ABSTRACT
Latent DNA replication of Kaposi's sarcoma-associated herpesvirus (KSHV) initiates at the terminal repeat (TR) element and requires trans-acting elements, both viral and cellular, such as ORCs, MCMs, and latency-associated nuclear antigen (LANA). However, how cellular proteins are recruited to the viral genome is not very clear. Here, we demonstrated that the host cellular protein, Bub1, is involved in KSHV latent DNA replication. We show that Bub1 constitutively interacts with proliferating cell nuclear antigen (PCNA) via a highly conserved PIP box motif within the kinase domain. Furthermore, we demonstrated that Bub1 can form a complex with LANA and PCNA in KSHV-positive cells. This strongly indicated that Bub1 serves as a scaffold or molecular bridge between LANA and PCNA. LANA recruited PCNA to the KSHV genome via Bub1 to initiate viral replication in S phase and interacted with PCNA to promote its monoubiquitination in response to UV-induced damage for translesion DNA synthesis. This resulted in increased survival of KSHV-infected cells.
IMPORTANCE During latency in KSHV-infected cells, the viral episomal DNA replicates once each cell cycle. KSHV does not express DNA replication proteins during latency. Instead, KSHV LANA recruits the host cell DNA replication machinery to the replication origin. However, the mechanism by which LANA mediates replication is uncertain. Here, we show that LANA is able to form a complex with PCNA, a critical protein for viral DNA replication. Furthermore, our findings suggest that Bub1, a spindle checkpoint protein, serves as a scaffold or molecular bridge between LANA and PCNA. Our data further support a role for Bub1 and LANA in PCNA-mediated cellular DNA replication processes as well as monoubiquitination of PCNA in response to UV damage. These data reveal a therapeutic target for inhibition of KSHV persistence in malignant cells.
INTRODUCTION
Kaposi's sarcoma-associated herpesvirus (KSHV) or human herpesvirus 8, an oncogenic member of the gammaherpesvirus subfamily, was first identified in 1994 from body cavity-based lymphoma (BCBL) and HIV-infected patients with Kaposi's sarcoma (KS) (1). KSHV is believed to be the etiological agent of several human cancers, including KS (1, 2), primary effusion lymphoma (PEL) or body cavity based lymphoma (3), and multicentric Castleman's disease (MCD) (4, 5). Additionally, there have been reports of KSHV-associated solid lymphomas in HIV-positive and -negative patients as well as KSHV-associated lymphomas in patients with primary immune deficiencies, such as common variable immune deficiency (6, 7). Similar to other members of the gammaherpesvirus family, KSHV establishes a predominantly latent infection in host cells after primary infection (8). During latent infections, the KSHV genome persists as a circular double-stranded DNA (episome) with most viral genes being silenced, except for a small number of latent genes, which include the viral cyclin (v-cyclin), the latency-associated nuclear antigen (LANA), and the viral FLICE inhibitory protein (vFLIP). These latent proteins are well known to be involved in the regulation of cell survival, cell cycle, and tumor suppressor pathways, such as P53, pRb, and VHL (9).
LANA, an important latent protein encoded by KSHV ORF73, is essential for cancer development, maintenance of viral latency, and segregation of the double-stranded DNA viral episomes (10, 11). LANA can perturb several cellular pathways to contribute to tumorigenesis and physically binds to and promotes suppression of the tumor suppressors p53 and VHL (12, 13). LANA also can suppress the tumor suppressor retinoblastoma (Rb) protein and releases E2F during transition of cells through the G1/S cell cycle checkpoint (14). To promote G1/S transition, LANA interacts with the bromodomain-containing protein RING3/Brd2 and sequesters glycogen synthase kinase 3β (GSK-3β) (15–19). LANA also cooperates with the oncoprotein H-Ras to transform primary rat embryo fibroblasts and render them tumorigenic (20). Transgenic mice expressing LANA under the endogenous LANA promoter developed splenic follicular hyperplasia with increased germinal centers as well as lymphomas (21). In addition to its role in oncogenesis, LANA can recruit a number of host proteins, such as origin recognition complex (ORC) proteins and minichromosome maintenance complex (MCM) proteins, to regulate replication of the viral episome and segregation of the freshly replicated episome copies to daughter nuclei by tethering to the host chromosomes (22–26).
The control of DNA replication is critical for the proper functioning of a cell and also may influence genome stability. Proliferating cell nuclear antigen (PCNA) is a highly conserved protein found in all eukaryotic species (27) and is characterized as a DNA sliding clamp for replicative DNA polymerases and as an essential component of the DNA replication machinery (28). Besides its role in DNA replication, it is well known that PCNA functions are associated with DNA repair and cell cycle control (29). PCNA, a processivity factor for replicative DNA polymerases, has a central role in the replisome. It functions as a platform for DNA polymerase δ/ε and other replication proteins. Only the presence of PCNA at the replication fork enables the exchange of DNA polymerase α for the other polymerases continuing DNA synthesis (29). During replication of DNA, synthesis across damaged templates is achieved by recruiting specific DNA polymerases in a process called DNA translesion synthesis (TLS). These TLS polymerases possess a spacious active site and are capable of accommodating a variety of DNA lesions that would block the high-fidelity replicative DNA polymerases (30, 31). Notably, the initial recruitment of TLS polymerases to the stalled replication fork occurs via their interaction with PCNA (32, 33). A key event in the regulation of TLS is the monoubiquitination of PCNA (34). In response to replication stress as well as DNA damage, PCNA is monoubiquitinated (35), and monoubiquitinated PCNA has been reported to have a much higher affinity than unmodified PCNA for TLS polymerases (36, 37).
In this report we now demonstrate that LANA can form a complex with PCNA, and this interaction is mediated by the cellular mitotic kinase Bub1. Furthermore, Bub1 enhances the monoubiquitination of PCNA in the presence of LANA, which is important for translesion synthesis.
MATERIALS AND METHODS
Plasmids and antibodies.
LANA, Bub1, and its deletion mutants in pA3M, pET23b-myc-Uba1-His, pRSETB-His-Ubc5a-Flg, and glutathione S-transferase-ubiquitin (GST-Ub) pGEX-4T have been described previously (38). Antibodies used in this study were either generated from hybridomas (Myc [9E10] and HA [12CA5]) or purchased from Santa Cruz Biotechnology (PCNA; Santa Cruz, CA), Abcam (Bub1; Cambridge, MA), and Sigma-Aldrich Corp. (Flag [M2]; St. Louis, MO).
GST protein purification.
Bacterially expressed GST and GST fusion proteins were purified by affinity purification (GE Healthcare, Pittsburgh, PA). Briefly, 100 ml of bacterial culture was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at log phase (optical density at 600 nm [OD600] 0.6) at either 37°C for 2 h or 25°C overnight, and bacteria were harvested by centrifugation at 5,000 rpm for 5 min. The pellets were washed twice with phosphate-buffered saline (PBS) and resuspended in 5 ml PBS and sonicated, and the insoluble material was removed by centrifugation at 15,000 rpm for 10 min at 4°C. The clarified lysate was mixed with glutathione-Sepharose 4B for 4 h at 4°C, and the beads were washed four times with ice-cold PBS. For GST pulldown assays, the protein-coated GST beads were mixed with cell lysate directly. For in vitro ubiquitination assays, GST fusion protein was eluted from GST beads with 10 mM reduced glutathione in 50 mM Tris, pH 8.0. The eluted proteins then were dialyzed overnight against PBS for in vitro ubiquitination assays.
Pulldown assay.
Purified GST-tagged proteins were incubated with the cell extracts for 6 h at 4°C. The mixture was centrifuged, and the pellet was washed four times with radioimmunoprecipitation assay (RIPA) buffer. The pellet was resuspended in Laemmli buffer after centrifugation and resolved by SDS-PAGE.
