ABSTRACT
Human T-cell leukemia virus type 1 (HTLV-1)-infected CD4+ T cells and dendritic cells (DCs) are present in peripheral blood from HTLV-1 carriers. While T-cell infection requires cell-cell contact, DCs might be infected with cell-free virus, at least in vitro. However, a thorough comparison of the susceptibilities of the two cell types to HTLV-1 infection using cell-associated and cell-free viral sources has not been performed. We first determined that human primary monocyte-derived dendritic cells (MDDCs) were more susceptible to HTLV-1 infection than their autologous lymphocyte counterparts after contact with chronically infected cells. Next, a comparison of infection efficiency using nonconcentrated or concentrated supernatants from infected cells as well as purified viral biofilm was performed. Integrated provirus was found after exposure of MDDCs or primary lymphocytes to viral biofilm but not to a viral supernatant. Using a large series of primary cell samples (n = 21), we demonstrated a higher proviral load in MDDCs exposed to viral biofilm than in lymphocytes. This higher susceptibility is correlated to a higher expression of neuropilin-1 on MDDCs than on autologous activated T lymphocytes. Moreover, we show that MDDCs infected with viral biofilm can transmit the virus to lymphocytes. In conclusion, MDDCs are more susceptible to HTLV-1 infection than autologous lymphocytes in vitro, supporting a model in which DC infection might represent an important step during primo-infection in vivo.
IMPORTANCE HTLV-1 is able to infect several cell types, but viral DNA is mainly found in T lymphocytes in vivo. This supports a model in which T lymphocytes are the main target of infection. However, during the primo-infection of new individuals, incoming viruses might first encounter dendritic cells (DCs), the specialized immune cells responsible for the antiviral response of the host. HTLV-1 cell-free purified viruses can infect dendritic cells in vitro, while T-cell infection is restricted to cell-to-cell transmission. In order to understand the sequence of HTLV-1 dissemination, we undertook a direct comparison of the susceptibilities of the two cell types using cell-associated and cell-free viral sources. We report here that MDDCs are more susceptible to HTLV-1 infection than autologous lymphocytes in vitro and are able to efficiently transmit the virus to lymphocytes. Our results suggest that DCs may represent a true viral reservoir, as the first cell type to be infected in vivo.
INTRODUCTION
Human T-cell leukemia virus type 1 (HTLV-1) infects 5 to 20 million people worldwide (1) and is the etiological agent of both an aggressive CD4+ T-cell leukemia named adult T-cell leukemia (ATL) (2) and a neurological disease named tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM) (3, 4). HTLV-1 is transmitted through contact with infected cells present in maternal milk, semen, or blood and is mainly found in CD4+ T cells, but other cell types might also be infected or functionally altered in vivo and thus involved in disease progression. As an example, monocytes obtained from ATL patients poorly differentiate into monocyte-derived dendritic cells (MDDCs) in vitro, have a reduced ability to present antigen, and have altered capacities to stimulate proliferation of allogeneic T lymphocytes (5). In contrast, monocytes isolated from TSP/HAM patients and differentiated in vitro into MDDCs have increased capabilities to stimulate proliferation of autologous CD4+ and CD8+ T lymphocytes (6), although their differentiation into MDDCs is also altered, with a lower expression of CD83, CD86, and CD1a (6, 7). Whether these defects are linked to their infection in vivo is not clear, since monocyte infection was not reported for ATL patients, and differentiation defects of MDDCs from TSP/HAM patients are not linked to their infection (7). In addition, monocytes seem to be refractory to HTLV-1 infection in vitro (8). This defect is not due to the absence of HTLV-1 receptor expression, since the two molecules involved in HTLV-1 binding and entry (neuropilin-1 [NRP-1] and hGlut-1, respectively) are present at the cell surface in both myeloid and activated T cells (9). Rather, the block seems to take place at a postentry step and leads to apoptosis of exposed monocytes (8). HTLV-1 was shown by other groups to replicate in vitro in MDDCs (10, 11) as well as in myeloid DCs purified from peripheral blood (10). Altogether, these results raise the question of the differential susceptibilities of DCs and CD4+ T cells to HTLV-1 infection. Whether DCs play a role in establishing a chronic infection in vivo is also not clear.
Furthermore, the nature of the viral entity accountable for infecting primary target cells, i.e., cell-free or cell-associated virus, is still a matter of debate. Since free viral particles are not detected in patient fluids (12), cell-free viruses should not represent the main route of viral infection in vivo. Consistently, studies have shown that T cells are poorly infected by cell-free purified viruses (13). In contrast, T-cell infection was observed when cells were cocultured with irradiated HTLV-1-infected T-cell lines (14) or when chronically infected irradiated T cells were used to infect animals (11, 15). Cell-to-cell transmission is thought to result from at least two nonexclusive mechanisms, i.e., formation of a virological synapse (16) and transfer of membrane-bound viruses that are embedded in biofilm-like structures at the surface of infected cells (17). According to this well-accepted model of viral transmission, during infection of new individuals and viral delivery by maternal milk or through sexual intercourse, infected cells from the donor must cross the epithelial barrier constituting the mucosa of the digestive or sexual tract before being able to transmit the virus to new targets. However, since T cells are not abundant in the mucosa, they may not be the first cells encountered by HTLV-1. In contrast, several DC subsets are located in all mucosa, in specific areas such as Peyer's patches or just beneath epithelial monolayers (18). Furthermore, recent data demonstrated that cell-free virus, but not infected cells, can cross the epithelial barrier by transcytosis without cell disruption (19). Epithelial cells are not infected but release viruses at the basal pole into the mucosa milieu, where mucosal DCs might be infected.
In this study, we used primary MDDCs as well as autologous lymphocytes derived from a cohort of 21 blood donors to determine the respective susceptibility of either cell type to HTLV-1 infection. Chronically infected cell lines, viral biofilm, or a viral supernatant was used as a source of virus. Infection efficiency was measured through the quantification of proviral DNA, viral DNA integration, viral protein expression, and viral transmission to reporter cells. We demonstrate that MDDCs are significantly more susceptible to HTLV-1 infection than their autologous primary T-cell counterparts, independently of the viral source. We also show that viral biofilm is a source of infectious virus that can truly infect and integrate in both primary T cells and MDDCs. MDDCs are productively infected with viral biofilm and transmit the virus to reporter T cells. Finally, we observed that NRP-1 expression is low in primary T cells compared to that in MDDCs, while Glut-1 levels are similar in both cell types. Thus, these results correlate with the level of HTLV-1 infection and suggest that NRP-1 expression levels could determine the fate of HTLV-1 infection in primary cells. Taken together, all of these findings suggest that productive infection of DCs is an important step for HTLV-1 transmission to T lymphocytes in vivo.
