ABSTRACT
Expression of the cytoprotective enzyme heme oxygenase-1 (HO-1) is significantly reduced in the brain prefrontal cortex of HIV-positive individuals with HIV-associated neurocognitive disorders (HAND). Furthermore, this HO-1 deficiency correlates with brain viral load, markers of macrophage activation, and type I interferon responses. In vitro, HIV replication in monocyte-derived macrophages (MDM) selectively reduces HO-1 protein and RNA expression and induces production of neurotoxic levels of glutamate; correction of this HO-1 deficiency reduces neurotoxic glutamate production without an effect on HIV replication. We now demonstrate that macrophage HO-1 deficiency, and the associated neurotoxin production, is a conserved feature of infection with macrophage-tropic HIV-1 strains that correlates closely with the extent of replication, and this feature extends to HIV-2 infection. We further demonstrate that this HO-1 deficiency does not depend specifically upon the HIV-1 accessory genes nef, vpr, or vpu but rather on HIV replication, even when markedly limited. Finally, antiretroviral therapy (ART) applied to MDM after HIV infection is established does not prevent HO-1 loss or the associated neurotoxin production. This work defines a predictable relationship between HIV replication, HO-1 loss, and neurotoxin production in MDM that likely reflects processes in place in the HIV-infected brains of individuals receiving ART. It further suggests that correcting this HO-1 deficiency in HIV-infected MDM could provide neuroprotection above that provided by current ART or proposed antiviral therapies directed at limiting Nef, Vpr, or Vpu functions. The ability of HIV-2 to reduce HO-1 expression suggests that this is a conserved phenotype among macrophage-tropic human immunodeficiency viruses that could contribute to neuropathogenesis.
IMPORTANCE The continued prevalence of HIV-associated neurocognitive disorders (HAND) underscores the need for adjunctive therapy that targets the neuropathological processes that persist in antiretroviral therapy (ART)-treated HIV-infected individuals. To this end, we previously identified one such possible process, a deficiency of the antioxidative and anti-inflammatory enzyme heme oxygenase-1 (HO-1) in the brains of individuals with HAND. In the present study, our findings suggest that the HO-1 deficiency associated with excess glutamate production and neurotoxicity in HIV-infected macrophages is a highly conserved phenotype of macrophage-tropic HIV strains and that this phenotype can persist in the macrophage compartment in the presence of ART. This suggests a plausible mechanism by which HIV infection of brain macrophages in ART-treated individuals could exacerbate oxidative stress and glutamate-induced neuronal injury, each of which is associated with neurocognitive dysfunction in infected individuals. Thus, therapies that rescue the HO-1 deficiency in HIV-infected individuals could provide additional neuroprotection to ART.
INTRODUCTION
Heme oxygenase-1 (HO-1) is a highly inducible phase II detoxifying enzyme that has emerged as a critical effector for limiting cellular injury associated with oxidative stress and inflammation within the central nervous system (CNS) and other tissues in several disease states, including HIV infection (1–3). It is a member of a family of antioxidant response element (ARE) promoter-driven genes that are induced by the transcriptional regulator Nrf2 in response to a variety of cellular insults (4). The protective functions of HO-1 have been linked to its degradation of heme and the subsequent generation of carbon monoxide, biliverdin, and bilirubin, which have immunomodulatory and antioxidative properties (5). In addition, HO-1 has nonenzymatic cytoprotective functions through activation of transcription factors (6, 7). A constitutively expressed heme oxygenase isoform, HO-2, catabolizes the same enzymatic reaction, although it is not regulated by the ARE, and unlike HO-1, HO-2 is not considered to be a critical mediator of acute cellular injury responses. Protective effects of HO-1 have been demonstrated in vitro and in vivo in animal models of oxidative, ischemic, and inflammatory diseases (8–11). Our in vitro studies have identified HO-1 as a protective host factor against HIV-associated excitotoxic injury, and our analysis of autopsy brain specimens from a large cohort of HIV-infected decedents further demonstrated an association between brain HO-1 deficiency and cognitive impairment, thus suggesting a role for HO-1 deficiency in HIV neuropathogenesis (1).
Because brain HO-1 deficiency is associated with cognitive impairment in HIV-infected individuals, we have proposed making HO-1 a therapeutic target for neuroprotection against HIV as an adjunctive therapy to antiretroviral therapy (ART) for preventing the pathogenic effects of HIV infection of the CNS (1, 3). HIV infection is associated with a syndrome of cognitive dysfunction (HIV-associated neurocognitive disorders [HAND]) in up to 50% of ART-treated HIV-infected individuals (12–14), which is thought to result in part from persistent inflammation and oxidative stress within both the CNS and systemic compartments (15). Within the CNS, such effects are driven largely by HIV infection of macrophages and microglia, long-lived HIV reservoirs that persist in ART-treated individuals (16).
The brain deficiency of HO-1 in HIV-infected individuals correlates with CNS HIV replication levels, immune activation markers, and the presence of HAND (3). In vitro, productive replication of HIV-1 in monocyte-derived macrophages (MDM) drastically reduces HO-1 protein expression while increasing the release of excitotoxins, including glutamate (2, 3, 17). Other direct and indirect excitotoxins associated with HIV infection and released from HIV-infected MDM include quinolinic acid (18), platelet-activating factor (19), and the viral proteins gp120 and Tat (20, 21), among others. Together, these observations suggest a heretofore unknown neuropathological link between HIV replication in macrophages and a failure of a normal host cytoprotection surveillance response (HO-1 induction), which can promote neurodegeneration through inflammation-associated excitotoxic injury.
To further define this neuropathological phenotype of HIV (ability to reduce HO-1 protein expression and induce glutamate production), we have expanded our previous studies to include an examination of a broader group of macrophage-tropic HIV-1 strains (n = 13), a macrophage-tropic clinical HIV-2 isolate, and an examination of the potential roles for the HIV-1 accessory proteins Nef, Vpr, and Vpu in modulating HO-1 expression in infected MDM. Finally, to address the association between HIV replication and HO-1 deficiency in the setting of ART use, we determined the ability of clinically relevant ART regimens applied pre- and postinfection to prevent HO-1 loss and the associated neurotoxicity in HIV-infected MDM. We found that induction of HO-1 loss in MDM is a consistent feature of macrophage-tropic HIV-1 strains, and we observed a similar effect with HIV-2 infection. HIV replication levels correlate negatively with HO-1 protein expression and positively with culture supernatant glutamate levels and associated neurotoxicity. Induction of HO-1 deficiency does not require the HIV-1 accessory genes nef, vpr, or vpu. Finally, ART ameliorated the neurotoxic effects of HO-1 deficiency by the prevention of HIV replication in newly infected MDM, but no such protection was seen in MDM with established HIV infection. These observations suggest that HO-1 deficiency in the pool of infected macrophages within the CNS of infected individuals represents a unique target for adjunctive therapies for neuroprotection in ART-treated individuals.
MATERIALS AND METHODS
Isolation and culture of human MDM.
Human monocytes were isolated from healthy donors by Ficoll density gradient centrifugation as previously described (2). Monocytes were plated at 10.5 × 103 cells/cm2 in 6-, 12-, or 24-well Cell-Bind plates (Corning) and cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Thermo Scientific), 10% horse serum (Invitrogen), 1% nonessential amino acids (Invitrogen), 2 mM glutamine (Invitrogen), and 50 U/ml penicillin-streptomycin at 37°C and 6% CO2. Cells were cultured for 7 days in vitro and visually inspected for differentiation of monocyte-derived macrophages (MDM) before use in HIV infection experiments.
HIV infection of MDM.
Differentiated MDM were exposed to HIV for 24 h (0.2 to 50 ng of HIV-1 p24 antigen content per 106 cells per 3 ml of medium). All HIV-1 virus stocks were prepared by the University of Pennsylvania Center for AIDS Research Virology Core (see Table S1 in the supplemental material) through transfection of 293T cells for molecularly cloned viruses or by amplification in primary human peripheral blood mononuclear cells. HIV-1 strain 89.6 accessory gene mutants have been described previously (22). HIV-2 CBL-20 (23) virus stock was obtained from Robin Weiss through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH. Supernatants from HIV-infected MDM (HIV-MDM) or noninfected MDM (mock-MDM) were collected every 3 days through 12 days postinfection and stored at −80°C. Supernatants were monitored for HIV replication by quantifying viral reverse transcriptase (RT) activity, as previously described (3), and by cell-associated HIV-1 p24 content, as analyzed by Western blotting.
MDM-mediated neurotoxicity.
