.8±19.1 | 94.4±9.1 | ||
| SW 20 | 89.2±20.0 | 79.4±9.9 | 63.1±13.7 | 114.6±21.4 | 131.5±14.1 | 151.9±9.7 | ||
| SW 21 | 88.9±14.3 | 62.8±8.2 | 61.1±8.1 | 116.5±26.3 | 155.0±37.7 | 163.4±25.4 | ||
| SW 22 | 73.9±25.0 | 71.8±28.7 | 71.0±4.6 | 135.7±18.7 | 127.9±5.1 | 137.7±33.5 | ||
| SW 23 | 110.1±6.6* | 119.0±3.2** | 104.2±2.8 | 92.5±8.1 | 80.5±10.3** | 99.2±7.0 | ||
Rat hippocampal cultures were grown in the presence of ethanol extracts of 23 different SW and the cultures were exposed to hypoxia (3 h) on DIV 9. After reoxygenation for 96 h (DIV 13), cell viabilities (PI staining) and cytotoxicities (LDH assay) were measured and expressed as percentages of the SW(−) no H/R control groups. Data points represent the means±SEMs of three independent experiments. H/R significantly decreased the viability and increased the cytotoxity of SW(−) cultures (#P<.05). Statistical significance of SW-treated cultures were made versus SW(−) H/R control groups on DIV 13: *P<.05, **P<.01 (ANOVA).
ANOVA, analysis of variance; DIV, days in vitro; H/R, hypoxia/reoxygenation; LDH, lactate dehydrogenase; PI, propidium iodide; SEMs, standard errors of means.
Neuroprotective activity of GCE in normoxia
Based on preliminary screening results, we further investigated the effects of GCE. Culture in vitro represents a stressful situation for brain cells, and some cells die even under normoxic conditions. Therefore, we first examined the effect of GCE on cell death under normoxia. Normoxic hippocampal cultures were treated with GCE at different concentrations, and PI staining of cells on DIV 13 (Fig. 3A) revealed that GCE significantly (P<.001) increased cell viability in a dose-dependent manner and that it exhibited a maximum effect (148.71%±5.73% of the control level) at a concentration of 15 μg/mL (Fig. 3B). This concentration matched that with maximal neuroprotective effect against H/R (3 h/96 h) (Table 2), and thus, this concentration was used in subsequent experiments.
FIG. 3.
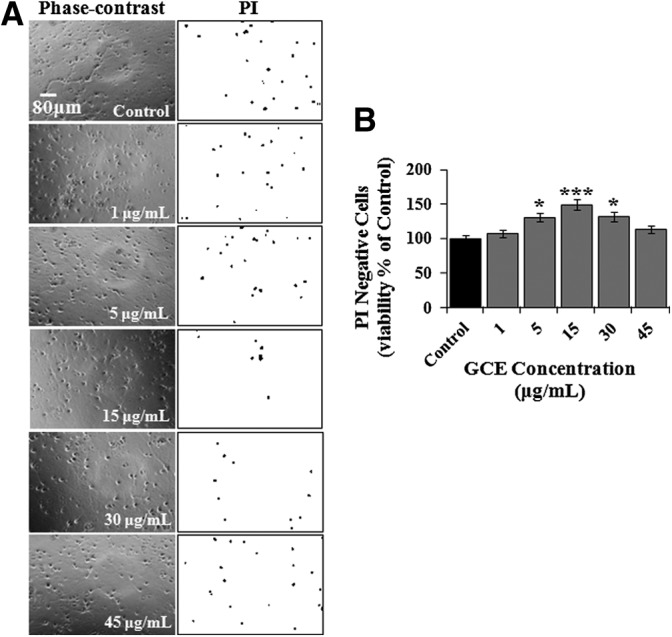
Neuroprotective effects of Gracilariopsis chorda (GCE) in normoxic culture. Hippocampal cultures were grown in vehicle- or GCE-treated (1–45 μg/mL) media. Cell viabilities were determined by PI staining on DIV 13. (A) Phase-contrast and images representative of PI staining at different GCE concentrations. The acquired red fluorescent PI images were gray scaled and inverted to make PI signals (i.e., dead cells) appear dark. Scale bar=80 μm in all images. (B) Viabilities, which are expressed as percentages of PI-negative cells (live neurons), were normalized versus vehicle-treated controls. Bars represent the means±SEMs of three independent experiments. Statistical significance of differences were determined between treated versus GCE untreated control: *P<.05 and ***P<.001 (ANOVA).