MATERIALS AND METHODS
Cells.
Jurkat, Jurkat-LTR-Luc (17), and HTLV-1-infected T-cell lines (MT-2 or C91PL) and activated primary blood lymphocytes (PBLs; referred to throughout as lymphocytes) were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS; Gibco Life Technologies) and penicillin-streptomycin (100 μg/ml; Gibco Life Technologies). Primary lymphocytes were stimulated for 3 days with phytohemagglutinin (PHA; 1 μg/ml; Sigma) and interleukin-2 (IL-2; 150 U/ml, Miltenyi Biotech). Monocyte-derived dendritic cells (MDDCs) were cultured in RPMI 1640 medium supplemented with 10% FCS (Gibco Life Technologies) and 100 μg/ml of penicillin-streptomycin (Gibco Life Technologies) supplemented with nonessential amino acids (2.5 mM; Gibco Life Technologies), sodium pyruvate (1 mM; Sigma), β-mercaptoethanol (0.05 mM; Gibco Life Technologies), and HEPES (10 mM; Gibco Life Technologies) in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF; 100 ng/ml; Eurobio) and interleukin-4 (100 ng/ml; Abcys). All cells were grown at 37°C in 5% CO2. Jurkat-LTR-Luc cells, stably transfected with a plasmid encoding luciferase under the control of the HTLV-1 long terminal repeat (LTR) promoter, were maintained under hygromycin (450 μg/ml, Sigma) selection. Jurkat-LTR-GFP cells were maintained under G418 (750 μg/ml; Gibco Life Technologies) selection.
Generation of Jurkat-LTR-GFP cells.
Jurkat cells were microporated with a plasmid encoding the green fluorescence protein (GFP) under the control of the LTR promoter. This plasmid was obtained by substituting the cytomegalovirus (CMV) promoter from pEgfp-N1 (Clontech) by the HTLV-1 LTR promoter amplified from molecular clone HT1M (a kind gift of D. Derse) with sense (5′-GCATTAATTGACAATGACCATGAGCCCC) and antisense (5′-GCCAAGCTTCTAGAGATCTGTAG) primers. The LTR promoter was cloned into pEgfp-N1 using HindIII/AseI restriction sites. Microporated cells were selected by G418 (750 μg/ml). Then single-cell dilution was performed to obtain a clone.
Primary human dendritic cells and lymphocytes.
Blood from healthy donors was collected by Etablissement Français du Sang (Lyon, France). Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood on Ficoll density gradients. Monocytes were purified from PBMCs on Percoll gradients. Monocytes were collected at the top of the density gradient, while PBLs were pelleted. Cells were cryopreserved in 10% dimethyl sulfoxide (DMSO)–50% FCS–40% culture medium before use. Primary cells were grown as described above. In order to obtain MDDCs, monocytes were resuspended in complete MDDC medium at a concentration of 1 × 106/ml and cultured for 7 days in MDDC medium supplemented with GM-CSF and IL-4. Fresh cytokines were added 72 h later. Twenty hours before infection, 1 × 105 MDDCs or lymphocytes were plated in 100 μl of complete medium in 96-well flat-bottom or round-bottom plates, respectively. Cells were incubated with viral preparations (biofilm or C91PL cell supernatant before or after sucrose cushion concentration; see below). Three hours after contact with virus, MDDCs were incubated with trypsin for 10 min at 37°C. Cells were then washed twice in complete medium before culture at 37°C and 5% CO2 for 3 days.
Viral preparations: biofilm, Sup, and [virus].
Viral preparations were obtained from C91PL cells. Supernatant (referred to as Sup) or concentrated virus (referred to as [virus]) was obtained from cells (1 × 106 cells/ml) that had been grown for 24 h. Supernatant were clarified by centrifugation (5 min at 800 × g) and filtered through a 0.45-μm-diameter pore filter (Millipore, MA) to eliminate cell debris. Supernatant (100 μl) was immediately used for MDDC or lymphocyte infection or purified by ultracentrifugation through a 20% (wt/vol) sucrose cushion at 100,000 × g in an SW28 rotor (Beckman) for 1 h 30 min at 4°C. Viral pellets were resuspended in RPMI 1640 medium without cytokines to obtain a 100-fold-concentrated viral suspension for 2 h at 4°C under gentle agitation. Five microliters of [virus] was immediately used for MDDC or lymphocyte infection.
Biofilm preparations were performed as previously described (17), with minor modifications. Briefly, C91PL cells were plated (3 × 105 cells/ml) and cultured for 4 days. HTLV-1-infected cells were washed 2 times in RPMI 1640 serum-free medium and incubated at 1 × 106 cells/ml for 1 h at 37°C. Every 10 min, cells were gently vortexed. Then FCS (10% final) and penicillin-streptomycin (final concentration, 100 μg/ml) were added. Cells were centrifuged and biofilm preparation was collected. HEPES (10 mM), nonessential amino acids (2.5 mM), sodium pyruvate (1 mM), β-mercaptoethanol (0.05 mM), and cytokines (GM-CSF and IL-4) were added, and 100 μl of biofilm was immediately used for MDDC infection. For lymphocyte infection, IL-2 and PHA were added into biofilm preparations before use. Where indicated below, MDDCs were infected with viral biofilms in the presence of zidovudine (AZT; 10 μg/ml; Sigma).
p19gag quantification in viral preparations.
All viral preparations (biofilm, supernatant, or [virus]) were kept at −80°C. The amount of p19gag was assessed using the Retrotek HTLV-1/2 p19 antigen enzyme-linked immunosorbent assay (ELISA) kit (Zeptometrix Corporation) by following the manufacturer's instructions as previously described (20).
Cell-to-cell infection.
Jurkat-LTR-GFP or Jurkat-LTR-Luc cells were cocultured (ratio, 1:1) with HTLV-1-infected T-cell lines (MT-2 or C91PL). Prior to coculture, C91PL or MT-2 cells were irradiated (77 Gy) from a 137Cs source (CIS BIO International; IBL 637) at 1.28 Gy/min. At each time point, cells were collected and reporter activities were assayed using the luciferase reporter assay system (Promega) for Jurkat-LTR-Luc cells or using flow cytometry with a FACSCalibur (BD Sciences) for Jurkat-LTR-GFP cells.