Immunoprecipitation (IP) analysis.
Cells were harvested and lysed in radioimmunoprecipitation assay buffer (10 mM Tris-HCl, pH 7.5, 1% NP-40, 2 mM EDTA, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin). After being precleared with 30 μl of protein A/G Sepharose beads, cell extracts were incubated with specific antibodies at 4°C overnight. Protein A/G Sepharose beads were added and incubated at 4°C for another 2 h. Beads were washed four times with RIPA buffer after centrifugation. Precipitates were resolved by SDS-PAGE for Western blot (WB) analysis.
Ubiquitination assays.
Bacterially expressed His fusion proteins Ubca1 and Ubc5a were purified by affinity purification with nickel-nitrilotriacetic acid (Ni-NTA) agarose (Qiagen Inc., Valencia, CA). Ubiquitination assays were carried out as previously described (22).
ChIP assay.
Chromatin immunoprecipitation (ChIP) was carried out as has been described (39). Briefly, cells were cross-linked with 1% formaldehyde for 30 min at room temperature and quenched in 0.125 M glycine. After the cells were cross-linked, genomic DNA was extracted and sheared to an average length of 500 to 1,000 bp by sonication. DNA was immunoprecipitated with anti-PCNA or goat IgG from the sonicated nuclear extracts and quantified by real-time PCR using primers designed for the terminal repeat (TR) region of the KSHV genome: TTATAGATGGTCCAAGGGAGGGG (sense) and TGGGCTTGGGCTTTTTGTC (antisense). Fold enrichment relative to the input was calculated based on the cycle threshold (CT) using 2−Δ(ΔCT), where ΔCT is CT IP minus CT input and Δ(ΔCT) is ΔCT antibody minus ΔCT IgG.
Immunofluorescence analysis (IFA).
Cells were washed twice with PBS, layered evenly onto a slide, air dried, and fixed and permeabilized with fixative solution (4% paraformaldehyde [PFA], 0.1% Triton X-100) for 30 min. Nonspecific protein binding sites were blocked with 5% milk. Cells were washed twice with PBS and blocked with 5% milk for 60 min. Cells then were incubated with appropriate primary antibodies at 4°C overnight in a humidity chamber. After 3 washes with PBS, the slides were incubated in the dark with appropriate secondary antibodies and 4′,6-diamidino-2-phenylindole (DAPI). Slides were examined with a FluoView FV300 confocal microscope (Olympus Inc., Melville, NY). To quantify the colocalization of different proteins, 50 cells were counted and analyzed with Image J.
FISH.
Fluorescence in situ hybridization assay (FISH) was carried out as has been described (39). Cells were arrested at G1 or G1/S phase. Cells were fixed and permeabilized with fixative solution (4% PFA plus 0.5% Triton X-100) at room temperature for 30 min. The cells were treated with RNase A (100 μg/ml) in 2× SSC (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate) at 37°C for 30 min. Subsequently, the slides were overlaid with in situ hybridization solution containing 20 ng of KSHV TR probe (the probe was generated according to the manual for the BioNick labeling system [Invitrogen, Inc., CA]). After denaturation of DNA at 90°C for 5 min, the slides were incubated in a humidity chamber at 37°C overnight. Slides were washed in 0.1× SSC at 42°C for 10 min and then with 2× SSC at 42°C for 10 min and then blocked with 5% milk for 60 min, and the remaining steps were carried out as described above.
Cell survival assays.
For Saos-2 cells, 10 million cells were transfected twice with pA3M or pA3M LANA. Twenty-four hours later, 1,000 cells were split and transferred into 60-mm dishes. Cells were incubated for 24 h before they were treated with UV as indicated. The medium was replaced 24 h later, and cells were incubated for 14 days. Resulting colonies were fixed and stained with crystal violet. For B cells, cells were treated with UV, and at 48 h, cells were counted.
Cell synchronization.
Cell synchronization in different cell cycle phases was performed as described previously (40). Briefly, cells were arrested at G1/S by culturing for 18 h in complete medium containing 2 mM thymidine, 10 h in fresh complete medium without thymidine, and then in thymidine-containing complete medium for an additional 17 h before release into complete medium. Cells were arrested at G1 phase by serum starvation for 72 h.
Chromatin fractionation.
After treatment with 50 J/m2 UV, cells were collected and washed with PBS. Cell pellets subsequently were resuspended in NETN buffer (20 mM Tris, pH 8.0, 100 mM NaCl, 1 mM EDTA, and 0.5% NP-40) and incubated on ice for 15 min. Nuclei then were recovered and resuspended in 0.2 M HCl. The soluble fraction was neutralized with 1 M Tris, pH 8.0, for further analysis.
Statistical analysis.
Each experiment was repeated at least 3 times. Statistical analysis was performed using Student's t test. Differences were classed as significant (*, P < 0.05) or highly significant (**, P < 0.01).
RESULTS
Bub1 mediates the interaction between LANA and replication processivity factor PCNA.
To persist in replicating cells, the KSHV viral episome must replicate once each cell cycle and precisely segregate to daughter cells. To gain insights into the molecular mechanism underlying these effects, we sought to identify host cell protein(s) interacting with LANA. To corroborate the LANA-protein interaction, we immunoprecipitated LANA from KSHV-positive cell lines BC-3 and BCBL-1 (Fig. 1A). The results showed that in addition to Bub1, ORC2 and MCM3 coimmunoprecipitated (co-IP) with LANA as expected (26, 38), and PCNA also coimmunoprecipitated with LANA.
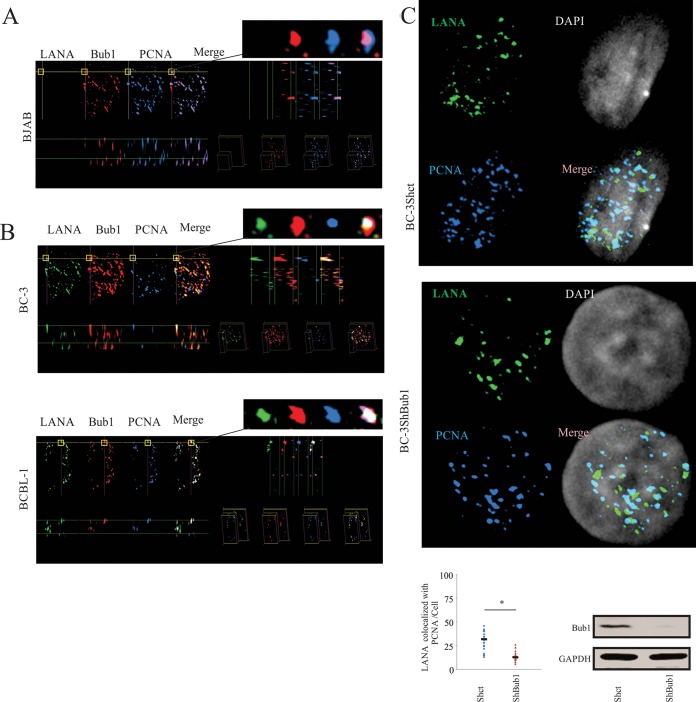
FIG 1.
