Abstract
Purpose.
To evaluate the in vivo changes in the Schlemm's canal (SC) in patients with primary angle-closure glaucoma (PACG) after trabeculectomy using spectral-domain optical coherence tomography (SD-OCT).
Methods.
Forty eyes of 40 patients with PACG who underwent trabeculectomy were included. All participants underwent SD-OCT. The diameter and area of SC were examined and measured before and within 1 month after trabeculectomy. All SD-OCT images were processed using adaptive compensation algorithm to improve contrast and image quality. Multivariate linear regression analysis was performed for predictors of percentage change in the mean SC diameter and area.
Results.
The mean age of participants was 60.5 ± 14.6 years. Adaptive compensation significantly increased the percentage of sections in which SC was observable in the subjects studied from 52.5% (21/40) to 75.0% (30/40), which has acceptable intraobserver and interobserver repeatability. There was a significant increase in the SC diameter and area at the follow-up examination compared with the baseline value (SC diameter: 34.2 ± 6.2 μm vs. 28.4 ± 6.1 μm; SC area: 8117 ± 1942 μm2 vs. 5200 ± 996 μm2; all P < 0.001). After multivariate analysis, the only variable related to changes in SC was percentage change in IOP (SC diameter, P = 0.002; SC area, P < 0.001). In addition, the magnitude of the change in the SC area also correlated with angle opening distance at 750 μm from the scleral spur at baseline.
Conclusions.
Expansion of SC was observed after trabeculectomy in PACG patients. The degree of SC expansion was related to the extent of the IOP decrease.
Keywords: trabeculectomy, primary angle closure glaucoma, Schlemm's canal
An adaptive compensation algorithm could facilitate the visibility of Schlemm's canal (SC) in the SD-OCT images. With this technique, expansion of SC was observed after trabeculectomy in PACG. The degree of SC expansion was related to the extent of the IOP decrease.
Introduction
Primary angle–closure glaucoma (PACG) is estimated to be a major cause of blindness worldwide.1 Controlling intraocular pressure (IOP) is the main goal of glaucoma treatment. Schlemm's canal (SC) was suggested to be important in regulating IOP in the human eye, whose size may correlate with the fluctuation in IOP.2 However, few reports that delineate the effect of the trabeculectomy on SC in glaucoma are currently available.
Technological advances in the imaging resolution and acquisition speed of spectral-domain optical coherence tomography (SD-OCT) have enabled observation of the anterior chamber angle architecture in greater detail and helped with our understanding of the in vivo structural and pathophysiological changes in SC. Using this technology, we recently reported that eyes with primary open-angle glaucoma have a decreased SC area compared with normal eyes, and a correlation between the SC area and the IOP also was observed.3 In addition, previous reports have suggested that canaloplasty, a procedure involving circumferential viscodilation and tensioning of Schlemm's canal, achieved a significant decrease in IOP at 12 months and the IOP decrease is sustained in adult patients with open-angle glaucoma.4–6 Since trabeculectomy could significantly decrease the IOP in PACG eyes, this surgical treatment could result in changes in the SC. However, the features of SC morphology in PACG eyes undergoing trabeculectomy are still unknown. The purpose of this study was to determine whether similar structural changes occurs in the SC in PACG eyes undergoing trabeculectomy, and to explore the potential factors associated with the change in the SC size.
Methods
Participants
This was a prospective study of 40 Chinese subjects with PACG at the glaucoma clinic of the Shanghai Eye, Ear, Nose and Throat Hospital. Written informed consent was obtained from all subjects. The study was approved by the Institutional Review Board of the hospital and was performed in accordance with the tenets of the Declaration of Helsinki. Each subject received comprehensive ophthalmic examinations that included visual acuity measurement, Goldmann applanation tonometry, slit-lamp biomicroscopy, and stereoscopic evaluation of the optic disc using a 90-diopter (D) lens (Volk Optical, Inc., Mentor, OH, USA). A-scan ultrasonography (Model US-800; Nidek Co., Ltd., Tokyo, Japan) was used to measure axial length and corneal thickness. The patients also underwent imaging of the angle using anterior segment optical coherence tomography (Visante; Carl Zeiss Meditec, Dublin, CA, USA). Finally, gonioscopy was performed in the dark in all patients by glaucoma specialists.