To determine the ability of infected MDDCs to transfer the virus, Jurkat-LTR-Luc cells were added in the culture (ratio, 1:1) 3 days after MDDC exposure to HTLV-1. Coculture lasted for 72 h. Then, cells were collected and lysed and luciferase activity was assayed. Where indicated below, this activity was normalized using the amount of p19gag used to infect Jurkat-LTR-Luc or primary cells.
Isolation of genomic DNA.
MDDCs or lymphocytes were collected 3 days postinfection and washed twice in phosphate-buffered saline (PBS). Genomic DNA was extracted from cell pellets using the Nucleospin blood kit (Macherey-Nagel, Düren, Germany) according to the manufacturer's instructions. DNA concentration was determined with a NanoDrop ND-1000 spectrophotometer (Thermo Scientific).
Real-time PCR.
Real-time quantitative PCR (qPCR) was performed on 10 ng of genomic DNA using the FastStart Universal SYBR green master (Roche, Mannheim, Germany) on a StepOnePlus system (Applied Biosystems, CA). The following primers were used: Tax-F (5′-GTTGTATGAGTGATTGGCGGGGTAA) and Tax-R (5′-TGTTTGGAGACTGTGTA CAAGGCG). Amplification of human β-actin (Act-F [5′-TGAGCTGCGTGTGGCTCC] and Act-R [5′-GGCATGGGGGAGGGCATACC]) was used for normalization. For ALU-PCR (15), the first round was carried out using an ALU-specific primer (ALU-F [5′-CCTCCCAAAGTGCTGGGATTACA]) and a gag-specific primer (Gag-R [5′-GGCTTGGGTTTGGATGAGTA]). The amplification product was then diluted in water (1:50) and used as the template for a second PCR using the Gag-F (5′-CCCTCCAGTTACGATTTCCA) and Gag-R primers. As a positive control, gag amplification was carried out on genomic DNA obtained from C91PL cells. PCR was performed using the Phusion enzyme (Finnzyme, Espoo, Finland) in a Piko thermocycler (Finnzyme).
HTLV-1 receptor expression.
To monitor neuropilin-1 expression, cells were first incubated with NRP-1 antibody (clone 12C2; Biolegend). They then were washed and fixed with 4% paraformaldhehyde (PFA). To detect Glut-1 expression, cells were first fixed with 4% PFA, washed, and then incubated with hGlut-1 antibody (clone 202915; R&D Systems) in PBS containing 0.1% saponin and 1% bovine serum albumin (BSA). Then cells were washed twice with PBS–1% BSA–0.1% saponin and once with PBS–1% BSA. Glut-1 expression was also monitored using an H2-RBD-GFP protein (5 μl/1 × 105 cells; Metafora) that was added in complete culture medium for 30 min. Cells were then washed in PBS and fixed in 4% PFA. Flow cytometry analysis was then performed (FACSCanto II; BD Biosciences).
p19gag detection.
MDDCs were labeled with a V450-coupled anti-CD11c antibody (clone B-Ly6; BD Biosciences). Then lymphocytes or CD11c-stained MDDCs were fixed in 4% PFA, permeabilized with PBS–1% BSA–0.1% saponin, and stained with a mouse anti-p19gag antibody (clone TP7; 1:1,000; Zeptometrix) followed by an allophycocyanin-coupled goat anti-mouse antibody (Southern Biotech; 1:1,000). Cells were then washed twice with PBS–1% BSA–0.1% saponin and once with PBS–1% BSA before flow cytometry analysis (FACSCanto II; BD Biosciences).
RESULTS
C91PL cells produce more infectious viral particles than MT-2 cells.
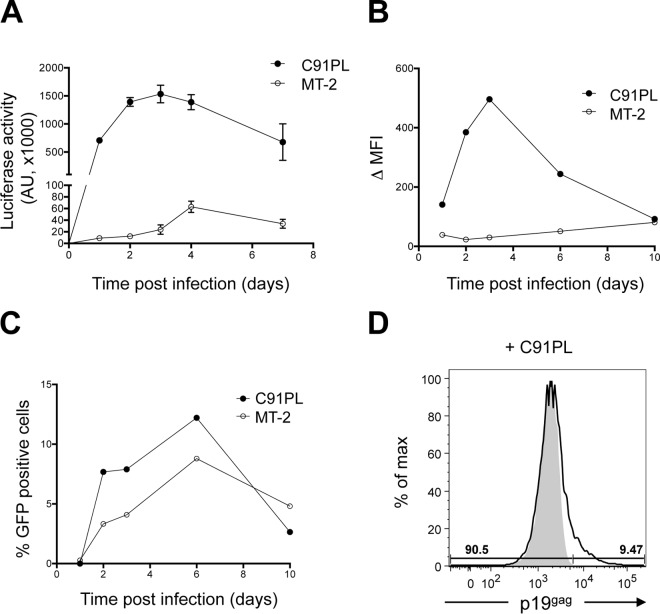
Several HTLV-1 chronically infected T-cell lines are available for in vitro studies and have been used to infect T lymphocytes (17, 20, 21). However, DC infection was performed using MT-2 cells only (10, 22), while HTLV-1 transcytosis experiments were done using C91PL cells only (19). In order to determine which HTLV-1 cell line produces infectious viral particles, we first compared the abilities of viruses produced by the MT-2 and C91PL cell lines to infect reporter T cells. Jurkat cells stably transfected with a plasmid expressing the luciferase gene under the control of the HTLV-1 LTR promoter (referred to here as Jurkat-LTR-Luc cells [17]) were cocultured with irradiated C91PL or MT-2 cells, and luciferase activity was measured over time (Fig. 1A). We have previously shown that passive Tax protein transfer from donor to target cells does not occur in this setting (20). Thus, luciferase activity represents a readout of productive infection. Although the amounts of cell-free virus present in C91PL and MT-2 cell supernatants were similar (data not shown), infection with C91PL cells was 30 to 50 times more efficient than with MT-2 (Fig. 1A). To better evaluate the number of infected cells, we also used Jurkat cells stably transfected with an HTLV-1 LTR-GFP construct (referred to here as Jurkat-LTR-GFP cells). When cocultured with C91PL cells, Jurkat-LTR-GFP cells also displayed a higher GFP mean fluorescence intensity (MFI) (Fig. 1B) and a higher proportion of GFP-positive cells (Fig. 1C) than when cocultured with MT-2 cells, indicating a higher infection rate. A high rate of infection of reporter cells upon coculture with C91PL cells was confirmed by flow cytometry, in which p19gag expression was detected in 9.47% of the Jurkat-LTR-Luc cell population cocultured with C91PL cells (Fig. 1D). This result is similar to the percentage of GFP-positive cells observed in Fig. 1C. Altogether, these results indicate that although MT-2 cells produce the same amount of cell-free viral particles as C91PL cells (data not shown), these virions are less infectious than those of C91PL. C91PL cells were therefore used as a source of virus for subsequent experiments.