GCE inhibited ROS formation
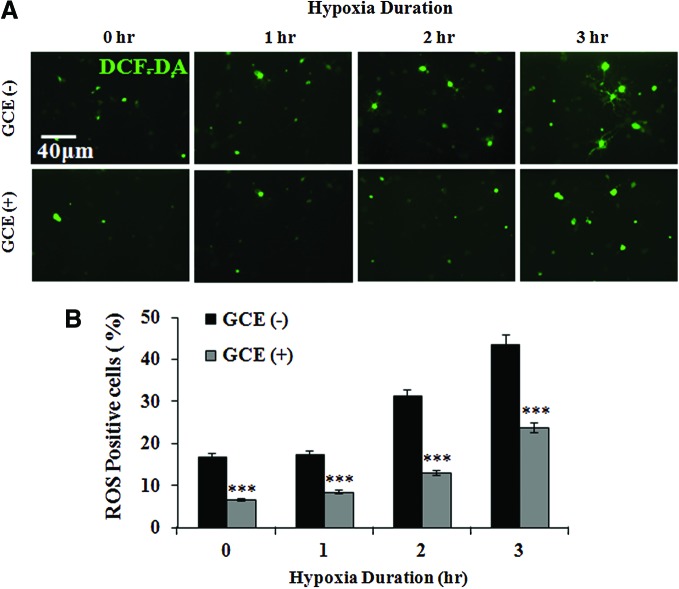
Excessive ROS generation has destructive effects in the brain when severe oxidative stress takes place. We, therefore, examined the effect of GCE on various levels of hypoxia-induced intracellular ROS formation in later stages of mature neurons. Hippocampal cultures were exposed to hypoxic conditions on DIV 9 and ROS levels were measured using DCF-DA (a fluorescent probe) after 24 h reoxygenation. As shown in Figure 4A, numbers of ROS-positive cells were increased by increasing hypoxia (0, 1, 2, and 3 h) in both control [GCE(−)] and GCE-treated [GCE(+)] cultures. However, treatment with GCE (15 μg/mL) significantly (P<.001) decreased the numbers of ROS-positive cells by 10.12%, 8.76%, 18.22%, and 19.96% (0, 1, 2, and 3 h hypoxia, respectively) compared with GCE(−) controls (Fig. 4B).
FIG. 4.
Effect of GCE on ROS production after H/R. Vehicle or GCE (15 μg/mL) were added to hippocampal cultures. On DIV 9, cultures were exposed to hypoxia for different times (0, 1, 2, or 3 h) and then to normoxia for 24 h. ROS levels were assessed by staining with the fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA). (A) Typical images of DCF-DA staining. Scale bar=40 μm in all images. (B) Percentages of ROS(+) cells were calculated. Bars represent the means±SEMs of three independent experiments. ***P<.001 [vs. the GCE(−) control] (Student's t-test). Color images available online at www.liebertpub.com/jmf
GCE attenuated DNA fragmentation
ROS-mediated chromatin dysfunctions such as single- and double-strand DNA fragmentation leads to cell death associated with apoptosis and necrosis. In view of that, hippocampal cultures were exposed to hypoxic conditions for 1.5 h on DIV 6, and immunostained with anti-phospho-H2AX antibody after 24 or 48 h of reoxygenation (Fig. 5A). In controls (DMSO), numbers of phospho-H2AX immunostained puncta per nucleus significantly (P<.001) increased from 10.5±4.9 (no-hypoxia) to 23.55±7.9 after 24 h of reoxygenation, and from 14.25±7.9 (no-hypoxia) to 29.65±10.5 after 48 h of reoxygenation. Pretreatment with GCE (15 μg/mL) significantly (P<.001) decreased numbers of puncta to 11.75±5.1 and 12.6±4.71 after 24 and 48 h of reoxygenation, respectively (Fig. 5B).
FIG. 5.
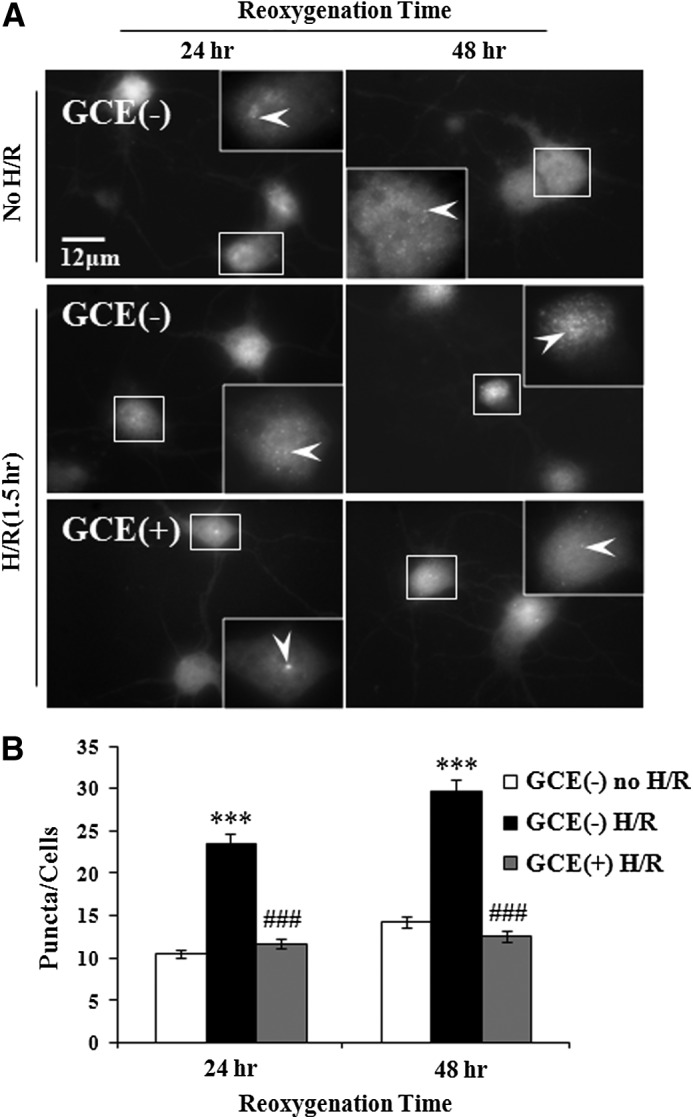
Protective effects of GCE on DNA fragmentation after H/R. Hippocampal cells were cultured under the conditions detailed in Figure 4. Cultures were exposed to hypoxia (1.5 h) on DIV 6, then normoxia for 24 or 48 h, and then fixed. Cells were immunostained with anti-phospho-H2AX antibody. (A) Representative immunofluorescence images. Boxed nuclei are enlarged to show individual puncta (inset, arrowheads). Scale bar=12 μm in all images. (B) Numbers of punctae per nucleus were counted. Bars represent means±SEMs (n=30 nuclei). ***P<.001 [vs. the GCE(−) control group], ###P<.001 (vs. H/R group) (ANOVA).
