ABSTRACT
We have previously shown that the addition of the raltegravir/elvitegavir (RAL/EVG) primary resistance mutation N155H to the R263K dolutegravir (DTG) resistance mutation partially compensated for the fitness cost imposed by R263K while also slightly increasing DTG resistance in vitro (K. Anstett, T. Mesplede, M. Oliveira, V. Cutillas, and M. A. Wainberg, J Virol 89:4681–4684, 2015, doi:10.1128/JVI.03485-14). Since many patients failing RAL/EVG are given DTG as part of rescue therapy, and given that the N155H substitution often is found in combination with other compensatory resistance mutations in such individuals, we investigated the effects of multiple such substitutions within integrase (IN) on each of integrase function, HIV-1 infectivity, and levels of drug resistance. To this end, each of the L74M, E92Q, T97A, E157Q, and G163R substitutions were introduced into NL4.3 subtype B HIV-1 vectors harboring N155H and R263K in tandem [termed NL4.3IN(N155H/R263K)]. Relevant recombinant integrase enzymes also were expressed, and purified and biochemical assays of strand transfer efficiency as well as viral infectivity and drug resistance studies were performed. We found that the addition of T97A, E157Q, or G163R somewhat improved the affinity of INN155H/R263K for its target DNA substrate, while the presence of L74M or E92Q had a negative effect on this process. However, viral infectivity was significantly decreased from that of NL4.3IN(N155H/R263K) after the addition of each tertiary mutation, and no increases in levels of DTG resistance were observed. This work shows that the compensatory mutations that evolve after N155H under continued DTG or RAL/EVG pressure in patients are unable to improve either enzyme efficiency or viral infectivity in an N155H/R263K background.
IMPORTANCE In contrast to other drugs, dolutegravir has not selected for resistance in HIV-positive individuals when used in first-line therapy. We had previously shown that HIV containing the primary raltegravir/elvitegravir resistance substitution N155H could select for R263K under dolutegravir pressure and that this virus was fit and displayed low-level resistance to dolutegravir (Anstett et al., J Virol 89:4681–4684). Therefore, the current study aimed to uncover whether accessory mutations that appear after N155H in response to raltegravir/elvitegravir were compatible with N155H and R263K. We found, however, that the addition of a third mutation negatively impacted both the enzyme and the virus in terms of activity and infectivity without large shifts in integrase inhibitor resistance. Thus, it is unlikely that these substitutions would be selected under dolutegravir pressure. These data support the hypothesis that primary resistance against DTG cannot evolve through RAL/EVG resistance pathways and that the selection of R263K leads HIV into an evolutionary dead-end.
INTRODUCTION
Integrase strand transfer inhibitors (INSTIs) constitute the newest class of drugs approved for the treatment of HIV. They act by inhibiting the HIV enzyme integrase (IN) at the essential step in the viral replication cycle of the insertion of viral DNA into the host cell genome (1). The older drugs of this class, i.e., raltegravir (RAL) and elvitegravir (EVG), can select for primary resistance mutations both in tissue culture and in the clinic, which allows for the virus to escape drug pressure. Furthermore, there is a high degree of cross-resistance between these two drugs (2–4). In contrast, dolutegravir (DTG), a newer INSTI, appears to have a higher genetic barrier to resistance and is active against many but not all RAL- and EVG-resistant viruses, because some of the RAL and EVG resistance mutations also can confer cross-resistance to DTG (5). However, DTG is able to select for an R263K substitution in IN, which yields a 3- to 4-fold level of resistance to this drug at the expense of ≈30% viral replicative capacity (6), and this has been reported both in tissue culture and in treatment-experienced, INSTI-naive patients (7, 8).
In the VIKING trial, INSTI-experienced patients with primary resistance mutations associated with RAL and EVG had lower success rates while on DTG than did patients who received DTG in first-line regimens, and resistance mutations at position 148 in IN were associated with the worst outcomes (9). There was, however, no selection of R263K in this trial, suggesting that RAL/EVG resistance substitutions are incompatible with the R263K DTG resistance pathway. Indeed, we have previously shown that the combination of R263K with the most common primary RAL/EVG resistance substitutions resulted in decreased enzymatic activity of IN as well as overall diminished viral infectivity (10). The only combination that appeared to increase viral infectivity and resistance to DTG compared to that of R263K was the combination of N155H with R263K (10). However, the changes associated with the addition of N155H to R263K were small. Moreover, the N155H substitution in the context of RAL/EVG resistance often is associated with other secondary mutations in INSTI-experienced patients (11). Therefore, we evaluated whether the addition of some of these secondary substitutions at positions L74M, E92Q, T97A, E157Q, and G163R to both N155H and R263K influence IN enzymatic activity as well as viral infectivity and levels of INSTI drug resistance. These secondary substitutions were selected because they are the five most commonly occurring in patients failing INSTI-based therapy with the N155H primary substitution (11).