FIG 1.
C91PL cells produce more infectious viral particles than MT-2 cells. (A) Jurkat-LTR-Luc cell infection after coculture with C91PL or MT-2 cells. The graph shows the means and the standard deviations from 3 independent experiments. (B) Time course infection of Jurkat-LTR-GFP cell infection after coculture with C91PL or MT-2 cells. Fluorescence intensity was measured by flow cytometry, and the difference in mean fluorescence intensity (Δ MFI) measured in uninfected and infected Jurkat-LTR-GFP cells was plotted over time. (C) Time course detection of GFP-positive cells after coculture with C91PL or MT-2 cells. (D) p19gag signal was measured in Jurkat-LTR-Luc cells after 3 days of coculture with C91PL cells. Jurkat-LTR-Luc cells were differentiated from C91PL cells based on their size. p19gag expression (solid line) was assessed in the Jurkat-LTR-Luc cell gated population. Noninfected Jurkat-LTR-Luc cells were used as a p19gag expression negative control (gray).
Monocyte-derived DCs are more susceptible to HTLV-1 infection than autologous T cells.
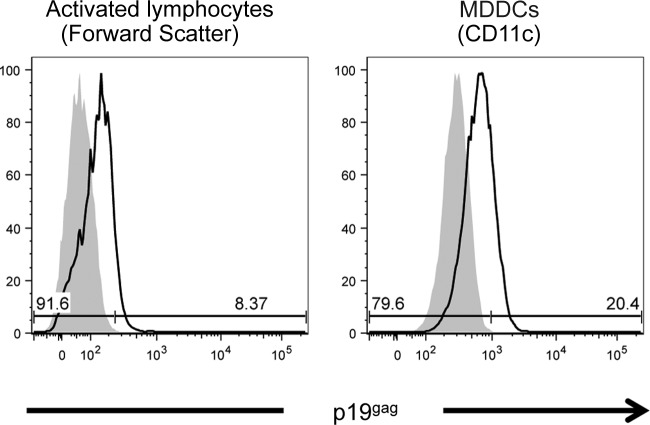
Autologous monocytes and lymphocytes were purified from healthy blood donors. Monocytes were cultured for 7 days in the presence of IL-4 and GM-CSF to generate immature dendritic cells (MDDCs). Monocyte differentiation was controlled by measuring DC-SIGN, CD11c, and NRP-1 upregulation concomitant with the loss of CD14 expression (data not shown). Lymphocytes were activated by IL-2 and PHA, and their activation was controlled by measuring CD69 upregulation (data not shown). The same number of primary MDDCs or activated lymphocytes was then cocultured with irradiated C91PL cells for 3 days, and infection was measured by flow cytometry (Fig. 2). Primary activated lymphocytes were discriminated from irradiated C91PL cells based on their smaller size and lower granularity, while MDDCs were gated on CD11c expression. p19gag expression was detected in 8% of primary lymphocytes (Fig. 2, left). In contrast, more than 20% MDDCs were p19gag positive (Fig. 2, right), suggesting that primary MDDCs are more susceptible to HTLV-1 infection than autologous activated lymphocytes.
FIG 2.
MDDCs are more susceptible than autologous activated primary lymphocytes to infection through cell-cell contact. Infection of MDDCs or activated lymphocytes was assessed by flow cytometry using p19gag antibody followed by anti-mouse-allophycocyanin staining after 3 days of coculture with C91PL cells. As a control, p19gag staining was also performed in the absence of coculture (gray). Lymphocyte infection was determined after gating on the lymphocyte population discriminated from the C91PL population based on its smaller size and lower granularity (left). Infection of MDDCs was determined after gating on CD11c expression (right).
Viral biofilm is the most infectious source of cell-free virus.
In order to better quantify the susceptibility to infection of MDDCs compared to that of activated lymphocytes, we then aimed at measuring the amount of viral DNA in infected cells using real-time quantitative PCR (qPCR). However, coculture with HTLV-1-infected cells leads to a complex cell population in which intimate cell-cell contacts preclude removal of donor cells (13, 23). Thus, we used either cell-free virus collected from C91PL culture supernatant (named Sup), culture supernatant concentrated on a sucrose cushion (named [virus]), or viral biofilm (Fig. 3A). The amount of virus present in each viral preparation was quantified (data not shown) and used to normalize luciferase results obtained after exposure of Jurkat-LTR-Luc cells to each source of virus (Fig. 3B). Interestingly, although it was less efficient than coculture with infected cells, viral biofilm was significantly more infectious than all other cell-free viral sources (compare Fig. 3B and C).
FIG 3.
Virus embedded in biofilm is more potent than cell-free virus for infection of T-lymphocytes. (A) Schematic representation of the experimental procedure. (B) Jurkat-LTR-Luc cells were exposed to biofilm, concentrated virus ([virus]), or supernatant (Sup) produced by C91PL cells. Luciferase activity was measured 3 days postexposure. Results were normalized according to the amount of p19gag present in each viral preparation. The median values from 15 independent experiments are indicated. Asterisks indicate statistically significant differences calculated using a paired t test: *, P < 0.1; ns, not significant. (C) Luciferase activity measured 24 h after coculture of Jurkat-LTR-luc cells with C91PL. The median values from 11 independent experiments are indicated. (D) Jurkat-LTR-Luc cells were exposed to freshly prepared biofilm or to a 3-day-old biofilm. Luciferase activity was measured after 3 days. The median values from 10 independent experiments are indicated. Asterisks indicate statistically significant differences calculated using a paired t test: ***, P < 0.001.
To ensure that preparations of viral biofilm were not contaminated with infected cells, biofilm samples were left for 3 days at 37°C in complete culture growth medium before being added to Jurkat-LTR-Luc reporter cells. Luciferase assays were then performed. Freshly prepared biofilm contained infectious virus, while biofilm incubated for 3 days in culture medium lost its ability to infect reporter T cells (Fig. 3D). This demonstrates that biofilm does not contain HTLV-1-infected cells. This was also controlled by visual observation under the microscope (data not shown). Altogether, these results demonstrate that purified biofilm is more efficient than other cell-free viral preparations for infecting reporter T cells.
Monocyte-derived DCs are more susceptible than autologous T cells to HTLV-1 biofilm infection.