Rat cerebrocortical mixed neuroglial cultures (∼80% neurons, 20% glia) were prepared from embryonic day 17 (E17) embryos of Sprague-Dawley rats, as previously described (17). Cells were plated in tissue culture plates precoated with poly-l-lysine (Peptides International) and maintained in neurobasal medium plus B27 supplement (Invitrogen) at 37°C and 5% CO2. After 7 days in vitro, approximately one-half volume of fresh medium was added to the cells to replace evaporation losses. All cultures were used between days 14 and 17 days in vitro. Cell-based microtubule-associated protein 2 (MAP2) enzyme-linked immunosorbent assays (ELISAs) were performed on primary rat cerebrocortical cells plated at a density of 1 × 104 cells/well in 96-well plates as previously described (3). Following a 24-h exposure to HIV-MDM supernatant (1:10 to 1:160 dilution; n = 6 technical replicates), neuronal cultures were fixed and fluorescently labeled using the following reagents: mouse anti-MAP2 (Covance), goat anti-mouse IgG β-lactamase TEM-1 conjugate (Invitrogen), and Fluorocillin green substrate (Invitrogen). Fluorescence intensity was measured using a fluorometric plate reader with a 480/520-nm filter set. Neuronal survival was expressed as a percentage of that of cultures treated with mock-MDM.
MDM extracellular glutamate.
The glutamate concentration in MDM supernatant was assayed in triplicate using the Amplex red glutamic acid/glutamate oxidase assay kit (Invitrogen) according to the manufacturer's directions.
Western blot analysis.
Cell cultures were rinsed with ice-cold phosphate-buffered saline (PBS), lysed in 75 mM Tris-HCl (pH 6.8) containing 15% glycerol, 3.75 mM EDTA, and 3% SDS, and supplemented with complete protease inhibitor cocktail (Roche) and PhosSTOP phosphatase inhibitor mixture (Roche). Cell protein lysates were subjected to Western blotting as previously described (3). Protein expression was probed using primary and secondary antibodies (see Tables S2 and S3 in the supplemental material), followed by analysis on an Odyssey CLx imager. Protein expression as determined by Western blotting was normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression.
RNA extraction and qPCR analysis.
Cell cultures were rinsed in ice-cold PBS, and RNA was isolated using an RNeasy kit (Qiagen) per the manufacturer's instructions. RNA concentration and quality were analyzed with a NanoDrop 2000c spectrophotometer (Thermo Scientific). Following cDNA generation using a reverse transcriptase kit (Applied Biosciences), quantitative real-time PCR (qPCR) was performed using RealMasterMix (5Prime) on a MasterCycler RealPlex 2 (Eppendorf) with the primer sets listed in Table S4 in the supplemental material.
ART preparation.
Stock solutions of tenofovir disoproxil fumarate, efavirenz, atazanavir sulfate, ritonavir, and raltegravir were prepared in dimethyl sulfoxide (DMSO), and a stock solution of emtricitabine was prepared in H2O and stored at −20°C until use. All ART drugs were provided by the NIH AIDS Research and Reference Reagent Program.
Statistical analysis.
All data are expressed as the mean ± standard error of the mean (SEM). One or two-way analysis of variance (ANOVA), followed by the Holm-Sidak post hoc test, was performed for the indicated comparisons. Analyses of linear trend were assessed by Pearson's correlation with the line of best fit determined by linear regression (α = 0.05). Statistical support was provided by the Biostatics and Data Management Core, Center for AIDS Research, Perelman School of Medicine, University of Pennsylvania.
Study approval.
All rodent studies and protocols for isolation of primary neuroglial cultures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (24) and approved by the University of Pennsylvania IACUC. All human studies and protocols for monocyte isolation were reviewed and approved by the University of Pennsylvania institutional review board, and all participants provided written informed consent.
RESULTS
Suppression of HO-1 expression in human macrophages is a consistent phenotype of infection by macrophage-tropic HIV-1 strains.
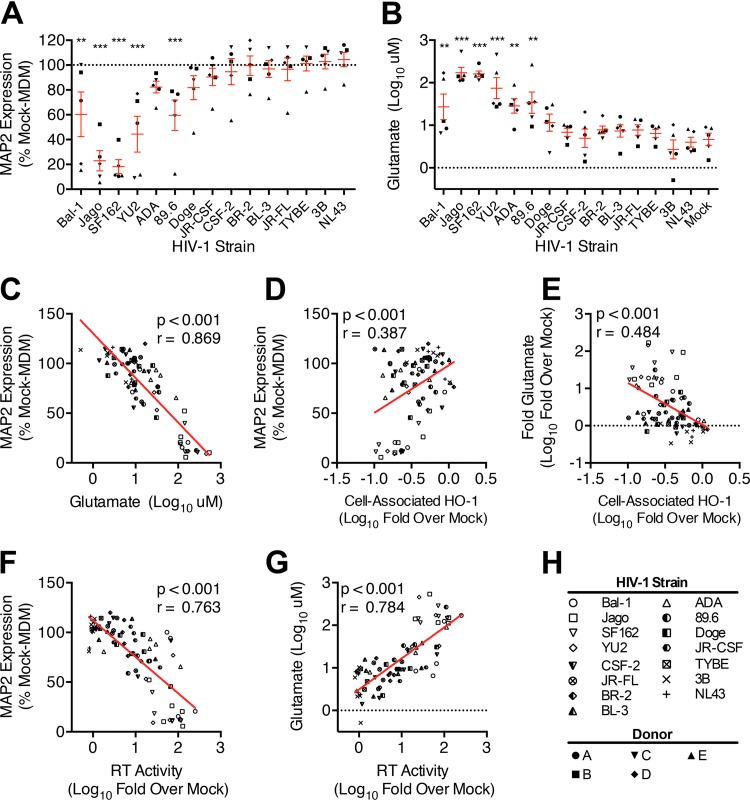
Our previous studies demonstrated the ability of several prototypic macrophage-tropic HIV-1 strains (89.6 and Jago) to selectively reduce expression of the antioxidant response enzyme HO-1 in human MDM, which is associated with a marked increase in MDM glutamate release and culture supernatant neurotoxicity (2, 3). This macrophage response suggests a plausible mechanism by which HIV-1 infection of brain macrophages could exacerbate oxidative stress and glutamate-induced neuronal injury, each of which is associated with neurocognitive dysfunction in infected individuals (25, 26). To determine whether this unique macrophage response to HIV is a consistent feature of macrophage-tropic HIV strains, we examined a broader panel of 13 HIV-1 strains used to infect MDM derived from five independent human donors. All donors were confirmed negative for the presence of the CCR5-Δ32 allele, a deletion mutation resulting in a nonfunctional CCR5 receptor that prevents infection by CCR5-using strains.
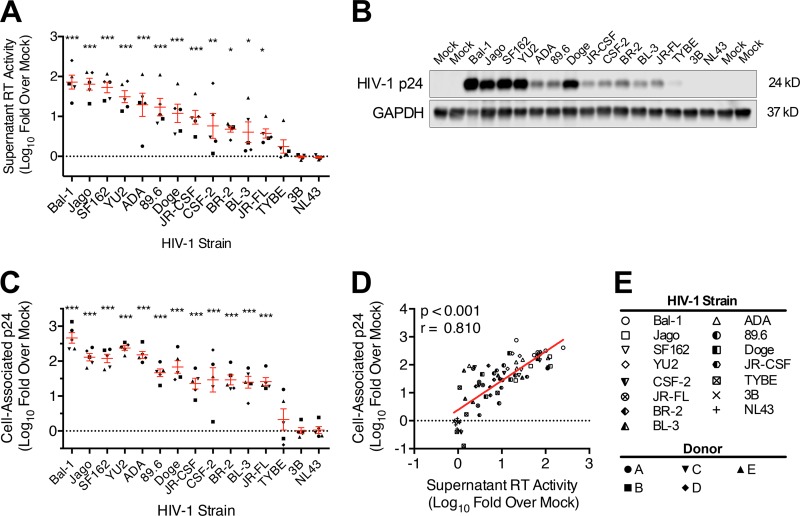
Among these 13 HIV-1 strains, we observed an ∼3-log range of replication in MDM derived from the different human MDM donors (Fig. 1; see Fig. S1 in the supplemental material). Two-way ANOVA by viral strain and MDM donor demonstrated that the variability in HIV replication level determined by supernatant reverse transcriptase (RT) activity (Fig. 1A) and cell-associated HIV-1 p24 antigen (Fig. 1B and C) was accounted for primarily by the viral strain (75.3% for supernatant RT, P < 0.001; 86.9% for cell-associated p24, P < 0.001) and much less by the donor (9.4% supernatant RT, P < 0.001; 1.2% cell-associated p24, P < 0.05). As predicted, supernatant RT activity had a strong positive correlation with cell-associated p24 content (Fig. 1D), suggesting a proportional production of intracellular viral proteins and release of viral particles and consistency among MDM cultures. Thus, an HO-1 response associated with infection of MDM with these strains can be attributed to an HIV replication-dependent phenotype.
