ABSTRACT
Macrophages are target cells of HIV/SIV infection that may play a role in AIDS pathogenesis and contribute to the long-lived reservoir of latently infected cells during antiretroviral therapy (ART). In previous work, we and others have shown that during pathogenic SIV infection of rhesus macaques (RMs), rapid disease progression is associated with high levels of in vivo macrophage infection. In contrast, during nonpathogenic SIV infection of sooty mangabeys (SMs), neither spontaneous nor experimental CD4+ T cell depletion results in substantial levels of in vivo macrophage infection. To test the hypothesis that SM macrophages are intrinsically more resistant to SIV infection than RM macrophages, we undertook an in vitro comparative assessment of monocyte-derived macrophages (MDMs) from both nonhuman primate species. Using the primary isolate SIVM949, which replicates well in lymphocytes from both RMs and SMs, we found that infection of RM macrophages resulted in persistent SIV-RNA production while SIV-RNA levels in SM macrophage cultures decreased 10- to 100-fold over a similar temporal course of in vitro infection. To explore potential mechanisms responsible for the lower levels of SIV replication and/or production in macrophages from SMs we comparatively assessed, in the two studied species, the expression of the SIV coreceptor as well as the expression of a number of host restriction factors. While previous studies showed that SM monocytes express lower levels of CCR5 (but not CD4) than RM monocytes, the level of CCR5 expression in MDMs was similar in the two species. Interestingly, we found that SM macrophages exhibited a significantly greater increase in the expression of tetherin (P = 0.003) and TRIM22 (P = 0.0006) in response to alpha interferon stimulation and increased expression of multiple host restriction factors in response to lipopolysaccharide stimulation and exposure to SIV. Overall, these findings confirm, in an in vitro infection system, that SM macrophages are relatively more resistant to SIV infection compared to RM macrophages, and suggest that a combination of entry and postentry restriction mechanisms may protect these cells from productive SIV infection.
IMPORTANCE This manuscript represents the first in vivo comparative analysis of monocyte-derived macrophages (MDMs) between rhesus macaques, i.e., experimental SIV hosts in which the infection is pathogenic and macrophages can be infected, and sooty mangabeys, i.e., natural SIV hosts in which the infection is nonpathogenic and macrophages are virtually never infected in vivo. This study demonstrates that mangabey-derived MDMs are more resistant to SIV infection in vitro compared to macaque-derived MDMs, and provides a potential explanation for this observation by showing increased expression of specific retrovirus restriction factors in mangabey-derived macrophages. Overall, this study is important as it contributes to our understanding of why SIV infection is nonpathogenic in sooty mangabeys while it is pathogenic in macaques, and is consistent with a pathogenic role for in vivo macrophage infection during pathogenic lentiviral infection.
INTRODUCTION
During pathogenic HIV-1 infection, macrophages comprise a potentially important target cell population that may support virus replication during the natural history of the infection, contribute to the immunopathogenesis of AIDS, and serve as a component of the reservoir of latently infected cells that persist under antiretroviral therapy (ART). Although CD4+ T cells are the major source of virus production in the majority of HIV-1-infected individuals, macrophages may contribute substantially to plasma viral load during late-stage disease when CD4+ T cell levels are very low (1–3). Of note, studies of the in vivo life span of productively infected cells are consistent with the presence of a pool of HIV-1-infected macrophages whose in vivo life span is substantially longer than CD4+ T cells, thus making the infection of these cells a potentially important mechanism for the long-term preservation of a virus reservoir under ART (4, 5).
Pathogenic SIV infection of the experimental, non-natural host rhesus macaque (RM) is associated with high levels of macrophage infection in the context of rapid disease progression (6). In two previous studies, we have shown that massive and widespread infection of tissue macrophages occurs in SIV-infected RMs in which experimental, antibody-induced CD4+ T cell depletion was performed at the time of SIV infection (7, 8). Of note, these SIV-infected, experimentally CD4-depleted RMs with high levels of macrophage infection show higher viral loads and faster disease progression than SIV-infected, undepleted RMs (7, 8). The observations that (i) the life span of productively infected cells is still relatively short in CD4-depleted, SIV-infected RMs when most virus replication occurs in macrophages (8) and (ii) rapid SIV disease progression is associated with faster in vivo turnover of macrophages (9) suggest that, in these instances, continuous rounds of macrophage infection are needed to support the observed high levels of plasma viremia. In stark contrast with the phenotype observed in RMs, nonpathogenic SIV infection of the natural host sooty mangabey (SM) is not associated with robust infection of macrophages even in the setting of CD4+ T cell depletion. Although the majority of SIV-infected SMs do not experience CD4+ T cell depletion despite high levels of virus replication (10–12), rare instances of dramatic CD4+ T cell depletion have been observed in the setting of both natural and experimental SIV infection (13, 14). However, these “CD4-low” SIV-infected SMs show lower levels of virus replication compared to SMs with normal CD4+ T cell counts and display no evidence of excessive in vivo virus replication in blood or tissue-derived macrophages (14). Moreover, we have shown that experimental CD4+ T cell depletion performed during the chronic phase of SIV infection in SMs is followed by a decline in plasma viral load, as opposed to the increase observed in SIV-infected RMs, and again no signs of increased virus replication in macrophages (15). Together, these data suggest that pathogenic SIV infection of RMs permits higher levels of macrophage infection compared to nonpathogenic SIV infection of SMs. Based on these results, we hypothesized that macrophages from SMs are intrinsically more resistant to SIV infection than their cellular counterparts in RMs.