LANA forms a complex with Bub1 and PCNA in KSHV-positive cells. (A) Twenty million BC-3 and BCBL-1 cells were harvested. After 3 washes with PBS, total protein was extracted and precipitated with LANA antibody or control IgG (normal mouse IgG). The immune complex and input (10% of total cell extracts) was examined by WB with antibodies as indicated. (B) Twenty million BC-3 Shct and ShBub1 cells were harvested. After 3 washes with PBS, total protein was extracted and precipitated with LANA antibody or control IgG (normal mouse IgG). The immune complex and input (10% of total cell extracts) was examined by Western blotting with antibodies as indicated. (C) Twenty million BC-3, BCBL-1, JSC-1, and BJAB cells were harvested. After 3 washes with PBS, total protein was extracted and precipitated with Bub1 antibody and control IgG (normal rabbit IgG). The immune complex and input (10% of total cell extracts) was examined by Western blotting with antibodies as indicated. (D) Twenty million BC-3, BCBL-1, JSC-1, and BJAB cells were harvested. After 3 washes with PBS, total protein was extracted and precipitated with PCNA antibody and control IgG (normal goat IgG). The immune complex and input (10% of total cell extracts) was examined by Western blotting with antibodies as indicated.
We previously found that Bub1 knockdown KSHV-positive cells showed significantly reduced KSHV episome copy numbers compared to those of CENP-F knockdown KSHV-positive cells (38). This suggested that Bub1 contributes to KSHV viral latent replication. The co-IP result showed that Bub1 coimmunoprecipitated with LANA antibodies as well as proteins known to be associated with DNA replication (Fig. 1A). Therefore, we wanted to investigate which proteins formed a complex with Bub1 and LANA during viral DNA replication and are dependent on the presence of Bub1. To confirm this, we performed similar coimmunoprecipitation assays in a KSHV-positive cell line knocked down for Bub1 expression (Fig. 1B). The results showed that there is a much weaker interaction between LANA and PCNA in Bub1 knockdown cells than between the controls. Importantly, there were no significant changes in the interactions between LANA and ORC2 or MCM3 (Fig. 1B). This result strongly suggests that Bub1 has an important role in contributing to the interaction between LANA and PCNA and can form a complex with PCNA and LANA. To confirm that LANA associates with PCNA and Bub1 in KSHV-positive cells, coimmunoprecipitation assays were performed to determine if LANA associated with Bub1 and PCNA in KSHV-positive cell lines (BCBL-1, JSC-1, and BC-3). The BJAB cell line was used as a negative control (Fig. 1C). The IP with anti-Bub1 antibody showed that endogenous LANA and PCNA were coimmunoprecipitated by anti-Bub1 antibody. The reverse IP with anti-PCNA antibody further validated that PCNA does associate with LANA and Bub1 in KSHV-positive cells (Fig. 1D). The immunoprecipitation results showed that Bub1 can associate with PCNA even in the absence of LANA (Fig. 1C and D, right).
To determine the colocalization of LANA with PCNA and Bub1 and to further determine the interaction of these three proteins under physiological conditions, a Z-stack analysis using immunofluorescence assays (IFA) in KSHV-positive cell lines (BC-3 and BCBL-1) and a KSHV-negative cell line (BJAB) were performed (Fig. 2A and B). The overlay pictures of all three dimensions (x, y, and z axes; termed an xyz stack) showed overlapped signals of LANA (green), Bub1 (red), and PCNA (blue). The white dots indicated the merge of Bub1, PCNA, and LANA overlapped signals, and the purple dots indicate the Bub1 and PCNA overlapped signals. Therefore, we observed strong colocalization of Bub1 and PCNA in all three dimensions in KSHV-negative cells (Fig. 2A) and strong colocalization of LANA, Bub1, and PCNA in KSHV-positive cells (Fig. 2B). To further support our evidence that Bub1 is important for interaction between PCNA and LANA, the colocalization of LANA and PCNA was further corroborated in BC-3 Shct and BC-3 ShBub1 cell lines by IFA. Quantitation of the overlapping signals clearly shows a significant decrease of greater than 50% colocalization of LANA and PCNA in BC-3 ShBub1 cells compared to the level for BC-3 Shct cells (Fig. 2C).
FIG 2.
Colocalization of LANA, Bub1, and PCNA. (A) Colocalization of PCNA, Bub1, and LANA in KSHV-negative (BJAB) cells shown by xyz stack with LANA (green), Bub1 (red), and PCNA (blue) staining. (B) Colocalization of PCNA, Bub1, and LANA in KSHV-positive (BC-3 and BCBL-1) cells shown by xyz stack with LANA (green), Bub1 (red), and PCNA (blue) staining. Colocalization of PCNA, Bub1, and LANA in KSHV-positive cells was distinctly observed as bright white dots. Enlarged sections are shown at the top right. (C) Endogenous LANA and PCNA were detected by using LANA and PCNA antibodies, followed by anti-mouse antibody and anti-goat antibody. Nuclei were counterstained by using DAPI. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Collectively, these results support our hypothesis that LANA, Bub1, and PCNA can form a complex in KSHV-positive cell lines. Furthermore, we found that Bub1 is important for interaction between LANA and PCNA, which suggested an involvement of LANA and Bub1 in PCNA-mediated cellular processes.
The PIP box of Bub1 is important for the association of PCNA and LANA.
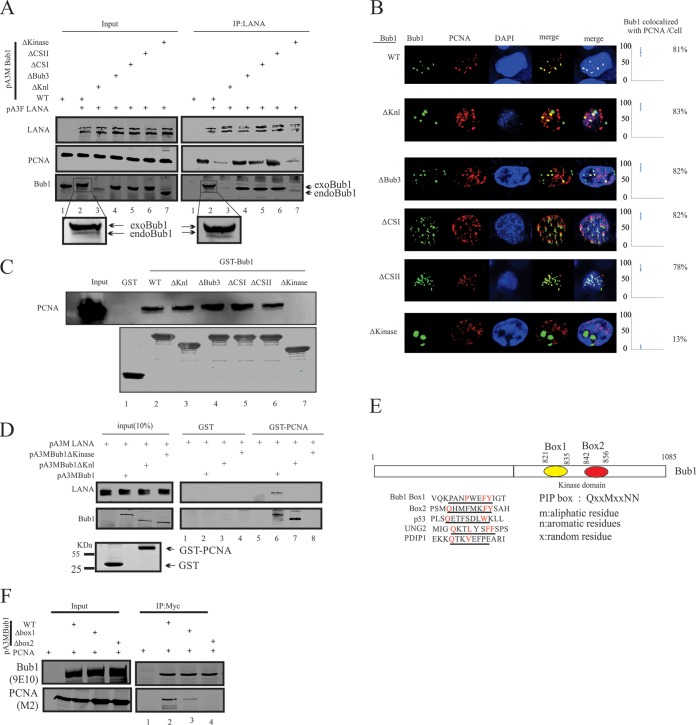
The results described above showed that LANA, Bub1, and PCNA formed a complex in KSHV-positive cells, and that the association between LANA and PCNA was dramatically decreased in Bub1 knockdown KSHV-positive cells. Therefore, we speculated that Bub1 is important for the association of PCNA and LANA in KSHV-positive cell lines. To define the domains of Bub1 required for association with LANA and PCNA, 293 cells were electroporated with pA3F-LANA and wild-type (WT) Bub1 or a series of Bub1 deletion mutants described before (38). Forty-eight hours posttransfection, coimmunoprecipitation assays were performed using anti-LANA antibodies. The coprecipitated proteins were detected by Bub1 and PCNA antibodies. In the precipitated complex, both exogenous Bub1 (exoBub1) and endogenous Bub1 (endoBub1) were detected. As expected, the Knl- and kinase domain-deleted Bub1 showed negligible interaction with LANA (Fig. 3A, lanes 3 and 7) (38). Interestingly, we found that interaction between LANA and PCNA was dramatically decreased in the presence of these two Bub1 mutants compared to that of the WT and other deletion mutants (Fig. 3A, lanes 3 and 7). Therefore, the Knl and kinase domains of Bub1 are required for interaction between LANA and PCNA. The Knl and kinase domains of Bub1 are required for the interaction between LANA and Bub1 (38). Therefore, we wanted to determine whether these two domains are required for the Bub1 and PCNA interaction. IFAs were performed in 293 cells transfected with Myc-tagged Bub1 WT or its deleted mutants (Fig. 3B). We found that the WT and a number of Bub1 deletion mutants, including the Knl deletion mutant, colocalized with PCNA, while Bub1 ΔKinase showed no colocalization with PCNA (Fig. 3B). These results were further validated by GST pulldown assays (Fig. 3C). Similarly, the pulldown assay clearly showed that interaction between PCNA and Bub1 was lost in the absence of the kinase domain (Fig. 3C, lane 7). To further confirm the requirement of the kinase domain of Bub1 for LANA-PCNA interaction, a pulldown assay was performed in purified Escherichia coli-expressed PCNA and mammalian cell-expressed LANA (Fig. 3D). The pulldown result showed LANA can be pulled down by PCNA only in the presence of WT Bub1 (Fig. 3D, compare lanes 5, 6, 7, and 8). These results strongly suggested that the kinase domain of Bub1 is required for interaction of LANA with PCNA.