FIG 1.
Supernatant reverse transcriptase activity and cell-associated HIV-1 p24 antigen content are highly correlated markers of HIV-1 replication in macrophages. MDM isolated from five healthy donors were infected with each of 15 HIV-1 strains (inoculum of 25 ng HIV-1 p24 content per 4 × 105 MDM). Replication was assessed on day 12 postinfection by supernatant reverse transcriptase (RT) and by cell-associated HIV-1 p24 expression as quantified by Western blotting. (A) Day 12 MDM supernatant RT activity (all 5 donors) stratified by strain and normalized to uninfected/mock-MDM. (B) Representative Western blot of cell-associated p24 on day 12 postinfection from donor A. (C) Densitometry analysis of cell-associated p24 expression (all 5 donors) stratified by strain and normalized to GAPDH and mock-MDM background. (D) Correlation between supernatant RT activity and cell-associated p24 expression on day 12 postinfection. (E) Key for HIV strain and MDM donor symbols. RT and p24 values for mock-MDM were set to 0 on a log scale (dotted line). Error bars indicate means ± SEMs. Statistical comparisons to mock-MDM were made by two-way ANOVA with the Holm-Sidak post hoc test. Correlations were assessed by Pearson's correlation with the line of best fit determined by linear regression. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
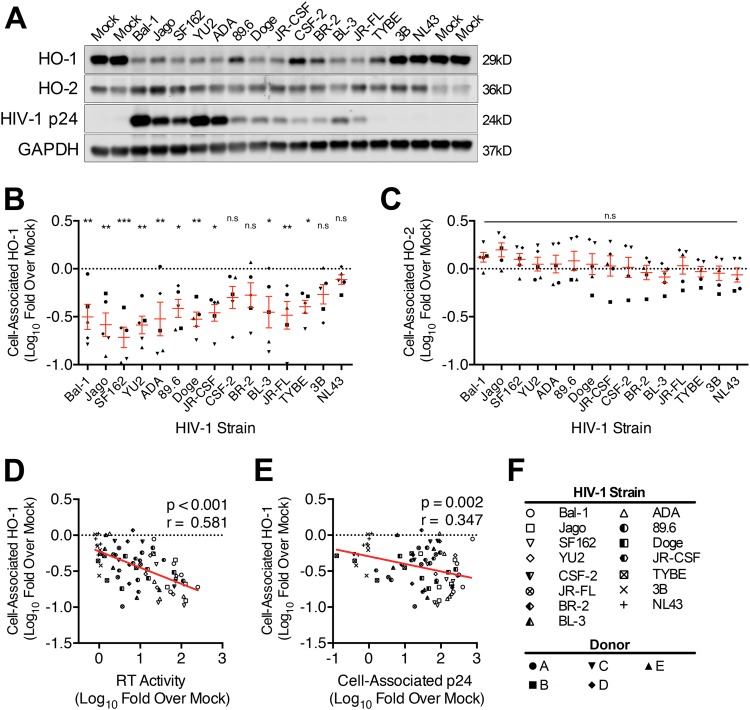
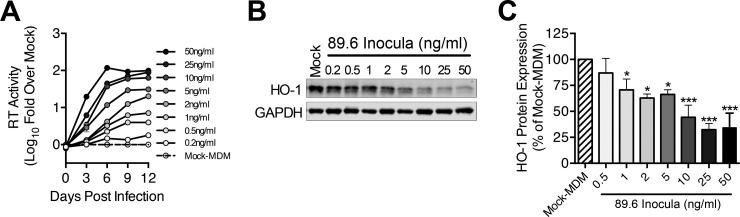
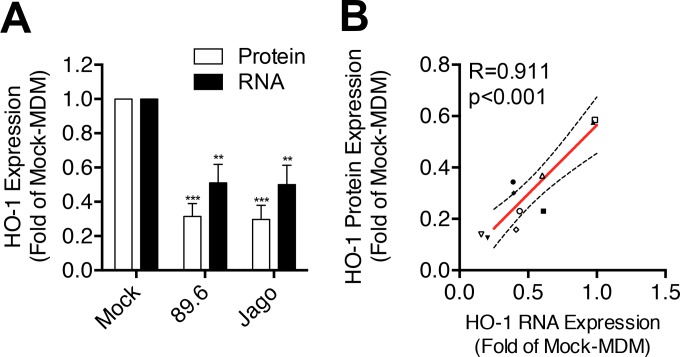
Productive infection of MDM with 11 of the 13 macrophage-tropic HIV-1 strains significantly decreased HO-1 protein expression (Fig. 2A and B), while infection with the two remaining strains (CSF-2 and BR2) did not. Nonproductive infection with the non-macrophage-tropic strain 3B viral swarm or the related strain NL43 also did not significantly reduce HO-1 expression. No effect on expression of the constitutive heme oxygenase isoform HO-2 by any HIV strain was observed (Fig. 2C), consistent with our previous observations of a selective suppression of HO-1 expression by the prototypic HIV-1 strains 89.6 and Jago. Expression of HO-1 correlated negatively with HIV replication level when analyzed across all five macrophage donors and all HIV strains (Fig. 2D and E), thus demonstrating consistency of this HO-1 deficiency with infection of macrophages by most, but not all, infecting strains. We confirmed the strong association between HIV replication level and HO-1 protein reduction seen among different HIV-1 strains by examining HO-1 reduction with different levels of replication by a single strain, 89.6 (Fig. 3). Achieving increasing levels of replication in MDM by inoculation with increasing amounts of virus (Fig. 3A) resulted in increasing HO-1 deficiency (Fig. 3B and C). This relationship was confirmed in each of three independent macrophage donors (see Fig. S2 in the supplemental material). This suggests that for individual macrophage-tropic strains, various levels of replication would predictably decrease HO-1 expression. Notably, the decrease in HO-1 protein expression is associated with a highly significant and strongly correlated decrease in HO-1 RNA expression (Fig. 4A and B). Consistent with Western blot analysis of HO-2 protein expression, we did not observe any changes in HO-2 RNA expression in HIV-MDM with strain 89.6 infection (data not shown). Thus, various levels of MDM infection by a viral swarm within an individual or among different individuals infected with genetically distinct macrophage-tropic HIV strains could be expected to result in different, albeit significant, reductions in levels of HO-1 expression within the MDM compartment.
FIG 2.
HIV-1 replication consistently induces HO-1 deficiency in macrophages. MDM isolated from five healthy donors were infected with each of 15 HIV-1 viral strains (inoculum of 25 ng HIV-1 p24 per 4 × 105 MDM). On day 12 postinfection, MDM lysates were assessed for HO-1, HO-2, HIV-1 p24, and GAPDH protein expression by Western blotting. Replication was assessed on day 12 postinfection by supernatant RT activity and by cell-associated HIV-1 p24. (A) Representative Western blot of HO-1, HO-2, HIV-1 p24, and GAPDH in HIV-MDM from one donor. (B and C) Densitometry analysis of HO-1 (B) and HO-2 (C) protein expression in HIV-MDM (all 5 donors) stratified by strain and normalized to GAPDH and presented as fold change from that for mock-MDM. HO-1 and HO-2 expression levels in mock-MDM were set to 0 on a log scale (dotted line). Error bars indicate means ± SEMs. (D and E) Correlation between macrophage HO-1 protein expression and supernatant RT (D) and cell-associated HIV-1 p24 (E). (F) Key for HIV strain and MDM donor symbols. Statistical comparisons to mock-MDM were made by two-way ANOVA with the Holm-Sidak post hoc test. Correlations were assessed by Pearson's correlation with the line of best fit determined by linear regression. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG 3.
HIV-1 replication level predicts HO-1 loss within an HIV-MDM culture. MDM were infected with different inocula (0.2 to 50 ng/ml HIV p24 per 4 × 105 cells) of HIV-1 89.6. (A and B) Representative data from one MDM donor infection showing HIV replication (n = 2 technical replicates) (A) and HO-1 and GAPDH protein Western blot (day 12 postinfection) (B) over the range of viral inocula tested. (C) Quantification of HO-1 protein expression normalized to GAPDH (n = 3 MDM donors). Error bars indicate means ± SEMs. Statistical comparisons to mock-MDM were made by one-way ANOVA with the Holm-Sidak post hoc test. *, P < 0.05; ***, P < 0.001.
FIG 4.