GCE prevented apoptosis
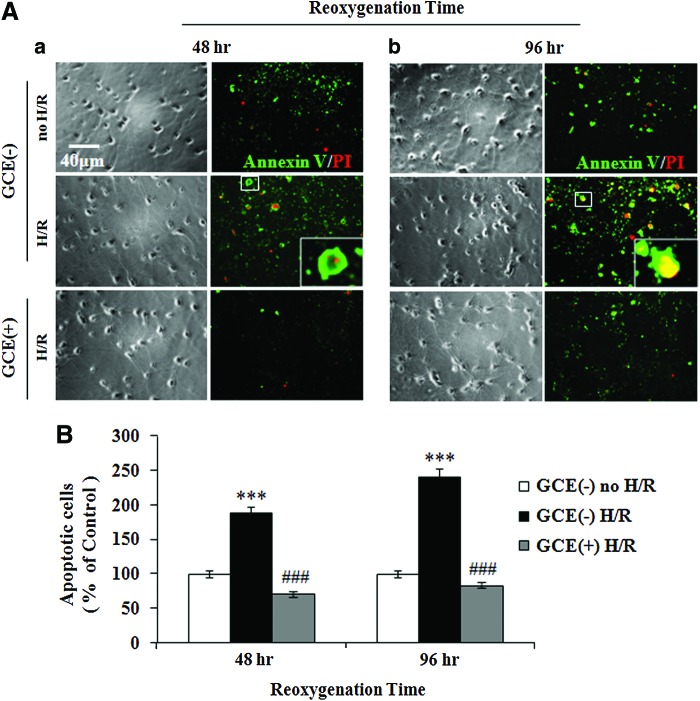
Apoptosis is a major mode of cell death after H/R,22 and therefore, we investigated whether GCE could protect cells from phosphorylation of the histone variant H2AX, which is a hallmark of apoptosis. Hippocampal cells were exposed to hypoxia (3 h) on DIV 9, and stained with Alexa Fluor 488 AV and PI after 48 or 96 h of subsequent reoxygenation (Fig. 6A). Statistical analysis showed that in control GCE(−) cultures, H/R increased the percentages of apoptotic cells [AV(+)/PI(+)] significantly (P<.001) to 188.7%±8.90% (48 h reoxygenation) and 241.14%±13.72% (96 h reoxygenation) [Fig. 6B, GCE(−) H/R]. However, when cells were pretreated with GCE, percentages of apoptotic cells were significantly (P<.001) reduced to 70.53%±7.19% (48 h reoxygenation) and 83.15%±6.23% (96 h reoxygenation) of the GCE(−) no H/R controls [Fig. 6B, GCE(+) H/R].
FIG. 6.
Protective effect of GCE on apoptosis after H/R. Hippocampal cells were grown under the culture conditions detailed in Figure 4. (A) Phase-contrast and typical AV/PI stain images. Cultures were exposed to hypoxia for 3 h and normoxia for 48 (a) or 96 h (b). Apoptotic cell percentages were determined by double-staining with Annexin V (AV; green) and PI (red). Typical AV(+)/PI(+) cells (boxed) are shown enlarged in insets. Scale bar=40 μm in all images. (B) The percentages of apoptotic cells [AV(+)/PI(+)] were normalized vs. GCE(−) no H/R cells. Bars represent the means±SEMs of three independent experiments. Statistically significant, ***P<.001 [vs. the GCE(−) control group], ###P<.001 (vs. H/R group) (ANOVA). Color images available online at www.liebertpub.com/jmf
GCE inhibited internucleosomal DNA fragmentation
Internucleosomal DNA fragmentation is a feature of apoptosis, which results in DNA laddering as determined by agarose gel electrophoresis. To investigate the effect of GCE on the chromosomal DNA, we extracted DNA from control and GCE-treated cultures 96 h after 3 h of hypoxia on DIV 9. Extracted DNA was electrophoresed on 1.5% agarose gels and visualized by ethidium bromide staining (Fig. 7A); the plot profiles of DNA intensities are shown in Figure 7B. H/R increased the intensity of DNA laddering in GCE(−) cultures and decreased the intensity of intact DNA (arrowhead) (Fig. 7A-middle lane; 7B-H/R) versus treatment-naive controls (Fig. 7 no H/R). In contrast, GCE-pretreated cells (Fig. 7A-right lane; 7B-H/R+GCE) showed less DNA laddering and greater intact DNA intensities (arrow in Fig. 7A) than GCE(−) H/R cells and naive no H/R controls.
