ABSTRACT
A novel highly pathogenic avian influenza (HPAI) H5N8 virus, first detected in January 2014 in poultry and wild birds in South Korea, has spread throughout Asia and Europe and caused outbreaks in Canada and the United States by the end of the year. The spread of H5N8 and the novel reassortant viruses, H5N2 and H5N1 (H5Nx), in domestic poultry across multiple states in the United States pose a potential public health risk. To evaluate the potential of cross-species infection, we determined the pathogenicity and transmissibility of two Asian-origin H5Nx viruses in mammalian animal models. The newly isolated H5N2 and H5N8 viruses were able to cause severe disease in mice only at high doses. Both viruses replicated efficiently in the upper and lower respiratory tracts of ferrets; however, the clinical symptoms were generally mild, and there was no evidence of systemic dissemination of virus to multiple organs. Moreover, these influenza H5Nx viruses lacked the ability to transmit between ferrets in a direct contact setting. We further assessed viral replication kinetics of the novel H5Nx viruses in a human bronchial epithelium cell line, Calu-3. Both H5Nx viruses replicated to a level comparable to a human seasonal H1N1 virus, but significantly lower than a virulent Asian-lineage H5N1 HPAI virus. Although the recently isolated H5N2 and H5N8 viruses displayed moderate pathogenicity in mammalian models, their ability to rapidly spread among avian species, reassort, and generate novel strains underscores the need for continued risk assessment in mammals.
IMPORTANCE In 2015, highly pathogenic avian influenza (HPAI) H5 viruses have caused outbreaks in domestic poultry in multiple U.S. states. The economic losses incurred with H5N8 and H5N2 subtype virus infection have raised serious concerns for the poultry industry and the general public due to the potential risk of human infection. This recent outbreak underscores the need to better understand the pathogenesis and transmission of these viruses in mammals, which is an essential component of pandemic risk assessment. This study demonstrates that the newly isolated H5N2 and H5N8 viruses lacked the ability to transmit between ferrets and exhibited low to moderate virulence in mammals. In human bronchial epithelial (Calu-3) cells, both H5N8 and H5N2 viruses replicated to a level comparable to a human seasonal virus, but significantly lower than a virulent Asian-lineage H5N1 (A/Thailand/16/2004) virus. The results of this study are important for the evaluation of public health risk.
INTRODUCTION
On 16 December 2014, the U.S. Department of Agriculture (USDA) confirmed the presence of a novel highly pathogenic avian influenza (HPAI) H5N8 virus in a captive gyrfalcon and H5N2 virus in a northern pintail duck from Whatcom County, Washington (1). This represented the first appearance of HPAI H5 virus in the United States since 2004 when H5N2 subtype virus was confirmed on a poultry farm in Texas (2). HPAI H5N8 viruses were first reported in duck farms in eastern China in 2010, and in early 2014 a novel reassortant H5N8 virus was detected in poultry in South Korea (3–5). The novel virus, belonging to Eurasian lineage clade 2.3.4.4 (formerly clade 2.3.4.6), subsequently spread to China, Japan, and five countries in Europe (6, 7). In November 2014, the virus was detected on chicken and turkey farms in British Columbia, Canada, followed by Washington State, USA, the following month (8). The novel H5N8 viruses continue to spread to multiple regions and have been found in three North America flyways (Pacific, Central, and Mississippi), where wild-bird migrations occur (USDA, Animal and Plant Health Inspection Service [http://www.aphis.usda.gov]). In addition, genetic reassortment with circulating North American avian influenza viruses has resulted in novel HPAI viruses, including H5N2 and H5N1 subtypes. These novel reassortant viruses carry a Eurasian-origin hemagglutinin (HA) gene genetically related to H5N8 viruses detected in South Korea in 2014 and the neuraminidase (NA) gene from N8, N2, and N1 subtypes (8) and are collectively referred to as H5Nx. Because these HPAI viruses can be spread by asymptomatic wild birds (9) and cause significant mortality in domestic poultry, they pose significant international trade issues and are a potential risk for public health.