Primary angle–closure glaucoma was diagnosed on the basis of narrow angles with glaucomatous optic neuropathy (vertical cup-to-disc ratio ≥ 0.7, cup-to-disc asymmetry > 0.2 and/or focal notching) with corresponding visual field loss on static automated perimetry (SITA standard algorithm with a 24-2 test pattern; Humphrey Visual Field Analyzer II; Carl Zeiss Meditec).4 The glaucoma hemifield test was outside normal limits including a cluster of three or more, nonedge, contiguous points on the pattern deviation plot, not crossing the horizontal meridian with a probability of <5% of being present in the age-matched normal (one of which was <1%), an abnormal pattern standard deviation with P < 5% occurring in the normal population, and fulfilling the test reliability criteria (fixation losses < 20%, false positives < 33% and/or false negatives < 33%).
Additional inclusion criteria were an IOP > 21 mm Hg despite maximally tolerated medications or requiring more than three topical medications for IOP control; at least 180 of angle-closure obliterating pigmented part of the trabecular meshwork, whether synechial or appositional, segmented or continuous; and eyes in which the degree of peripheral anterior synechiae is too extensive to be managed by laser peripheral iridotomy. All ocular topical medications were continued up to the time of the surgery. Participants were excluded if they had previous uveitis, trauma or prior intraocular surgery, laser iridotomies, penetrating eye injury, or corneal disorders such as corneal endothelial dystrophy or severe corneal opacity.
Surgical Trabeculectomy Procedure
The surgical procedure details of trabeculectomy in the current study have been described previously.5 Intraocular lens surgery was not performed simultaneously in each patient. Briefly, trabeculectomy was performed under peribulbar anesthesia. A limbus-based conjunctival flap was prepared, which was followed by a rectangular half-thickness scleral flap measuring 4 × 4 mm. A sponge soaked in 0.04% mitomycin C was applied under the scleral flap and subconjunctival space for 4 minutes. After careful rinsing with approximately 50 mL of physiological saline, a 2- × 2-mm section of corneoscleral tissue was excised, and a peripheral iridectomy was performed. The scleral flap was then closed with two 10-0 nylon sutures at its corners, whereas the Tenon capsule and conjunctiva were reapproximated with 8-0 vicryl continuous sutures so that they were watertight.
SC Measurements Using Optical Coherence Tomography
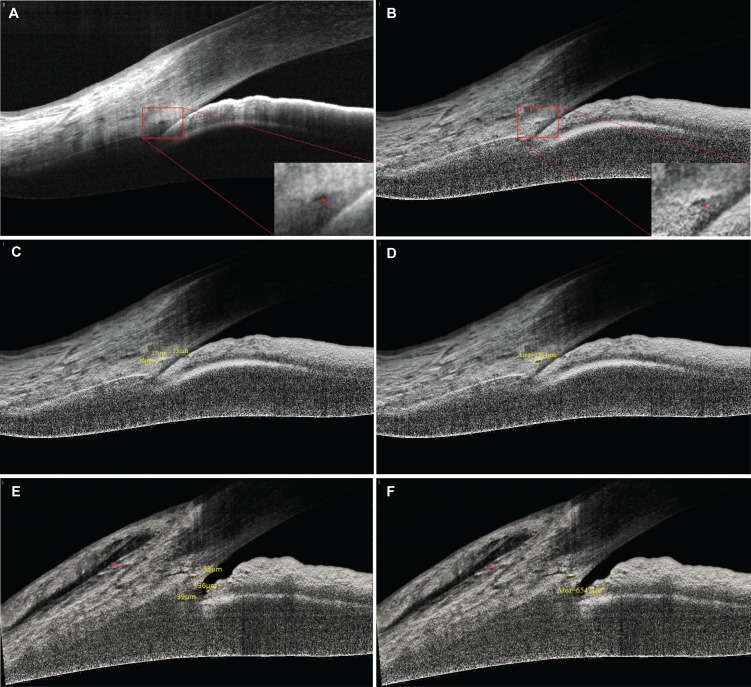
Baseline SC scanning was performed 1 day before trabeculectomy. Follow-up angle scanning was performed within 1 month after the surgery. Measurements of IOP were recorded at all follow-up visits. For SC scanning, all subjects underwent SD-OCT imaging (RTVue OCT, software version 4.0.7.5; Optovue, Inc., Fremont, CA, USA) in a dark room. Scans centered on the pupil and were obtained using the standard anterior-segment single-scan protocol that included one image scanning the angle at the 3 and 9 o'clock positions (horizontal meridian). The details of the SC imaging protocol were previously reported.3 In order to remove image artifacts caused by light attenuation and to enhance SD-OCT image quality, all scans (nasal and temporal) were postprocessed using adaptive compensation (Figs. A, B) with a threshold exponent of 9 and a contrast exponent of 2. Adaptive compensation has been demonstrated to improve tissue visibility at high depth; remove shadow artifacts (through decreases in intralayer contrast); enhance tissue boundary visibility (through increases in inter-layer contrast); and reduce noise over-amplification at high depth.6 Following adaptive compensation postprocessing, SD-OCT image quality was evaluated by two independent observers (JH, YY), and scans with poor resolution and/or nonvisible SC were excluded.
