Abstract
We demonstrate that novel bat HL17NL10 and HL18NL11 influenza virus NS1 proteins are effective interferon antagonists but do not block general host gene expression. Solving the RNA-binding domain structures revealed the canonical NS1 symmetrical homodimer, and RNA binding required conserved basic residues in this domain. Interferon antagonism was strictly dependent on RNA binding, and chimeric bat influenza viruses expressing NS1s defective in this activity were highly attenuated in interferon-competent cells but not in cells unable to establish antiviral immunity.
TEXT
The complete genomes of two novel influenza A-like viruses (IAVs) were recently identified in Central and South American bat species and were provisionally designated the unique subtypes H17N10 and H18N11 due to their high sequence divergence from other IAVs (1, 2). Remarkably, the surface glycoproteins of bat IAVs (hemagglutinin [HA] and neuraminidase [NA]) lack canonical features normally associated with these proteins (3–6) and were recently proposed to be renamed HA-like (HL17 and HL18) and NA-like (NL10 and NL11) proteins (7). Although basic functions of the RNA replicative machinery appear largely conserved (1, 8–10), properties of other bat IAV proteins have yet to be determined. NS1 is a multifunctional virulence factor that acts as a major interferon (IFN) antagonist during IAV infection and has been associated with host range restriction (11–13). The mechanisms by which NS1 can pre- and posttranscriptionally inhibit cellular antiviral defenses are known to be highly strain specific (14–17). Here, we sought to characterize the structure and IFN-antagonistic functions of bat IAV NS1 proteins, which share only ∼50% sequence identity with human and avian IAV NS1 proteins (1, 2, 18).
Novel bat IAV NS1 proteins are IFN antagonists.
A recent study revealed that transient expression of the HL17NL10 (HL17) NS1 protein is sufficient to antagonize the interferon-β (IFN-β) response in human cells (18). Given that the HL17 and HL18NL11 (HL18) NS1 proteins differ by ∼10%, we compared the abilities of these two IAV proteins to antagonize IFN-β promoter induction, along with the well-characterized NS1 from the PR8 strain.
293T cells in 24-well plates were cotransfected with plasmids expressing V5-tagged glutathione S-transferase (GST), HL17 NS1, HL18 NS1, or PR8 NS1 proteins (1, 10, or 100 ng), together with an IFN-β promoter-dependent firefly luciferase (FF-Luc) expression plasmid (p125-FFLuc [25 ng]) and a constitutively active Renilla luciferase (Ren-Luc) expression plasmid (pRL-TK [25 ng]), which was used for normalization. Total plasmid DNA was kept constant with an empty expression vector. Twenty-four hours posttransfection, cells were infected with a defective interfering (DI) particle-rich stock of Sendai virus (SeV) for 16 h to stimulate the IFN-β promoter. High induction of FF-Luc activity occurred in SeV-infected GST-expressing cells compared to mock-infected GST-expressing cells. However, this induction was antagonized in a dose-dependent manner in cells expressing PR8 NS1, HL17 NS1, or HL18 NS1 (Fig. 1A). The extents of IFN antagonism by each NS1 protein were largely comparable, although we note that the bat IAV NS1s were slightly less efficient than PR8 NS1 in this system. The mechanism of IFN-antagonism by the bat IAV NS1 proteins is likely independent of CPSF30 inhibition as, like PR8 NS1, both HL17 and HL18 NS1s were unable to block expression from the constitutively active Renilla luciferase reporter plasmid (Fig. 1B). This observation is consistent with the fact that both HL17 and HL18 NS1s, unlike our control H5N1 (Ch/Nig/07) NS1, lack consensus amino acid residues essential for this function in other IAV strains (14, 19–21) (Fig. 1C). No striking differences were observed between human, bat, or avian IAV NS1s when the intracellular distribution of each protein was assessed in human lung fibroblasts transiently transfected with 0.5 μg of each V5-tagged NS1 construct: immunofluorescence detecting the V5 tag revealed that HL17 and HL18 NS1 proteins localize predominantly to the nucleus, with an absence from the nucleolus (Fig. 1D). These data indicate that both HL17 and HL18 NS1 proteins can similarly act as IFN antagonists in human cells, and likely inhibit induction at a pretranscriptional level.
FIG 1.