To determine whether SM macrophages are intrinsically more resistant to SIV infection than RM macrophages we conducted an in vitro comparative assessment of monocyte-derived macrophages (MDMs) from both nonhuman primate species. We found that SIV infection of RM macrophages resulted in persistent virus production, whereas SM macrophages exposed to the same SIV inoculum showed a 10- to 100-fold decrease in virus concentration over the same time course. In addition, we found that SM macrophages exhibited a significantly greater increase in the expression of tetherin (P = 0.003) and TRIM22 (P = 0.0006) in response to alpha interferon (IFN-α) stimulation, as well as increased expression of multiple restriction factors in response to lipopolysaccharide (LPS) stimulation or exposure to SIV. Based on these findings, we directly confirmed that SM macrophages are relatively more resistant to SIV infection compared to RM macrophages, and we propose that a combination of entry and postentry restriction mechanisms may protect these cells from productive SIV infection. These data are compatible with the hypothesis that the minimal-to-absent macrophage infection in SIV-infected SM contributes to the typically nonpathogenic outcome observed in these animals.
MATERIALS AND METHODS
Animals.
Twelve healthy SIV-uninfected RMs of Indian origin and twelve healthy SIV-uninfected SMs were included in the present study, and peripheral blood samples were collected by venipuncture according to standard procedures. All animals were anesthetized prior to the performance of any procedure, and proper steps were taken to ensure the welfare and to minimize the suffering of all animals in these studies. The animals were housed at the Yerkes National Primate Research Center of Emory University and maintained in accordance with U.S. National Institutes of Health guidelines. Anesthesia was used for all blood collections.
Generation and stimulation of monocyte-derived macrophages.
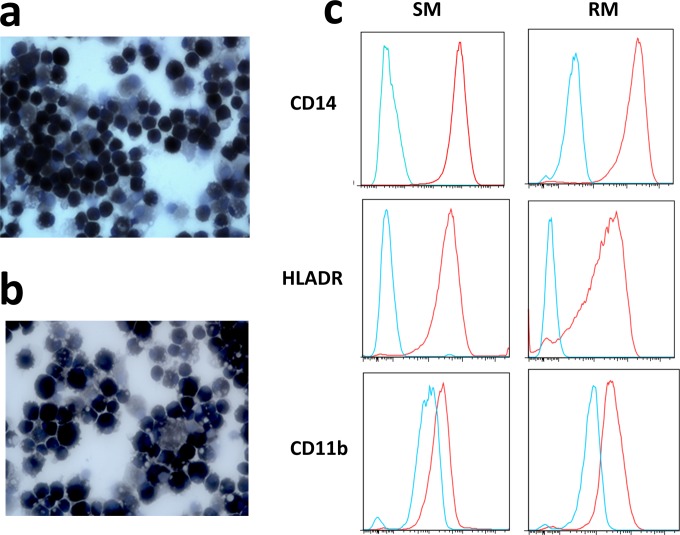
Peripheral blood mononuclear cells (PBMCs) were harvested from whole blood by density gradient centrifugation, and monocytes were isolated by positive selection via anti-CD14 magnetic beads (Miltenyi). A total of 4 × 105 monocytes were plated in 24-well TC-treated plates in 1 ml of complete RPMI (20% fetal bovine serum [FBS], 10% human A/B serum, 2 mM l-glutamine, 10 U of penicillin-streptomycin/ml) and incubated at 37°C overnight. The next day, nonadherent cells were removed by gentle washing, and the medium was replaced with 1 ml of macrophage growth medium (RPMI 1640 1[RPMI], 20% FBS, 2 mM l-glutamine, 10 U of penicillin-streptomycin/ml, 25 U of macrophage colony-stimulating factor [M-CSF]/ml [Cell Signaling Technology]). Adherent cells were cultured at 37°C for 10 to 14 days, with fresh macrophage growth media added every third day. Mature macrophages expressed CD14, HLA-DR, and CD11b, as confirmed by flow cytometry, and displayed diffuse nonspecific esterase activity (shown in Fig. 1). Macrophages were stimulated with 1,000 U of IFN-α/ml, 100 ng of LPS/ml, or medium alone for 24 h.
FIG 1.
Nonspecific esterase activity and surface marker expression in SM and RM macrophages. (a and b) Nonspecific esterase staining in SM (a) and RM (b) monocyte-derived macrophages. (c) CD14, HLA-DR, and CD11b staining (red lines) relative to unstained controls (blue lines) in monocyte-derived macrophages from SM and RM.
Generation of SIV stocks.
SIVM949 stocks were generated by expansion in primary SM PBMCs activated with concanavalin A and interleukin-2 for 3 days. Activated PBMCs were infected by spinoculation with 5,000 pg p27 equivalents for 2 h, followed by 7 days of incubation at 37°C. Culture supernatants were sampled every other day, and the p27 concentration was determined by an enzyme-linked immunosorbent assay (Zeptometrix). Supernatants with p27 > 10,000 pg/ml were collected and stored at −80°C.
In vitro infections.
Macrophages were infected with a 5,000 pg p27 equivalents of SIVM949 by spinoculation for 2 h, followed by 4 h of incubation at 37°C. After three washes with RPMI, a final volume of 1 ml of RPMI plus M-CSF was added, and the cells were cultured for 21 days with intermittent sampling of supernatants in which one-half of the volume of medium was carefully collected without disturbing cells and replaced with fresh RPMI plus M-CSF. The collected volume of supernatant was centrifuged to pellet any contaminating cells prior to measurement of SIV gag.
Measurement of SIV gag RNA in culture supernatants.
Quantitative real-time reverse transcription-PCR (RT-PCR) to measure SIV gag RNA was performed as previously described (16). The sensitivity of the assay is 50 copies/ml of culture supernatant.
Quantitative real-time PCR.
The total RNA was isolated and utilized as a template to generate cDNA by reverse transcription. Real-time PCR was undertaken with gene-specific primers (Table 1) and SYBR green. The quantity of each gene of interest was normalized to the housekeeping gene GAPDH. The fold change of mRNA production was calculated by normalizing stimulated values to unstimulated controls. Statistical significance was determined by Mann-Whitney U test. A total of 0.2 μl of cDNA was used for real-time SYBR green PCR analysis with an ABI 7900 HT instrument (Applied Biosystems). Serial dilutions of pcDNA3.1-smGPR15, CXCR6, CCR5 plasmids (kindly provided by R. Collman) and GAPDH plasmid were used for the quantification of SIV coreceptors. The results were normalized to GAPDH expression.