Although the addition of either T97A, E157Q, or G163R improved the affinity of IN for its DNA substrate, we found that none of the tertiary mutations that were tested could compensate for the decreased strand transfer activity of the double N155H/R263K mutant in biochemical assays. This finding was mirrored in tissue culture infections that assessed the infectivity of subtype B HIV-1 viruses harboring these same substitutions. In regard to resistance to INSTIs, all of the triply mutated viruses were highly resistant to EVG, while resistance to RAL and DTG was mostly unchanged compared to the effect of the N155H/R263K mutational combination.
(This work was largely performed by K. Anstett in partial fulfillment of the requirements for a Ph.D. from McGill University, Montreal, Canada.)
MATERIALS AND METHODS
Experimental design.
The research objectives of this study were to evaluate the strand transfer activity of recombinant integrase proteins expressing different combinations of point mutations that confer resistance to RAL, EVG, and/or DTG. We also wished to assess the viral infectivity of equivalent NL4.3 viruses in the presence or absence of RAL, EVG, and DTG.
Cells and reagents.
TZM-bl and 293T cells were cultured as reported previously (7). Merck & Co., Inc., Gilead Sciences, Inc., and ViiV Healthcare, Ltd., supplied raltegravir, elvitegravir, and dolutegravir, respectively.
Integrase strand transfer activity assay.
Site-directed mutagenesis was performed as described previously to introduce the secondary mutations L74M, E92Q, T97A, E157Q, and G163R into the pET15bN155H/R263K integrase subtype B expression vectors (6). Sense and antisense primer sequences are listed in Table 1. The generation of the construct bearing the N155H/R263K mutations has been described already (10). Recombinant integrase proteins were expressed in BL21(DE3) bacterial cells and purified as previously published (7). Strand transfer assays were performed using preprocessed HIV-1 long terminal repeat (LTR) DNA as reported previously (6). Briefly, DNA Bind 96-well plates (Corning) were coated with LTR DNA and then incubated with purified protein. Afterwards, biotinylated target DNA was added and plates were incubated at 37°C for 1 h to allow the strand transfer reaction to occur. Integrated target DNA was quantified through the use of europium (Eu)-labeled streptavidin, which stably binds to the biotin tag of the target DNA molecules.
TABLE 1.
Primer sequences used to introduce mutations in HIV-1 integrase in both the pNL4.3 and pET15b vectors
| Integrase mutation | 5′ to 3′ primer sequence |
|---|---|
| L74 M | |
| Sense | TGGCTACATGAACTGCTACCATGATAACTTTTCCTTCTAAATG |
| Antisense | CATTTAGAAGGAAAAGTTATCATGGTAGCAGTTCATGTAGCCA |
| E92Q | |
| Sense | GCAGAAGTAATTCCAGCACAGACAGGGCAAGAAA |
| Antisense | TTTCTTGCCCTGTCTGTGCTGGAATTACTTCTGC |
| T97A | |
| Sense | AGCAGAGACAGGGCAAGAAGCAGCATACTTCCTC |
| Antisense | GAGGAAGTATGCTGCTTCTTGCCCTGTCTCTGCT |
| N155H/E157Q | |
| Sense | CCCAAAGTCAAGGAGTAATAGAATCTATGCATAAACAGTTAAAGAAAATTATAGGACAGGTAAGAGA |
| Antisense | TCTCTTACCTGTCCTATAATTTTCTTTAACTGTTTATGCATAGATTCTATTACTCCTTGACTTTGGG |
| N155H/G163R | |
| Sense | GGAGTAATAGAATCTATGCATAAAGAATTAAAGAAAATTATAAGACAGGTAAGAGATCAGGC |
| Antisense | GCCTGATCTCTTACCTGTCTTATAATTTTCTTTAATTCTTTATGCATAGATTCTATTACTCC |
Generation of NL4.3 HIV-1 viral clones.
The generation of the pNL4.3IN(N155H/R263K) plasmid has been reported previously (10). Similar methods were used to generate other pNL4.3IN(mutant) plasmids through site-directed mutagenesis using the primers listed in Table 1. Genetically homogenous viral stocks were produced as described previously (7). Briefly, 293T cells were transfected with the various pNL4.3 plasmids using Lipofectamine 2000. At 48 h after transfection, cell culture supernatants were collected and filtered at 0.45 μm to remove plasmids and cell debris. Viruses then were aliquoted and stored at −80°C. Viral stocks were quantified by measuring cell-free reverse transcriptase (RT) activity in culture fluids.