FIG. 7.
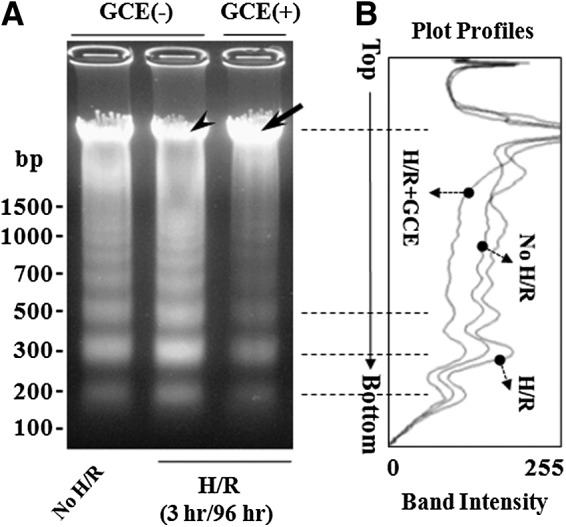
Protective effect of GCE on internucleosomal DNA fragmentation after H/R. Hippocampal cultures were grown using the culture conditions detailed in Figure 4. Cultures were exposed to hypoxia for 3 h on DIV 9 and normoxia for 96 h. DNA was isolated, electrophoresed in agarose gel (1.5%), and visualized by EtBr staining. (A) Representative agarose gel image showing DNA laddering. The positions of intact DNA are marked with an arrow or an arrowhead. Molecular sizes are marked on the far left in base pairs (bp). (B) Profiles for each lane are overlapped to allow comparisons of relative band intensities (arbitrary numbers).
GCE prevented loss of MMP
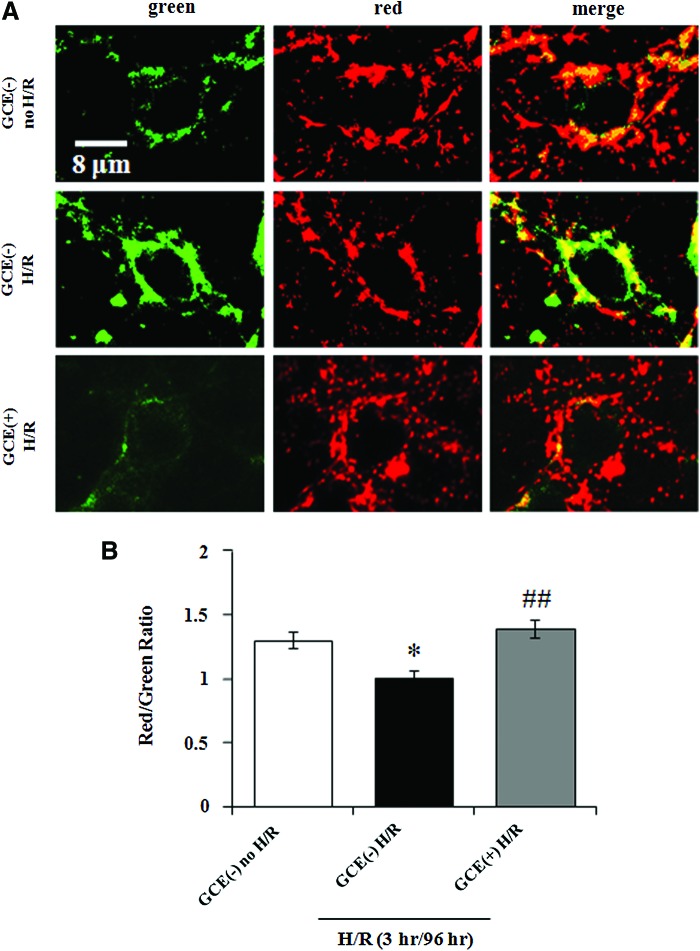
H/R-induced MMP loss plays a key role in the initiation and execution of apoptosis.23 To observe the effect of GCE on MMP maintenance after H/R, hippocampal cultures were exposed to 3 h of hypoxia on DIV 9 and MMP levels were measured by staining with JC-1 after 96 h of reoxygenation. Green (representing JC-monomer), red fluorescent (representing JC-aggregate) images, and merge images are shown in Figure 8A. Before H/R, cells grown without GCE [GCE(−) no H/R] showed a mixture of green (i.e., low MMP) and red fluorescing (i.e., high MMP) mitochondria. When GCE(−) cultures were exposed to hypoxia, they showed mostly green fluorescence [GCE(−) H/R], whereas GCE-treated cultures [GCE(+) H/R] showed mostly red fluorescence. The overall field intensities were measured using ImageJ software, and the intensity ratios of red to green fluorescence are shown in Figure 8B. H/R (3 h/96 h) decreased the red/green ratio significantly to 1.01±0.23 [Fig. 8B, GCE(−) H/R] from 1.31±0.29 in GCE(−) no H/R control cultures [Fig. 8B, GCE(−) no H/R]. In contrast, the red/green ratio was 1.41±0.46 in GCE(+) H/R cultures under the same conditions, which represented a significant (P<.01) increase. These results indicate that GCE inhibits MMP dissipation from mitochondria after H/R shock.