HIV-MDM HO-1 protein expression correlates with HO-1 RNA expression. Protein and RNA expression of HO-1 and GAPDH in HIV-MDM lysates were assessed by Western blotting and qPCR, respectively, on day 12 after HIV infection (89.6 or Jago strain). (A) Protein and RNA expression of HO-1 normalized to GAPDH expression in HIV-MDM from 5 or 6 independent donors. (B) Correlation between HO-1 protein and RNA expression levels in HIV-MDM from 5 independent donors (distinguished by symbol shape) infected with HIV-1 strain 89.6 (closed symbols) or Jago (open symbols). Mock-MDM HO-1 protein and RNA expression levels were set to 1. Error bars indicate means ± SEMs. Statistical comparisons to mock-MDM were made by one-way ANOVA with the Holm-Sidak post hoc test. Correlations were assessed by Pearson's correlation with the line of best fit determined by linear regression. **, P < 0.01; ***, P < 0.001.
HO-1 deficiency in HIV-MDM correlates with supernatant glutamate level and associated neurotoxicity.
Previously, we demonstrated that modulating HO-1 in HIV-MDM modulates supernatant neurotoxicity (2, 3). Thus, we assessed the association between HIV-driven HO-1 reduction and neurotoxin production in our panel of HIV-1 strains. Replication in MDM by five of the six highest-replicating viral strains (Bal-1, Jago, SF162, YU2, and 89.6) produced significant supernatant neurotoxicity in our neuronal/glial cell-based ELISA (Fig. 5A). Notably, MDM derived from one of the five donors produced significantly higher levels of HIV-MDM supernatant neurotoxicity than the other donors (Fig. 5A, upright filled triangles; see Fig. S3A in the supplemental material). A two-way ANOVA demonstrated that while most supernatant neurotoxicity was accounted for by the viral strain (69.6%, P < 0.001), neurotoxicity variability could also be attributed to donor variability (16.0%, P < 0.001). These data suggest that host macrophage determinants that affect neurotoxic potential, potentially such as differential donor HO-1 inducibility or basal HO-1 expression, can play a significant role in determining neurotoxin output in response to HIV infection.
FIG 5.
HIV-1 infection of MDM induces glutamate-associated supernatant neurotoxicity. Neurotoxicity of HIV-MDM supernatants was assessed by applying supernatants (day 12, 1:20 dilution) to primary rat neuroglial cultures for 24 h, followed by quantification of culture MAP2 content by ELISA. Supernatant glutamate content was quantified by the Amplex red glutamate assay. (A and B) Supernatant neurotoxicity (loss of MAP2; mock-MDM set to 100% expression of MAP2) (A) and supernatant glutamate concentrations (all 5 donors) (B) stratified by strain. (C) Correlation between supernatant neurotoxicity and glutamate level. (D and E) Correlations between MDM HO-1 protein expression and supernatant neurotoxicity (D) and glutamate concentration (E). (F and G) Correlation between supernatant RT activity and supernatant neurotoxicity (F) and glutamate concentration (G). (H) Key for HIV strain and MDM donor symbols. Error bars indicate means ± SEMs. Statistical comparisons to mock-MDM were made by two-way ANOVA with the Holm-Sidak post hoc test. Correlations were assessed by Pearson's correlation with the line of best fit determined by linear regression. **, P < 0.01; ***, P < 0.001.
We and others previously identified glutamate as one of the major neurotoxins released from HIV-infected macrophages and microglia (3, 17, 27, 28). The six most robustly replicating HIV strains (Bal-1, Jago, SF162, YU2, ADA, and 89.6) significantly increased MDM supernatant glutamate content, in comparison with mock-MDM (Fig. 5B). Although there was significant variability in supernatant glutamate levels among different donors (see Fig. S3B in the supplemental material), these differences were minimized when supernatant glutamate content was expressed as a fold increase over matched mock-MDM glutamate content (see Fig. S3C and D in the supplemental material). These data suggest that while basal levels of glutamate production differ among donors, the proportional HIV induction of MDM glutamate content and associated neurotoxicity are similar among donors. Supernatant neurotoxicity positively correlated (P < 0.001) with supernatant glutamate content across all donors and strains (Fig. 5C), which is consistent with glutamate toxicity being a primary component of HIV-MDM supernatant neurotoxicity (17, 27). Despite this highly significant and highly robust correlation, the differing levels of neurotoxicity of HIV-MDM supernatants with similar glutamate levels (Fig. 5C) suggest the presence of other neurotoxins in HIV-MDM in addition to glutamate.
Within our HIV-1 panel, the HIV-MDM supernatant neurotoxicity and glutamate level correlated with HO-1 deficiency (Fig. 5D and E) and HIV strain replication (Fig. 5F and G; see Fig. S3E and F in the supplemental material), consistent with our previous studies (2, 3). Despite these correlations between HO-1 loss, neurotoxicity, and replication, however, several poorly replicating HIV-1 strains significantly reduced HO-1 protein expression without significantly increasing supernatant neurotoxicity or glutamate content. This suggests that a “threshold” level of HIV replication is necessary for increasing glutamate release from HO-1-deficient HIV-MDM. Consistent with this, HO-1 reduction in MDM by small interfering RNA (siRNA) knockdown in the absence of HIV infection did not increase supernatant glutamate content or supernatant neurotoxicity (see Fig. S4A to C in the supplemental material). Thus, HIV replication concomitant with HO-1 reduction appears necessary to increase glutamate release, suggesting critical virus-specific effects that create a permissive environment in MDM for enhanced glutamate release in a state of HO-1 deficiency.
Antiretroviral treatment of established HIV infection of MDM fails to limit HO-1 deficiency and associated neurotoxicity.
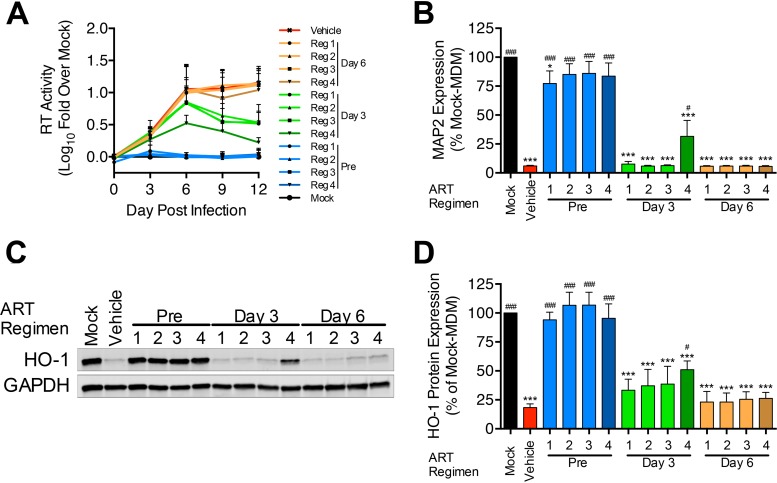
Given the strong correlation between HIV replication and HO-1 loss, we determined the ability of ART treatment to prevent the HO-1 loss and neurotoxicity associated with HIV-MDM. We utilized three ART regimens recommended for initial treatment in ART-naive patients (29). Each regimen included the two nucleoside reverse transcriptase inhibitors (NRTIs), tenofovir disoproxil fumarate (TDF; 20 nM) and emtricitabine (FTC; 500 nM), in addition to (i) efavirenz (EFZ; 40 nM), a nonnucleoside reverse transcriptase inhibitor (NNRTI)-based regimen (regimen 1); (ii) atazanavir boosted with ritonavir (ATV/r; 15 nM/10 nM), a protease inhibitor (PI)-based regimen (regimen 2); or (iii) raltegravir (RAL; 50 nM), an integrase strand transfer inhibitor (INSTI)-based regimen (regimen 3). The chosen drug concentrations were based upon reported cerebrospinal fluid drug concentrations in ART-treated patients (30). To more effectively suppress HIV replication, we also used a high-dose PI-based regimen with 5-fold-higher ATV/r concentrations (75 nM/50 nM) and unchanged TDF and FTC concentrations (regimen 4).
To attempt to fully determine the effectiveness of ART in preventing HO-1 loss in HIV-MDM, we applied the treatment before and after establishing HIV infection in MDM. We pretreated MDM with each ART regimen 1 h prior to HIV infection and replenished all ART drugs with each medium change. To model the ability of ART to impact already-infected macrophages, we also treated HIV-MDM with each ART regimen after HIV inoculation (beginning days 3 and 6 postinfection) and replenished all ART drugs with each medium change. We analyzed HIV replication over the course of infection, and we analyzed HO-1 expression and supernatant neurotoxicity from the last day of infection (Fig. 6). Pretreatment with each ART regimen resulted in no detectable viral replication or HO-1 loss and dramatically reduced supernatant neurotoxicity (Fig. 6A to C). Notably, supernatants from ART-pretreated HIV-MDM showed a nonsignificant trend for enhanced neurotoxicity compared to mock-MDM supernatants, although only those derived from MDM treated with the NNRTI-based regimen (regimen 1) reached a statistically significant level of neurotoxicity compared to that of mock-MDM (Fig. 6B).