Bat IAV NS1 proteins antagonize IFN-β induction but do not block general host gene expression. (A) Impact of bat IAV NS1s on IFN-β induction. 293T cells were cotransfected with increasing amounts of pLVX-based plasmids expressing a V5-tagged version of the indicated NS1 protein (or GST), together with an IFN-β promoter-driven firefly luciferase (FF-Luc)-expressing plasmid (p125luc [25 ng]) and an HSV-TK promoter-driven Renilla luciferase (Ren-Luc) plasmid (pRL-TK [25 ng]). Twenty-four hours posttransfection, cells were infected with SeV (+) or mock infected (−) for 16 h to stimulate the IFN-β promoter. Relative FF-Luc activity was determined as the ratio between FF- and Ren-Luc. Values were normalized to GST plus SeV (set to 100% for each GST dose). (B) Impact of bat IAV NS1s on general host gene expression. 293T cells were cotransfected with 0.5 μg V5-tagged GST or the indicated NS1 expression plasmid and 50 ng Renilla luciferase-expressing plasmid (pRL-TK). Twenty-four hours later, total Ren-Luc levels were determined, and values were normalized to GST (set to 100%). For panels A and B, bars represent means and standard deviations from three independent experiments. Lysates from parallel samples were analyzed by Western blotting for the indicated proteins and are shown from a representative experiment. Significance was calculated using the Student's t test comparing the appropriate GST dose with each respective NS1 dose (****, P < 0.0001; ***, P < 0.001; **, P < 0.01; *, P < 0.05; ns, not significant). (C) Lack of CPSF30-binding consensus residues in the bat IAV NS1 proteins. The table highlights the amino acid residues for each NS1 studied. #, residue numbering based on PR8 NS1 due to bat IAV NS1 amino acid insertions. Pink-shaded residues indicate CPSF30-binding consensus. (D) Intracellular localization of bat IAV NS1s in human lung fibroblasts. MRC5-hTERT cells were transfected with 0.5 μg of plasmid expressing the indicated V5-tagged NS1 protein or empty vector. Cells were fixed and permeabilized 24 h posttransfection, immunostained for the V5 tag, and visualized by confocal microscopy.
Structural and functional analyses of RNA binding by novel bat IAV NS1 proteins.
Previous data revealed that C-terminal truncations to the PR8 NS1 effector domain (ED) dramatically reduce its IFN-antagonistic ability in the context of a recombinant virus and lead to attenuation of virus replication in vitro and pathogenicity in vivo (18). Intriguingly, the same truncations to HL17 NS1 in the context of a chimeric bat IAV had negligible impacts on IFN antagonism and viral replication (18). This may suggest that the IFN-antagonistic property of bat IAV NS1s resides predominantly in their N-terminal RNA-binding domain (RBD).