HIV susceptibility to integrase strand transfer inhibitors.
HIV susceptibilities to DTG, RAL, and EVG were measured by the infection of 30,000 TZM-bl cells using 100,000 RT units per well of each virus in the presence of 1:10 serial dilutions of drugs. After 48 h, cells were lysed and luciferase production was measured using the luciferase assay system (Promega, Madison, WI).
HIV infectivity and replication capacity.
HIV-1 infectivity was measured through the infection of 30,000 TZM-bl cells per well using serial 1:4 dilutions of the various NL4.3 viral clones. Levels of infection were measured as described above.
Statistical analysis.
Each experiment is an average of at least two replicates performed in triplicate (n = 6). Biochemical assays of strand transfer activity were normalized to wild-type (WT) activity at 1,600 nM protein (Fig. 1A) or 128 nM target DNA (Fig. 1B). The relative infectivity index was normalized to the wild-type 50% effective concentration (EC50) (Table 2). Fold change (FC) was normalized to the wild-type IC50 (Table 3). Km, Vmax, infectivity index, IC50, standard errors of the means (SEM), and 95% confidence intervals were calculated using Prism 6.0 software, and all figures were visualized using the same software. Student's t tests were performed using the OpenEpi toolkit, accessible for free online at www.openepi.com.
FIG 1.
Strand transfer activities of purified recombinant integrase proteins in a biochemical assay. Biotinylated target DNA incorporation is measured by streptavidin fluorescence as a function of protein concentration (a) or target DNA concentration (b). (c) Km values for each mutant were obtained from the slope of the curves shown in panel b. An asterisk indicates P ≤ 0.05 by Student's t test. (d) Enzyme proficiency is quantified by dividing the Vmax (taken from the plateau in panel b) by the Km. Error bars display SEM. The E92Q/N155H/R263K enzyme was too inactive to accurately permit calculation of Km and was left out of panels c and d. RFU, relative fluorescence units.
TABLE 2.
Effects of tertiary INSTI resistance mutations on the infectivity of HIV-1 in TZM-bl cells relative to that of the wild type
| Genotype | Infectivity indexa | 95% CIa |
|---|---|---|
| WT | 1.00 | 0.86–1.17 |
| N155H/R263K | 1.60* | 1.35–1.88 |
| L74 M/N155H/R263K | 4.04*† | 3.51–4.66 |
| E92Q/N155H/R263K | 4.99*† | 4.50–5.54 |
| T97A/N155H/R263K | 4.05*† | 3.58–4.57 |
| N155H/E157Q/R263K | 3.75*† | 3.38–4.16 |
| N155H/G163R/R263K | 2.73*† | 2.25–3.32 |
Infectivity index (relative to that of the WT) and 95% confidence intervals (CI) are reported. An asterisk denotes an infectivity index that is statistically different from that of the WT by Student's t test (P ≤ 0.05). A dagger denotes an infectivity index that is statistically different from that of N155H/R263K by Student's t test (P ≤ 0.05).
TABLE 3.
Effects of tertiary INSTI resistance mutations on IC50s in TZM-bl cells for DTG and RAL
| Genotype | Effects fora: |
|||||
|---|---|---|---|---|---|---|
| DTG |
RAL |
|||||
| IC50 (nM) | 95% CI | FC | IC50 (nM) | 95% CI | FC | |
| WT | 0.284 | 0.164–0.494 | 1.00 | 6.797 | 5.580–8.279 | 1.00 |
| N155H/R263K | 4.202* | 1.820–9.700 | 14.78 | 105.7* | 59.76–186.8 | 15.55 |
| L74 M/N155H/R263K | 2.463* | 1.167–5.199 | 8.66 | 136.9* | 66.98–279.9 | 20.14 |
| E92Q/N155H/R263K | 4.922* | 1.051–23.04 | 17.31 | >340* | >50 | |
| T97A/N155H/R263K | 4.889* | 0.997–23.97 | 17.20 | 192.4* | 117.2–315.9 | 28.31 |
| N155H/E157Q/R263K | 4.019* | 0.897–18.02 | 14.14 | 177.7* | 102.4–308.6 | 26.14 |
| N155H/G163R/R263K | 3.770* | 1.431–9.930 | 13.26 | 88.83* | 60.76–129.9 | 13.07 |
95% Confidence intervals (CI) and fold changes (FC) are shown. An asterisk denotes IC50s significantly greater than that of the WT by Student's t test (P ≤ 0.05).
RESULTS
The effect of accessory mutations within INN155H/R263K in biochemical assays.