FIG. 8.
Prevention of mitochondrial membrane potential (MMP) loss by GCE after H/R. Hippocampal cultures were grown under the culture conditions detailed in Figure 4. Cultures were exposed to hypoxia for 3 h on DIV 9 and to normoxia for 96 h. MMP levels in cultured cells were assessed by staining with the fluorescence probe 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1). (A) Representative images of JC-1 staining. Scale bar=8 μm in all images. (B) Red/green intensity ratios. Bars represent the means±SEMs of three independent experiments. *P<.05 [vs. the GCE(−) control group], ##P<.01 (vs. H/R group) (ANOVA). Color images available online at www.liebertpub.com/jmf
Identification and quantification of AA in G. chorda
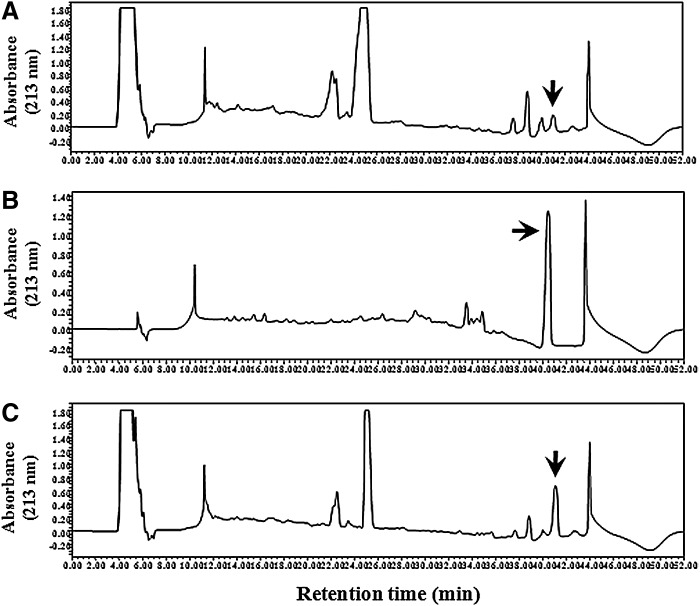
The presence of AA in GCE was identified by the retention time in RP-HPLC compared with standard AA peak, and also quantified by comparing the peak height of the sample with those of the AA standards. Using the calibration curve, AA content was calculated as 0.64% (w/w) of GCE and 1.5 mg/100 g dry weight of G. chorda powder (three repeats) (Fig. 9). Our estimated AA content in G. chorda sample was comparable to the previous report.24
FIG. 9.
Identification and quantification of arachidonic acid (AA). Representative reverse-phase high-pressure liquid chromatography (RP-HPLC) profiles of GCE (A), AA (B), and the mixture of GCE and AA (C). The positions of the AA peak are indicated by arrows.
AA protects hippocampal neurons against H/R injury
AA is reported to be in high amounts in G. chorda,24 and afforded neuroprotection in hippocampal slices insulted by oxidative stress in vitro.25 To investigate whether AA can protect neurons from death in H/R, hippocampal cultures were exposed to hypoxia for 3 h on DIV 9. Trypan blue exclusion assay was performed to assess the cell viability after 96 h reoxygenation, in the presence of AA at different concentrations and/or GCE (15 μg/mL). As shown in Figure 10, AA protected neurons from death induced by H/R, as compared to GCE, and even increased the cell viability in a dose-dependent manner at an optimum concentration of 7.5 μM (Fig. 10A, B). However, at higher concentrations (>7.5 μM), AA caused cell death, as previously reported by Wang et al.25
FIG. 10.

AA protects hippocampal neurons against H/R. Hippocampal cultures were grown in the presence of vehicle (dimethyl sulfoxide), GCE (15 μg/mL), or AA (1–30 μM). Cultures were subjected to hypoxia for 3 h on DIV 9 and returned to normoxia for 96 h. Cell viabilities were determined by Trypan blue exclusion assay on DIV 13. (A) Representative images of Trypan blue exclusion assays. Arrowheads indicate dead cells. Scale bar=40 μm. (B) Statistics. Viabilities are expressed as percentages of Trypan blue excluded cells (live neurons), and the data were normalized versus vehicle-treated controls. Bars represent the means±SEMs of three independent experiments. **P<.01 (vs. control group), and ###P<.001 (vs. H/R group) (ANOVA).