FIG 6.
Antiretroviral drug treatment prior to, but not after, HIV infection of MDM prevents HO-1 loss and associated neurotoxicity. MDM were treated with ART regimens either 1 h prior to, 3 days after, or 6 days after HIV-1 (89.6) infection. ART drugs were replenished every 3 days with medium changes. ART regimens were as follows: regimen (reg) 1, 20 nM TDF, 500 nM FTC, and 40 nM EFZ; regimen 2, 20 nM TDF, 500 nM FTC, and 15 nM/10 nM ATV/r; regimen 3, 20 nM TDF, 500 nM FTC, and 50 nM RAL; regimen 4, 20 nM TDF, 500 nM FTC, and 75 nM/50 nM ATV/r. (A and B) HIV replication (A) and supernatant neurotoxicity (B) in HIV-MDM (n = 4). (C and D) Representative Western blot (C) and densitometry quantification (D) of HO-1 protein expression from the last day of infection in all 4 donors. Error bars indicate means ± SEMs. Statistical comparisons to mock-MDM (*) and vehicle (#) were made by one-way ANOVA with the Holm-Sidak post hoc test. * or #, P < 0.05; *** or ###, P < 0.001.
Treatment of HIV-MDM on day 3 postinfection significantly reduced HIV replication, but only regimen 4 (high-dose PI) treatment significantly reduced HO-1 loss and supernatant neurotoxicity (Fig. 6). No treatment regimen applied on day 6 postinfection reduced replication (Fig. 6A), supernatant neurotoxicity (Fig. 6B), or HO-1 loss (Fig. 6C and D). These data confirm the correlations between viral replication, HO-1 loss, and supernatant neurotoxicity in HIV-MDM. Furthermore, these findings strongly suggest that although physiologically relevant CNS concentrations of ART can protect against HO-1 loss by preventing HIV infection, they cannot prevent the HO-1 loss and associated neurotoxicity from HIV-MDM. This failure of ART to protect HIV-MDM from HO-1 loss and increased neurotoxicity underscores the potential importance of a persistently infected CNS macrophage reservoir in mediating neuronal injury even in ART-treated individuals.
Accessory genes nef, vpr, and vpu are not required for HIV-1-mediated HO-1 reduction in infected macrophages.
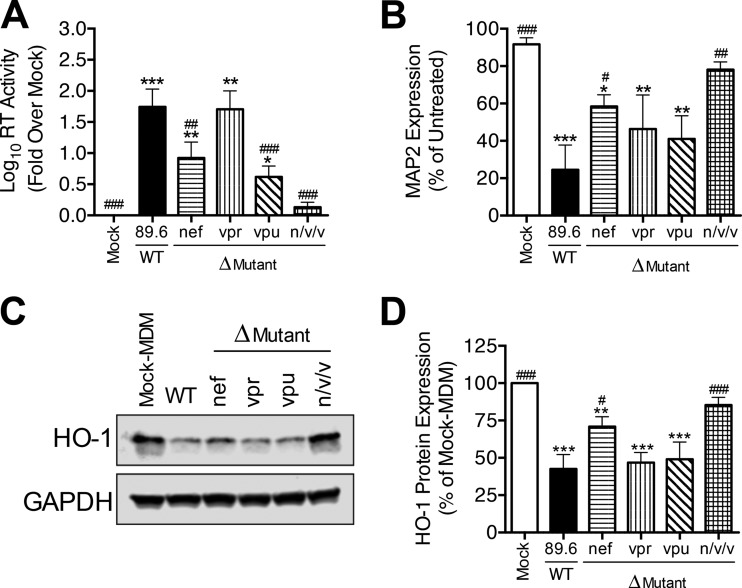
In addition to viral proteins required for genome replication and viral particle assembly, HIV-1 expresses several accessory proteins, Nef, Vpr, and Vpu, which can downregulate, degrade, or functionally inactivate certain host cell proteins, particularly viral restriction factors (31). To determine whether nef, vpr, or vpu might directly modulate HO-1 protein loss in HIV-MDM, we analyzed the effects of infectious HIV mutant strains deficient in each (or all) of these accessory genes. We infected MDM with the wild-type (WT) molecular clone 89.6 or with 89.6 clones with individual deletions of nef, vpr, or vpu (Δnef, Δvpr, and Δvpu, respectively) or with deletion of a combination of all three genes (89.6 Δnef/vpr/vpu) (22).
The 89.6 Δnef, Δvpr, and Δvpu mutants each replicated productively in MDM, albeit to different levels (Fig. 7A). We found that 89.6 Δnef and 89.6 Δvpu replicated less robustly than WT 89.6, while 89.6 Δvpr replicated to levels comparable to those of WT 89.6. As previously reported (22), 89.6 Δnef/vpr/vpu did not replicate to detectable levels in MDM. Each replicating strain significantly reduced the MDM HO-1 expression (Fig. 7C and D) and the increased associated supernatant neurotoxicity (Fig. 7B), while the nonreplicating strain 89.6 Δnef/vpr/vpu did not. Furthermore, as predicted by our observations across all HIV-1 strains examined (Fig. 5), the profile of HO-1 loss in infected MDM (Fig. 7D) closely resembled the profile of neurotoxicity of the corresponding culture supernatants (Fig. 7B). These data thus demonstrate that the nef, vpr, and vpu accessory genes are not themselves directly required for HIV-1-mediated HO-1 loss in infected MDM, although their effects on HIV-1 replication do indeed modulate the level of HO-1 expression and associated supernatant neurotoxicity.
FIG 7.
HIV-1 accessory genes nef, vpr, and vpu are not required for induction of HO-1 deficiency or associated neurotoxicity in HIV-MDM. MDM from 5 different donors were infected with wild-type (WT) 89.6 or mutant 89.6 lacking either the nef, vpr, or vpu gene or all three genes in combination (n/v/v). (A and B) Supernatants (day 12 postinfection) were analyzed for HIV replication (A) and supernatant neurotoxicity (B). (C and D) Representative Western blot (C) and summary densitometry (D) analysis of HO-1 expression in HIV-MDM (day 12). Errors bars indicate means ± SEMs (n = 5). Statistical comparisons to mock-MDM (*) or WT 89.6 (#) were made by one-way ANOVA with the Holm-Sidak post hoc test. * or #, P < 0.05; ** or ##, P < 0.01; *** or ###, P < 0.001.
Macrophage-tropic HIV-2 strain CBL-20 reduces HO-1 protein expression in infected MDM comparably to HIV-1 strain 89.6.
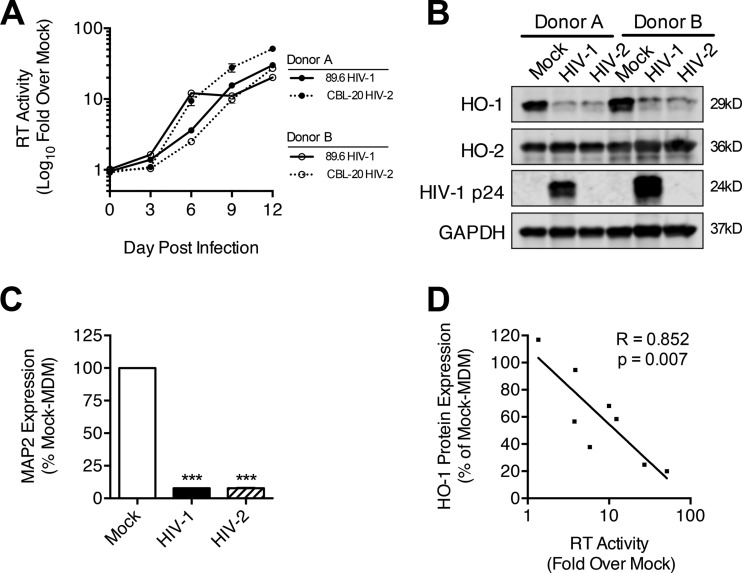
The observation that 11 of 13 macrophage-tropic HIV-1 strains tested significantly reduce HO-1 protein expression in MDM suggests that HO-1 loss is a highly conserved response to HIV-1 infection. This finding along with the lack of HIV-1 accessory gene requirement for HO-1 loss suggests that the other major human immunodeficiency virus group, HIV-2, which also causes immune deficiency, albeit less severely (32), might also induce HO-1 loss in MDM. We infected MDM derived from eight different human donors with HIV-2 CBL-20 (23) and HIV-1 89.6. While 89.6 replicated robustly and reduced MDM HO-1 protein expression in all donor cultures, HIV-2 CBL-20 replicated as robustly as 89.6 in MDM derived from only two of the eight donors (Fig. 8). In these HIV-2-infected MDM cultures, HO-1 protein expression was reduced similarly to that of HIV-1 89.6-infected MDM, with comparable levels of supernatant neurotoxicity and glutamate content (Fig. 8B and C; see Fig. S5A to C in the supplemental material). Moreover, among all MDM donors, HO-1 protein loss in MDM correlated significantly with the HIV-2 replication level (Fig. 8D). Similar to the HO-2-sparing effects of HIV-1 replication, we did not observe changes in HO-2 protein expression with HIV-2 replication (Fig. 8B). These data suggest that HIV-driven HO-1 deficiency, glutamate release, and associated neurotoxicity are consistent features of HIV-1 and HIV-2 MDM infection.