Primary MDDCs and IL-2/PHA-activated T cells obtained from a series of blood donors (n = 21) were exposed to viral biofilm, Sup, or [virus]. The presence of viral DNA was monitored by qPCR 3 days after exposure (Fig. 4), and qPCR data were normalized to the amount of p19gag in the input. Consistent with results presented above, the amount of viral DNA was significantly higher when cells were exposed to biofilm than to other sources of cell-free virus (Fig. 4A and B). The amounts of viral DNA did not differ significantly when primary activated lymphocytes (Fig. 4A) and MDDCs (Fig. 4B) were exposed to [virus] or to Sup. Interestingly, MDDCs were significantly more susceptible to HTLV-1 infection with viral biofilm than their autologous activated lymphocyte counterparts (Fig. 4C, left). MDDC susceptibility was not dependent upon biofilm preparation, or in vitro MDDC generation, since repeated infections of different MDDC batches obtained from a given donor (Fig. 5, donors 12, 24, and 30) led to similar results. In contrast, exposure to [virus] or Sup led to a very limited MDDC or activated T-cell infection (Fig. 4C, middle and right).
FIG 4.
MDDCs are more susceptible to HTLV-1 infection with biofilm than activated primary lymphocytes. Primary activated lymphocytes (A) and MDDCs (B) were exposed to biofilm, concentrated virus ([virus]), or supernatant (Sup). Genomic DNA was extracted 3 days postinfection and analyzed by real-time PCR. The percentage of positive cells was normalized according to the amount of HTLV-1 p19gag present in each viral preparation. (C) The same results were plotted for each viral preparation. For panels A to C, the median values from 25 independent experiments obtained with 21 different blood donors are indicated. Asterisks indicate statistically significant differences calculated using a paired t test: ***, P < 0.001; ****, P < 0.0001; ns, not significant. (D) ALU-PCR was carried out on genomic DNA extracted from biofilm-exposed cells (lanes 1 and 4), concentrated virus-exposed cells (lanes 2 and 5), and viral supernatant-exposed cells (lanes 3 and 6). The length of the amplified gag sequence is shown on the right (arrow). DNA extracted from C91PL cells was used as a positive control (lane 8). Negative control, no DNA (lane 7). MW, molecular weight. (E) Results of the ALU-PCR analyses performed on genomic DNA extracted from cells from 5 different blood donors (donors 7, 10, 12, 23, and 30).
FIG 5.
HTLV-1 infection of MDDCs and activated primary autologous lymphocytes. Primary activated lymphocytes (A) or autologous MDDCs (B) obtained from 21 different blood donors were incubated with biofilm (circles), concentrated supernatants ([virus]) (squares) or supernatant (Sup) (diamonds) for 3 days. Genomic DNA was extracted and real-time PCR performed. Data were normalized according to the p19gag amount present in each viral preparation used to infect the target cells. Results were plotted for each blood donor. Lines indicate experimental duplicates using donors 12 and 30 or a triplicate using donor 24.
Detection of viral DNA by qPCR does not formally demonstrate the presence of integrated provirus. We therefore used a previously described ALU-PCR protocol (15) that allows the amplification of HTLV-1 provirus integrated in genomic DNA using nested PCR. Interestingly, a gag amplification signal was detected in samples from lymphocytes or MDDCs exposed to biofilm, but not when cells were incubated with other cell-free virus preparations (Fig. 4D, compare lane 1 to lanes 2 and 3 and compare lane 4 to lanes 5 and 6). As a positive control, DNA from C91PL cells was used (Fig. 4D, lane 8). The experiment was repeated using primary cells from five blood donors for which the amount of viral DNA detected by qPCR was the highest (donors 7, 12, 10, 23, and 30) (Fig. 5A and B). Integrated proviruses were detected in 5/5 MDDC DNA samples that had been exposed to biofilm (Fig. 4E) but in only 2/5 DNA samples obtained from activated lymphocytes from donors 7 and 12, for which the amount of viral DNA detected by qPCR was the highest (Fig. 5A). Viral integration was rarely detected (1 out of 20 samples) when cells were exposed to [virus] or Sup (Fig. 4E). Altogether, these results confirm that MDDCs are more susceptible to HTLV-1 infection than autologous activated lymphocytes. Interestingly, the higher susceptibility of MDDCs over lymphocytes was observed with both cell-associated (Fig. 2) and cell-free (Fig. 4) sources of virus.
Monocyte-derived DCs infected with viral biofilm can transfer HTLV-1 to lymphocytes.
We wanted to quantify the ability of MDDCs exposed to biofilm or [virus] to transmit HTLV-1 to lymphocytes. To this end, MDDCs were exposed to biofilm or to [virus], treated with trypsin to remove particles that could be bound to the cell membrane, and grown for 3 days (Fig. 6A). MDDC infection was monitored by qPCR. As expected, viral biofilm was able to infect MDDCs, while [virus] was not (Fig. 6B). MDDCs were then cocultured with Jurkat-LTR-Luc reporter cells, and luciferase activity was measured (Fig. 6C). MDDCs exposed to viral biofilm were able to induce a significant luciferase activity after contact with Jurkat-LTR-Luc reporter cells (Fig. 6C, middle). In contrast, the luciferase signal from Jurkat-LTR-Luc cells cocultivated with MDDCs exposed to [virus] was very low and not significantly different from that from noninfected Jurkat cells (Fig. 6C, right). Since no viral DNA was detected in [virus]-exposed MDDCs (Fig. 6B, right), the low luciferase signal could be the result of a passive viral transfer from HTLV-1-exposed MDDCs to Jurkat-LTR-Luc cells, a phenomenon reminiscent of trans-infection. To demonstrate that the luciferase activity observed after biofilm-exposed MDDC coculture resulted from transmission of newly synthesized virus from productively infected MDDCs (cis-infection), and not capture of viral particles followed by transmission to Jurkat-LTR-Luc cells (trans-infection), the experiment was repeated in the presence of AZT (20). Under those conditions, MDDC infection, measured by viral DNA qPCR, was reduced (20 to 80%) compared to that of untreated MDDCs, with some variability depending on the blood donor (Fig. 6D). Consistent with those results, viral transmission to Jurkat-LTR-Luc cells was also reduced by 50 to 80% when MDDCs were treated with AZT before coculture (Fig. 6E). In one sample (20-2), a decreased luciferase activity was not observed, even if the amount of viral DNA present in MDDCs was strongly diminished in the presence of AZT (Fig. 6D). This suggests again that a passive viral transfer from HTLV-1-exposed MDDCs to Jurkat-LTR-Luc cells may have occurred. Altogether, our results show that although the number of infected MDDCs is low (around 0.1% of exposed MDDCs [Fig. 4C, left]), it is sufficient to allow transmission of infectious HTLV-1 viruses to lymphocytes. Thus, cis-infection of MDDCs with HTLV-1 is the main route of viral transmission to lymphocytes.