FIG 3.
PIP box of Bub1 is important for the association of PCNA and LANA. (A) Ten million HEK 293 cells were cotransfected with the indicated plasmids expressing Flag-tagged LANA and Myc-tagged WT or deletion mutants. At 48 h posttransfection, cells were harvested and subjected to IP using anti-LANA followed by WB with the indicated antibodies. Ten percent of cell lysates was used as the input. (B) GST-tagged full-length Bub1 or its deletion mutants were incubated with the cell lysates from 293 cells transfected with Flag-tagged PCNA. The pulldown of GST-tagged proteins was detected by WB using PCNA antibodies. (C) 293 cells were electroporated with wild-type (pA3M-Bub1) Bub1 or its deletion mutants, and 36 h later cells were collected and stained with 9E10 antibodies and PCNA antibodies. Nuclei were counterstained by using DAPI. (D) Purified GST-PCNA was incubated with cell lysate generated from 293T cells that were cotransfected with the indicated plasmid for GST pulldown assays. The pulldown proteins were detected with 9E10 antibodies. (E) The kinase domain of Bub1 contains 2 putative PIP box motifs. (F) Ten million 293 cells were electroporated with the indicated plasmid, and 48 h later cells were lysed with RIPA buffer and subjected to IP with 9E10 antibodies. Input and IP complexes were resolved by SDS-PAGE and subjected to Western blotting with the indicated antibodies.
Previous studies showed that proteins interact with PCNA via a motif known as a PCNA-interacting protein motif (PIP box). In silico analysis showed there are two possible PIP boxes in the kinase domain of Bub1 (Fig. 3E). To confirm whether these two putative PIP boxes are required for the interaction between PCNA and Bub1, 293 cells were transfected with PCNA and Bub1 or the PIP box-deleted mutants (Fig. 3F). The results showed that the PIP box 2-deleted Bub1 had no detectable interaction with PCNA (Fig. 3F, lane 4). The PIP box 1-deleted Bub1 retained some of the capability of binding to PCNA (Fig. 3F, lane 3). However, the interaction signal was less than 50% compared to that of WT Bub1 (Fig. 3F, lane 2). The decrease in interaction signal most likely is due to the deletion of PIP box 1, which may affect the conformation of PIP box 2 required for interaction between Bub1 and LANA, as these two motifs are in close proximity to each other in Bub1.
Collectively, we showed that Bub1 not only interacted with LANA through its Knl and kinase domains (40) but also interacts with PCNA through its kinase domain via the PIP box. Bub1 may function as a scaffold or bridge which enhances the proximity of LANA and PCNA. Bub1ΔKnl lost the ability to interact with LANA, resulting in the loss of PCNA's ability to interact with LANA (Fig. 3A, lane 3). However, Bub1ΔKnl still was able to interact with PCNA (Fig. 3B, C, lane 3, and D, lane 7). The weak PCNA signal shown with LANA IP likely was due to the presence of endogenous Bub1 (Fig. 3A, lane 3). The presence of endogenous Bub1 in 293 cells also can explain why there was still some PCNA interaction with LANA, although Bub1ΔKinase was unable to interact with both LANA and PCNA (Fig. 3A, lane 7).
Bub1 is important for LANA to recruit PCNA for loading onto the KSHV genome.
One major role of PCNA is to participate in DNA replication. To achieve this goal, PCNA is loaded onto DNA during S phase. To investigate if Bub1 has a role in the recruitment of PCNA to the KSHV genome mediated by LANA, we first investigated the loading of PCNA to the KSHV genome in different cell cycle phases. BC3 cells were synchronized in G1 and G1/S phases, and they were subjected to FISH analysis (Fig. 4A). In the G1 phase, only 15% of the TRs were colocalized with PCNA, compared to about 82% colocalized with PCNA in G1/S (Fig. 4A). The result of the FISH analysis clearly demonstrated that PCNA is recruited to the KSHV genome during KSHV latent replication. The recruitment of PCNA to the KSHV genome was further corroborated by CHIP assays using anti-PCNA antibody in BC-3 and BCBL-1 cell lines after cells were synchronized in G1 and G1/S phases (Fig. 4B). Consistent with our results from the FISH analysis, we found that PCNA was greatly enriched (about 10-fold) in association with the TRs of the KSHV genome in G1/S phase in both BC3 and BCBL1 cell lines (Fig. 4B). To determine the role of Bub1 in recruitment of PCNA to the KSHV genome, BC-3 Shct and BC-3 ShBub1 cells were synchronized in G1/S using the double thymidine (TdR) block. The synchronized cells then were subjected to additional FISH and ChIP analyses (Fig. 4C and D). The FISH results showed that the percentage of colocalization between PCNA and TR was decreased from about 85% to 40% (Fig. 4C). Consistent with the FISH data, the enrichment of PCNA at the TR also was decreased in Bub1 knockdown cells (Fig. 4D).
FIG 4.
Bub1 is important for LANA to recruit PCNA to the TR of KSHV. (A) BC-3 cells were synchronized in G1 or G1/S phase and immuno-FISH was performed. (B) BC-3 and BCBL-1 cells were synchronized in G1 or G1/S phase, and the synchronized cells were harvested and subjected to ChIP with anti-PCNA antibodies or control serum. (C) BC-3-derived cells were synchronized in G1/S phase and immuno-FISH was performed. (D) BC-3-derived cells were synchronized in G1/S phase, and the synchronized cells were harvested and subjected to ChIP with anti-PCNA antibodies or control serum.
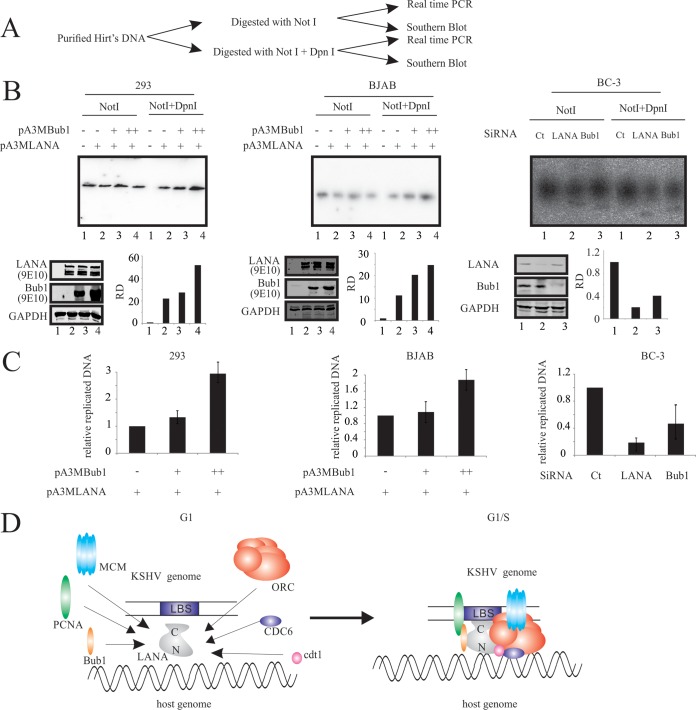
Bub1 is important for LANA recruitment of PCNA and KSHV genome replication.