Discussion
As compared with other tissues and organs, the brain is particularly prone to ischemic damage. In animal models, even brief periods of ischemia/reoxygenation cause massive neuronal death.26 Excitotoxicity, defined as toxicity due to the excessive activation of glutamate receptors, is known to be the main mechanism of cell death after ischemia/reperfusion. The excessive activation of glutamate receptors depolarizes neurons, which increases [Ca2+]i levels and triggers a variety of death pathways, which include mitochondrial dysfunction, protease activation, ROS accumulation, and NO release.27,28 The hippocampus contains the highest densities of excitatory amino acid receptors in the mammalian brain, which renders hippocampal cells more susceptible to oxidative stress and neurodegenerative complications.29 Therefore, the development of a therapeutic strategy aimed to the prevention of neuronal damage by oxidative stress is much sought after to combat neurological disorders.
With this objective in mind, we adopted an H/R model of rat hippocampal cultures, to examine the effects of marine seaweed extracts, which are known to have many bioactive metabolites.30 In view of that, we screened 23 different types of seaweeds on the basis of PI staining and LDH assay. Among the seaweeds examined in the present study, G. chorda was found to exhibit the most potent neuroprotective activity. In particular, the ethanol GCE increased neuronal cell viability after H/R-induced oxidative stress by reducing ROS levels, double-strand DNA breakages, apoptosis, internucleosomal DNA fragmentation, and MMP loss. Attenuation of ROS formation was a promising approach to inhibit the H/R-mediated cellular apoptosis.4 Edible seaweeds have a potent antioxidant activity to scavenge free radicals so that it could inhibit apoptosis.31,32 In this study, we showed that GCE and its active compound possessed protective actions, which inhibited ROS accumulation in hippocampal cells. In accordance with our observation, protection of DNA damage by Gracilaria was also reported by Yang et al.33 who showed that the red alga G. tenuistipitata had a protective effect against H2O2-induced oxidative DNA damage and other cellular responses.
Several lines of evidence have provided insights into the health-modulating potentials of the genus Gracilaria (Gracilariales, Rhodophyta), notably, regarding the anti-hypercholesterolemic properties of G. tenuistipitata,34 the antioxidative properties of G. tenuistipitata,35 Gracilaria edulis,36 Gracilaria salicornia,37 G. biridae, and G. cornea,38 the anti-inflammatory properties of Gracilaria verrucosa,39 and G. cornea,40 and the antimicrobial properties of G. salicornia,36 and G. tenuistipitata.41 These various health-promoting effects are probably due to the presence of diverse bioactive compounds, such as, polyphenols, polysaccharides, and pigments, and thus, seaweeds offer a vast resource of compounds with health-improving effects. Gracilaria spp. contains numerous phytochemicals such as, terpenoids, proteins, flavonoids, saponins, tannins, lignins, phenolics, alkaloids, and polyunsaturated fatty acids (PUFAs).42 PUFAs, particularly AA, are well known for their diverse range of beneficial roles in human health. The AA content of our G. chorda sample (0.64% [w/w] of GCE or 1.5 mg/100 g dry weight of G. chorda powder) was comparable to the previously reported data.24 AA has antioxidative activity and can defend against oxidative stress by glutamate, NaN3, or H2O2 in hippocampal slices.25 Moreover, the AA significantly protects the rat retinal ganglion cells against glutamate neurotoxicity43 and inhibits apoptosis in serum-starved Neuro-2A and PC-12 cells.44 Therefore, the antioxidative and neuroprotective properties of GCE might be due to, at least in part, the presence of AA. We are currently identifying the active components by fractionation of GCE and HPLC separation.
The red alga G. chorda is distributed throughout the world, especially in tropical and warm-water regions, and has been used for the large-scale production of food grade agar and phycocolloids.45 To the best of our knowledge, this is the first report to be issued on the neuroprotective efficacy of G. chorda in CNS neurons. Due to its popularity as a foodstuff and the lack of any toxic effects, our results support the notion that G. chorda is a good source for biomolecules with the potential to treat neurological disorders.
Acknowledgment
This research was supported by the MKE (the Ministry of Knowledge Economy), Korea, under the RIS (Regional Innovation System) support program (R0002920) supervised by the KIAT (Korean Institute for Advancement of Technology).
Author Disclosure Statement
The authors have no potential conflict of interest to declare.