TABLE 1.
Oligonucleotide primers used for real-time PCR
| Target gene | Primer sequence (5′–3′) |
|
|---|---|---|
| Forward | Reverse | |
| APOBEC3G | ACCTTTGTGGACTGCCA | CACTCAGGGCTTGGCT |
| APOBEC3H | TGGACGAAACGCAGTGCTAC | CAGATGGTCGTGAGCCTTGAT |
| CCR5 | AGGGCTGTGAGGCTTATCTTC | CACCTGCATGGCTTGGTCCA |
| CD14 | TAGACCTCAGCCACAACTCG | CGCTGGACCACATACATCTC |
| CXCR6 | ACCCTGTGCTCTATGCCTTTGTCA | AAGGGAGACAGCCAATGTCCTTCA |
| GAPDH | GAAGGTGAAGGTCGGAGTC | CAAGCTTCCCGTTCTCAGCC |
| GPR15 | ACTGCAGTGTCTTCCTGCTCACTT | AAACCAGATGCTGGCGCAAACTAC |
| MD2 | AAGCTCAGAAGCACTATTGGG | CAACAATCCTCTGGATCCCT |
| MX1 | AGGAGTTGCCCTTCCCAGA | TCGTTCACAAGTTTCTTCAGTTTCA |
| MX2 | CAGAGGCAGCGGAATCGTAA | CTGAAGCTCTAGCTCGGTGT |
| OAS2 | CAGTCCTGGTGAGTTTGCAGT | GCCAGTGCTTTATCAAGAGGAT |
| SAMHD1 | TGCCAGAGAAATTTGCAGAGCAGC | TGGTGAAATTTCTGTCTGCGCACC |
| Tetherin | CTAATGGCTTCCCTGGAT | GTTCAATGTAGTGATCTCCCC |
| TLR4 | AGACTTTATTCCCGGTGTGG | AAAGATACACCAGCGGCTCT |
| TRIM5α | GATGGTTCCTCACATACTCC | CGAAAACTCCAACACGATCAG |
| TRIM22 | CTGTCCTGTGTGTCAGACCAG | TGGGCTCATCTTGACCTCTTT |
RESULTS
Generation of MDMs from RMs and SMs.
To examine the susceptibility of SM and RM macrophages to SIV infection and explore potential mechanisms responsible for interspecies differences in permissiveness to the virus, we first generated monocyte-derived macrophages from both species using a previously described protocol relying on M-CSF as the key differentiation factor (17). To the best of our knowledge, this is the first study using in vitro-generated SM monocyte-derived macrophages (MDMs). As shown in Fig. 1A and B, we found that MDMs generated from SMs and RMs were morphologically similar and displayed comparable diffuse staining for nonspecific esterase activity, a hallmark of mature cells of the monocyte/macrophage lineage (18). In addition, macrophages from both species expressed the canonical macrophage lineage markers CD14, HLA-DR, and CD11b (Fig. 1C). Collectively, these data confirm that MDMs can be generated in vitro from RM PBMCs and indicate, for the first time, that a similar protocol can be successfully used to generate MDMs from SMs.
SM MDMs are relatively resistant to in vitro infection with SIVM949.
Previous in vivo studies by us and others indicated that SIV replication in macrophages is substantially higher in RMs than in SMs (7, 8). To test whether a similar difference is seen when these cells are infected in vitro, we undertook a comparative assessment of SIV infection of SM and RM MDMs using the primary isolate SIVM949, a virus strain that was isolated from a naturally infected SM as described previously (4). Importantly, SIVM949 infects PBMCs from both SM and RM with similar efficiency and kinetics and has been shown to infect RM lymphocytes and MDMs (17), thus making this virus a useful tool to compare the relative permissiveness of MDMs isolated from these two species to SIV infection. As expected based on previous studies (17), SIVM949 infection of RM macrophages was characterized by an increase in the levels of SIV-gag RNA of between 5- and 100-fold by day 4 from the initial inoculation that was maintained over the course of infection (Fig. 2A). In contrast, in vitro SIVM949 infection of SM macrophages resulted in very different SIV-gag RNA kinetics. As shown in Fig. 2B, the concentration of SIV-gag RNA in the culture supernatants of SM macrophages exposed to SIVM949 declined progressively over the course of infection, and by day 14 the fold change in virus levels compared to day 0 was between 0.1 and 0.01, thus representing a 10- to 100-fold decrease in SIV-gag RNA concentration. Of note, in most experiments we observed no increase in SIV-gag RNA levels in the supernatants throughout the course of in vitro SM macrophage infection, therefore suggesting an almost complete absence of productive virus replication in these cells. In these experiments, the SIV-gag RNA detected in the supernatants represents likely a remnant of the initial inoculum, which was steadily degraded and/or diluted out as the macrophage culture was sampled sequentially over the course of the experiment. Collectively, these results demonstrate that SM macrophages are substantially less susceptible to in vitro SIV infection compared to RM macrophages.
FIG 2.