The SD-OCT images of SC were imported in ImageJ (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA) for analysis after they were enhanced with the adaptive compensation algorithm. For each image, the SC diameter and area were measured manually by masked operators (JH and AJ), as shown in Figures C and D. The SC diameter was defined as the average of three measurements of the sagittal axial length of the thin, black, lucent space on the SD-OCT images. The SC area was drawn freehand and depicted the area surrounded by the outline of SC. The mean of the nasal and temporal SC was used in the analysis.
Repeatability and Reproducibility of SC Measurements.
To measure intraobserver variation, 20 SC images before and after surgeries were chosen from 10 PACG patients using a randomization table generated from a block randomization table. These images were evaluated in random order by a single masked observer (JH) on two separate occasions at an interval of 2 days, and agreement between the two observations was determined. To measure interobserver variation, the same images were evaluated by two independent and well-trained observers (JH, AW), and the agreement between them were analyzed. The order of the observers was randomized. The participants were given a 15-minute pause between the SC measurements taken by the two clinicians. All the evaluators were unaware of the subjects' demographic details and diagnosis.
Statistical Analysis
All analyses were performed using a statistical software package (SPSS for Windows, version 17.0; SPSS, Inc., Chicago, IL, USA) and a spreadsheet program (Microsoft Excel 2003; Microsoft Corp., Redmond, WA, USA). Data are shown as mean ± standard deviation. Repeatability and reproducibility were analyzed by using the coefficient of variation (CV) and the intraclass correlation coefficient (ICC) between measurements. The CV is the standard deviation of the measurements divided by their mean, expressed as a percentage. The ICC measures the proportion of total variability in measurements contributed by variability in measurements between different subjects and was determined using the random-effects mixed model. For comparison, differences in the mean values of the parametric data were examined using the Student's t-test or the Mann–Whitney U test. The nonparametric Spearman correlation was used to compare the data between the parameters. The following variables were analyzed: age, sex, refraction, central corneal thickness, axial length of eye, mean deviation, number of preoperative IOP-lowering drugs, visual outcomes, baseline and postoperative IOP, angle opening distance (AOD750), angle recess area (ARA750), trabecular iris surface area (TISA750), and percentage change in IOP (ΔIOP). Multivariate linear regression analysis with stepwise methods was performed for the predictors of “percentage change in mean Schlemm's canal diameter or area” (hereafter denoted as “ΔSCD” or “ΔSCA”) using parameters that showed significance at less than the 0.1 level in univariate analysis, excluding those that showed multicollinearity. All P values were two-sided and considered statistically significant when < 0.05.
Results
Demographics
Of the 40 patients with PACG who were initially enrolled, the percentage of sections in which the SC was observable in the subjects studied increased from 52.5% (21/40) to 75.0% (30/40) before and after we used adaptive compensation (Figs. A, B), showing a statistically significant difference (P = 0.03). Ten subjects were excluded in the final analysis because of poor scan image quality nonvisibility of SC. The reasons of exclusion included dense arcus senilis (4/10), pterygium (4/10), and pinguecula (2/10).