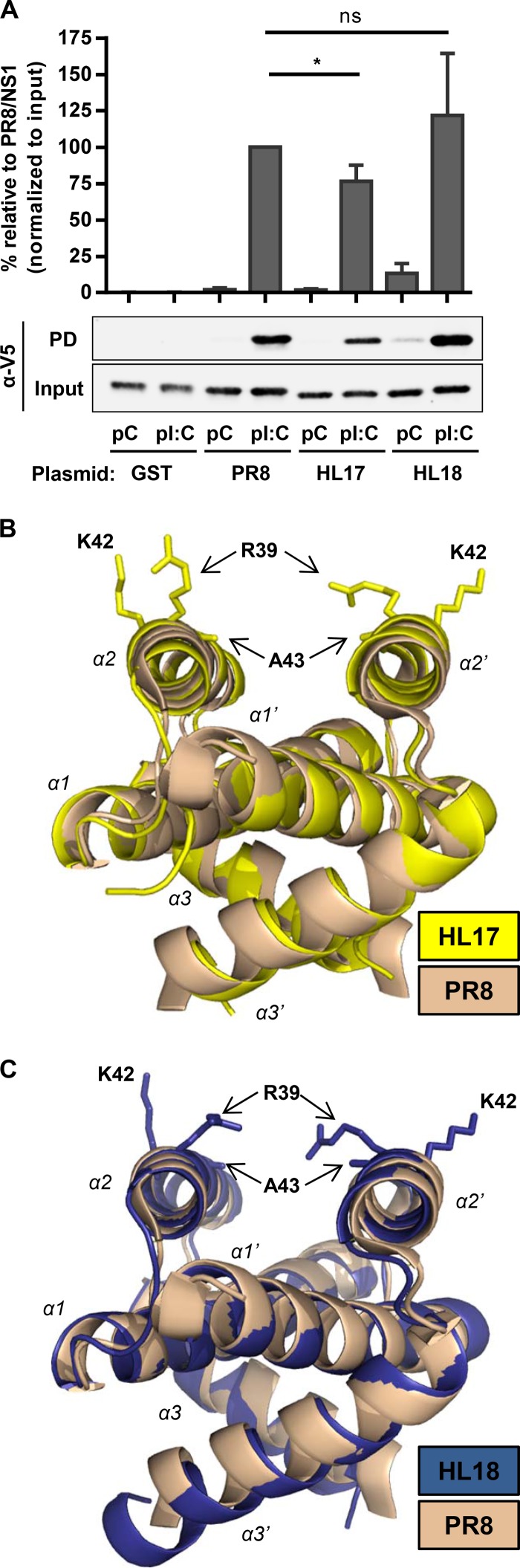
To determine RNA-binding properties of the bat IAV NS1 proteins, 293T cells in 25-cm2 flasks were transfected for 48 h with 1 μg of each V5-tagged NS1 (or GST)-expressing plasmid. Cells were lysed in buffer A (20 mM Tris-HCl [pH 7.8], 650 mM NaCl, 5 mM EDTA and 0.5% [vol/vol] IGEPAL CA-6300 supplemented with protease inhibitors [Complete mini-EDTA free from Roche]), and lysates were passed through a 29G needle six times. Following centrifugation at 14,000 rpm for 50 min at 4°C, soluble fractions were incubated with end-over-end mixing overnight at 4°C with either poly(C) (synthetic single-stranded RNA [ssRNA]) or poly(I-C) (synthetic double-stranded RNA [dsRNA]) beads, which were generated essentially as described previously (22). Beads were collected by centrifugation at 4,000 rpm for 1 min and washed six times with 1 ml of fresh buffer A. Western blot analysis of proteins bound to the beads revealed that V5-tagged GST was not precipitated by either poly(C) or poly(I-C) beads, while all V5-tagged NS1s bound strongly to the poly(I-C) but not poly(C) beads (Fig. 2A). Variations in poly(I-C) binding between the different NS1s were minimal, although notably the HL18 NS1 consistently showed some weak binding to poly(C) beads.
FIG 2.
Characterization of the bat IAV NS1 protein RNA-binding domain. (A) RNA binding by wild-type NS1 proteins. 293T cells were transfected with 1 μg of a plasmid expressing the indicated V5-tagged NS1 protein (or GST). Cells were lysed 48 h later, and soluble fractions were precipitated with either poly(C) or poly(I-C) beads. Input and bound (PD, pulldown) fractions were analyzed by Western blotting using anti-V5 antibody. Quantified bound protein was normalized to input, and bound PR8 NS1 was arbitrarily set to 100%. Bars represent means and standard deviations from three independent experiments. Significance was calculated using Student's t test (*, P < 0.05; ns, not significant). (B and C) Crystal structures of the HL17 NS1 (B) and HL18 NS1 (C) RBDs. Highlighted are the corresponding residues responsible for RNA binding in the bat IAV NS1 proteins (R39 and K42), as well as A43. The previously described PR8 NS1 RBD (beige) is superpositioned on both structures to indicate highly similar folds. Figures were generated with PyMOL using PDB IDs 2ZKO (PR8), 5BXZ (HL17), and 5BY1 (HL18).