Figure 1A shows the strand transfer activities of all of the recombinant proteins that were studied as a function of protein concentration. All proteins displayed maximal activity at a concentration of 400 nM, except for E92Q/N155H/R263K, which was maximally active at 200 nM. The N155H/R263K and N155H/G163R/R263K proteins displayed similar activities at both concentrations listed above. The N155H/R263K mutant was less active than the WT at 400 nM, and this decrease was exacerbated by the addition of each of the tertiary mutations, especially L74M, E92Q, and G163R. All proteins lost activity at higher concentrations, and this may be due to the formation of higher-order inactive integrase oligomers.
Figure 1B depicts the strand transfer activities of the recombinant proteins when target DNA concentration was varied. As the substrate of the enzyme increases, so does the activity of the protein until attainment of maximal activity, i.e., Vmax. All mutants in this study exhibited both a lower Vmax and a shallower curve compared to that of the WT; the E92Q/N155H/R263K-containing protein was especially inactive, while the other triple mutants appeared to perform in a similar fashion to each other.
From the curves in Fig. 1B, we derived Vmax and Km values, the latter being a measure of enzyme affinity for substrate. Figure 1C shows the changes in Km upon the addition of each mutation. An increased Km is indicative of a decrease in affinity for the target DNA. The results show that Km was significantly increased (P ≥ 0.05) upon the addition of any mutation compared to the level of the WT. Compared to N155H/R263K, the addition of T97A, E157Q, or G163R each was able to significantly reduce the Km of integrase, whereas the addition of L74M or E92Q was not. In fact, the E92Q/N155H/R263K-containing enzyme was so inactive that an accurate Km could not be determined. When Vmax/Km, which is a measure of enzyme proficiency, was considered, however, we saw less of a difference among the various combinations of mutations. Although all decreases in enzyme efficiency were significant compared to the level of the WT, the results depicted in Fig. 1D show that the addition of each tertiary mutation did not have a significant effect on the efficiency of the N155H/R263K-containing protein, although both the T97A/N155H/R263K and N155H/E157Q/R263K mutant proteins had increased Vmax/Km values. These results suggest that the addition of certain tertiary mutations to integrase containing the N155H/R263K resistance mutations increased the affinity of the enzyme for its substrate but generally were unable to improve enzyme performance.
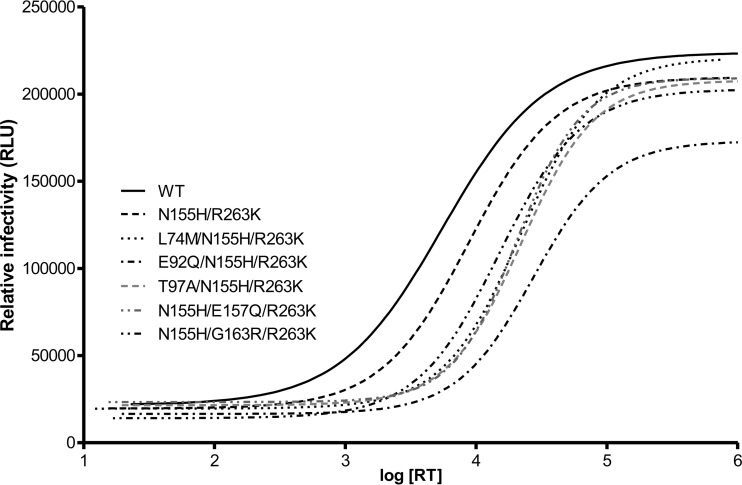
The addition of accessory mutations to NL4.3IN(N155H/R263K) makes HIV-1 less infectious.
Table 2 summarizes the results of experiments assessing the infectivity of NL4.3 viruses harboring the N155H/R263K mutations [termed NL4.3IN(N155H/R263K)], both alone and in combination with any additional tertiary resistance substitution that was studied. These values are derived from the infectivity curves displayed in Fig. 2 for each mutant. Similar to what was seen biochemically, the data show that the addition of each single mutation caused a significant decrease in infectivity (here represented as an increase in the infectivity index relative to the WT) when comparisons were made to either the WT or to the N155H/R263K double mutant. Thus, the decreased strand transfer activities described in Fig. 1 correlate with a general negative effect on the analogous viruses in tissue culture. This trend also can be observed in Fig. 2, which shows changes in luciferase reporter gene activity as a function of increasing concentrations of virus.
FIG 2.
Infectivity in TZM-bl cells of WT, N155H/R263K, and triply mutated NL4.3 viruses. Relative infectivity measured by the luciferase fluorescence of cells 48 h after infection. RLU, relative luciferase units.