Although no human infections with the novel H5Nx viruses have yet been reported in the United States, human infections with other subtypes of avian influenza viruses have occurred following direct or close contact with infected poultry. Such virus infections in humans have been associated with a number of symptoms ranging from mild influenza-like illness to potentially fatal respiratory disease (10–12). Since information about biologic and molecular properties is not sufficient to predict virulence and transmissibility of HPAI viruses in humans, it is imperative that their pathobiological properties be examined using mammalian models. We evaluated the virulence and transmission of two novel HPAI viruses: A/northern pintail/Washington/40964/2014 (Pin/WA/40964; H5N2) and A/gyrfalcon/Washington/41088-6/2014 (Gyr/WA/41088-6; H5N8) isolated in Washington State in mid-December 2014. The H5N8 and H5N2 viruses were capable of causing severe disease in mice at high inoculation doses; however, compared to highly virulent Asian H5N1 viruses (13), these novel H5Nx viruses showed less infectivity and lethality in mice and ferrets. Unlike seasonal influenza H3N2 viruses, which mainly infect the upper respiratory tract of ferrets (14), both H5N8 and H5N2 viruses could be detected in lung tissues. The H5Nx viruses lacked the ability to transmit among cohoused ferrets, a characteristic feature of influenza A H5 viruses. Understanding the pathogenesis of H5Nx viruses and their capacity for human-to-human transmission is a critical requirement for guidance of public health responses.
MATERIALS AND METHODS
Viruses.
Virus stocks of HPAI A/northern pintail/Washington/40964/2014 H5N2 (Pin/WA/40964), A/Gyrfalcon/Washington/41088-6/2014 H5N8 (Gyr/WA/41088-6), and A/Thailand/16/2004 H5N1 (Th/16) virus were propagated in the allantoic cavity of 10-day-old embryonated hens' eggs at 37°C for 24 to 26 h. Allantoic fluid was pooled from multiple eggs, clarified by centrifugation, and frozen in aliquots at −80°C. The seasonal H1N1 virus A/Brisbane/59/2007 (Bris/59) was propagated in Madin-Darby canine kidney (MDCK; American Type Culture Collection [ATCC], Manassas, VA) cells at 37°C for 48 h. To determine the 50% egg infectious dose (EID50) for each virus stock, eggs were inoculated with serially diluted virus and EID50 was calculated using the Reed and Muench method (15). Viruses were additionally tested by standard plaque assay in MDCK cells for determination of the titer in PFU/ml (16). The virus stocks were sequenced, and real-time reverse transcription-PCR exclusivity tests were performed to rule out the presence of other subtypes of influenza virus. All research with HPAI viruses was conducted under biosafety level 3 containment, including enhancements required by the U.S. Department of Agriculture and Select Agent Program outlined in Biosafety in Microbiological and Biomedical Laboratories (17).
Mouse experiments.
All animal experiments were performed under the guidance of the Centers for Disease Control and Prevention's Institutional Animal Care and Use Committee and were conducted in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited animal facility. Female BALB/c mice (Charles River Laboratories, Wilmington, MA), 6 to 8 weeks old, were anesthetized intraperitoneally with 0.2 ml of 2,2,2-tribromoethanol in tert-amyl alcohol (Avertin; Aldrich Chemical Co., Milwaukee, WI) and inoculated intranasally (i.n.) with 50 μl of virus diluted in phosphate-buffered saline (PBS). The 50% mouse infectious dose (MID50) and the 50% mouse lethal dose (MLD50) were determined by inoculating groups of eight mice with serial 10-fold dilutions of virus ranging from 100 to 107 EID50. Three mice from each group were euthanized on day 3 postinoculation (p.i.), and the lung and brain tissues were collected to determine the virus titers. The remaining mice from each group were monitored daily for clinical signs and weight loss for 14 days p.i. Weight loss was determined from five mice per group on the dates indicated. The percent weight loss was determined from the mean original starting weight. The MID50 values, as determined by the presence of virus in lung tissues, and the MLD50 values were calculated using Reed and Muench method (15). Additional groups of four mice were inoculated i.n. with 103, 105, and 106 EID50 of each virus and euthanized 6 days p.i., with lung and brain tissues collected for virus titration in eggs. Any mouse which lost ≥25% of initial body weight was euthanized.