To further confirm the nature of dsRNA binding by bat IAV NS1s, we purified the HL17 and HL18 NS1 RBDs (residues 1 to 75), essentially as described previously (23). Protein was concentrated to 10 to 15 mg/ml and crystallized by vapor diffusion in a 1:1 ratio with mother liquor: for HL17, 0.1 M HEPES (pH 7.5) and 25% polyethylene glycol (PEG) 1000, and for HL18, 0.2 M sodium acetate, 0.1 M MES (morpholineethanesulfonic acid [pH 6.5]), and 30% PEG 400. X-ray diffraction data were collected in house and were processed and refined as described previously (23). Structures were elucidated by molecular replacement using Ud NS1 RBD as a model (PDB ID no. 1AIL) (24) (Table 1). Both bat IAV NS1 RBDs have similar secondary, tertiary, and quaternary structures to other NS1 RBDs, with the HL17 and HL18 RBDs consisting of a symmetrical dimeric unit formed by 3 interlocking α-helices from each monomer (Fig. 2B and C). An amino acid insertion at position 5 of the bat IAV NS1 proteins means residues known to be important for dsRNA binding by other NS1s (e.g., R38, K41, and S42) (25–27) are shifted in the bat IAV NS1 proteins (i.e., to positions R39, K42, and A43); although consistent with their dsRNA-binding properties, these residues still lie exposed on antiparallel “tracks” formed by helix 2 (α2) of each monomer. Overall, these structural and functional data indicate that the bat IAV NS1 proteins share the dsRNA-binding property of other IAV NS1s.
TABLE 1.
Data collection and refinement statistics for bat IAV NS1 RNA-binding domains
| Parameter | Result for PDB ID no.a: |
|
|---|---|---|
| 5BXZ | 5BY1 | |
| Protein | HL17 NS1 RBD | HL18 NS1 RBD |
| Space group | P41 | P21 |
| Cell dimensions, a, b, c (Å) | 46.7, 46.7, 60.2 | 30.7, 56.8, 39.8 |
| Resolution (Å) | 30.00–2.60 (2.64–2.60) | 30.00–1.75 (1.78–1.75) |
| Rmerge (%) | 13.1 (43.2) | 5.8 (41.6) |
| I/σ〈I〉 | 24.9 (8.7) | 33.3 (2.4) |
| Completeness (%) | 100.0 (100.0) | 94.9 (57.8) |
| No. of unique reflections | 4,036 | 12,373 |
| Redundancy | 7.2 | 3.2 |
| Rwork (%) | 17.3 | 16.0 |
| Rfree (%) | 27.4 | 20.9 |
| No. of atoms | ||
| Protein | 1,121 | 1,155 |
| Water | 25 | 75 |
| RMSDb | ||
| Bond length (Å) | 0.08 | 0.07 |
| Bond angle (°) | 1.11 | 0.996 |
Values in parentheses are for the highest-resolution shell.
RMSD, root mean square deviation.
dsRNA-binding residues of the bat IAV NS1 protein are critical for IFN antagonism.
In order to identify residues crucial for dsRNA binding by the bat IAV NS1 proteins, we repeated our RNA-binding assays using a panel of selected V5-tagged HL17 NS1 mutants. The panel included mutants with alanine substitutions at the suspected direct dsRNA-binding residues R39 and K42, as well as W189, a residue responsible for NS1 ED dimerization that plays an important role in full-length NS1 oligomerization, dsRNA binding, and virulence (28–31). We confirmed that residues R39 and K42 in HL17 NS1 are essential for interactions with dsRNA, and furthermore, that potential HL17 NS1 ED dimerization via W189 also contributes to efficient dsRNA binding (Fig. 3A). As a previous study has shown that substitution of residue S42 for alanine in a human IAV NS1 protein results in a 10-fold decrease in dsRNA-binding ability (26), we sought to determine whether the naturally occurring A43 at the equivalent position in HL17 NS1 is suboptimal for dsRNA binding in this context. In our assays, an HL17 NS1 A43S construct displayed minimally increased dsRNA-binding ability that was not statistically significant (Fig. 3A).
FIG 3.
RNA binding and IFN antagonism by bat IAV NS1 mutants. (A) RNA binding by HL17 NS1 mutants. RNA binding for the indicated V5-tagged HL17 NS1 constructs was performed as described in Fig. 2A. Quantified bound protein (PD, pulldown) was normalized to input, and the bound HL17 NS1 wild type was arbitrarily set to 100%. Bars represent means and standard deviations from three independent experiments. Significance was calculated using Student's t test (****, P < 0.0001; **, P < 0.01; ns, not significant). (B) IFN antagonism by HL17 NS1 mutants. IFN-β reporter assays using the indicated V5-tagged HL17 NS1 constructs were performed as described in Fig. 1A. Significance was calculated using Student's t test comparing the appropriate HL17 NS1 WT dose with each respective mutant dose (***, P < 0.001; **, P < 0.01; *, P < 0.05; ns, not significant). (C) Intracellular localization of HL17 NS1 mutants. Immunofluorescence assays in transfected MRC5-hTERT cells using the indicated V5-tagged HL17 NS1 constructs were performed as described in the legend to Fig. 1D.