FIG 6.
HTLV-1-infected MDDCs transmit the virus to reporter T cells. (A) Schematic representation of the experimental procedure. (B) Real-time PCR performed on genomic DNA extracted from biofilm- or [virus]-exposed MDDCs. Results were normalized according to the amount of p19gag present in each viral preparation. The median values from 5 independent experiments obtained with 5 different blood donors are indicated. Asterisks indicate statistically significant differences calculated using a paired t test: *, P < 0.1; ns, not significant. (C) Luciferase activity was measured after coculture of reporter T cells with biofilm-exposed MDDCs. Noninfected reporter cells were used as a negative control (NI). The median values from 5 independent experiments obtained with 5 different blood donors are indicated. Asterisks indicate statistically significant differences calculated using a paired t test: **, P < 0.01; *, P < 0.1; ns, not significant. (D) Real-time PCR performed on genomic DNA extracts from biofilm-exposed MDDCs in the absence (black bars) or in presence (gray bars) of AZT. Results from independent experiments using cells from 3 different blood donors are represented as percentages of Tax-positive MDDCs in the absence of AZT. (E) Luciferase activity measured after coculture of reporter T cells with biofilm-exposed MDDCs in the absence (black bars) or in presence (gray bars) of AZT. Results from independent experiments using cells from 3 different blood donors are represented as percentages of the luciferase activity obtained with MDDCs in the absence of AZT.
Autologous T cells express low levels of NRP-1 but a level of hGlut-1 similar to that of MDDCs.
The presence of integrated viral DNA and expression of p19gag in both MDDCs and lymphocytes suggests that the difference in their susceptibilities results not from a postentry step restriction but rather from a binding and/or fusion defect in lymphocytes. Thus, we compared the levels of HTLV-1 receptor expression in IL-2/PHA-activated lymphocytes and MDDCs (Fig. 7). Consistent with a previous result (24), both primary activated lymphocytes and MDDCs expressed hGlut-1, although the level of expression was 2-fold higher in MDDCs than in activated T cells (Fig. 7A). Since antibodies directed against hGlut-1 may not efficiently bind to endogenous protein (25), we also used the isolated receptor-binding domain of HTLV enveloped proteins (26) and obtained similar results (data not shown). In contrast, neuropilin-1 expression was barely detectable in primary activated lymphocytes, even after additional stimulation with phorbol myristate acetate (PMA) and ionomycin (data not shown), or in the Jurkat-LTR-Luc cell line, while it was highly expressed in MDDCs (60-fold difference in MFI [Fig. 7B]). This suggests that the amount of neuropilin-1 present at the cell membrane is one critical parameter for the ability of HTLV-1 to infect target cells.
FIG 7.
HTLV-1 receptor expression in Jurkat-LTR-Luc cells, primary activated lymphocytes, and MDDCs. Cells were labeled with fluorochrome-coupled antibodies (black lines) directed against hGlut-1 (A) or NRP-1 (B). Unstained cells were used as negative controls (solid gray). Flow cytometry analysis was performed using FACSCanto II. Quantification of the mean fluorescence intensity was performed using FlowJo software and is presented on the right as the difference in mean fluorescence intensity detected in stained versus unstained cells.
DISCUSSION
The lack of an immunocompetent small-animal model currently precludes the identification of the route of infection followed by HTLV-1 in vivo and the identification of the first host cells encountered by the virus. Apart from circulating CD4+ T lymphocytes that represent the main infected cell population in chronically infected individuals, HTLV-1 proviral DNA is detected in antigen-presenting cells (APCs) such as DCs (27), monocytes, and pDCs (28). Because APCs are thought to be the first cells encountered by pathogens, they could then be involved in viral transmission to T cells (14), either by transmission after virus capture in the absence of infection (trans-infection) or by production of new virions after productive infection (cis-infection) (10). While cell-free virus is poorly efficient for infecting T cells, it was successfully used to infect DCs (10), thus suggesting that these cell types may differ in their susceptibilities to HTLV-1 infection. Using coculture of autologous primary IL-2/PHA-activated lymphocytes or MDDCs with HTLV-1 chronically infected cells, we have shown in this study that MDDCs are more susceptible to HTLV-1 infection than autologous primary activated lymphocytes. We have also used cell-free viral preparations, i.e., biofilm or viral particles. Consistent with previous studies, we observed that virus contained in a supernatant is very inefficient for infecting activated T cells, even when concentrated. However, we demonstrated that, unexpectedly, it also fails to efficiently infect MDDCs. These results are not consistent with reports showing MDDC infection using cell-free viruses obtained from chronically infected cells (10, 22). However, it is worth noting that these studies used highly concentrated viral preparations for infecting 1 × 105 DCs (i.e., 200 ng [10] and 300 ng [22], compared to the 10 ng we used in our experiments), amounts which are not naturally found in vitro or in vivo.
HTLV-1 is known to accumulate at the plasma membrane of infected cells in biofilm-like structures (17). Viral biofilm is formed by the accumulation of highly glycosylated proteins forming the extracellular matrix (17). This matrix could provide a physical protection to the viral envelope proteins, which are known to be highly sensitive to thiol reduction in biological medium (29, 30). In addition, proteins present in the extracellular matrix are involved in cell-substrate or cell-cell adhesion. In particular, heparin sulfate glycoproteins present in the viral biofilm are ligands of neuropilin-1, which is a member of the HTLV receptor complex. Thus, besides providing physical protection, such a biofilm could also favor interactions with surface proteins present in target cells and could concentrate virus in close proximity to its receptor, allowing a more efficient entry. Removing biofilm decreases the ability of chronically infected cell lines to transfer HTLV-1 to other cells (17). Since biofilm represents aggregates of HTLV-1 particles embedded in extracellular matrix, it can be detached from the cells. It might also be naturally released in culture supernatant when culture is prolonged at a high cell density. Therefore, one cannot exclude that particles embedded in biofilm might have been present together with bona fide free particles in supernatants used to infect DCs in those studies (10, 22). Therefore, MDDC infection might have been due not to bona fide free virus but to viral biofilm. Our results clearly show that HTLV-1 biofilm represents an infectious entity that accounts for MDDC infection in vitro.