To determine the role of Bub1 in PCNA-dependent KSHV genome replication mediated by LANA, purified Hirt's DNA was analyzed with Southern blot analysis and real-time PCR (Fig. 5A). Transient replication assays were performed in 293-, BJAB-, and BC-3-derived cell lines (Fig. 5B and C). Results of Southern blot assays showed that Bub1 can lead to enhanced replication of TR DNA in the presence of LANA in both 293 and the KSHV-negative BJAB cell line in a dose-dependent manner (Fig. 5B, left and middle). The role of Bub1 and LANA in KSHV latent replication was further confirmed in BC-3-derived cell lines (Fig. 5B, right). As expected, depletion of LANA resulted in inhibition of TR plasmid replication (Fig. 5B, left, lane 2). Furthermore, depletion of Bub1 also showed a dramatic decrease of replicated TR DNA (Fig. 5B, left, lane 3). Similar results were found by real-time PCR assay (Fig. 5C). These data strongly suggested that Bub1 can induce KSHV replication in the presence of LANA and that Bub1 alone cannot promote KSHV replication. Recruitment of replication factors in G1/S by LANA strongly suggests a role for Bub1 as well as PCNA (Fig. 5D).
FIG 5.
Bub1 is important for LANA to recruit PCNA to mediate KSHV latent replication. (A) Schematic representation of analysis of the in vivo transient replication assay. (B) 293-, BJAB-, and BC-3-derived cells were electroporated with TR plasmid or the indicated plasmid. Ninety-six hours later, Hirt's DNA was extracted and analyzed with Southern blotting. (C) 293-, BJAB-, and BC-3-derived cells were electroporated with TR plasmid or the indicated plasmid. Ninety-six hours later, Hirt's DNA was extracted and analyzed with real-time PCR. (D) Model depicting the molecular function of Bub1 in recruiting PCNA to the KSHV genome. RD, relative density; Ct, control.
Bub1 is important for LANA recruitment of PCNA to mediate DNA TLS.
Another important role of PCNA is mediating DNA translesion synthesis (TLS). Monoubiquitination of PCNA induced by UV is thought to promote direct DNA lesion bypass by recruiting TLS polymerases to the stalled replication forks. The association of LANA with PCNA raised the intriguing possibility that LANA plays an important role in PCNA monoubiquitination. To determine the role of LANA and Bub1 in PCNA monoubiquitination in response to UV, we performed in vitro ubiquitination assays (Fig. 6A). In agreement with this hypothesis, we found that PCNA can be monoubiquitinated by a LANA immune complex (LANA IC) purified from UV-treated 293 cells transfected with Myc-tagged LANA (Fig. 6A). As expected, the monoubiquitination of PCNA was shown by either the appearance of an electrophoretically shifted form of PCNA (Fig. 6A, part I) or Ub signal (Fig. 6A, part II). Interestingly, the ubiquitin-modified PCNA was significantly enhanced on addition of the LANA immune complex in a dose-dependent manner (Fig. 6A, lanes 6, 7, and 8). In addition, PCNA was monoubiquitinated in the presence of LANA IC without UV treatment, albeit at a reduced level (Fig. 6A, lanes 2, 3, and 4). These results were further verified by ubiquitination assays performed in Saos-2 cells (Fig. 6B). To further corroborate the above-described data that LANA can enhance the monoubiquitination of PCNA, in vivo ubiquitination assays were carried out again in Saos-2 cells transfected with an increasing amount of LANA (Fig. 6C). Together with the in vitro ubiquitination assay shown in Fig. 6A, the results showed that LANA can induce the monoubiquitination of the PCNA response to UV (Fig. 6A, lanes 6, 7, and 8, and C, lanes 8, 9, and 10). Interestingly, Bub1 alone had no appreciable effect on the monoubiquitination of PCNA in response to UV (Fig. 6C, compare lanes 1 and 5 to lanes 7 and 11). However, it can enhance the monoubiquitination of PCNA in response to UV in the presence of LANA (Fig. 6C, compare lanes 10 and 12). The role of LANA and Bub1 in PCNA monoubiquitination in response to UV was further supported in BC-3-derived cells (Fig. 6D). The monoubiquitination signal of PCNA was detected with anti-PCNA antibody and quantitated using Odyssey software. We found that in LANA knockdown BC-3 cells, PCNA was not efficiently monoubiquitinated, with a reduction of about 40% of the signal compared to that of its control (Fig. 6D, compare lanes 2 and 6). In Bub1 knockdown BC-3 cells, the PCNA monoubiquitination in response to UV also was reduced, although only to about 20% compared to that of the control (Fig. 6D, compare lanes 2 and 4). Furthermore, this suppression was not as dramatic in LANA knockdown BC-3 cells (Fig. 6D, compare lanes 4 and 6).
FIG 6.
Bub1 is important for LANA to recruit PCNA to mediate DNA translesion synthesis. (A) In vitro ubiquitination assays were carried out as described in Material and Methods. The product was divided into two fractions. Fraction I was resolved by SDS-PAGE and Western blot analysis against PCNA. Fraction II was subjected to IP with Ub and then resolved using SDS-PAGE and WB analysis against PCNA. (B) In vivo ubiquitination assays were carried out in Saos-2 cells. Saos-2 cells were electroporated with the indicated plasmids. Forty-eight hours later, cells were left untreated or were treated with 50 J/m2 for 4 h. Nuclear protein was extracted and subjected to IP with the indicated antibodies. Lysates and IP complexes were resolved by SDS-PAGE and subjected to WB with the indicated antibodies. (C) Saos-2 cells were electroporated with the indicated plasmid. Forty-eight hours later, cells were mock treated or treated with 50 J/m2 for 4 h. Nuclear protein was extracted and Western blotting performed with the indicated antibodies. CT, control. (D) Indicated BC-3-derived cell lines were left untreated or were treated with 50 J/m2 for 4 h. Nuclear protein was extracted and subjected to Western blotting with the indicated antibodies. (E) Saos-2 cells with or without LANA expression were treated with the indicated dose of UV. (Top) After UV treatment, cells were grown for 14 days before staining. The indicated BC-3-derived cell lines were treated with the indicated dose of UV. (Middle and bottom) After UV treatment, cells were grown for 3 days before counting. (F) Model depicting the role of Bub1 in enhancing PCNA monoubiquitination in response to the DNA lesion induced by UV and bypass of the lesion through replication, resulting in replicated DNA with an intact lesion.
To investigate the cellular function of LANA and Bub1, we expressed LANA in Saos-2 cells and exposed them to an increasing dose of UV. The clonogenic survival assays showed that LANA can efficiently protect cells from UV with a doubling of survival in the presence of LANA compared to that with the control (Fig. 6E, upper). To further investigate the role of Bub1 and LANA in cell survival, we analyzed the KSHV-positive cell line BC-3 knocked down for their expression. BC-3 shLANA and BC-3 ShBub1 cells showed significant sensitivity to UV treatment compared to that of control BC-3 Shct cells (Fig. 6E, middle). Furthermore, the Bub1 stable expression cell line BC3 Bub1 showed less sensitivity to UV treatment than control BC3 green fluorescent protein (GFP) (Fig. 6E, bottom).