dsRNA binding by the IAV NS1 protein is essential for some strains to inhibit activation of the IFN induction pathway (32). We therefore tested the ability of our HL17 NS1 RNA-binding mutants to limit induction of the IFN-β promoter in response to SeV infection in a transfection-based assay. In contrast to wild-type (WT) HL17 NS1, both the single and double HL17 NS1 RNA-binding mutants (R39A and R39A/K42A, respectively) were highly defective in limiting activation of the IFN-β promoter. IFN-antagonism by the HL17 NS1 A43S and W189A mutants, which possess only slightly altered affinities for dsRNA binding, was not grossly affected (Fig. 3B). As expected given that the dsRNA-binding residues overlap the sole nuclear localization signal of HL17 NS1 (33), immunofluorescence analysis revealed that both the R39A and R39A/K42A mutants had an increased cytoplasmic distribution in addition to strong nuclear staining (Fig. 3C). Overall, these data indicate that the ability of the bat IAV NS1 proteins to antagonize IFN-β induction in human cells correlates with their ability to bind dsRNA.
dsRNA-binding residues of NS1 support replication of an infectious chimeric bat IAV in IFN-competent cells.
The importance of dsRNA binding by bat IAV NS1s was investigated in the context of a recombinant chimeric bat IAV featuring the six internal gene segments from the HL17NL10 virus and the HA/NA surface glycoproteins from a prototypic H7N7 IAV (strain bat C3 PAS550R+M2N31S;T70A, as described in reference 34). The multicycle growth kinetics of a chimeric bat IAV expressing wild-type HL17 NS1 were identical in both A549 cells and A549 cells stably expressing the BVDV-NPro protein, which causes the proteasomal degradation of IRF3 and thereby abrogates IFN induction (35, 36). Infectious titer yields reached ∼109 PFU/ml in both cell-lines, suggesting that the IFN system does not restrict replication of the wild-type chimeric bat IAV (Fig. 4A). However, isogenic viruses expressing HL17 NS1 with either the single (R39A) or double (R39A/K42A) dsRNA-binding mutations were highly attenuated in IFN-competent A549 cells, reaching peak titers of only ∼107 or ∼105 PFU/ml, respectively (Fig. 4B and C). This attenuation was statistically significant for both mutant viruses, particularly at 48 and 60 h postinfection (P < 0.001, Student's t test). Remarkably, replication of these dsRNA-binding-defective mutants was enhanced 100- to 1,000-fold in IFN-defective A549-NPro cells (Fig. 4B and C). For the R39A mutant virus, this was a statistically significant enhancement (P < 0.005 by Student's t test at 24, 36, and 48 h postinfection) and resulted in a replication phenotype indistinguishable from that of the wild-type virus in IFN-competent cells. These infection-based observations support our transfection-based reporter assay data that dsRNA binding by the bat IAV NS1s is required for counteracting IFN-β induction, thus preventing viral restriction by the host innate immune system.
FIG 4.
Multicycle replication kinetics of chimeric bat IAVs expressing wild-type or mutant HL17 NS1 proteins in A549 and A549-NPro cells. Cells were infected at a multiplicity of infection of 0.001 PFU/cell with the indicated virus, and supernatants were harvested at various time points prior to titration by plaque assay. (A) Bat-HA/NASC35M expressing wild-type HL17 NS1. (B) Bat-HA/NASC35M expressing HL17 NS1-R39A. (C) Bat-HA/NASC35M expressing HL17 NS1 R39A/K42A. Means and standard deviations from three independent experiments are shown.
Concluding remarks.
Despite extensive sequence differences from other IAVs, the novel bat HL17 and HL18 IAV NS1 proteins possess IFN-antagonistic abilities in human cells. This function maps to dsRNA binding by the bat IAV NS1 RBD, which is structurally highly similar to other IAV NS1 RBDs. These data emphasize the highly conserved functional importance of dsRNA binding by IAV NS1 proteins in circumventing host innate immune responses. The bat IAV NS1 protein ED lacks the ability to antagonize general host gene expression, as well as other sequences associated with IFN antagonism, such as E196 (16) or a histone mimic motif (37). Together with the surprising recent data indicating that deletion of the bat IAV NS1 ED has limited impact on virus replication or virulence in human and mouse systems (18), it may be that this domain has unique functional properties apparent only in bat hosts. Future studies on the novel bat IAV NS1s may therefore reveal new mechanisms by which these proteins modulate the bat host cell environment and could uncover the ways in which NS1 has functionally evolved in the ancient bat host.