Using a large series of human primary cells obtained from healthy blood donors, we have demonstrated in this study that MDDCs are more susceptible to infection than autologous activated lymphocytes. This result was unexpected for several reasons. First, MDDCs do not divide, implying that the HTLV-1 genome needs to be actively transported through an intact nuclear membrane; this process has not yet been described for retroviruses other than lentiviruses, with the exception of murine leukemia virus, which can infect primary macrophages when they differentiate from monocytes in vitro (31). Second, because these cells do not proliferate, viral clonal expansion within infected MDDCs cannot occur. Thus, the number of infected MDDCs represents the number of cells in which a complete viral cycle has occurred. In contrast, lymphocytes do proliferate, and in patients, clonal amplification of infected T lymphocytes is the main mechanism for viral expansion (32, 33).
In our experimental system, activated lymphocytes exposed to HTLV-1 divided, thus allowing in principle an increase in proviral load without a need for new infectious cycles. Nevertheless, proviral load values were lower in lymphocytes than in their autologous MDDC counterparts. We have shown that T cells express very low levels of NRP-1. Previous reports have shown that NRP-1 is required for binding of free virions to target cells (34) and that competition with a natural ligand of NRP-1 decreases this binding and subsequently the infection of target cells (35). This led to a model in which HTLV-1 first uses heparan sulfate proteoglycan as an attachment factor and then NRP-1 as a binding receptor. Then it facilitates envelope binding to hGlut-1, a molecule involved in the fusion step (9). MDDCs express high levels of NRP-1 and are more susceptible to HTLV-1 infection than lymphocytes, thus suggesting that NRP-1 is important for allowing a productive infection in those cells. In addition, MDDCs also express high levels of DC-SIGN, also reported to mediate infection of MDDCs (22) and not present on T cells. Thus, it would be important to determine whether increased NRP-1 and/or DC-SIGN expression in lymphocytes would suffice to increase their susceptibility to HTLV-1.
Altogether, our results suggest that MDDCs are much more susceptible to HTLV-1 infection than primary lymphocytes. In addition, although the number of infected MDDCs was low, these cells were able to efficiently transmit the virus to lymphocytes, thus demonstrating that they may represent a true viral reservoir. In conclusion, the higher susceptibility to HTLV-1 infection of MDDCs than of lymphocytes strongly supports a model in which these cells would be the first to be infected in vivo.
ACKNOWLEDGMENTS
R.M. is supported by ENS Lyon and by a Contrat Hospitalier de recherche translationnelle (AP-HP Paris). S.A. and H.D. are supported by INSERM.
We thank Maria-Isabel Thoulouze (Institut Pasteur, Paris) for technical advice on biofilm removal from infected cells and for the gift of Jurkat-LTR-Luc cells. We thank Chloé Journo and Patrick Lécine for critical reading of the manuscript. We acknowledge the support of ARC and of La Ligue Contre le Cancer (the program Équipe Labellisée). The figures were produced by Servier Medical Art.
REFERENCES
- 1.Gessain A, Cassar O. 2012. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol 3:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yodoi J, Maeda M. 2011. Discovery of ATL: an odyssey in restrospect [sic]. Int J Hematol 94:423–428. doi: 10.1007/s12185-011-0957-x. [DOI] [PubMed] [Google Scholar]
- 3.Gessain A, Barin F, Vernant J, Gout O, Maurs L, Calender A, de Thé G. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407–410. [DOI] [PubMed] [Google Scholar]
- 4.Osame M, Igata A, Matsumoto M, Izumo S, Usuku K, Rosales RL, Tara M. 1988. HTLV-1 associated myelopathy (HAM). Kansenshogaku Zasshi 62(Suppl):240–248. [PubMed] [Google Scholar]
- 5.Makino M, Wakamatsu S, Shimokubo S, Arima N, Baba M. 2000. Production of functionally deficient dendritic cells from HTLV-I-infected monocytes: implications for the dendritic cell defect in adult T cell leukemia. Virology 274:140–148. doi: 10.1006/viro.2000.0445. [DOI] [PubMed] [Google Scholar]
- 6.Makino M, Shimokubo S, Wakamatsu SI, Izumo S, Baba M. 1999. The role of human T-lymphotropic virus type 1 (HTLV-1)-infected dendritic cells in the development of HTLV-1-associated myelopathy/tropical spastic paraparesis. J Virol 73:4575–4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nascimento CR, Lima MA, de Andrada Serpa MJ, Espindola O, Leite AC, Echevarria-Lima J. 2011. Monocytes from HTLV-1-infected patients are unable to fully mature into dendritic cells. Blood 117:489–499. doi: 10.1182/blood-2010-03-272690. [DOI] [PubMed] [Google Scholar]
- 8.Sze A, Belgnaoui SM, Olagnier D, Lin R, Hiscott J, van Grevenynghe J. 2013. Host restriction factor SAMHD1 limits human T cell leukemia virus type 1 infection of monocytes via STING-mediated apoptosis. Cell Host Microbe 14:422–434. doi: 10.1016/j.chom.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Ghez D, Lepelletier Y, Jones KS, Pique C, Hermine O. 2010. Current concepts regarding the HTLV-1 receptor complex. Retrovirology 7:99. doi: 10.1186/1742-4690-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones KS, Petrow-Sadowski C, Huang YK, Bertolette DC, Ruscetti FW. 2008. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat Med 14:429–436. doi: 10.1038/nm1745. [DOI] [PubMed] [Google Scholar]
- 11.Valeri V, Hryniewicz A, Andresen V, Jones K, Fenizia C, Bialuk I, Chung H, Fukumoto R, Parks R, Ferrari M, Nicot C, Cecchinato V, Ruscetti F, Franchini G. 2010. Requirement of the human T-cell leukemia virus p12 and p30 products for infectivity of human dendritic cells and macaques but not rabbits. Blood 116:3809–3817. doi: 10.1182/blood-2010-05-284141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demontis MA, Sadiq MT, Golz S, Taylor GP. 15 May 2015. HTLV-1 viral RNA is detected rarely in plasma of HTLV-1 infected subjects. J Med Virol doi: 10.1002/jmv.24264. [DOI] [PubMed] [Google Scholar]