Therefore, our data suggested that LANA is able to recruit PCNA to promote monoubiquitination in response to UV-induced damage for translesion DNA synthesis. This results in increased survival of KSHV-infected cells. Importantly, Bub1 can enhance this process but cannot promote monoubiquitination of PCNA independent of LANA.
DISCUSSION
In KSHV latently infected cells, the viral episomal DNA replicates once during each cell cycle. Although KSHV does not express proteins that are required for DNA replication during latency, KSHV-encoded LANA recruits host cell DNA replication machinery to the replication origin. However, the mechanism by which LANA recruits the host protein and mediates replication is uncertain. In this study, similar to what had been reported (41), we provided several lines of evidence to show that Bub1 is important for LANA to recruit PCNA to the TR of KSHV to mediate KSHV latent replication as well as monoubiquitination of PCNA in response to UV damage.
It is well known that LANA physically binds to the LANA binding sites (LBS) of the KSHV TR region and supports KSHV episome replication (42, 43). The components of the prereplication complex (pre-RC), such as ORCs, Cdc6, Cdt, and MCMs, are recruited to the TR region in a LANA-dependent manner (24, 26, 44–46). Here, we showed that LANA also can recruit PCNA, another protein that is required for DNA replication, to the TR through Bub1. The recruitment of PCNA through Bub1 was shown by co-IP and IFA. We showed that LANA can recruit PCNA to the TR of KSHV during S phase, during which the KSHV episome is duplicated. Our data are consistent with a model in which Bub1 serves as a scaffold or molecular bridge between LANA and PCNA which promotes the loading of PCNA to the KSHV genome during S phase (Fig. 5D).
A large number of viruses, including KSHV, could induce chromosomal lesions in infected cells (47). These DNA lesions can block genome replication and transcription and, if not repaired, yield mutations or larger-scale genome aberrations that threaten the survival of the individual cells and the organism as a whole (48, 49). To counteract the deleterious effects of damaged DNA, PCNA is recruited to stalled replication forks to perform TLS (50–52). A key event in regulation of TLS is the monoubiquitination of PCNA (34, 37, 53, 54). PCNA is monoubiquitinated in response to DNA damage induced by UV or other chemical reagents, such as mitomycin C (MMC), hydroxyurea (HU), methyl methanesulfonate (MMS), and aphidicolin (55–57). We found that monoubiquitination of PCNA in response to UV treatment is enhanced in the presence of LANA. Furthermore, we found that Bub1 can enhance the UV-induced monoubiquitination of PCNA in the presence of LANA in a dose-dependent manner. Bub1 alone showed no enhancement of PCNA monoubiquitination induced by UV. However, in the absence of Bub1, although PCNA is still responsive to UV damage, it fails to efficiently interact with LANA in KSHV-positive cells. This led to defects in PCNA monoubiquitination and subsequent KSHV-positive cell survival. We present a model which shows that Bub1 functions as a scaffold to recruit LANA and other proteins to the site of damage. PCNA is monoubiquitinated by this complex, which is required for replication of damaged DNA without repair (Fig. 6F).
Monoubiquitination of PCNA results in efficient TLS, an error-prone replication of DNA. It is a DNA damage tolerance process that allows the DNA replication machinery to bypass DNA lesions (31, 58–61). Although TLS is important for cell survival, it also results in increased mutation frequencies (62, 63). To ensure the high fidelity of DNA replication, a deubiquitinating (DUB) enzyme is recruited to deubiquitinate the monoubiquitinated PCNA (64). The EBV DUB enzyme encoded by BPLF1 targets monoubiquitinated PCNA and can disrupt TLS (65). Although the DUB homologue ORF64 encoded by KSHV had not been reported previously to target PCNA, it recently had been shown to decrease retinoic acid-inducible gene I (RIG-I) product ubiquitination and is substantially enhanced early during KSHV infection (66). Bioinformatic analysis showed that ORF64 contains a potential PIP box, which indicates that ORF64 also has the ability to bind PCNA and deubiquitinate the ubiquitinated PCNA to inhibit TLS.
The relationship between the DNA replication and spindle checkpoints of the cell cycle is unclear and not well understood. In eukaryotes, spindle formation occurs only after DNA replication is complete. A few reports showed that DNA replication control is mediated by the spindle checkpoint protein Mad2 during the S phase (67–69). In agreement with these findings, we showed that another important spindle checkpoint protein, Bub1, also was involved in KSHV genome replication as well as response to replication stress caused by UV in KSHV-positive cell lines. These findings will increase our understanding of the cross talk between DNA replication and mitotic spindle checkpoint.
This work defines LANA's interaction with PCNA as a critical component of KSHV DNA latent replication and DNA translesion syntheses in response to UV. Furthermore, we investigated the role of Bub1 in LANA-mediated latent replication of KSHV DNA. We also showed that Bub1 can substantially enhance LANA-mediated PCNA recruitment to the KSHV genome as well as PCNA monoubiquitination in response to UV. This work implicates Bub1 as an attractive target for disruption and therapy. Bub1's enhancement of PCNA loading to KSHV is critical for efficient viral replication and PCNA monoubiquitination. Therefore, strategies that inhibit the function of Bub1 may effectively disrupt the formation of the LANA-PCNA complex, which may be effective for viral genome eradication.
ACKNOWLEDGMENTS
This project was supported by Public Health Service grants R01-CA-171979, R01-CA-177423, P30-DK-050306, and P01-CA-174439 (to E.S.R.).
E.S.R. is a scholar of the Leukemia and Lymphoma Society of America. Jie Lu and Shuovomoy Banerjee provided guidance and technical support for these studies.