ACKNOWLEDGMENTS
We thank Silke Stertz (University of Zurich, Switzerland) for providing Sendai virus stocks, Denis Kainov (University of Helsinki, Finland) for providing cDNA encoding A/chicken/Nigeria/OG10/2007 (H5N1) NS1, and Chris Boutell (University of Glasgow, United Kingdom) for MRC5-hTERT cells.
Some of the experiments described were begun while H.L.T. and B.G.H. were at the MRC-University of Glasgow Centre for Virus Research, United Kingdom, and were partially funded by the MRC. The work was also supported by CRIP (Center for Research on Influenza Pathogenesis), an NIAID-funded Center of Excellence for Influenza Research and Surveillance (CEIRS; contract HHSN272201400008C to A.G.-S.), the Deutsche Forschungsgemeinschaft (grant SCHW 632/15-1 to M.S.), and the Swiss National Science Foundation (grant 31003A_159993 to B.G.H.).
REFERENCES
- 1.Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, Recuenco S, Ellison JA, Davis CT, York IA, Turmelle AS, Moran D, Rogers S, Shi M, Tao Y, Weil MR, Tang K, Rowe LA, Sammons S, Xu X, Frace M, Lindblade KA, Cox NJ, Anderson LJ, Rupprecht CE, Donis RO. 2012. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci U S A 109:4269–4274. doi: 10.1073/pnas.1116200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, Chen LM, Johnson A, Tao Y, Dreyfus C, Yu W, McBride R, Carney PJ, Gilbert AT, Chang J, Guo Z, Davis CT, Paulson JC, Stevens J, Rupprecht CE, Holmes EC, Wilson IA, Donis RO. 2013. New World bats harbor diverse influenza A viruses. PLoS Pathog 9:e1003657. doi: 10.1371/journal.ppat.1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Sun X, Li Z, Liu Y, Vavricka CJ, Qi J, Gao GF. 2012. Structural and functional characterization of neuraminidase-like molecule N10 derived from bat influenza A virus. Proc Natl Acad Sci U S A 109:18897–18902. doi: 10.1073/pnas.1211037109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun X, Shi Y, Lu X, He J, Gao F, Yan J, Qi J, Gao GF. 2013. Bat-derived influenza hemagglutinin H17 does not bind canonical avian or human receptors and most likely uses a unique entry mechanism. Cell Rep 3:769–778. doi: 10.1016/j.celrep.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 5.Zhu X, Yang H, Guo Z, Yu W, Carney PJ, Li Y, Chen LM, Paulson JC, Donis RO, Tong S, Stevens J, Wilson IA. 2012. Crystal structures of two subtype N10 neuraminidase-like proteins from bat influenza A viruses reveal a diverged putative active site. Proc Natl Acad Sci U S A 109:18903–18908. doi: 10.1073/pnas.1212579109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu X, Yu W, McBride R, Li Y, Chen LM, Donis RO, Tong S, Paulson JC, Wilson IA. 2013. Hemagglutinin homologue from H17N10 bat influenza virus exhibits divergent receptor-binding and pH-dependent fusion activities. Proc Natl Acad Sci U S A 110:1458–1463. doi: 10.1073/pnas.1218509110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma W, Garcia-Sastre A, Schwemmle M. 2015. Expected and unexpected features of the newly discovered bat influenza A-like viruses. PLoS Pathog 11:e1004819. doi: 10.1371/journal.ppat.1004819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pflug A, Guilligay D, Reich S, Cusack S. 2014. Structure of influenza A polymerase bound to the viral RNA promoter. Nature 516:355–360. doi: 10.1038/nature14008. [DOI] [PubMed] [Google Scholar]
- 9.Reich S, Guilligay D, Pflug A, Malet H, Berger I, Crepin T, Hart D, Lunardi T, Nanao M, Ruigrok RW, Cusack S. 2014. Structural insight into cap-snatching and RNA synthesis by influenza polymerase. Nature 516:361–366. doi: 10.1038/nature14009. [DOI] [PubMed] [Google Scholar]