As shown in Table 1, of the 30 eyes diagnosed as PACG, 10 (33%) had previous acute primary angle closure. All eyes had been treated with maximally tolerated IOP-lowering medication and then underwent trabeculectomy. The mean age was 60.5 ± 14.6 years (range, 22–95 years); 17 patients were women, and 13 were men. Their visual acuity ranged from <6/60 to 6/6, and the refractive error (spherical equivalent) was −0.36 ± 3.36 D (range, −5.25 to +3.75 D). The visual field mean deviation was −19.8 ± 8.1 dB (range, −31.2 to −6.2 dB).
Table 1.
Characteristics of the Study Subjects
|
Parameters |
Descriptive Statistics* |
| Age, y | 60.5 ± 14.6, (22, 95) |
| Sex, male:female | 13:17 |
| Refraction, D | −0.36 ± 3.36, (−5.25, 3.75) |
| Baseline IOP, mm Hg | 33.6 ± 10.2, (20, 59.6) |
| Central corneal thickness, μm | 549 ± 46, (490, 692) |
| Axial length of eye, mm | 22.5 ± 1.3, (20.6, 26.2) |
| Mean deviation, dB | −19.8 ± 8.1, (−31.2, −6.2) |
| Number of preoperative IOP-lowering drugs |
3 ± 1, (2, 5) |
| Best corrected visual acuity | |
| 6/6 to 6/18 | 16 |
| 6/24 to 6/60 | 3 |
| <6/60 | 11 |
Values are shown in mean ± SD, range (minimum, maximum).
For intraobserver repeatability, the CV and ICC values were 7.5% and 0.95% for the SC diameter, and 11.6% and 0.85% for the SC area, respectively. For interobserver repeatability, the CV and ICC values were 12.3% and 0.87% for the SC diameter, and 13.0% and 0.81% for the SC area, respectively.
Changes in Anterior Segment Angle Morphology After Trabeculectomy
In all 30 eyes, the IOP decreased from 33.6 ± 10.2 mm Hg (range, 20–59.6 mm Hg) to 14.3 ± 3.5 mm Hg (range, 8–21 mm Hg; P < 0.001). After trabeculectomy, AOD750 (0.29 ± 0.15 mm vs. 0.49 ± 0.15 mm, P < 0.001); ARA750 (0.18 ± 0.08 mm2 vs. 0.27 ± 0.09 mm2, P < 0.001); and TISA750 (0.14 ± 0.07 mm2 vs. 0.23 ± 0.06 mm2, P < 0.001) increased (Table 2). Interestingly, the mean SC diameter (28.4 ± 6.1 μm vs. 34.2 ± 6.2 μm, P = 0.001) and the mean SC area (5200 ± 996 μm2 vs. 8117 ± 1942 μm2; P < 0.001) increased significantly after the surgical treatment.
Table 2.
Intraocular Pressure, Anterior Chamber Angle Parameters, and SC Morphology at Baseline and Follow-up Using OCT Examinations
|
Parameters |
Baseline |
Follow-up |
PValues* |
| IOP, mm Hg | 33.6 ± 10.2 | 14.3 ± 3.5 | <0.001 |
| AOD750, mm | 0.29 ± 0.15 | 0.49 ± 0.15 | <0.001 |
| ARA750, mm2 | 0.18 ± 0.08 | 0.27 ± 0.09 | <0.001 |
| TISA750, mm2 | 0.14 ± 0.07 | 0.23 ± 0.06 | <0.001 |
| SC diameter, μm | 28.4 ± 6.1 | 34.2 ± 6.2 | 0.001 |
| SC area, μm2 | 5200 ± 996 | 8117 ± 1942 | <0.001 |
Independent t-test was performed for these parameters
Multivariate Logistic Regression Analysis for Potential Predictors Associated With SC Changes
As shown in Table 3, the only variable related to changes in SC was ΔIOP (ΔSCD, P = 0.002; ΔSCA, P < 0.001). In addition, ΔSCA was also correlated to AOD750 at baseline (P = 0.047). The multivariate linear regression model found no significant associations for changes in SC with age, sex, refraction, central corneal thickness, axial length of eye, mean deviation, number of preoperative IOP-lowering drugs, visual outcomes, baseline and postoperative IOP, ARA750, and TISA750.