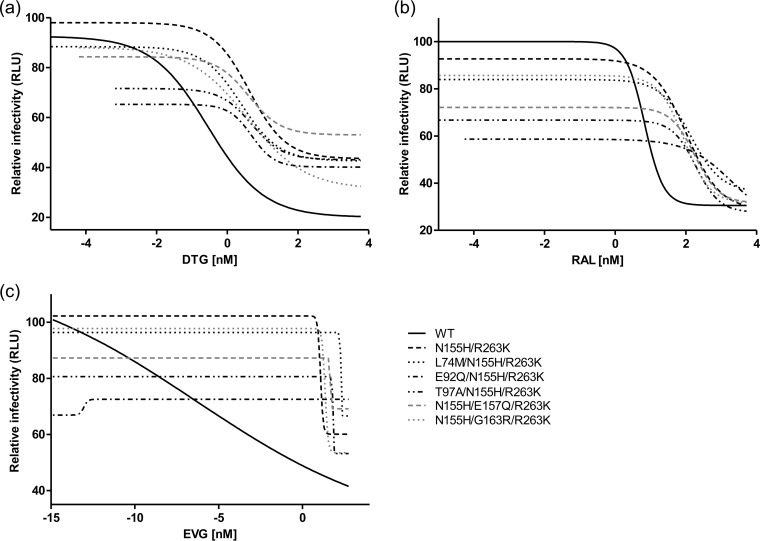
Accessory mutations have small effects on INSTI drug resistance in tissue culture.
Figure 3 displays the results of resistance assays in tissue culture with the various drugs used in this study, i.e., DTG, RAL, and EVG. When the concentration of drug was low, the negative effect of each mutation on viral replication was clearly seen. As drug concentration was increased, all of the mutants attained a growth advantage in the presence of drug and were able to replicate at higher drug levels than the WT virus. The IC50s depicted in Fig. 3A and B are summarized in Table 3. The addition of L74M to N155H/R263K virus increased HIV susceptibility to DTG by ∼2-fold compared to that of NL4.3IN(N155H/R263K). Although resistance to DTG was relatively unchanged upon the addition of the other tertiary mutations, these same viruses displayed increased IC50s for RAL compared to those of either the WT or the N155H/R263K double mutant, with the exception of the virus that contained G163R.
FIG 3.
Infectivity of wild-type, N155H/R263K, and triply mutated NL4.3 HIV-1 in the presence of increasing concentrations of dolutegravir (DTG) (a), raltegravir (RAL) (b), and elvitegravir (EVG) (c). Error bars indicate SEM.
Similar findings are displayed in Fig. 3C, with higher concentrations of EVG leading to a stepwise decline in infectivity of WT virus but not of the triple mutants, except at the highest concentration of EVG that was used. The calculation of IC50s for EVG was not technically possible, because very high levels of resistance against this drug had been reached. Although the WT virus exhibits a nonstandard dose-response curve in Fig. 3C, it is still evident that these mutants all are highly resistant, as their infectivity in increasing concentrations of EVG is unaffected, except at the highest concentration tested. This points to an extremely high level of resistance to this drug in our assay for both the N155H/R263K double mutant and all triply mutated viruses.
DISCUSSION
Although combined antiretroviral therapy has revolutionized treatment for HIV-positive individuals, drug resistance has been observed for every class of drugs currently available (12). DTG is a promising new INSTI that may defy this paradigm but only if it used in first-line therapy. In general, drug resistance is characterized by the initial presence of primary mutations that increase levels of resistance at the expense of viral replication capacity. Secondary mutations that arise after primary mutations generally are compensatory in nature and act to increase drug resistance while also restoring viral fitness (13). However, previous results have shown that the secondary mutations associated with DTG may counterintuitively diminish viral fitness following the selection of a first mutation at position R263K.
Here, we investigated the effects of adding common INSTI secondary mutations to an N155H/R263K background that we had previously characterized as a relatively fit, DTG-resistant virus (10). We now show that the addition of any of five different tertiary substitutions had different effects on the strand transfer activity of IN. In our biochemical analyses, three mutations (T97A, E157Q, and G163R) improved the ability of IN to bind target DNA but did not make a significant overall difference to enzymatic activity. Mirroring this, we saw a statistically significant negative effect on infectivity upon addition of each accessory mutation relative to both WT virus and to viruses containing N155H/R263K.
There are varying and sometimes conflicting reports on how these mutations act to increase fitness and/or drug resistance in response to RAL or EVG. One study showed that the addition of E92Q or G163R to N155H was able to increase viral fitness in both the absence and presence of RAL, whereas the accessory mutation L74M increased replication capacity only in the presence of the drug (14). A different study showed that the E92Q substitution in combination with N155H was deleterious to HIV-1 replication in the absence of INSTI pressure (15). The E157Q substitution has been shown to have variable effects on INSTI activity (16, 17), while a T97A substitution has been reported to cause a 70% decrease in viral replication relative to that of the WT (15).