REFERENCES
- 1.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 2.Neipel F, Fleckenstein B. 1999. The role of HHV-8 in Kaposi's sarcoma. Semin Cancer Biol 9:151–164. doi: 10.1006/scbi.1999.0129. [DOI] [PubMed] [Google Scholar]
- 3.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 4.Gessain A, Sudaka A, Briere J, Fouchard N, Nicola MA, Rio B, Arborio M, Troussard X, Audouin J, Diebold J, de The G. 1996. Kaposi sarcoma-associated herpes-like virus (human herpesvirus type 8) DNA sequences in multicentric Castleman's disease: is there any relevant association in non-human immunodeficiency virus-infected patients? Blood 87:414–416. [PubMed] [Google Scholar]
- 5.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay MF, Clauvel JP, Raphael M, Degos L, Sigaux F. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276–1280. [PubMed] [Google Scholar]
- 6.Carbone A, Gloghini A, Vaccher E, Cerri M, Gaidano G, Dalla-Favera R, Tirelli U. 2005. Kaposi's sarcoma-associated herpesvirus/human herpesvirus type 8-positive solid lymphomas: a tissue-based variant of primary effusion lymphoma. J Mol Diagn 7:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheat WH, Cool CD, Morimoto Y, Rai PR, Kirkpatrick CH, Lindenbaum BA, Bates CA, Ellison MC, Serls AE, Brown KK, Routes JM. 2005. Possible role of human herpesvirus 8 in the lymphoproliferative disorders in common variable immunodeficiency. J Exp Med 202:479–484. doi: 10.1084/jem.20050381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boshoff C, Chang Y. 2001. Kaposi's sarcoma-associated herpesvirus: a new DNA tumor virus. Annu Rev Med 52:453–470. doi: 10.1146/annurev.med.52.1.453. [DOI] [PubMed] [Google Scholar]
- 9.Jarviluoma A, Ojala PM. 2006. Cell signaling pathways engaged by KSHV. Biochim Biophys Acta 1766:140–158. [DOI] [PubMed] [Google Scholar]
- 10.Kaul R, Verma SC, Robertson ES. 2007. Protein complexes associated with the Kaposi's sarcoma-associated herpesvirus-encoded LANA. Virology 364:317–329. doi: 10.1016/j.virol.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballestas ME, Kaye KM. 2011. The latency-associated nuclear antigen, a multifunctional protein central to Kaposi's sarcoma-associated herpesvirus latency. Future Microbiol 6:1399–1413. doi: 10.2217/fmb.11.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friborg J Jr, Kong W, Hottiger MO, Nabel GJ. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889–894. [DOI] [PubMed] [Google Scholar]
- 13.Cai QL, Knight JS, Verma SC, Zald P, Robertson ES. 2006. EC5S ubiquitin complex is recruited by KSHV latent antigen LANA for degradation of the VHL and p53 tumor suppressors. PLoS Pathog 2:e116. doi: 10.1371/journal.ppat.0020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monge A, Chorghade M, Erhardt PW, Ganellin CR, Koga N, Lindberg P, Perun TJ, Topliss JG, Trivedi BK, Wermuth CG. 2000. Medicinal chemistry in the development of societies. Biodiversity and natural products. Eur J Med Chem 35:1121–1125. [DOI] [PubMed] [Google Scholar]
- 15.Denis GV, Vaziri C, Guo N, Faller DV. 2000. RING3 kinase transactivates promoters of cell cycle regulatory genes through E2F. Cell Growth Differ 11:417–424. [PMC free article] [PubMed] [Google Scholar]
- 16.Guo N, Faller DV, Denis GV. 2000. Activation-induced nuclear translocation of RING3. J Cell Sci 113(Part 17):3085–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Platt GM, Simpson GR, Mittnacht S, Schulz TF. 1999. Latent nuclear antigen of Kaposi's sarcoma-associated herpesvirus interacts with RING3, a homolog of the Drosophila female sterile homeotic (fsh) gene. J Virol 73:9789–9795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boshoff C. 2003. Kaposi virus scores cancer coup. Nat Med 9:261–262. doi: 10.1038/nm0303-261. [DOI] [PubMed] [Google Scholar]
- 19.Fujimuro M, Hayward SD. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus manipulates the activity of glycogen synthase kinase-3beta. J Virol 77:8019–8030. doi: 10.1128/JVI.77.14.8019-8030.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radkov SA, Kellam P, Boshoff C. 2000. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat Med 6:1121–1127. doi: 10.1038/80459. [DOI] [PubMed] [Google Scholar]
- 21.Fakhari FD, Jeong JH, Kanan Y, Dittmer DP. 2006. The latency-associated nuclear antigen of Kaposi sarcoma-associated herpesvirus induces B cell hyperplasia and lymphoma. J Clin Investig 116:735–742. doi: 10.1172/JCI26190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garber AC, Shu MA, Hu J, Renne R. 2001. DNA binding and modulation of gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J Virol 75:7882–7892. doi: 10.1128/JVI.75.17.7882-7892.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim C, Sohn H, Gwack Y, Choe J. 2000. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) binds ATF4/CREB2 and inhibits its transcriptional activation activity. J Gen Virol 81:2645–2652. [DOI] [PubMed] [Google Scholar]
- 24.Lim C, Sohn H, Lee D, Gwack Y, Choe J. 2002. Functional dissection of latency-associated nuclear antigen 1 of Kaposi's sarcoma-associated herpesvirus involved in latent DNA replication and transcription of terminal repeats of the viral genome. J Virol 76:10320–10331. doi: 10.1128/JVI.76.20.10320-10331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakakibara S, Ueda K, Nishimura K, Do E, Ohsaki E, Okuno T, Yamanishi K. 2004. Accumulation of heterochromatin components on the terminal repeat sequence of Kaposi's sarcoma-associated herpesvirus mediated by the latency-associated nuclear antigen. J Virol 78:7299–7310. doi: 10.1128/JVI.78.14.7299-7310.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stedman W, Deng Z, Lu F, Lieberman PM. 2004. ORC, MCM, and histone hyperacetylation at the Kaposi's sarcoma-associated herpesvirus latent replication origin. J Virol 78:12566–12575. doi: 10.1128/JVI.78.22.12566-12575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuka I, Hata S, Matsuoka M, Kosugi S, Hashimoto J. 1991. Highly conserved structure of proliferating cell nuclear antigen (DNA polymerase delta auxiliary protein) gene in plants. Eur J Biochem 195:571–575. doi: 10.1111/j.1432-1033.1991.tb15739.x. [DOI] [PubMed] [Google Scholar]
- 28.Kelman Z, O'Donnell M. 1995. Structural and functional similarities of prokaryotic and eukaryotic DNA polymerase sliding clamps. Nucleic Acids Res 23:3613–3620. doi: 10.1093/nar/23.18.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maga G, Hubscher U. 2003. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci 116:3051–3060. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- 30.Ohmori H, Friedberg EC, Fuchs RP, Goodman MF, Hanaoka F, Hinkle D, Kunkel TA, Lawrence CW, Livneh Z, Nohmi T, Prakash L, Prakash S, Todo T, Walker GC, Wang Z, Woodgate R. 2001. The Y-family of DNA polymerases. Mol Cell 8:7–8. doi: 10.1016/S1097-2765(01)00278-7. [DOI] [PubMed] [Google Scholar]