- 13.Derse D, Hill SA, Lloyd PA, Chung H, Morse BA. 2001. Examining human T-lymphotropic virus type 1 infection and replication by cell-free infection with recombinant virus vectors. J Virol 75:8461–8468. doi: 10.1128/JVI.75.18.8461-8468.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pique C, Jones KS. 2012. Pathways of cell-cell transmission of HTLV-1. Front Microbiol 3:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villaudy J, Wencker M, Gadot N, Gillet NA, Scoazec JY, Gazzolo L, Manz MG, Bangham CR, Dodon MD. 2011. HTLV-1 propels thymic human T cell development in “human immune system” Rag2(−)/(−) gamma c(−)/(−) mice. PLoS Pathog 7:e1002231. doi: 10.1371/journal.ppat.1002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igakura T, Stinchcombe JC, Goon PK, Taylor GP, Weber JN, Griffiths GM, Tanaka Y, Osame M, Bangham CR. 2003. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 299:1713–1716. doi: 10.1126/science.1080115. [DOI] [PubMed] [Google Scholar]
- 17.Pais-Correia AM, Sachse M, Guadagnini S, Robbiati V, Lasserre R, Gessain A, Gout O, Alcover A, Thoulouze MI. 2010. Biofilm-like extracellular viral assemblies mediate HTLV-1 cell-to-cell transmission at virological synapses. Nat Med 16:83–89. doi: 10.1038/nm.2065. [DOI] [PubMed] [Google Scholar]
- 18.Niedergang F, Didierlaurent A, Kraehenbuhl J-P, Sirard J-C. 2004. Dendritic cells: the host Achille's heel for mucosal pathogens? Trends Microbiol 12:79–88. doi: 10.1016/j.tim.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Latil S, Gnadig NF, Mallet A, Desdouits M, Guivel-Benhassine F, Jeannin P, Prevost MC, Schwartz O, Gessain A, Ozden S, Ceccaldi PE. 2012. Transcytosis of HTLV-1 across a tight human epithelial barrier and infection of subepithelial dendritic cells. Blood 120:572–580. doi: 10.1182/blood-2011-08-374637. [DOI] [PubMed] [Google Scholar]
- 20.Cachat A, Chevalier SA, Alais S, Ko NL, Ratner L, Journo C, Dutartre H, Mahieux R. 2013. Alpha interferon restricts human T-lymphotropic virus type 1 and 2 de novo infection through PKR activation. J Virol 87:13386–13396. doi: 10.1128/JVI.02758-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang S-WW, Chen C-YY, Klase Z, Zane L, Jeang K-TT. 2013. The cellular autophagy pathway modulates human T-cell leukemia virus type 1 replication. J Virol 87:1699–1707. doi: 10.1128/JVI.02147-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain P, Manuel SL, Khan ZK, Ahuja J, Quann K, Wigdahl B. 2009. DC-SIGN mediates cell-free infection and transmission of human T-cell lymphotropic virus type 1 by dendritic cells. J Virol 83:10908–10921. doi: 10.1128/JVI.01054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazurov D, Ilinskaya A, Heidecker G, Lloyd P, Derse D. 2010. Quantitative comparison of HTLV-1 and HIV-1 cell-to-cell infection with new replication dependent vectors. PLoS Pathog 6:e1000788. doi: 10.1371/journal.ppat.1000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manel N, Kim FJ, Kinet S, Taylor N, Sitbon M, Battini JL. 2003. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 115:449–459. doi: 10.1016/S0092-8674(03)00881-X. [DOI] [PubMed] [Google Scholar]
- 25.Kinet S, Swainson L, Lavanya M, Mongellaz C, Montel-Hagen A, Craveiro M, Manel N, Battini JL, Sitbon M, Taylor N. 2007. Isolated receptor binding domains of HTLV-1 and HTLV-2 envelopes bind Glut-1 on activated CD4+ and CD8+ T cells. Retrovirology 4:31. doi: 10.1186/1742-4690-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manel N, Battini JL, Taylor N, Sitbon M. 2005. HTLV-1 tropism and envelope receptor. Oncogene 24:6016–6025. doi: 10.1038/sj.onc.1208972. [DOI] [PubMed] [Google Scholar]
- 27.Macatonia S, Cruickshank J, Rudge P, Knight S. 1992. Dendritic cells from patients with tropical spastic paraparesis are infected with HTLV-1 and stimulate autologous lymphocyte proliferation. AIDS Res Hum Retroviruses 8:1699–1706. doi: 10.1089/aid.1992.8.1699. [DOI] [PubMed] [Google Scholar]
- 28.Hishizawa M, Imada K, Kitawaki T, Ueda M, Kadowaki N, Uchiyama T. 2004. Depletion and impaired interferon-alpha-producing capacity of blood plasmacytoid dendritic cells in human T-cell leukaemia virus type I-infected individuals. Br J Haematol 125:568–575. doi: 10.1111/j.1365-2141.2004.04956.x. [DOI] [PubMed] [Google Scholar]
- 29.Shinagawa M, Jinno-Oue A, Shimizu N, Roy BB, Shimizu A, Hoque SA, Hoshino H. 2012. Human T-cell leukemia viruses are highly unstable over a wide range of temperatures. J Gen Virol 93:608–617. doi: 10.1099/vir.0.037622-0. [DOI] [PubMed] [Google Scholar]
- 30.Li K, Zhang S, Kronqvist M, Wallin M, Ekstrom M, Derse D, Garoff H. 2008. Intersubunit disulfide isomerization controls membrane fusion of human T-cell leukemia virus Env. J Virol 82:7135–7143. doi: 10.1128/JVI.00448-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jarrosson-Wuilleme L, Goujon C, Bernaud J, Rigal D, Darlix JL, Cimarelli A. 2006. Transduction of nondividing human macrophages with gammaretrovirus-derived vectors. J Virol 80:1152–1159. doi: 10.1128/JVI.80.3.1152-1159.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillet NA, Malani N, Melamed A, Gormley N, Carter R, Bentley D, Berry C, Bushman FD, Taylor GP, Bangham CR. 2011. The host genomic environment of the provirus determines the abundance of HTLV-1-infected T-cell clones. Blood 117:3113–3122. doi: 10.1182/blood-2010-10-312926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wattel E, Cavrois M, Gessain A, Wain-Hobson S. 1996. Clonal expansion of infected cells: a way of life for HTLV-I J Acquir Immune Defic Syndr Hum Retrovirol 13(Suppl 1):S92–S99. [DOI] [PubMed] [Google Scholar]
- 34.Ghez D, Lepelletier Y, Lambert S, Fourneau JM, Blot V, Janvier S, Arnulf B, van Endert PM, Heveker N, Pique C, Hermine O. 2006. Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry. J Virol 80:6844–6854. doi: 10.1128/JVI.02719-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambert S, Bouttier M, Vassy R, Seigneuret M, Cari P-S, Janvier S, Heveker N, Ruscetti FW, Perret G, Jones KS, Pique C. 2009. HTLV-1 uses HSPG and neuropilin-1 for entry by molecular mimicry of VEGF165. Blood 113:5176–5185. doi: 10.1182/blood-2008-04-150342. [DOI] [PMC free article] [PubMed] [Google Scholar]