A recent study documented that viruses that contained the N155H mutation did not select for resistance to DTG via the acquisition of common compensatory substitutions; this finding is in agreement with our results. Interestingly, the selection of E92Q/N155H/R263K in response to EVG was reported in that study (18), and we show here that this virus is highly resistant to this INSTI.
It is generally agreed that the five accessory mutations discussed here contribute to higher levels of resistance to the older INSTIs RAL and EVG than do the primary mutations for these two drugs on their own (14, 15, 19, 20). In this study, we have expanded on these results by showing that each triply mutated virus is just as or more resistant to each INSTI than the N155H/R263K double mutant, with the exception of L74M/N155H/R263K for DTG and N155H/G163R/R263K for RAL. Most important, we have not observed significant changes in levels of resistance to DTG or RAL following the addition of any of these five compensatory mutations to backbone viruses. It appears that the presence of the R263K substitution that confers low-level resistance to DTG prevents the development of further INSTI drug resistance substitutions, and this likely is due to its effects on viral fitness. Investigations are under way in our laboratory to identify the precise mechanism in the context of the three-dimensional structure of IN through which the R263K substitution confers low-level resistance to DTG while preventing the generation of additional substitutions.
E157Q is particularly interesting because it is a naturally occurring polymorphism in HIV-1 subtype B (21), implying that it presents in some patients at treatment initiation. E157Q is important because it confers some resistance to RAL and EVG on its own (20); thus, it may facilitate the selection of further resistance to these drugs. Investigations in our laboratory also are under way to deduce whether this polymorphism has an effect on the emergence of R263K and resistance to DTG. Indeed, a RAL-experienced patient who possessed numerous RAL-related mutations was recently reported to have failed DTG while also possessing E157Q (17).
A recent report from the P1093 DTG dose-ranging study identified a potentially nonadherent, treatment-experienced but INSTI-naive adolescent with low-level viremia and the emergence of the R263K substitution. In this case study, secondary resistance mutations were observed that modestly increased the level of DTG resistance. However, none of these substitutions were able to compensate for the decrease in replicative capacity conferred by R263K, consistent with the data presented here and with previous results (6, 22–24).
DTG is approved for use in both first- and second-line therapy. In initial therapy, DTG is unique in that no case of resistance against it or the nucleoside drugs with which it has been coutilized has yet been reported (25, 26). However, when DTG is used as a second-line drug or in salvage therapy after prior failure on either RAL or EVG, it is far less effective due to cross-resistance against DTG that is conferred by RAL/EVG classical resistance mutations. The absence of resistance to DTG in initial therapy may be due to a relatively low level of resistance that is conferred by R263K coupled with a significant diminution in viral replication ability. We hypothesize that the N155H/R263K DTG-resistant virus is not able to develop additional resistance substitutions or to acquire increased fitness due to constraints on viral mutability and replication that are primarily imposed by the R263K mutation (10). Indeed, the N155H substitution has been associated with DTG failure in treatment-experienced patients (11, 27–29), including two INSTI-naive subjects recently reported in the SAILING trial. Although this is the first documentation of N155H in patients treated with DTG and not RAL, it is noteworthy that these two individuals possessed non-subtype B viruses and showed only very modest decreases in DTG susceptibility (30). Importantly, the combination of N155H and R263K has never been documented in individuals receiving DTG therapy, but previous work has shown that these two mutations may not be incompatible in the context of the virus in tissue culture (10). The current study suggests that this combination of mutations will not yield high-level resistance against DTG.
ACKNOWLEDGMENTS
We thank Maureen Oliveira for technical assistance and Estrella Moyal for help with manuscript preparation.
This research was funded by grants from the Canadian Institutes for Health Research (CIHR). K.A. is the recipient of a studentship from CIHR.