References
- 1.Castellani RJ, Rolston RK, Smith MA: Alzheimer disease. Dis Mon 2010;56:484–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee Y, Park HW, Park SG, et al. : Proteomic analysis of glutamate-induced toxicity in HT22 cells. Proteomics 2007;7:185–193 [DOI] [PubMed] [Google Scholar]
- 3.Orrenius S, Gogvadze V, Zhivotovsky B: Mitochondrial oxidative stress: Implications for cell death. Annu Rev Pharmacol Toxicol 2007;47:143–183 [DOI] [PubMed] [Google Scholar]
- 4.Bredesen DE: Neural apoptosis. Ann Neurol 1995;38:839–851 [DOI] [PubMed] [Google Scholar]
- 5.Willie AH: Cell death: The significance of apoptosis. Int Rev Cytol 1980;68:251–306 [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Kim CN, Yang J, Jemmerson R, Wang X: Induction of apoptotic program in cell free extracts: Requirement for dATP and cytochrome c. Cell 1996;86:147–157 [DOI] [PubMed] [Google Scholar]
- 7.Susin SA, Zamzami N, Castedo M, et al. : Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med 1996;184:1331–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swing JT: What future for the oceans? Foreign Aff 2003;82:139–152 [Google Scholar]
- 9.Kim KA, Kim SM, Kang SW, Jeon SI, Um BH, Jung SH: Edible seaweed, Eisenia bicyclis, protects retinal ganglion cells death caused by oxidative stress. Mar Biotechnol 2012;14:383–395 [DOI] [PubMed] [Google Scholar]
- 10.Gao Y, Dong C, Yin J, Shen J, Tian J, Li C: Neuroprotective effect of fucoidan on H2O2 induced apoptosis in PC12 cells via activation of PI3K/Akt pathway. Cell Mol Neurobiol 2012;32:523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannan MA, Kang JY, Hong YK, et al. : A brown alga Sargassum fulvellum facilitates neuronal maturation and synaptogenesis. In Vitro Cell Dev Biol Anim 2012;48:535–544 [DOI] [PubMed] [Google Scholar]
- 12.Hannan MA, Kang JY, Hong YK, et al. : The marine alga Gelidium amansii promotes the development and complexity of neuronal cytoarchitecture. Phytother Res 2013;27:21–29 [DOI] [PubMed] [Google Scholar]
- 13.Hannan MA, Mohibbullah M, Hong YK, Nam JH, Moon IS: Gelidium amansii promotes dendritic spine morphology and synaptogenesis, and modulates NMDA receptor-mediated postsynaptic current. In Vitro Cell Dev Biol Anim 2014;50:445–452 [DOI] [PubMed] [Google Scholar]
- 14.Shigeno T, Mima T, Takakura K, et al. : Amelioration of delayed neuronal death in the hippocampus by nerve growth factor. J Neurosci 1991;11:2914–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimohama S, Ogawa N, Tamura Y, et al. : Protective effect of nerve growth factor against glutamate-induced neurotoxicity in cultured cortical neurons. Brain Res 1993;632:296–302 [DOI] [PubMed] [Google Scholar]
- 16.Suh KT: Classification of herbs in decoction part of Donguibogam [PhD Thesis], Kyungsung University, Busan, Korea, 1997;138 [Google Scholar]
- 17.Brewer GJ, Torricelli JR, Evege EK, Price PJ: Optimized survival of hippocampal neurons in B27-supplemented neurobasal™, a new serum-free medium combination. J Neurosci Res 1993;35:567–576 [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Ding AS, Wu LY, Ma ZM, Fan M: Establishment of the model of oxygen-glucose deprivation in vitro rat hippocampal neurons. Chin J Appl Physiol 2003;19:197–200 [PubMed] [Google Scholar]
- 19.Zhu FF, Yin YY, Li WP, et al. : Protective effect of extract of astragalus against injury induced by hypoxia/reoxygenation in hippocampus neuron. Chin Pharmacol Bull 2009;25:213–216 [Google Scholar]
- 20.Moon IS, Cho SJ, Jin I, Walikonis R: A simple method for combined fluorescence in situ hybridization and immunocytochemistry. Mol Cells 2007;24:76–82 [PubMed] [Google Scholar]
- 21.Tamatani M, Che YH, Matsuzaki H, et al. : Tumor necrosis factor induces Bcl-2 and Bcl-x expression through NFκB activation in primary hippocampal neurons. J Biol Chem 1999;274:8531–8538 [DOI] [PubMed] [Google Scholar]
- 22.Bossenmeyer C, Chihab R, Muller S, Schroeder H, Daval JL: Hypoxia/reoxygenation induces apoptosis through biphasic induction of protein synthesis in cultured rat brain neurons. Brain Res 1998;787:107–116 [DOI] [PubMed] [Google Scholar]
- 23.Lemasters JJ, Qian T, Bradham CA, et al. : Mitochondrial dysfunction in the pathogenesis of necrotic and apoptotic cell death. J Bioenerg Biomembr 1999;31:305–319 [DOI] [PubMed] [Google Scholar]
- 24.Araki S, Sakurai T, Oohusa T, Kayama M, Nisizawa K: Content of arachidonic and eicosapentaenoic acids in polar lipids from Gracilaria (Gracilariales, Rhodophyta). Hydrobiologia 1990;204–205:513–519 [Google Scholar]