Table 3.
Univariate and Multivariate Linear Regression Analysis for Predictors of Percentage Change in Mean SC after the IOP Decrease
|
Parameters* |
ΔSCD, PValue |
ΔSCA, PValue |
||
|
Univariate |
Multivariate |
Univariate |
Multivariate |
|
| Age, y | 0.404 | n/a | 0.972 | n/a |
| Sex | 0.744 | n/a | 0.886 | n/a |
| Refraction, D | 0.092 | n/a | 0.197 | n/a |
| Central corneal thickness, μm | 0.471 | n/a | 0.014 | 0.373 |
| Axial length of eye, mm | 0.071 | 0.473 | 0.678 | n/a |
| Mean deviation, dB | 0.854 | 0.367 | 0.385 | n/a |
| Number of preoperative IOP-lowering drugs | 0.233 | n/a | 0.128 | n/a |
| Baseline IOP, mm Hg | 0.056 | 0.187 | 0.002 | 0.268 |
| Postoperative IOP, mm Hg | 0.138 | n/a | 0.153 | n/a |
| Baseline AOD750, mm | 0.078 | 0.069 | 0.040 | 0.047 |
| Baseline ARA750, mm2 | 0.146 | n/a | 0.131 | n/a |
| Baseline TISA750, mm2 | 0.150 | n/a | 0.119 | n/a |
| Percentage Change in IOP, mm Hg | 0.005 | 0.002 | <0.001 | <0.001 |
n/a, not applicable.
All anterior segment parameters are the mean of nasal and temporal measurements.
Discussion
We found that SD-OCT equipped with the adaptive compensation algorithm yielded acceptable visualization of SC in patients with PACG. Our results demonstrate the expansion of SC after the trabeculectomy in PACG eyes. In addition, the multivariate linear regression analysis also showed a significant relationship between the degree in SC changes and the magnitude of the decrease in the IOP. The percentage of eyes in which SC was observable (75%) in the PACG patients was lower than that (86%) in normal subjects, but was similar to that (78%) in patients with primary open-angle glaucoma patients.3 One possible reason is that the patients with PACG were significantly older than the two other groups, who tend to develop arcus senilis and pterygium.7,8
Previous studies have demonstrated the advantages of adaptive compensation, including removal of noise overamplification in the deep layers of the tissue and improved visibility of the posterior tissue boundary (such as SC in our study).6,9 However, this technique is not available for all subjects. In our experience, the SC in patients with dense arcus senilis, pterygium, and pinguecula may be hard to investigate. This issue must be addressed in a future study.
The reproducibility and repeatability of SC measurement with SD-OCT have been examined previously. Kagemann et al10 reported a CV of 11.4% for the SC area between two observers in healthy subjects. Our study in primary open angle glaucoma showed that both the SC diameter and area measurements by SD-OCT have good intraobserver and interobserver repeatability.3 Similarly, our new results indicated acceptable agreement for SC measurement with SD-OCT in PACG patients.
To our knowledge, this is the first detailed assessment of the in vivo change in the SC in PACG eyes before and after glaucoma surgery. Based on our previous study of the SC morphology in normal subjects,3 the SC in PACG eyes seems to be smaller. One might suspect that the SC would collapse after trabeculectomy because a path of lesser resistance has been created, and the driving force across the trabecular meshwork has been eliminated. However, in the current study, we found that the mean SC diameter and area increased significantly after surgical treatment. The SC increase in our study might be explained by the decreased mechanical expression due to the IOP decrease, the relief of the pupillary block, and the widening of the anterior chamber angle, facilitating the access of aqueous humor to the drainage system in PACG eyes. Based on this finding, SC compression may exit in PACG eyes. However, the SC expanded after the surgery within 1 month in our study, and the IOP and the chamber depth may not have been stable yet. Further studies are necessary to determine the long-term changes in the SC to further support these findings.