REFERENCES
- 1.Delelis O, Carayon K, Saib A, Deprez E, Mouscadet JF. 2008. Integrase and integration: biochemical activities of HIV-1 integrase. Retrovirology 5:114. doi: 10.1186/1742-4690-5-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanco JL, Varghese V, Rhee SY, Gatell JM, Shafer RW. 2011. HIV-1 integrase inhibitor resistance and its clinical implications. J Infect Dis 203:1204–1214. doi: 10.1093/infdis/jir025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mesplede T, Quashie PK, Wainberg MA. 2012. Resistance to HIV integrase inhibitors. Curr Opin HIV AIDS 7:401–408. doi: 10.1097/COH.0b013e328356db89. [DOI] [PubMed] [Google Scholar]
- 4.Mesplede T, Quashie PK, Zanichelli V, Wainberg MA. 2014. Integrase strand transfer inhibitors in the management of HIV-positive individuals. Ann Med 46:123–129. doi: 10.3109/07853890.2014.883169. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi M, Yoshinaga T, Seki T, Wakasa-Morimoto C, Brown KW, Ferris R, Foster SA, Hazen RJ, Miki S, Suyama-Kagitani A, Kawauchi-Miki S, Taishi T, Kawasuji T, Johns BA, Underwood MR, Garvey EP, Sato A, Fujiwara T. 2011. In vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother 55:813–821. doi: 10.1128/AAC.01209-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mesplede T, Quashie PK, Osman N, Han Y, Singhroy DN, Lie Y, Petropoulos CJ, Huang W, Wainberg MA. 2013. Viral fitness cost prevents HIV-1 from evading dolutegravir drug pressure. Retrovirology 10:22. doi: 10.1186/1742-4690-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quashie PK, Mesplede T, Han YS, Oliveira M, Singhroy DN, Fujiwara T, Underwood MR, Wainberg MA. 2012. Characterization of the R263K mutation in HIV-1 integrase that confers low-level resistance to the second-generation integrase strand transfer inhibitor dolutegravir. J Virol 86:2696–2705. doi: 10.1128/JVI.06591-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cahn P, Pozniak AL, Mingrone H, Shuldyakov A, Brites C, Andrade-Villanueva JF, Richmond G, Buendia CB, Fourie J, Ramgopal M, Hagins D, Felizarta F, Madruga J, Reuter T, Newman T, Small CB, Lombaard J, Grinsztejn B, Dorey D, Underwood M, Griffith S, Min S. 2013. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 382:700–708. doi: 10.1016/S0140-6736(13)61221-0. [DOI] [PubMed] [Google Scholar]
- 9.Eron JJ, Clotet B, Durant J, Katlama C, Kumar P, Lazzarin A, Poizot-Martin I, Richmond G, Soriano V, Ait-Khaled M, Fujiwara T, Huang J, Min S, Vavro C, Yeo J. 2013. Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING study. J Infect Dis 207:740–748. doi: 10.1093/infdis/jis750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anstett K, Mesplede T, Oliveira M, Cutillas V, Wainberg MA. 2015. Dolutegravir resistance mutation R263K cannot coexist in combination with many classical integrase inhibitor resistance substitutions. J Virol 89:4681–4684. doi: 10.1128/JVI.03485-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurt CB, Sebastian J, Hicks CB, Eron JJ. 2014. Resistance to HIV integrase strand transfer inhibitors among clinical specimens in the United States, 2009-2012. Clin Infect Dis 58:423–431. doi: 10.1093/cid/cit697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wainberg MA, Zaharatos GJ, Brenner BG. 2011. Development of antiretroviral drug resistance. N Engl J Med 365:637–646. doi: 10.1056/NEJMra1004180. [DOI] [PubMed] [Google Scholar]
- 13.Shafer RW. 2006. Rationale and uses of a public HIV drug-resistance database. J Infect Dis 194(Suppl 1):S51–S58. doi: 10.1086/505356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu X, Kuritzkes DR. 2010. Effect of raltegravir resistance mutations in HIV-1 integrase on viral fitness. J Acquir Immune Defic Syndr 55:148–155. doi: 10.1097/QAI.0b013e3181e9a87a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abram ME, Hluhanich RM, Goodman DD, Andreatta KN, Margot NA, Ye L, Niedziela-Majka A, Barnes TL, Novikov N, Chen X, Svarovskaia ES, McColl DJ, White KL, Miller MD. 2013. Impact of primary elvitegravir resistance-associated mutations in HIV-1 integrase on drug susceptibility and viral replication fitness. Antimicrob Agents Chemother 57:2654–2663. doi: 10.1128/AAC.02568-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malet I, Soulie C, Tchertanov L, Derache A, Amellal B, Traore O, Simon A, Katlama C, Mouscadet JF, Calvez V, Marcelin AG. 2008. Structural effects of amino acid variations between B and CRF02-AG HIV-1 integrases. J Med Virol 80:754–761. doi: 10.1002/jmv.21169. [DOI] [PubMed] [Google Scholar]