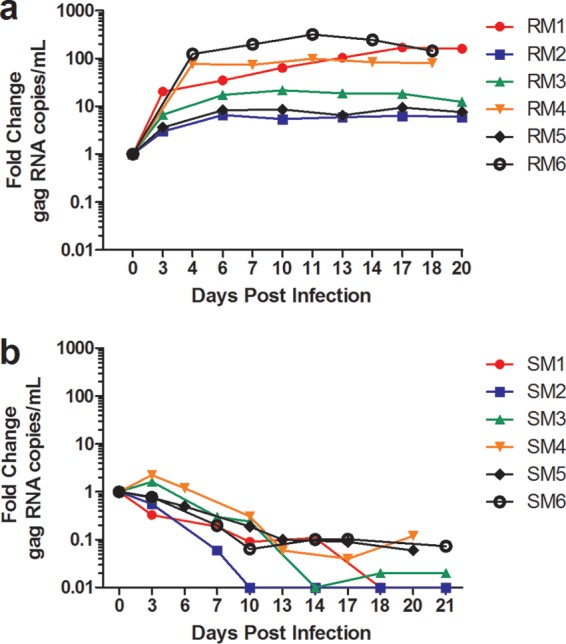
SIVM949 infection of RM and SM macrophages. (a and b) Fold change in SIV gag RNA levels in the supernatant of monocyte-derived macrophages from RMs (a) and SMs (b) after in vitro infection with SIVM949. SIV gag RNA level at day 0 represents the amount of virus in the initial inoculation.
CCR5 expression in SM monocytes and macrophages.
The lower levels of macrophage infection that we have observed both in vivo and in vitro in SMs compared to RMs could be explained, at least in part, by a reduced virus entry due to decreased surface expression of CD4 and CCR5. In previous studies we have shown that monocytes derived from SMs express lower surface levels of CCR5, but not CD4, compared to human or RM monocytes (19; unpublished results). Here, we examined the expression of SIV coreceptors, including CCR5, CXCR6, and GPR15, by RT-PCR quantification of the relevant mRNA in MDMs from RMs and SMs. As shown in Fig. 3, the levels of CCR5 expression, as well as alternative coreceptors CXCR6 and GPR15, in macrophages were in fact similar between RMs and SMs. Although this observation is not per se consistent with the fact that SM monocytes show lower surface levels of CCR5 by flow cytometric analysis, it is possible that the cytokine-induced in vitro differentiation of monocytes to macrophages results in increased CCR5 mRNA expression in both species. Indeed, it has been documented that differentiation of human monocytes to macrophages is accompanied by increased expression of CCR5 (20). Overall, these data suggest that the lower in vivo and in vitro levels of SIV infection in SM macrophages are unlikely to be explained solely by lower expression levels of virus coreceptors such as CCR5.
FIG 3.
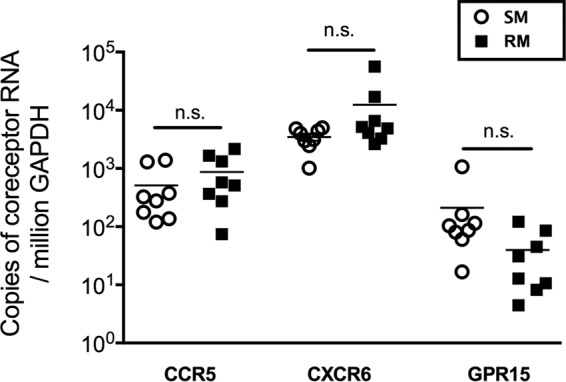
CCR5, CXCR6, and GPR15 expression in SM and RM macrophages. The relative CCR5, CXCR6, and GPR15 expression in SM and RM monocyte-derived macrophages was measured via real-time PCR and normalized to the housekeeping gene GAPDH.
Higher expression of host restriction factors in IFN-α stimulated macrophages from SMs.
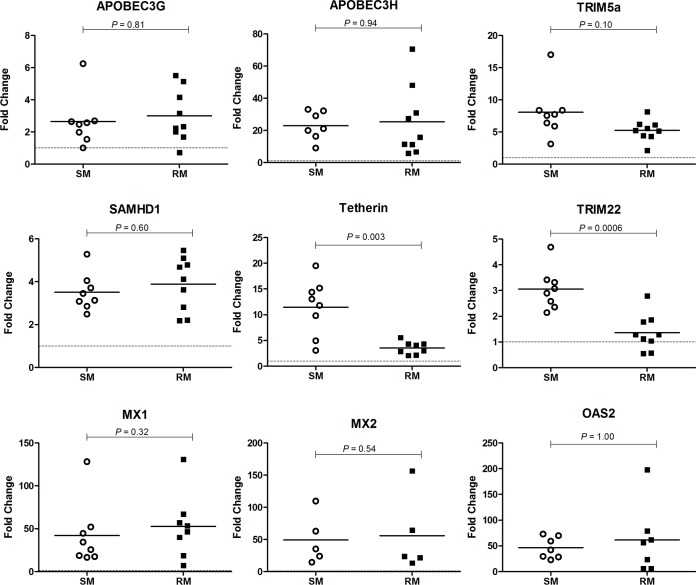
To further investigate potential mechanisms responsible for the relative resistance of SM macrophages to SIV infection, we next investigated the expression of a number of host restriction factors in MDMs from SMs and RMs. Since the expression of restriction factors is typically induced as part of the type-I IFN-mediated innate antiviral host response, we first utilized the prototypical type-IFN, i.e., IFN-α, to investigate the in vitro expression of these genes in macrophages from the two studied species. In these experiments, macrophages from SMs and RMs were stimulated with IFN-α for 24 h, and the mRNA levels for several well-characterized host restriction factors were measured by real-time PCR. The choice of a 24-h time point was based on a preliminary set of experiments showing that (i) the kinetics of restriction factor expression at 2, 4, and 24 h after IFN-a stimulation are comparable in MDMs of SMs and RMs and that (ii) the fold change of expression was highest at 24 h poststimulation (data not shown). Of note, the expression of all genes of interest was normalized to the housekeeping gene GAPDH, and the fold change after IFN-α stimulation was calculated relative to sample-matched, mock-stimulated cells. The relative expression levels of all measured restriction factors were similar in unstimulated MDMs from SMs and RMs, and no differences were observed in the levels of GAPDH expression after these in vitro stimulations (data not shown). As a positive control, we first measured in both SM and RM macrophages the expression of the classical IFN response genes (ISGs) MX1 and OAS2 after IFN-α stimulation (Fig. 4). As expected, both MX1 and OAS2 transcripts were strongly induced by IFN-α, with an average fold change near 50 in both species. This comparable high induction of MX1 and OAS2 in RM and SM macrophages indicates that there is no difference in the intrinsic ability of these cells to sense and respond to IFN-α in this in vitro experimental system. We next measured the expression of known retroviral host restriction factors, and