FIG 8.
HIV-2 replication in MDM induces HO-1 deficiency and associated supernatant neurotoxicity. MDM (8 independent donors) were infected with HIV-2 CBL-20 and HIV-1 89.6. (A) HIV-2 infection of MDM from 2 of 8 donors showed replication levels comparable to those of HIV-1 89.6. (B and C) Western blot analysis of HO-1, HO-2, and HIV-1 p24 (B) and supernatant neurotoxicity (C) in these representative infections. Errors bars indicate means ± SEMs. (D) Correlation between HO-1 protein expression and supernatant RT activity for HIV-2 MDM infections (all 8 donors). Statistical comparisons to mock-MDM were made by one-way ANOVA with the Holm-Sidak post hoc test. Correlations were assessed by Pearson's correlation with the line of best fit determined by linear regression. ***, P < 0.001.
DISCUSSION
The continued prevalence of HAND and associated inflammation and oxidative stress in ART-treated HIV-infected individuals highlights the need for adjunctive therapies that target these neuropathological processes, which persist in both the CNS and systemic compartments. To this end, we have identified the anti-inflammatory and antioxidative enzyme HO-1 as a targetable host factor for adjunctive neuroprotective therapy for HAND. We previously demonstrated a significant deficiency of HO-1 protein within the brains of HIV-infected individuals with HAND, and we further showed that this deficiency correlated with brain and CSF viral loads and markers of immune activation (3). Furthermore, using two HIV-1 viruses (Jago and 89.6), we demonstrated that HIV infection of macrophages reduces HO-1 protein expression and that this deficiency is directly linked to increased toxic levels of glutamate (3), a HAND-associated neurotoxin (25). This loss of HO-1 protein is specific, as other members of the ARE-driven gene family and the heme oxygenase isoform HO-2 are unaffected (3).
To further define this neuropathological phenotype of HIV, we have expanded our previous studies to examine a broader group of macrophage-tropic HIV-1 strains (n = 13). In this study, we demonstrated that most (11 of 13) HIV-1 macrophage-tropic strains induce a loss of HO-1 protein and RNA, in contrast to the unaltered expression of HO-2 protein and RNA. The HIV-1 accessory genes nef, vpr, and vpu, which are implicated in downregulating multiple host factors, are not required for this HO-1 deficiency phenotype, as mutant HIV-1 molecular clones lacking expression of these accessory genes maintained the ability to reduce HO-1 protein expression and induce supernatant neurotoxicity in infected macrophages to an extent commensurate with their overall replication levels. The degree of HO-1 loss in HIV-1-infected macrophages correlated significantly with supernatant glutamate levels and associated neurotoxicity, consistent with our previous findings demonstrating that HO-1 negatively regulates glutamate release and supernatant neurotoxicity (2, 3).
We further demonstrated that the other major human immunodeficiency virus, HIV-2, also reduces HO-1 protein expression and increases neurotoxin release in infected macrophages. Although HIV-1 and HIV-2 share many similarities, including basic gene organization, replication pathways, and modes of transmission, HIV-2 shares only 50 to 60% nucleic acid homology with HIV-1 and it is more closely related to the simian immunodeficiency virus. Compared to HIV-1, HIV-2 causes less severe disease, as determined by lower plasma viral loads, less CD4+ T lymphocyte loss, reduced risk of progression to AIDS, and decreased mortality (32). Despite its reduced pathogenic potential, HIV-2 infiltrates into the CNS and can cause neurological impairment and neuropathological changes similar to those of HIV-1 (33). Our data suggest that this HO-1-deficient neurotoxic phenotype in macrophages is conserved across HIV-1 and HIV-2 strains and thus HO-1 loss may have a conserved role in neurological disease and neurocognitive impairment in both HIV-1- and HIV-2-infected individuals. In each of our HIV-1 and HIV-2 macrophage infection experiments, HO-1 deficiency correlated with viral replication. Despite this correlation, our data clearly demonstrate that even low-level HIV replication in macrophages can promote HO-1 loss. The conservation of this HO-1 deficiency phenotype across human immunodeficiency viruses suggests that reduction in HO-1 protein expression might provide a selective advantage for HIV survival in macrophages and ultimately therefore contribute to HIV pathogenesis.
A role for HO-1 in the pathogenesis of virus infections has been suggested not only for HIV-1 but also for other viruses. Induction of HO-1 expression in uninfected macrophages has been shown to reduce subsequent HIV-1 infection and replication (34, 35). An antiviral effect of HO-1 induction has been observed in infection studies of hepatitis C virus (HCV) (36), hepatitis B virus (HBV) (37), Ebola virus (38), enterovirus 71 (EV71) (39), vaccinia virus (40), and porcine reproductive and respiratory syndrome virus (41). Interestingly, HCV has been reported to downregulate HO-1 protein expression (42, 43), although contrasting studies report HO-1 induction by HCV (44, 45).
How HO-1 expresses antiviral effects is unknown. Recent work suggests that HO-1 expression in myeloid cells is required for the induction of type I interferon expression and the associated innate immune responses (46, 47). These effects might occur through signaling by biliverdin, an HO-1 enzymatic product (48). Thus, HO-1 might promote an interferon-mediated antiviral state. Other proposed HO-1 antiviral effects include posttranscriptional destabilization of viral core proteins, as for HBV (37), and inhibition of viral enzymes, as for the HIV protease by biliverdin and bilirubin (49). The consistent ability of HIV to decrease HO-1 expression in macrophages suggests an adaptive benefit for the virus; however, the HIV-mediated loss of HO-1 in MDM seems unlikely to have a significant effect on HIV replication, as HO-1 deficiency is not observed until 6 to 9 days postinfection, after robust infection is established (3). Although induction of HO-1 in macrophages prior to HIV infection has been reported to reduce subsequent HIV replication, enzymatic inhibition of target cell HO-1 prior to infection does not augment subsequent HIV replication (35). Moreover, we demonstrated that potent HO-1 knockdown, HO-1 enzymatic inhibition, or induction of HO-1 expression in macrophages in which productive HIV replication is established does not alter replication (3). Thus, a role for HO-1 in modulating HIV replication in macrophages has not been clearly established, in contrast to a clear role for HIV replication in macrophages in reducing HO-1 expression. The consequences and full effects of HIV-mediated HO-1 deficiency in macrophages clearly require further study.
Some evidence suggests a link between HO-1 expression and systemic disease progression in HIV-infected individuals (50). The HO-1 promoter region contains a microsatellite dinucleotide GT(n) repeat polymorphism that predictably modulates HO-1 inducibility in in vitro cell culture systems (51, 52). In a human HIV cohort study, Seu et al. demonstrated associations between GT(n) repeat length, low HO-1 expression in peripheral blood mononuclear cells, increased plasma sCD14 expression, and elevated viral load in HIV-infected African-Americans (50). These findings are consistent with an immune-modulatory role for HO-1 in HIV-1 infection. Additionally, HIV/HCV-coinfected individuals have lower liver HO-1 expression than that observed in HCV-monoinfected individuals; this loss of liver HO-1 is particularly evident in subjects with high HIV loads and low CD4+ T lymphocyte counts (44). These findings, in conjunction with our observation of HO-1 deficiency in brain tissue from HIV-infected subjects, suggest that HIV infection modulates HO-1 expression in the CNS and in peripheral tissues (3).
To address the association between HIV replication and HO-1 deficiency in the context of ART use, we determined the ability of clinically relevant ART regimens applied pre- and postinfection to prevent HO-1 loss and associated neurotoxicity in HIV-MDM. We demonstrated that ART exposure at CNS-relevant concentrations fails to prevent HO-1 loss and neurotoxicity in HIV-MDM once infection is already established. This suggests that long-lived CNS macrophage reservoirs in HIV-infected individuals, even those on suppressive ART, can maintain an HO-1-deficient, neurotoxic phenotype.