- 25.Wang ZJ, Liang CL, Li GM, Yu CY, Yin M: Neuroprotective effects of arachidonic acid against oxidative stress on rat hippocampal slices. Chem Biol Interact 2006;163:207–217 [DOI] [PubMed] [Google Scholar]
- 26.Rees S, Harding R, Walker D: The biological basis of injury and neuroprotection in the fetal and neonatal brain. Int J Dev Neurosci 2011;29:551–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puyal J, Ginet V, Clarke PG: Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage: A challenge for neuroprotection. Prog Neurobiol 2013;105:24–48 [DOI] [PubMed] [Google Scholar]
- 28.Kostandy BB: The role of glutamate in neuronal ischemic injury: The role of spark in fire. J Neurol Sci 2012;33:223–237 [DOI] [PubMed] [Google Scholar]
- 29.Liu DS, Zhou YH, Liang ES, et al. : Neuroprotective effects of the Chinese Yi-Qi-Bu Shen recipe extract on injury of rat hippocampal neurons induced by hypoxia/reoxygenation. J Ethnopharmacol 2013;145:168–174 [DOI] [PubMed] [Google Scholar]
- 30.Jha RK, Zi-rong X: Biomedical compounds from marine organisms. Marine Drugs 2004;2:123–146 [Google Scholar]
- 31.Fallarero A, Loikkanen JJ, Männistö PT, Castañeda O, Vidal A: Effects of aqueous extracts of Halimeda incrassate (Ellis) Lamouroux and Bryothamnion triquetrum (SG Gmelim) Howe on hydrogen peroxide and methyl mercury-induced oxidative stress in GT1-7 mouse hypothalamic immortalized cells. Phytomedicine 2003;10:39–47 [DOI] [PubMed] [Google Scholar]
- 32.Fallarero A, Peltoketo A, Loikkanen J, Tammela P, Vidal A, Vuorela P: Effects of the aqueous extract of Bryothamnion triquetrum on chemical hypoxia and aglycemia-induced damage in GT1-7 mouse hypothalamic immortalized cells. Phytomedicine 2006;13:240–245 [DOI] [PubMed] [Google Scholar]
- 33.Yang JI, Yeh CC, Lee JC, et al. : Aqueous extracts of the edible Gracilaria tenuistipitata are protective against H2O2-induced DNA damage, growth inhibition, and cell cycle arrest. Molecules 2012;17:7241–7254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin YH, Tsai JS, Hung LB, Pan BS: Hypocholesterolemic effect of compounded freshwater clam protein hydrolysate and Gracilaria. Food Chem 2010;123:395–399 [Google Scholar]
- 35.Yangthong M, Hutadilok-Towatana N, Phromkunthong W: Antioxidant activities of four edible seaweeds from the southern coast of Thailand. Plant Food Human Nutr 2009;64:218–223 [DOI] [PubMed] [Google Scholar]
- 36.Ganesan P, Kumar CS, Bhaskar N: Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresource Technol 2008;99:2717–2723 [DOI] [PubMed] [Google Scholar]
- 37.Vijayavel K, Martinez JA: In vitro antioxidant and antimicrobial activities of two Hawaiian marine Limu: Ulva fasciata (Chlorophyta) and Gracilaria salicornia (Rhodophyta). J Med food 2010;13:1494–1499 [DOI] [PubMed] [Google Scholar]
- 38.Souza BW, Cerqueira MA, Martins JT, et al. : Antioxidant potential of two red seaweeds from the Brazilian coasts. J Agric Food chem 2011;59:5589–5594 [DOI] [PubMed] [Google Scholar]
- 39.Dang HT, Lee HJ, Yoo ES, Shinde PB, Lee YM, Hong J, Jung JH: Anti-inflammatory constituents of the red alga Gracilaria verrucosa and their synthetic analogues. J Nat Prod 2008;71:232–240 [DOI] [PubMed] [Google Scholar]
- 40.Coura CO, de Araújo IW, Vanderlei ES, et al. : Antinociceptive and anti-inflammatory activities of sulphated polysaccharides from the red seaweed Gracilaria cornea. Basic Clin Pharmacol 2012;110:335–341 [DOI] [PubMed] [Google Scholar]
- 41.Yeh ST, Lin YC, Huang CL, Chen JC: White shrimp Litopenaeus vannamei that received the hot-water extract of Gracilaria tenuistipitata showed protective innate immunity and up-regulation of gene expressions after low-salinity stress. Fish Shellfish Immunol 2010;28:887–894 [DOI] [PubMed] [Google Scholar]
- 42.De Almeida CLF, Falcão DS, Lima DM, et al. : Bioactivities from marine algae of the genus Gracilaria. Int J Mol Sci 2011;12:4550–4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawasaki A, Han MH, Wei JY, Hirata K, Otori Y, Barnstable CJ: Protective effect of arachidonic acid on glutamate neurotoxicity in rat retinal ganglion cells. Invest Ophthalmol Vis Sci 2002;43:1835–1842 [PubMed] [Google Scholar]
- 44.Kim HY, Akbar M, Kim KY: Inhibition of neuronal apoptosis by polyunsaturated fatty acids. J Mol Neurosci 2001;16: 223–227 [DOI] [PubMed] [Google Scholar]
- 45.Gargiulo GM, De Masi F, Tripodi G: Morphology, reproduction and taxonomy of the Mediterranean species of Gracilaria (Gracilariales, Rhodophyta). Phycologia 1992;31:53–80 [Google Scholar]