The IOP change was associated with SCD and SCA changes. Very few reports address the relationship between IOP and SC parameters. The current general belief is that the aqueous drainage system has a great chance of improvement with canaloplasty alone or combined with phacoemulsification.10–12 Canaloplasty is a surgical alternative that involves viscodilation of the Schlemm's canal to enlarge the drainage canal, relieving pressure in open-angle glaucoma to decrease the IOP.11,12 The decrease in IOP is mainly attributable to the successful plasticity of SC in canaloplasty. Recently, SC compression has been shown to be a result of IOP elevation alone and has been demonstrated in healthy eyes with open angles.13 Our report findings complement these previous studies that the change in SC size correlated to the IOP fluctuation, not only for open-angle glaucoma but also potentially for angle-closure glaucoma. An enlarged SC might be an additional mechanism IOP decrease in trabeculectomy in PACG. In addition, we also found that the association between the ΔSCA and AOD750 was statistically significant (P = 0.047). This may support the hypothesis that mechanical compression of the trabecular meshwork may be contributing to the low AOD baseline values. However, the statistical P value is very close to 0.05, which may be the result of the relatively small number of investigated eyes and a possible variation in the linear model. A larger, prospective, long-term study is still warranted to address this issue. Nevertheless, our study is a preliminary step in deriving a mechanism that eventually elucidates the SC changes after trabeculectomy.
Several limitations of the present study should be noted. First, despite the good quality of the OCT images, 25% (10/40) of eyes were excluded because the SC was not observed. This may have resulted from the effect of the position of collector channels. Kagemann et al.10,13 and Usui et al.14 found that the SC is larger at the site of a collector channel than at sites close to but not at the collector channel. The status of the collector channel may change the SC size. Second, only the nasal and temporal sections were used for analysis in our study, because the superior and inferior section images could not be imaged without manipulation of the eyelid, which may compress the angle structures. In addition, the superior SC was absent because the incision of the surgery mostly located in the superior limbus. We have to admit that two single cross-sectional slices of the SC may not represent the entire SC anatomy. A more systematic investigation of the SC morphology throughout a region would enhance our findings. Third, because only the eyes undergoing trabeculectomy of most of the enrolled subjects were measured, we did not investigate changes in the lateral eyes; this is the subject of an ongoing study. Fourth, we did not collect information regarding the duration of glaucoma therapy, which may be associated with the baseline status of SC, because some patients were referred to our unit with limited data. Finally, the current study included subjects with medically uncontrolled severe PACG who have large visual field defects and higher IOP. Therefore, this finding might not be extrapolated to mild PACG or the other types of glaucoma.
In summary, we have demonstrated the in vivo expansion of SC after trabeculectomy in patients with PACG. Our data also indicated that the adaptive compensation algorithm provided significant improvement in the visibility of SC in the SD-OCT images. The current study suggests that the IOP decrease might also be correlated to the changes in SC in some of the PACG eyes, because the increase in SC was associated with the magnitude of the IOP decrease. Further study is needed to determine the influence of the SC changes after glaucoma surgery on disease progress.
Figure.
Enhancement of SC visibility in OCT images of PACG using adaptive compensation. (A) Baseline OCT image of the SC in patient 12. The SC is only partially visible. Note the presence of shadows as indicated by the red arrow. (B) Adaptive compensation was applied to the baseline image to remove noise overamplification. Note that shadows are removed and the SC becomes more visible (red arrow). The SC sagittal diameter (C) and area (D) are marked manually (yellow line). The SC parameter values were generated automatically by the digital imaging software (yellow marker). For this subject at baseline, the diameter was 35.3 μm [(36 + 33 + 37)/3], and the area was 3564 μm2. After the patients underwent trabeculectomy, the IOP in this patient decreased from 30 to 14 mm Hg. (E, F) Note that the increase in the SC diameter (36 μm) and area (6542 μm2) of SC. Clear fluid-filled space can be identified in the subconjunctival space above the level of the episclera (red arrow).