we observed a comparable induction of gene expression in response to IFN-α stimulation between SM and RM macrophages for APOBEC3G, APOBEC3H, TRIM5α, and SAMHD1 (Fig. 4). Of note, the effect of this stimulation on gene expression varied significantly among the measured restriction factors, with an average fold change in gene expression for these restriction factors ranging from 2.5 (APOBEC3G) to 21 (APOBEC3H) and no detectable difference between SM and RM macrophages. In contrast, the induction of tetherin and TRIM22 was significantly greater in SM macrophages compared to RM macrophages (P = 0.003 and 0.0006, respectively). We found that after IFN-α stimulation, tetherin mRNA levels in SM macrophages were, on average, 12-fold higher than controls (range, 3- to 19-fold), whereas RM macrophages displayed comparatively little tetherin induction, with an average increase of 3-fold versus controls (Fig. 4). In the case of TRIM22, we observed an average 3-fold increase in SM macrophages compared to the 1.5-fold increase in RM macrophages. Interestingly, in some RM macrophages, the fold change of TRIM22 was ≤1, indicating that there was no increase in the transcription of this restriction factor in response to IFN-α. Taken together, these data suggest a potential role for the IFN-α-induced restriction factors tetherin and TRIM22 in protecting SM macrophages from SIV infection.
FIG 4.
IFN-α-induced restriction factors in SM and RM macrophages. MDMs from SMs and RMs were stimulated with IFN-α, and the fold change in mRNA levels was quantified via real-time PCR relative to unstimulated controls for APOBEC3G, APOBEC3H, TRIM5α, SAMHD1, tetherin, TRIM22, MX1, MX2, and OAS2. All genes were normalized to the housekeeping gene GAPDH.
Higher expression of host restriction factors in LPS stimulated macrophages from SMs.
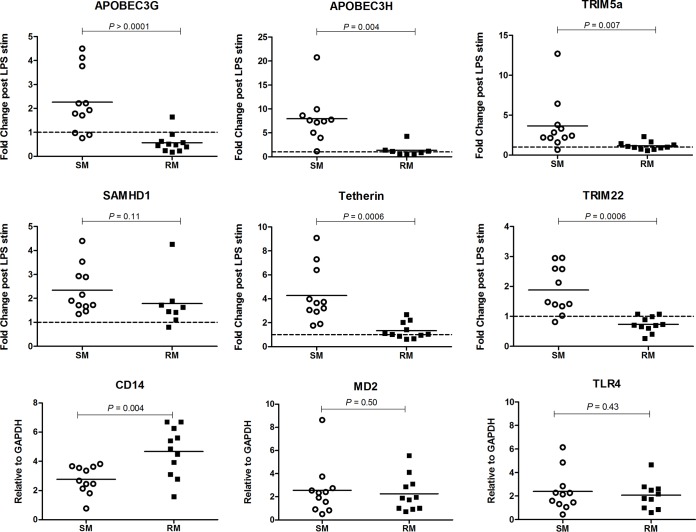
To further probe how macrophages from SMs and RMs may differentially modulate the expression of antiviral host restriction factors, we stimulated the cells with the Gram-negative bacterial antigen LPS. After 24 h of LPS or mock stimulation, total RNA was harvested and gene specific mRNA levels were quantified by real-time PCR as described above. Similar to what we described above for the IFN-α stimulation experiments, we observed a range of responses to LPS among the measured restriction factors, although the magnitude of changes elicited by LPS were on the whole lower than those stimulated by IFN-α. Interestingly, LPS stimulation resulted in significantly higher fold changes in the transcription of APOBEC3G (P > 0.0001), APOBEC3H (P = 0.004), TRIM5α (P = 0.007), tetherin (P = 0.0006), and TRIM22 (P = 0.0006) in SM macrophages compared to RM macrophages (Fig. 5). The average fold change of each restriction factor assessed in RM macrophages was at or very close to 1, indicating little change in the gene expression after 24 h of LPS stimulation. These minimal responses observed in RM macrophages were likely not due to distinct kinetics of the LPS response, as we did not detect any induction of the mRNA levels for these restriction factors at 4 or 6 h after LPS stimulation (data not shown). Moreover, RM macrophages displayed comparable or increased expression of the LPS receptors CD14, TLR4, and MD2 relative to SM macrophages (Fig. 5), suggesting that the lack of LPS-mediated restriction factor induction in RM macrophages was unlikely due to an intrinsic species-specific defect in LPS sensing. Collectively, these data indicate that LPS stimulation induces the gene expression of several retroviral restriction factors in macrophages derived from SMs but not from RMs.
FIG 5.
LPS-induced restriction factors in SM and RM macrophages. MDMs from SMs and RMs were stimulated with LPS, and the fold change in mRNA levels was quantified via real-time PCR relative to unstimulated controls for APOBEC3G, APOBEC3H, TRIM5α, SAMHD1, tetherin, and TRIM22. The relative expression of the LPS receptors CD14, MD2, and TLR4 was assessed in unstimulated MDMs from SMs and RMs. All genes were normalized to the housekeeping gene GAPDH.
Higher expression of host restriction factors in macrophages from SMs after exposure to SIV.