This inability of ART to attenuate this phenotype once established may be especially relevant given the recent evidence for plasma and CSF viral blipping (intermittent HIV replication) in ART-treated individuals and its association with immune activation. Recent studies suggest that despite apparently prolonged plasma HIV suppression by ART (<50 HIV RNA copies/ml), intermittent viral replication is detectable in the plasma and/or cerebrospinal fluid (53). Moreover, CSF viral blipping, which can occur independently from plasma blipping, associates with monocyte activation (neopterin) and emergence of CNS-compartmentalized ART resistance mutations (54, 55). Intermittent viral replication within the CNS macrophage compartment could promote the persistence of an HO-1-deficient neurotoxic phenotype that is resistant to suppressive ART therapy. This suggests a plausible mechanism by which HIV infection of brain macrophages in ART-treated individuals could exacerbate oxidative stress and glutamate-induced neuronal injury, each of which is associated with neurocognitive dysfunction in infected individuals (25, 26).
The conservation of the ability to induce HO-1 deficiency and associated neurotoxin production across macrophage-tropic HIV strains and the association of brain HO-1 deficiency with neurocognitive impairment make it an attractive therapeutic target in HIV-infected individuals with HAND (3). The failure of ART to prevent this HO-1 loss in established infection highlights the potential value of HO-1-inducing drugs as adjunctive therapy to ART. Previously, we showed that application of an enzymatic inhibitor or genetic knockdown of HO-1 in HIV-MDM augments glutamate production and associated neurotoxicity, confirming that this HIV-mediated HO-1 loss promotes neurotoxin production (2, 3). Moreover, we demonstrated that HO-1 induction in HIV-MDM reduces glutamate production and the associated neurotoxicity (2, 3), thus supporting a role for HO-1 induction as a neuroprotective strategy against HIV. Our current findings suggest that the induction of HO-1 deficiency associated with excess glutamate production and neurotoxicity is a highly conserved phenotype of macrophage-tropic HIV strains, which can persist in the macrophage compartment in the presence of ART. Our studies further suggest a role for HO-1 deficiency in ongoing neuronal injury in HAND, which is only partially prevented by ART. Therapies that target HO-1 deficiency in chronic HIV infection could provide additional neuroprotection to ART.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the support of the Center for AIDS Research (CFAR) Virology Core (Farida Shaheen, Technical Director) and the Biostatistics and Data Management Core and the Mahoney Institute of Neurological Sciences (MINS) Neuron Culture Service Center (Margie Maronski, Technical Director) at the University of Pennsylvania Perelman School of Medicine.
This work was supported in part by National Institutes of Health R01 grants MH095671 (D.L.K.), MH104134 (D.L.K.), and MH061139 (R.G.C.), and F30 grant MH102120 (A.J.G.).
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01495-15.
REFERENCES
- 1.Ambegaokar SS, Kolson DL. 2014. Heme oxygenase-1 dysregulation in the brain: implications for HIV-associated neurocognitive disorders. Curr HIV Res 12:174–188. doi: 10.2174/1570162X12666140526122709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cross SA, Cook DR, Chi AW, Vance PJ, Kolson LL, Wong BJ, Jordan-Sciutto KL, Kolson DL. 2011. Dimethyl fumarate, an immune modulator and inducer of the antioxidant response, suppresses HIV replication and macrophage-mediated neurotoxicity: a novel candidate for HIV neuroprotection. J Immunol 187:5015–5025. doi: 10.4049/jimmunol.1101868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gill AJ, Kovacsics CE, Cross SA, Vance PJ, Kolson LL, Jordan-Sciutto KL, Gelman BB, Kolson DL. 2014. Heme oxygenase-1 deficiency accompanies neuropathogenesis of HIV-associated neurocognitive disorders. J Clin Invest 124:4459–4472. doi: 10.1172/JCI72279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. 2000. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem 275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 5.Gozzelino R, Jeney V, Soares MP. 2010. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 6.Lin Q, Weis S, Yang G, Weng YH, Helston R, Rish K, Smith A, Bordner J, Polte T, Gaunitz F, Dennery PA. 2007. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem 282:20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- 7.Hori R, Kashiba M, Toma T, Yachie A, Goda N, Makino N, Soejima A, Nagasawa T, Nakabayashi K, Suematsu M. 2002. Gene transfection of H25A mutant heme oxygenase-1 protects cells against hydroperoxide-induced cytotoxicity. J Biol Chem 277:10712–10718. doi: 10.1074/jbc.M107749200. [DOI] [PubMed] [Google Scholar]
- 8.Yet SF, Tian R, Layne MD, Wang ZY, Maemura K, Solovyeva M, Ith B, Melo LG, Zhang L, Ingwall JS, Dzau VJ, Lee ME, Perrella MA. 2001. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res 89:168–173. doi: 10.1161/hh1401.093314. [DOI] [PubMed] [Google Scholar]
- 9.Otterbein LE, Kolls JK, Mantell LL, Cook JL, Alam J, Choi AM. 1999. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J Clin Invest 103:1047–1054. doi: 10.1172/JCI5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panahian N, Yoshiura M, Maines MD. 1999. Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J Neurochem 72:1187–1203. [DOI] [PubMed] [Google Scholar]
- 11.Hashiba T, Suzuki M, Nagashima Y, Suzuki S, Inoue S, Tsuburai T, Matsuse T, Ishigatubo Y. 2001. Adenovirus-mediated transfer of heme oxygenase-1 cDNA attenuates severe lung injury induced by the influenza virus in mice. Gene Ther 8:1499–1507. doi: 10.1038/sj.gt.3301540. [DOI] [PubMed] [Google Scholar]
- 12.Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, CHARTER Group. 2010. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology 75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nightingale S, Winston A, Letendre S, Michael BD, McArthur JC, Khoo S, Solomon T. 2014. Controversies in HIV-associated neurocognitive disorders. Lancet Neurol 13:1139–1151. doi: 10.1016/S1474-4422(14)70137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. 2007. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen MF, Gill AJ, Kolson DL. 2014. Neuropathogenesis of HIV-associated neurocognitive disorders: roles for immune activation, HIV blipping and viral tropism. Curr Opin HIV AIDS 9:559–564. doi: 10.1097/COH.0000000000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burdo TH, Lackner A, Williams KC. 2013. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev 254:102–113. doi: 10.1111/imr.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Donnell LA, Agrawal A, Jordan-Sciutto KL, Dichter MA, Lynch DR, Kolson DL. 2006. Human immunodeficiency virus (HIV)-induced neurotoxicity: roles for the NMDA receptor subtypes. J Neurosci 26:981–990. doi: 10.1523/JNEUROSCI.4617-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones SP, Franco NF, Varney B, Sundaram G, Brown DA, de Bie J, Lim CK, Guillemin GJ, Brew BJ. 2015. Expression of the kynurenine pathway in human peripheral blood mononuclear cells: implications for inflammatory and neurodegenerative disease. PLoS One 10:e0131389. doi: 10.1371/journal.pone.0131389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelbard HA, Nottet HS, Swindells S, Jett M, Dzenko KA, Genis P, White R, Wang L, Choi YB, Zhang D, Lipton SA, Tourtellotte WW, Epstein LG, Gendelman HE. 1994. Platelet-activating factor: a candidate human immunodeficiency virus type 1-induced neurotoxin. J Virol 68:4628–4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mocchetti I, Bachis A, Avdoshina V. 2012. Neurotoxicity of human immunodeficiency virus-1: viral proteins and axonal transport. Neurotox Res 21:79–89. doi: 10.1007/s12640-011-9279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King JE, Eugenin EA, Buckner CM, Berman JW. 2006. HIV tat and neurotoxicity. Microbes Infect 8:1347–1357. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Balliet JW, Kolson DL, Eiger G, Kim FM, McGann KA, Srinivasan A, Collman R. 1994. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology 200:623–631. doi: 10.1006/viro.1994.1225. [DOI] [PubMed] [Google Scholar]