- 10.Tefsen B, Lu G, Zhu Y, Haywood J, Zhao L, Deng T, Qi J, Gao GF. 2014. The N-terminal domain of PA from bat-derived influenza-like virus H17N10 has endonuclease activity. J Virol 88:1935–1941. doi: 10.1128/JVI.03270-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Sastre A, Egorov A, Matassov D, Brandt S, Levy DE, Durbin JE, Palese P, Muster T. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324–330. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- 12.Hale BG, Randall RE, Ortin J, Jackson D. 2008. The multifunctional NS1 protein of influenza A viruses. J Gen Virol 89:2359–2376. doi: 10.1099/vir.0.2008/004606-0. [DOI] [PubMed] [Google Scholar]
- 13.Noronha JM, Liu M, Squires RB, Pickett BE, Hale BG, Air GM, Galloway SE, Takimoto T, Schmolke M, Hunt V, Klem E, Garcia-Sastre A, McGee M, Scheuermann RH. 2012. Influenza virus sequence feature variant type analysis: evidence of a role for NS1 in influenza virus host range restriction. J Virol 86:5857–5866. doi: 10.1128/JVI.06901-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayllon J, Domingues P, Rajsbaum R, Miorin L, Schmolke M, Hale BG, Garcia-Sastre A. 2014. A single amino acid substitution in the novel H7N9 influenza A virus NS1 protein increases CPSF30 binding and virulence. J Virol 88:12146–12151. doi: 10.1128/JVI.01567-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hale BG, Steel J, Medina RA, Manicassamy B, Ye J, Hickman D, Hai R, Schmolke M, Lowen AC, Perez DR, Garcia-Sastre A. 2010. Inefficient control of host gene expression by the 2009 pandemic H1N1 influenza A virus NS1 protein. J Virol 84:6909–6922. doi: 10.1128/JVI.00081-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo RL, Zhao C, Malur M, Krug RM. 2010. Influenza A virus strains that circulate in humans differ in the ability of their NS1 proteins to block the activation of IRF3 and interferon-beta transcription. Virology 408:146–158. doi: 10.1016/j.virol.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajsbaum R, Albrecht RA, Wang MK, Maharaj NP, Versteeg GA, Nistal-Villan E, Garcia-Sastre A, Gack MU. 2012. Species-specific inhibition of RIG-I ubiquitination and IFN induction by the influenza A virus NS1 protein. PLoS Pathog 8:e1003059. doi: 10.1371/journal.ppat.1003059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou B, Ma J, Liu Q, Bawa B, Wang W, Shabman RS, Duff M, Lee J, Lang Y, Cao N, Nagy A, Lin X, Stockwell TB, Richt JA, Wentworth DE, Ma W. 2014. Characterization of uncultivable bat influenza virus using a replicative synthetic virus. PLoS Pathog 10:e1004420. doi: 10.1371/journal.ppat.1004420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kainov DE, Muller KH, Theisen LL, Anastasina M, Kaloinen M, Muller CP. 2011. Differential effects of NS1 proteins of human pandemic H1N1/2009, avian highly pathogenic H5N1, and low pathogenic H5N2 influenza A viruses on cellular pre-mRNA polyadenylation and mRNA translation. J Biol Chem 286:7239–7247. doi: 10.1074/jbc.M110.203489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kochs G, Garcia-Sastre A, Martinez-Sobrido L. 2007. Multiple anti-interferon actions of the influenza A virus NS1 protein. J Virol 81:7011–7021. doi: 10.1128/JVI.02581-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Twu KY, Kuo RL, Marklund J, Krug RM. 2007. The H5N1 influenza virus NS genes selected after 1998 enhance virus replication in mammalian cells. J Virol 81:8112–8121. doi: 10.1128/JVI.00006-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sumpter R Jr, Loo YM, Foy E, Li K, Yoneyama M, Fujita T, Lemon SM, Gale M Jr. 2005. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol 79:2689–2699. doi: 10.1128/JVI.79.5.2689-2699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hale BG, Barclay WS, Randall RE, Russell RJ. 2008. Structure of an avian influenza A virus NS1 protein effector domain. Virology 378:1–5. doi: 10.1016/j.virol.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Lynch PA, Chien CY, Montelione GT, Krug RM, Berman HM. 1997. Crystal structure of the unique RNA-binding domain of the influenza virus NS1 protein. Nat Struct Biol 4:896–899. doi: 10.1038/nsb1197-896. [DOI] [PubMed] [Google Scholar]