- 31.Sale JE, Lehmann AR, Woodgate R. 2012. Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nat Rev Mol Cell Biol 13:141–152. doi: 10.1038/nrm3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haracska L, Johnson RE, Unk I, Phillips B, Hurwitz J, Prakash L, Prakash S. 2001. Physical and functional interactions of human DNA polymerase eta with PCNA. Mol Cell Biol 21:7199–7206. doi: 10.1128/MCB.21.21.7199-7206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haracska L, Johnson RE, Unk I, Phillips BB, Hurwitz J, Prakash L, Prakash S. 2001. Targeting of human DNA polymerase iota to the replication machinery via interaction with PCNA. Proc Natl Acad Sci U S A 98:14256–14261. doi: 10.1073/pnas.261560798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. 2002. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 35.Lehmann AR. 2011. Ubiquitin-family modifications in the replication of DNA damage. FEBS Lett 585:2772–2779. doi: 10.1016/j.febslet.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Terai K, Abbas T, Jazaeri AA, Dutta A. 2010. CRL4(Cdt2) E3 ubiquitin ligase monoubiquitinates PCNA to promote translesion DNA synthesis. Mol Cell 37:143–149. doi: 10.1016/j.molcel.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe K, Tateishi S, Kawasuji M, Tsurimoto T, Inoue H, Yamaizumi M. 2004. Rad18 guides poleta to replication stalling sites through physical interaction and PCNA monoubiquitination. EMBO J 23:3886–3896. doi: 10.1038/sj.emboj.7600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao B, Verma SC, Cai Q, Kaul R, Lu J, Saha A, Robertson ES. 2010. Bub1 and CENP-F can contribute to Kaposi's sarcoma-associated herpesvirus genome persistence by targeting LANA to kinetochores. J Virol 84:9718–9732. doi: 10.1128/JVI.00713-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jha HC, Upadhyay SK, M AJP, Lu J, Cai Q, Saha A, Robertson ES. 2013. H2AX phosphorylation is important for LANA-mediated Kaposi's sarcoma-associated herpesvirus episome persistence. J Virol 87:5255–5269. doi: 10.1128/JVI.03575-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Z, Xiao B, Jha HC, Lu J, Banerjee S, Robertson ES. 2014. Kaposi's sarcoma-associated herpesvirus-encoded LANA can induce chromosomal instability through targeted degradation of the mitotic checkpoint kinase Bub1. J Virol 88:7367–7378. doi: 10.1128/JVI.00554-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Q, Tsurimoto T, Juillard F, Li L, Li S, De Leon Vazquez E, Chen S, Kaye K. 2014. Kaposi's sarcoma-associated herpesvirus LANA recruits the DNA polymerase clamp loader to mediate efficient replication and virus persistence. Proc Natl Acad Sci U S A 111:11816–11821. doi: 10.1073/pnas.1404219111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong LY, Wilson AC. 2005. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen induces a strong bend on binding to terminal repeat DNA. J Virol 79:13829–13836. doi: 10.1128/JVI.79.21.13829-13836.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu F, Tsai K, Chen HS, Wikramasinghe P, Davuluri RV, Showe L, Domsic J, Marmorstein R, Lieberman PM. 2012. Identification of host-chromosome binding sites and candidate gene targets for Kaposi's sarcoma-associated herpesvirus LANA. J Virol 86:5752–5762. doi: 10.1128/JVI.07216-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohsaki E, Ueda K, Sakakibara S, Do E, Yada K, Yamanishi K. 2004. Poly(ADP-ribose) polymerase 1 binds to Kaposi's sarcoma-associated herpesvirus (KSHV) terminal repeat sequence and modulates KSHV replication in latency. J Virol 78:9936–9946. doi: 10.1128/JVI.78.18.9936-9946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verma SC, Choudhuri T, Kaul R, Robertson ES. 2006. Latency-associated nuclear antigen (LANA) of Kaposi's sarcoma-associated herpesvirus interacts with origin recognition complexes at the LANA binding sequence within the terminal repeats. J Virol 80:2243–2256. doi: 10.1128/JVI.80.5.2243-2256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uppal T, Banerjee S, Sun Z, Verma SC, Robertson ES. 2014. KSHV LANA–the master regulator of KSHV latency. Viruses 6:4961–4998. doi: 10.3390/v6124961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nichols WW, Bradt CI, Toji LH, Godley M, Segawa M. 1978. Induction of sister chromatid exchanges by transformation with simian virus 40. Cancer Res 38:960–964. [PubMed] [Google Scholar]
- 48.Jackson SP, Bartek J. 2009. The DNA-damage response in human biology and disease. Nature 461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ciccia A, Elledge SJ. 2010. The DNA damage response: making it safe to play with knives. Mol Cell 40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedberg EC. 2005. Suffering in silence: the tolerance of DNA damage. Nat Rev Mol Cell Biol 6:943–953. doi: 10.1038/nrm1781. [DOI] [PubMed] [Google Scholar]
- 51.Lehmann AR, Niimi A, Ogi T, Brown S, Sabbioneda S, Wing JF, Kannouche PL, Green CM. 2007. Translesion synthesis: Y-family polymerases and the polymerase switch. DNA Repair 6:891–899. doi: 10.1016/j.dnarep.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Moldovan GL, Pfander B, Jentsch S. 2007. PCNA, the maestro of the replication fork. Cell 129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 53.Kannouche PL, Wing J, Lehmann AR. 2004. Interaction of human DNA polymerase eta with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol Cell 14:491–500. doi: 10.1016/S1097-2765(04)00259-X. [DOI] [PubMed] [Google Scholar]
- 54.Stelter P, Ulrich HD. 2003. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature 425:188–191. doi: 10.1038/nature01965. [DOI] [PubMed] [Google Scholar]
- 55.Tian F, Sharma S, Zou J, Lin SY, Wang B, Rezvani K, Wang H, Parvin JD, Ludwig T, Canman CE, Zhang D. 2013. BRCA1 promotes the ubiquitination of PCNA and recruitment of translesion polymerases in response to replication blockade. Proc Natl Acad Sci U S A 110:13558–13563. doi: 10.1073/pnas.1306534110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang W, Qin Z, Zhang X, Xiao W. 2011. Roles of sequential ubiquitination of PCNA in DNA-damage tolerance. FEBS Lett 585:2786–2794. doi: 10.1016/j.febslet.2011.04.044. [DOI] [PubMed] [Google Scholar]
- 57.Niimi A, Brown S, Sabbioneda S, Kannouche PL, Scott A, Yasui A, Green CM, Lehmann AR. 2008. Regulation of proliferating cell nuclear antigen ubiquitination in mammalian cells. Proc Natl Acad Sci U S A 105:16125–16130. doi: 10.1073/pnas.0802727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Yuan F, Wu X, Rechkoblit O, Taylor JS, Geacintov NE, Wang Z. 2000. Error-prone lesion bypass by human DNA polymerase eta. Nucleic Acids Res 28:4717–4724. doi: 10.1093/nar/28.23.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loeb LA, Monnat RJ Jr. 2008. DNA polymerases and human disease. Nat Rev Genet 9:594–604. doi: 10.1038/nrg2345. [DOI] [PubMed] [Google Scholar]
- 60.Lehmann AR. 2000. Replication of UV-damaged DNA: new insights into links between DNA polymerases, mutagenesis and human disease. Gene 253:1–12. doi: 10.1016/S0378-1119(00)00250-X. [DOI] [PubMed] [Google Scholar]
- 61.Matsuda T, Bebenek K, Masutani C, Hanaoka F, Kunkel TA. 2000. Low fidelity DNA synthesis by human DNA polymerase-eta. Nature 404:1011–1013. doi: 10.1038/35010014. [DOI] [PubMed] [Google Scholar]
- 62.Machida Y, Kim MS, Machida YJ. 2012. Spartan/C1orf124 is important to prevent UV-induced mutagenesis. Cell Cycle 11:3395–3402. doi: 10.4161/cc.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han J, Liu T, Huen MS, Hu L, Chen Z, Huang J. 2014. SIVA1 directs the E3 ubiquitin ligase RAD18 for PCNA monoubiquitination. J Cell Biol 205:811–827. doi: 10.1083/jcb.201311007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang TT, Nijman SM, Mirchandani KD, Galardy PJ, Cohn MA, Haas W, Gygi SP, Ploegh HL, Bernards R, D'Andrea AD. 2006. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat Cell Biol 8:339–347. [DOI] [PubMed] [Google Scholar]
- 65.Whitehurst CB, Vaziri C, Shackelford J, Pagano JS. 2012. Epstein-Barr virus BPLF1 deubiquitinates PCNA and attenuates polymerase eta recruitment to DNA damage sites. J Virol 86:8097–8106. doi: 10.1128/JVI.00588-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jha HC, Lu J, Verma SC, Banerjee S, Mehta D, Robertson ES. 2014. Kaposi's sarcoma-associated herpesvirus genome programming during the early stages of primary infection of peripheral blood mononuclear cells. mBio 5:e02261-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Magiera MM, Gueydon E, Schwob E. 2014. DNA replication and spindle checkpoints cooperate during S phase to delay mitosis and preserve genome integrity. J Cell Biol 204:165–175. doi: 10.1083/jcb.201306023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garber PM, Rine J. 2002. Overlapping roles of the spindle assembly and DNA damage checkpoints in the cell-cycle response to altered chromosomes in Saccharomyces cerevisiae. Genetics 161:521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sugimoto I, Murakami H, Tonami Y, Moriyama A, Nakanishi M. 2004. DNA replication checkpoint control mediated by the spindle checkpoint protein Mad2p in fission yeast. J Biol Chem 279:47372–47378. doi: 10.1074/jbc.M403231200. [DOI] [PubMed] [Google Scholar]