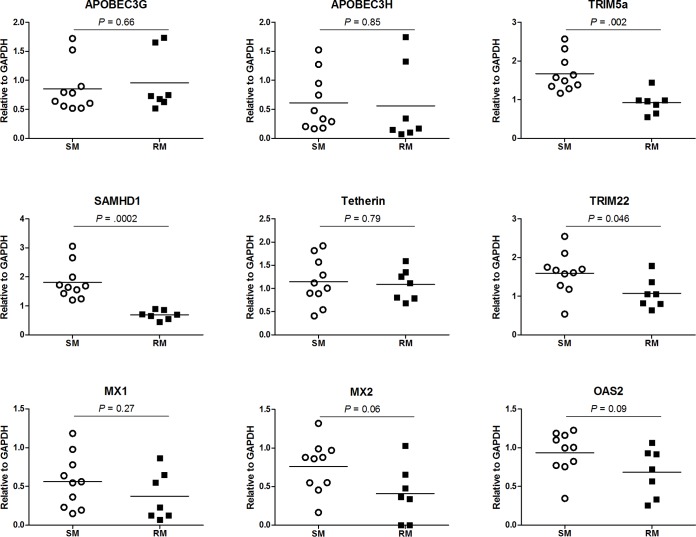
To further investigate potential determinants of the different levels of virus replication after in vitro SIV infection of SM and RM macrophages, we measured the expression of multiple restriction factors and classical ISGs at day 21 after the initial exposure to SIVM949. We observed no difference in the relative expression of MX1, MX2, or OAS2 in SM versus RM macrophages (Fig. 6), which is consistent with our findings in IFN-stimulated macrophages (Fig. 4). In addition, we detected no difference in the relative expression of APOBEC3G, APOBEC3H, or tetherin in SM and RM macrophages. Similar to the pattern of induction we measured following IFN stimulation, we found that the relative expression of TRIM22 was significantly higher in SM macrophages after SIV infection (P = 0.046). SM macrophages also displayed significantly higher expression levels of SAMHD1 (P = 0.0002) and TRIM5α (P = 0.002) after exposure to SIV. These data support the hypothesis that SM and RM macrophages exhibit a different pattern of restriction factor expression upon various in vitro stimuli.
FIG 6.
Relative expression of restriction factors in SM and RM macrophages after SIV infection. The expression of restriction factors and classical ISGs MX1, MX2, and OAS2 were measured relative to the housekeeping gene GAPDH at day 21 after in vitro infection with SIVM949 in MDMs of SMs and RMs. In this part of the study, we examined a total of five RM-derived MDMs and six SM-derived MDMs that were exposed to different doses of virus (i.e., ranging between 400 and 3,200 pg p27 equivalents) for a total of 7 and 10 data points for RM and SM, respectively.
DISCUSSION
The role of macrophages in the pathogenesis of primate lentiviral infections, including HIV infection of humans and SIV infection of macaques, remains incompletely understood. In particular, there is still significant controversy regarding the extent to which direct virus infection of macrophages, which clearly occurs in many in vitro experimental systems, is (i) a determinant of the in vivo pathogenesis of these infections, and (ii) a contributor to the reservoirs of latently infected cells that persist after active virus replication is fully suppressed by antiretroviral therapy (ART). In previous studies, we and others have shown that, in SIV or SHIV-infected rhesus macaques (RMs), virus-induced or experimental depletion of CD4+ T cells is associated with massive virus replication in tissue macrophages (6–8). In contrast, the nonpathogenic SIV infection of the natural host sooty mangabeys (SMs) is not associated with detectable levels of virus replication in macrophages even in the setting of severe CD4+ T cell depletion, either naturally occurring or experimentally induced (13–15). Overall, these comparative studies of RMs and SMs are compatible with the hypothesis that SIV infection of macrophages is a correlate of disease progression during primate lentiviral infections.
Based on the above-described studies, we hypothesized that SM macrophages are intrinsically less susceptible to SIV infection than RM macrophages. To directly test this hypothesis in vitro, monocyte-derived macrophages were infected with SIVM949, a strain of SIVsmm that replicates equally well in PBMCs isolated from both RMs and SMs (17). These experiments showed that while RM macrophages were productively and stably infected in vitro, SM macrophages were relatively resistant to SIVM949 infection. In an attempt to define mechanisms responsible for this resistance, we measured the expression of several well-characterized host restriction factors that block retroviral replication in RM and SM macrophages upon two different in vitro stimuli, i.e., IFN-α and LPS, and found that SM macrophages express significantly higher levels of specific restriction factors compared to RMs. In particular, the expression of higher levels of both tetherin and TRIM22 in response to IFN-α suggests that, in SMs, macrophages are more resistant to SIV replication due, at least in part, to postentry mechanisms that limit the production and release of infectious virions. In this context, the contribution of entry mechanisms cannot be ruled out, although its significance remains unclear given the fact that lower CCR5 expression was observed in SM monocytes but not in SM monocyte-derived macrophages (18). In addition, we found that the relative expression of TRIM22, SAMHD1, and TRIM5α was significantly higher in SM macrophages after exposure to SIV. Although we detected no difference in tetherin expression between SM and RM macrophages on day 21 after SIV infection, it is possible that by then the expression of tetherin had subsided substantially. Indeed, Rahmberg et al. found that the in vivo peak of tetherin expression in SIV-infected RMs coincided with the peak of IFN production, which occurred at 10 days postchallenge (21). Moreover, we cannot rule out a role for differences in SAMHD1 phosphorylation, which has been shown to regulate its antiviral activity (22), nor can we exclude a potential contribution of additional factors known to restrict lentiviral infection in myeloid cells, including p21, CypA, and multiple micro RNAs (23). In addition, while the SM and RM monocyte-derived macrophages displayed comparable morphology and expression of classical macrophage markers, we cannot exclude potential species-specific differences in M1 and M2 polarization of the cells.