- 25.Cheng A, Wong SM, Yuan YA. 2009. Structural basis for dsRNA recognition by NS1 protein of influenza A virus. Cell Res 19:187–195. doi: 10.1038/cr.2008.288. [DOI] [PubMed] [Google Scholar]
- 26.Hsiang TY, Zhou L, Krug RM. 2012. Roles of the phosphorylation of specific serines and threonines in the NS1 protein of human influenza A viruses. J Virol 86:10370–10376. doi: 10.1128/JVI.00732-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W, Riedel K, Lynch P, Chien CY, Montelione GT, Krug RM. 1999. RNA binding by the novel helical domain of the influenza virus NS1 protein requires its dimer structure and a small number of specific basic amino acids. RNA 5:195–205. doi: 10.1017/S1355838299981621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aramini JM, Ma LC, Zhou L, Schauder CM, Hamilton K, Amer BR, Mack TR, Lee HW, Ciccosanti CT, Zhao L, Xiao R, Krug RM, Montelione GT. 2011. Dimer interface of the effector domain of non-structural protein 1 from influenza A virus: an interface with multiple functions. J Biol Chem 286:26050–26060. doi: 10.1074/jbc.M111.248765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayllon J, Russell RJ, Garcia-Sastre A, Hale BG. 2012. Contribution of NS1 effector domain dimerization to influenza A virus replication and virulence. J Virol 86:13095–13098. doi: 10.1128/JVI.02237-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hale BG. 2014. Conformational plasticity of the influenza A virus NS1 protein. J Gen Virol 95:2099–2105. doi: 10.1099/vir.0.066282-0. [DOI] [PubMed] [Google Scholar]
- 31.Kerry PS, Ayllon J, Taylor MA, Hass C, Lewis A, Garcia-Sastre A, Randall RE, Hale BG, Russell RJ. 2011. A transient homotypic interaction model for the influenza A virus NS1 protein effector domain. PLoS One 6:e17946. doi: 10.1371/journal.pone.0017946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramos I, Carnero E, Bernal-Rubio D, Seibert CW, Westera L, Garcia-Sastre A, Fernandez-Sesma A. 2013. Contribution of double-stranded RNA and CPSF30 binding domains of influenza virus NS1 to the inhibition of type I interferon production and activation of human dendritic cells. J Virol 87:2430–2440. doi: 10.1128/JVI.02247-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melen K, Kinnunen L, Fagerlund R, Ikonen N, Twu KY, Krug RM, Julkunen I. 2007. Nuclear and nucleolar targeting of influenza A virus NS1 protein: striking differences between different virus subtypes. J Virol 81:5995–6006. doi: 10.1128/JVI.01714-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juozapaitis M, Aguiar Moreira E, Mena I, Giese S, Riegger D, Pohlmann A, Hoper D, Zimmer G, Beer M, Garcia-Sastre A, Schwemmle M. 2014. An infectious bat-derived chimeric influenza virus harbouring the entry machinery of an influenza A virus. Nat Commun 5:4448. doi: 10.1038/ncomms5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hale BG, Knebel A, Botting CH, Galloway CS, Precious BL, Jackson D, Elliott RM, Randall RE. 2009. CDK/ERK-mediated phosphorylation of the human influenza A virus NS1 protein at threonine-215. Virology 383:6–11. doi: 10.1016/j.virol.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Hilton L, Moganeradj K, Zhang G, Chen YH, Randall RE, McCauley JW, Goodbourn S. 2006. The NPro product of bovine viral diarrhea virus inhibits DNA binding by interferon regulatory factor 3 and targets it for proteasomal degradation. J Virol 80:11723–11732. doi: 10.1128/JVI.01145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marazzi I, Ho JS, Kim J, Manicassamy B, Dewell S, Albrecht RA, Seibert CW, Schaefer U, Jeffrey KL, Prinjha RK, Lee K, Garcia-Sastre A, Roeder RG, Tarakhovsky A. 2012. Suppression of the antiviral response by an influenza histone mimic. Nature 483:428–433. doi: 10.1038/nature10892. [DOI] [PMC free article] [PubMed] [Google Scholar]