Acknowledgments
Supported by grants from the California Institute for Regenerative Medicine (TR2-01768) and the National Eye Institute (R01EY021797, 2T3EY007026); the Key Clinic Medicine Research Program, the Ministry of Health, China (201302015); the National Science and Technology Research Program, the Ministry of Science and Technology, China (2012BAI08B01); the National Natural Science Foundation of China (81170817, 81200658, 81300735, 81270978, U1205025, and 81330022); and the Scientific Research Program, Science and Technology Commission of Shanghai Municipality, Shanghai (13441900900, 13430720400, 134119a8800, 13430710500); Singapore Ministry of Education, Academic Research Funds, Tier 1, Singapore. The authors alone are responsible for the content and writing of the paper.
Disclosure: J. Hong, None; Y. Yang, None; A. Wei, None; S.X. Deng, None; X. Kong, None; J. Chen, None; M.J.A. Girard, None; J.M. Mari, None; J. Xu, None; X. Sun, None
References
- 1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allingham RR, de Kater AW, Ethier CR. Schlemm's canal and primary open angle glaucoma: correlation between Schlemm's canal dimensions and outflow facility. Exp Eye Res. 1996; 62: 101–109. [DOI] [PubMed] [Google Scholar]
- 3. Hong J, Xu J, Wei A, et al. Spectral-domain optical coherence tomographic assessment of Schlemm's canal in Chinese subjects with primary open-angle glaucoma. Ophthalmology. 2013; 120: 709–715. [DOI] [PubMed] [Google Scholar]
- 4. Guzman CP, Gong T, Nongpiur ME, et al. Anterior segment optical coherence tomography parameters in subtypes of primary angle closure. Invest Ophthalmol Vis Sci. 2013; 54: 5281–5286. [DOI] [PubMed] [Google Scholar]
- 5. Tang Y, Qian S, Wang J, et al. Effects of combined phacoemulsification and viscogoniosynechialysis versus trabeculectomy in patients with primary angle-closure glaucoma and coexisting cataract. Ophthalmologica. 2012; 228: 167–173. [DOI] [PubMed] [Google Scholar]
- 6. Girard MJ, Strouthidis NG, Ethier CR, Mari JM. Shadow removal and contrast enhancement in optical coherence tomography images of the human optic nerve head. Invest Ophthalmol Vis Sci. 2011; 29; 52: 7738–7748. [DOI] [PubMed] [Google Scholar]
- 7. Sun LP, Lv W, Liang YB, et al. The prevalence of and risk factors associated with pterygium in a rural adult Chinese population: the Handan Eye Study. Ophthalmic Epidemiol. 2013; 20: 148–154. [DOI] [PubMed] [Google Scholar]
- 8. Vurgese S, Panda-Jonas S, Saini N, Sinha A, Nangia V, Jonas JB. Corneal arcus and its associations with ocular and general parameters: the Central India Eye and Medical Study. Invest Ophthalmol Vis Sci. 2011; 52: 9636–9643. [DOI] [PubMed] [Google Scholar]
- 9. Girard MJ, Strouthidis NG, Ethier CR, Mari JM. Shadow removal and contrast enhancement in optical coherence tomography images of the human optic nerve head. Invest Ophthalmol Vis Sci. 2011; 52: 7738–7748. [DOI] [PubMed] [Google Scholar]
- 10. Kagemann L, Wollstein G, Ishikawa H, et al. Identification and assessment of Schlemm's canal by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2010; 51: 4054–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm's canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults: interim clinical study analysis. J Cataract Refract Surg. 2007; 33: 1217–1226. [DOI] [PubMed] [Google Scholar]
- 12. Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults: two-year interim clinical study results. J Cataract Refract Surg. 2009; 35: 814–824. [DOI] [PubMed] [Google Scholar]
- 13. Kagemann L, Wang B, Wollstein G, et al. IOP elevation reduces Schlemm's canal cross-sectional area. Invest Ophthalmol Vis Sci. 2014; 55: 1805–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Usui T, Tomidokoro A, Mishima K, et al. Identification of Schlemm's canal and its surrounding tissues by anterior segment Fourier domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011; 52: 6934–6939. [DOI] [PubMed] [Google Scholar]