- 17.Danion F, Belissa E, Peytavin G, Thierry E, Lanternier F, Scemla A, Lortholary O, Delelis O, Avettand-Fenoel V, Duvivier C. 2015. Non-virological response to a dolutegravir-containing regimen in a patient harbouring a E157Q-mutated virus in the integrase region. J Antimicrob Chemother 70:1921–1923. [DOI] [PubMed] [Google Scholar]
- 18.Seki T, Suyama-Kagitani A, Kawauchi-Miki S, Miki S, Wakasa-Morimoto C, Akihisa E, Nakahara K, Kobayashi M, Underwood MR, Sato A, Fujiwara T, Yoshinaga T. 2015. Effects of raltegravir or elvitegravir resistance signature mutations on the barrier to dolutegravir resistance in vitro. Antimicrob Agents Chemother 59:2596–2606. doi: 10.1128/AAC.04844-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quashie PK, Mesplede T, Wainberg MA. 2013. HIV drug resistance and the advent of integrase inhibitors. Curr Infect Dis Rep 15:85–100. doi: 10.1007/s11908-012-0305-1. [DOI] [PubMed] [Google Scholar]
- 20.Ghosn J, Mazet AA, Avettand-Fenoel V, Peytavin G, Wirden M, Delfraissy JF, Chaix ML. 2009. Rapid selection and archiving of mutation E157Q in HIV-1 DNA during short-term low-level replication on a raltegravir-containing regimen. J Antimicrob Chemother 64:433–434. doi: 10.1093/jac/dkp182. [DOI] [PubMed] [Google Scholar]
- 21.Lataillade M, Chiarella J, Kozal MJ. 2007. Natural polymorphism of the HIV-1 integrase gene and mutations associated with integrase inhibitor resistance. Antivir Ther 12:563–570. [PubMed] [Google Scholar]
- 22.Mesplede T, Osman N, Wares M, Quashie PK, Hassounah S, Anstett K, Han Y, Singhroy DN, Wainberg MA. 2014. Addition of E138K to R263K in HIV integrase increases resistance to dolutegravir, but fails to restore activity of the HIV integrase enzyme and viral replication capacity. J Antimicrob Chemother doi: 10.1093/jac/dku199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wares M, Mesplede T, Quashie PK, Osman N, Han Y, Wainberg MA. 2014. The M50I polymorphic substitution in association with the R263K mutation in HIV-1 subtype B integrase increases drug resistance but does not restore viral replicative fitness. Retrovirology 11:7. doi: 10.1186/1742-4690-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vavro C, Palumbo P, Wiznia A, Alvero C, Graham B, Fenton T, Hazra R, Townley E, Buchanan A, Horton J, Viani R, P1093 Study Group . 2015. Evolution of HIV-1 integrase following selection of R263K with further dolutegravir treatment: a case report from the P1093 study. Abstr 8th IAS Conf HIV Pathog Treatment Prevent. IAS, Geneva, Switzerland. [Google Scholar]
- 25.Katlama C, Murphy R. 2012. Dolutegravir for the treatment of HIV. Expert Opin Investig Drugs 21:523–530. doi: 10.1517/13543784.2012.661713. [DOI] [PubMed] [Google Scholar]
- 26.Raffi F, Jaeger H, Quiros-Roldan E, Albrecht H, Belonosova E, Gatell JM, Baril JG, Domingo P, Brennan C, Almond S, Min S. 2013. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis 13:927–935. doi: 10.1016/S1473-3099(13)70257-3. [DOI] [PubMed] [Google Scholar]
- 27.Hardy IBB, Quashie P, Thomas R, Petropoulos C, Huang W, Moisi D, Wainberg MA, Roger M. 2014. Evolution of a novel pathway leading to dolutegravir resistance in a patient harbouring N155H and multiclass drug resistance. J Antimicrob Chemother 70:405–411. [DOI] [PubMed] [Google Scholar]
- 28.Carganico ADS, Ehret R, Berg T, Baumgarten A, Obermeier M, Walter H. 2014. New dolutegravir resistance pattern identified in a patient failing antiretroviral therapy. J Int AIDS 17(4 Suppl 3):19749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malet I, Thierry E, Wirden M, Lebourgeois S, Subra F, Katlama C, Deprez E, Calvez V, Marcelin AG, Delelis O. 23 July 2015. Combination of two pathways involved in raltegravir resistance confers dolutegravir resistance. J Antimicrob Chemother doi: 10.1093/jac/dkv197. [DOI] [PubMed] [Google Scholar]
- 30.Underwood M, DeAnda F, Dorey D, Hightower K, Wang R, Griffith S, Horton J. 2015. Resistance post week 48 in ART-experienced, integrase inhibitor-naïve subjects with dolutegravir (DTG) versus raltegravir (RAL) in SAILING (ING111762). Abstr 13th Eur HIV Hepat Workshop Virology Education, Utrecht, The Netherlands. [Google Scholar]