- 23.Schulz TF, Whitby D, Hoad JG, Corrah T, Whittle H, Weiss RA. 1990. Biological and molecular variability of human immunodeficiency virus type 2 isolates from The Gambia. J Virol 64:5177–5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 25.Ferrarese C, Aliprandi A, Tremolizzo L, Stanzani L, De Micheli A, Dolara A, Frattola L. 2001. Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology 57:671–675. doi: 10.1212/WNL.57.4.671. [DOI] [PubMed] [Google Scholar]
- 26.Uzasci L, Nath A, Cotter R. 2013. Oxidative stress and the HIV-infected brain proteome. J Neuroimmune Pharmacol 8:1167–1180. doi: 10.1007/s11481-013-9444-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang ZG, Piggee C, Heyes MP, Murphy C, Quearry B, Bauer M, Zheng J, Gendelman HE, Markey SP. 2001. Glutamate is a mediator of neurotoxicity in secretions of activated HIV-1-infected macrophages. J Neuroimmunol 117:97–107. doi: 10.1016/S0165-5728(01)00315-0. [DOI] [PubMed] [Google Scholar]
- 28.Huang Y, Zhao L, Jia B, Wu L, Li Y, Curthoys N, Zheng JC. 2011. Glutaminase dysregulation in HIV-1-infected human microglia mediates neurotoxicity: relevant to HIV-1-associated neurocognitive disorders. J Neurosci 31:15195–15204. doi: 10.1523/JNEUROSCI.2051-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panel on Antiretroviral Guidelines for Adults and Adolescents, Department of Health and Human Services. 2015. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, Washington, DC: http://aidsinfo.nih.gov/guidelines. [Google Scholar]
- 30.Calcagno A, Di Perri G, Bonora S. 2014. Pharmacokinetics and pharmacodynamics of antiretrovirals in the central nervous system. Clin Pharmacokinet 53:891–906. doi: 10.1007/s40262-014-0171-0. [DOI] [PubMed] [Google Scholar]
- 31.Collins DR, Collins KL. 2014. HIV-1 accessory proteins adapt cellular adaptors to facilitate immune evasion. PLoS Pathog 10:e1003851. doi: 10.1371/journal.ppat.1003851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyamweya S, Hegedus A, Jaye A, Rowland-Jones S, Flanagan KL, Macallan DC. 2013. Comparing HIV-1 and HIV-2 infection: lessons for viral immunopathogenesis. Rev Med Virol 23:221–240. doi: 10.1002/rmv.1739. [DOI] [PubMed] [Google Scholar]
- 33.Choi Y, Townend J, Vincent T, Zaidi I, Sarge-Njie R, Jaye A, Clifford DB. 2011. Neurologic manifestations of human immunodeficiency virus-2: dementia, myelopathy, and neuropathy in West Africa. J Neurovirol 17:166–175. doi: 10.1007/s13365-011-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devadas K, Dhawan S. 2006. Hemin activation ameliorates HIV-1 infection via heme oxygenase-1 induction. J Immunol 176:4252–4257. doi: 10.4049/jimmunol.176.7.4252. [DOI] [PubMed] [Google Scholar]
- 35.Devadas K, Hewlett IK, Dhawan S. 2010. Lipopolysaccharide suppresses HIV-1 replication in human monocytes by protein kinase C-dependent heme oxygenase-1 induction. J Leukoc Biol 87:915–924. doi: 10.1189/jlb.0307172. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Z, Wilson AT, Mathahs MM, Wen F, Brown KE, Luxon BA, Schmidt WN. 2008. Heme oxygenase-1 suppresses hepatitis C virus replication and increases resistance of hepatocytes to oxidant injury. Hepatology 48:1430–1439. doi: 10.1002/hep.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Protzer U, Seyfried S, Quasdorff M, Sass G, Svorcova M, Webb D, Bohne F, Hosel M, Schirmacher P, Tiegs G. 2007. Antiviral activity and hepatoprotection by heme oxygenase-1 in hepatitis B virus infection. Gastroenterology 133:1156–1165. doi: 10.1053/j.gastro.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 38.Hill-Batorski L, Halfmann P, Neumann G, Kawaoka Y. 2013. The cytoprotective enzyme heme oxygenase-1 suppresses Ebola virus replication. J Virol 87:13795–13802. doi: 10.1128/JVI.02422-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tung WH, Hsieh HL, Lee IT, Yang CM. 2011. Enterovirus 71 induces integrin beta1/EGFR-Rac1-dependent oxidative stress in SK-N-SH cells: role of HO-1/CO in viral replication. J Cell Physiol 226:3316–3329. doi: 10.1002/jcp.22677. [DOI] [PubMed] [Google Scholar]
- 40.Meseda CA, Srinivasan K, Wise J, Catalano J, Yamada KM, Dhawan S. 2014. Non-coding RNAs and heme oxygenase-1 in vaccinia virus infection. Biochem Biophys Res Commun 454:84–88. doi: 10.1016/j.bbrc.2014.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao S, Zhang A, Zhang C, Ni H, Gao J, Wang C, Zhao Q, Wang X, Wang X, Ma C, Liu H, Li N, Mu Y, Sun Y, Zhang G, Hiscox JA, Hsu WH, Zhou EM. 2014. Heme oxygenase-1 acts as an antiviral factor for porcine reproductive and respiratory syndrome virus infection and over-expression inhibits virus replication in vitro. Antiviral Res 110:60–69. doi: 10.1016/j.antiviral.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Abdalla MY, Britigan BE, Wen F, Icardi M, McCormick ML, LaBrecque DR, Voigt M, Brown KE, Schmidt WN. 2004. Down-regulation of heme oxygenase-1 by hepatitis C virus infection in vivo and by the in vitro expression of hepatitis C core protein. J Infect Dis 190:1109–1118. doi: 10.1086/423488. [DOI] [PubMed] [Google Scholar]
- 43.Wen F, Brown KE, Britigan BE, Schmidt WN. 2008. Hepatitis C core protein inhibits induction of heme oxygenase-1 and sensitizes hepatocytes to cytotoxicity. Cell Biol Toxicol 24:175–188. doi: 10.1007/s10565-007-9027-9. [DOI] [PubMed] [Google Scholar]
- 44.Jablonowska E, Wojcik K, Szymanska B, Omulecka A, Cwiklinska H, Piekarska A. 2014. Hepatic HMOX1 expression positively correlates with Bach-1 and miR-122 in patients with HCV mono and HIV/HCV coinfection. PLoS One 9:e95564. doi: 10.1371/journal.pone.0095564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghaziani T, Shan Y, Lambrecht RW, Donohue SE, Pietschmann T, Bartenschlager R, Bonkovsky HL. 2006. HCV proteins increase expression of heme oxygenase-1 (HO-1) and decrease expression of Bach1 in human hepatoma cells. J Hepatol 45:5–12. doi: 10.1016/j.jhep.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 46.Tzima S, Victoratos P, Kranidioti K, Alexiou M, Kollias G. 2009. Myeloid heme oxygenase-1 regulates innate immunity and autoimmunity by modulating IFN-beta production. J Exp Med 206:1167–1179. doi: 10.1084/jem.20081582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koliaraki V, Kollias G. 2011. A new role for myeloid HO-1 in the innate to adaptive crosstalk and immune homeostasis. Adv Exp Med Biol 780:101–111. doi: 10.1007/978-1-4419-5632-3_9. [DOI] [PubMed] [Google Scholar]
- 48.Lehmann E, El-Tantawy WH, Ocker M, Bartenschlager R, Lohmann V, Hashemolhosseini S, Tiegs G, Sass G. 2010. The heme oxygenase 1 product biliverdin interferes with hepatitis C virus replication by increasing antiviral interferon response. Hepatology 51:398–404. doi: 10.1002/hep.23339. [DOI] [PubMed] [Google Scholar]
- 49.McPhee F, Caldera PS, Bemis GW, McDonagh AF, Kuntz ID, Craik CS. 1996. Bile pigments as HIV-1 protease inhibitors and their effects on HIV-1 viral maturation and infectivity in vitro. Biochem J 320:681–686. doi: 10.1042/bj3200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seu L, Burt TD, Witte JS, Martin JN, Deeks SG, McCune JM. 2012. Variations in the heme oxygenase-1 microsatellite polymorphism are associated with plasma CD14 and viral load in HIV-infected African-Americans. Genes Immun 13:258–267. doi: 10.1038/gene.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, Sasaki H. 2000. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet 66:187–195. doi: 10.1086/302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen YH, Lin SJ, Lin MW, Tsai HL, Kuo SS, Chen JW, Charng MJ, Wu TC, Chen LC, Ding YA, Pan WH, Jou YS, Chau LY. 2002. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Genet 111:1–8. doi: 10.1007/s00439-002-0769-4. [DOI] [PubMed] [Google Scholar]
- 53.Grennan JT, Loutfy MR, Su D, Harrigan PR, Cooper C, Klein M, Machouf N, Montaner JS, Rourke S, Tsoukas C, Hogg B, Raboud J, CANOC Collaboration. 2012. Magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: a recurrent events analysis. J Infect Dis 205:1230–1238. doi: 10.1093/infdis/jis104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Canestri A, Lescure FX, Jaureguiberry S, Moulignier A, Amiel C, Marcelin AG, Peytavin G, Tubiana R, Pialoux G, Katlama C. 2010. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis 50:773–778. doi: 10.1086/650538. [DOI] [PubMed] [Google Scholar]
- 55.Eden A, Fuchs D, Hagberg L, Nilsson S, Spudich S, Svennerholm B, Price RW, Gisslen M. 2010. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis 202:1819–1825. doi: 10.1086/657342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.