During in vivo SIV infection of SMs, type I IFN production and upregulation of ISGs typically occurs during the acute phase of infection, while the chronic phase is associated with lower levels of both parameters. The fact that SM macrophages express more tetherin and TRIM22 in response to IFN-α is consistent with the possibility that this mechanism of virus restriction occurs predominantly during the acute infection, thus avoiding the rapid infection of a large number of macrophages at a time in which adaptive antiviral immune responses are still largely absent. On the other hand, it is also possible that such mechanisms play a role even during chronic infection if focal and/or transient bursts of type I IFN production are still present in SIV-infected SMs in specific organs and tissues.
The observation that RM macrophages are more susceptible to SIV infection both in vivo and in vitro compared to macrophages of natural host SMs is clearly compatible with the hypothesis that virus production and/or replication in macrophages is a contributor to AIDS pathogenesis. HIV/SIV infection of macrophages may result in depletion and functional dysfunction of these cells, thus contributing to the virus-induced immunodeficiency. In addition, virus-infected macrophages can be important mediators of the HIV/SIV-associated chronic immune activation, which is well-established marker of disease progression. Furthermore, macrophage infection may be an important component of the neuropathogenesis of AIDS, and indeed massive infection of both perivascular macrophages and microglial cells is observed in CD4-depleted SIV-infected RMs in which most in vivo virus replication is supported by nonlymphoid cells (8). In the setting of ART-mediated suppression of virus replication, latently and/or chronically infected macrophages may contribute to the persistent virus reservoir that represents the main obstacle to “cure” HIV infection via conventional antiviral approaches. Ongoing experiments in which SIV-infected RMs, both CD4 depleted and undepleted, as well as SIV-infected SMs are treated with long-term ART and then undergo a structured treatment interruption will help determine whether the presence of higher levels of in vivo macrophage infection are associated with a more stable virus reservoir and/or to a more rapid rebound of viremia upon ART interruption.
In conclusion, this set of data strongly supports the hypothesis that a differential susceptibility of macrophages from RMs and SMs to SIV infection is a contributor to the strikingly different outcomes of SIV infection in these two species. Together with the previous observations that SIV infection of SMs is associated with lower immune activation during the chronic phase of infection and lower levels of virus replication in CD4+ central-memory T cells and memory stem cells (24, 25), these results define a further mechanistic aspect of the AIDS resistance of SMs and provide rationale for additional investigation of the role of macrophages in the pathogenesis of HIV infection in humans.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grants R37 AI66998 to G.S., RR000165/OD011132 to the Yerkes National Primate Research Center, and P30 AI050409 to the Emory Center for AIDS Research (Virology Core).
We thank the animal care and veterinary staff at the Yerkes National Primate Research Center.
REFERENCES
- 1.Hockett RD, Kilby JM, Derdeyn CA, Saag MS, Sillers M, Squires K, Chiz S, Nowak MA, Shaw GM, Bucy RP. 1999. Constant mean viral copy number per infected cell in tissues regardless of high, low, or undetectable plasma HIV RNA. J Exp Med 189:1545–1554. doi: 10.1084/jem.189.10.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Igarashi T, Imamichi H, Brown CR, Hirsch VM, Martin MA. 2003. The emergence and characterization of macrophage-tropic SIV/HIV chimeric viruses (SHIVs) present in CD4+ T cell-depleted rhesus monkeys. J Leukoc Biol 74:772–780. doi: 10.1189/jlb.0503196. [DOI] [PubMed] [Google Scholar]
- 3.Pantaleo G, Graziosi C, Butini L, Pizzo PA, Schnittman SM, Kotler DP, Fauci AS. 1991. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci U S A 88:9838–9842. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho DD, Rota TR, Hirsch MS. 1986. Infection of monocyte/macrophages by human T lymphotropic virus type III. J Clin Invest 77:1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholson JK, Cross GD, Callaway CS, McDougal JS. 1986. In vitro infection of human monocytes with human T lymphotropic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV). J Immunol 137:323–329. [PubMed] [Google Scholar]
- 6.Brown CR, Czapiga M, Kabat J, Dang Q, Ourmanov I, Nishimura Y, Martin MA, Hirsch VM. 2007. Unique pathology in simian immunodeficiency virus-infected rapid progressor macaques is consistent with a pathogenesis distinct from that of classical AIDS. J Virol 81:5594–5606. doi: 10.1128/JVI.00202-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortiz AM, Klatt NR, Li B, Yi Y, Tabb B, Hao XP, Sternberg L, Lawson B, Carnathan PM, Cramer EM, Engram JC, Little DM, Ryzhova E, Gonzalez-Scarano F, Paiardini M, Ansari AA, Ratcliffe S, Else JG, Brenchley JM, Collman RG, Estes JD, Derdeyn CA, Silvestri G. 2011. Depletion of CD4 T cells abrogates post-peak decline of viremia in SIV-infected rhesus macaques. J Clin Invest 121:4433–4445. doi: 10.1172/JCI46023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Micci L, Alvarez X, Iriele RI, Ortiz AM, Ryan ES, McGary CS, Deleage C, McAtee BB, He T, Apetrei C, Easley K, Pahwa S, Collman RG, Derdeyn CA, Davenport MP, Estes JD, Silvestri G, Lackner AA, Paiardini M. 2014. CD4 depletion in SIV-infected macaques results in macrophage and microglia infection with rapid turnover of infected cells. PLoS Pathog 10:e1004467. doi: 10.1371/journal.ppat.1004467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasegawa A, Liu H, Ling B, Borda JT, Alvarez X, Sugimoto C, Vinet-Oliphant H, Kim WK, Williams KC, Ribeiro RM, Lackner AA, Veazey RS, Kuroda MJ. 2009. The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood 114:2917–2925. doi: 10.1182/blood-2009-02-204263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silvestri G, Sodora DL, Koup RA, Paiardini M, O'Neil SP, McClure HM, Staprans SI, Feinberg MB. 2003. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18:441–452. doi: 10.1016/S1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 11.Sumpter B, Dunham R, Gordon S, Engram J, Hennessy M, Kinter A, Paiardini M, Cervasi B, Klatt N, McClure H, Milush JM, Staprans S, Sodora DL, Silvestri G. 2007. Correlates of preserved CD4+ T cell homeostasis during natural, nonpathogenic simian immunodeficiency virus infection of sooty mangabeys: implications for AIDS pathogenesis. J Immunol 178:1680–1691. doi: 10.4049/jimmunol.178.3.1680. [DOI] [PubMed] [Google Scholar]
- 12.Taaffe J, Chahroudi A, Engram J, Sumpter B, Meeker T, Ratcliffe S, Paiardini M, Else J, Silvestri G. 2010. A five-year longitudinal analysis of sooty mangabeys naturally infected with simian immunodeficiency virus reveals a slow but progressive decline in CD4+ T-cell count whose magnitude is not predicted by viral load or immune activation. J Virol 84:5476–5484. doi: 10.1128/JVI.00039-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milush JM, Mir KD, Sundaravaradan V, Gordon SN, Engram J, Cano CA, Reeves JD, Anton E, O'Neill E, Butler E, Hancock K, Cole KS, Brenchley JM, Else JG, Silvestri G, Sodora DL. 2011. Lack of clinical AIDS in SIV-infected sooty mangabeys with significant CD4+ T cell loss is associated with double-negative T cells. J Clin Invest 121:1–9. doi: 10.1172/JCI46024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milush JM, Reeves JD, Gordon SN, Zhou D, Muthukumar A, Kosub DA, Chacko E, Giavedoni LD, Ibegbu CC, Cole KS, Miamidian JL, Paiardini M, Barry AP, Staprans SI, Silvestri G, Sodora DL. 2007. Virally induced CD4+ T cell depletion is not sufficient to induce AIDS in a natural host. J Immunol 179:3047–3056. doi: 10.4049/jimmunol.179.5.3047. [DOI] [PubMed] [Google Scholar]
- 15.Klatt NR, Villinger F, Bostik P, Gordon SN, Pereira L, Engram JC, Mayne A, Dunham RM, Lawson B, Ratcliffe SJ, Sodora DL, Else J, Reimann K, Staprans SI, Haase AT, Estes JD, Silvestri G, Ansari AA. 2008. Availability of activated CD4+ T cells dictates the level of viremia in naturally SIV-infected sooty mangabeys. J Clin Invest 118:2039–2049. doi: 10.1172/JCI33814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaur A, Alexander L, Staprans SI, Denekamp L, Hale CL, McClure HM, Feinberg MB, Desrosiers RC, Johnson RP. 2001. Emergence of cytotoxic T lymphocyte escape mutations in nonpathogenic simian immunodeficiency virus infection. Eur J Immunol 31:3207–3217. doi:. [DOI] [PubMed] [Google Scholar]
- 17.Gautam R, Carter AC, Katz N, Butler IF, Barnes M, Hasegawa A, Ratterree M, Silvestri G, Marx PA, Hirsch VM, Pandrea I, Apetrei C. 2007. In vitro characterization of primary SIVsmm isolates belonging to different lineages: in vitro growth on rhesus macaque cells is not predictive for in vivo replication in rhesus macaques. Virology 362:257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monahan RA, Dvorak HF, Dvorak AM. 1981. Ultrastructural localization of nonspecific esterase activity in guinea pig and human monocytes, macrophages, and lymphocytes. Blood 58:1089–1099. [PubMed] [Google Scholar]
- 19.Pandrea I, Apetrei C, Gordon S, Barbercheck J, Dufour J, Bohm R, Sumpter B, Roques P, Marx PA, Hirsch VM, Kaur A, Lackner AA, Veazey RS, Silvestri G. 2007. Paucity of CD4+ CCR5+ T cells is a typical feature of natural SIV hosts. Blood 109:1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufmann A, Salentin R, Gemsa D, Sprenger H. 2001. Increase of CCR1 and CCR5 expression and enhanced functional response to MIP-1 alpha during differentiation of human monocytes to macrophages. J Leukoc Biol 69:248–252. [PubMed] [Google Scholar]
- 21.Rahmberg AR, Neidermyer WJ, Breed MW, Alvarez X, Midkiff CC, Piatak M, Lifson JD, Evans DT. 2013. Tetherin upregulation in simian immunodeficiency virus-infected macaques. J Virol 87:13917–13921. doi: 10.1128/JVI.01757-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White TE, Brandariz-Nuñez A, Valle-Casuso JC, Amie S, Nguyen LA, Kim B, Tuzova M, Diaz-Griffero F. 2013. The retroviral restriction ability of SAMHD1, but not its deoxynucleotide triphosphohydrolase activity, is regulated by phosphorylation. Cell Host Microbe 13:441–451. doi: 10.1016/j.chom.2013.1003.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cobos Jiménez V, Booiman T, de Taeye SW, van Dort KA, Rits MAN, Hamann J, Kootstra NA. 2012. Differential expression of HIV-1 interfering factors in monocyte-derived macrophages stimulated with polarizing cytokines or interferons. Sci Rep 2:763. doi: 10.1038/srep00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cartwright EK, McGary CS, Cervasi B, Micci L, Lawson B, Elliott ST, Collman RG, Bosinger SE, Paiardini M, Vanderford TH, Chahroudi A, Silvestri G. 2014. Divergent CD4+ T memory stem cell dynamics in pathogenic and nonpathogenic simian immunodeficiency virus infections. J Immunol 192:4666–4673. doi: 10.4049/jimmunol.1303193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chahroudi A, Bosinger SE, Vanderford TH, Paiardini M, Silvestri G. 2012. Natural SIV hosts: showing AIDS the door. Science 335:1188–1193. doi: 10.1126/science.1217550. [DOI] [PMC free article] [PubMed] [Google Scholar]