Ferret experiments.
Male Fitch ferrets (Triple F Farms, Sayre, PA) 6 months of age were used for the study. Each animal was serologically negative for currently circulating influenza viruses as determined by standard hemagglutination inhibition assay. During experimentation ferrets were housed in Duo-Flo Bioclean mobile units (Lab Products Incorporated, Seaford, DE). Ferrets (three ferrets per virus) were inoculated i.n. with 106 EID50 of virus diluted in 1 ml of PBS. The following day, a serologically naive ferret was placed in the same cage as each inoculated ferret for the assessment of virus transmission between ferrets in direct contact (18). The ferret pairs were observed daily for clinical signs of infection, and nasal washes and rectal swabs were collected every 2 days for 2 weeks. Three additional ferrets were inoculated and then euthanized on day 3 p.i. for the assessment of virus replication and systemic spread, as previously described (13).
Cell culture and viral replication.
Human airway epithelial Calu-3 cells, obtained from the ATCC, were cultured on 12-well plate transwells as described previously (19). Briefly, polarized Calu-3 cells grown on transwells were inoculated apically in triplicate with HPAI H5 or seasonal H1N1 viruses at a multiplicity of infection (MOI) of 0.01 for 1 h, washed, and then incubated at 33°C or 37°C in a 5% CO2 atmosphere. Culture supernatants were collected at 2, 16, 24, 48, and 72 h p.i., and virus titers were determined in MDCK cells by standard plaque assay.
Nucleotide sequence accession numbers.
The GenBank accession numbers are as follows: A/northern pintail/Washington/40964/2014 (H5N2), AJE30344; and A/Gyrfalcon/Washington/41088-6/2014 (H5N8), AJE30333.
RESULTS
Pathogenicity of H5N2 and H5N8 viruses in mice.
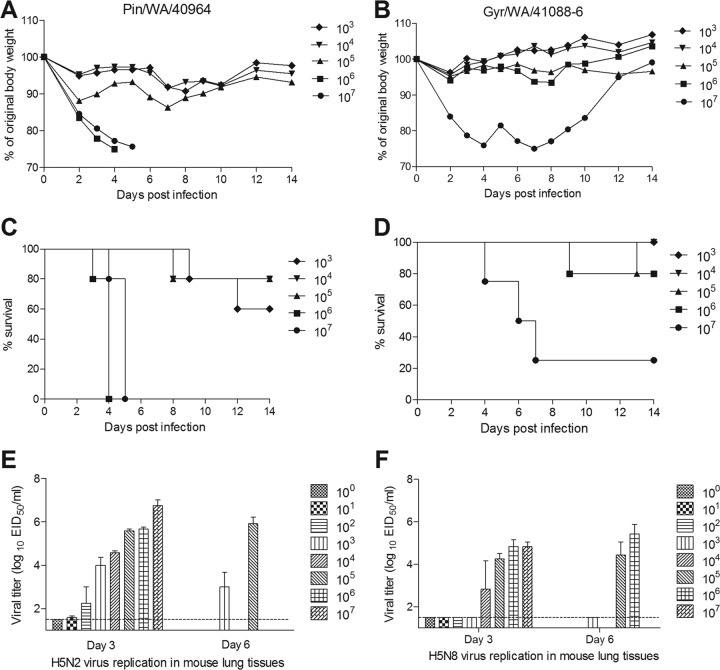
Mice provide a reliable mammalian model for the study of HPAI H5 viral pathogenesis (20). Groups of BALB/c mice were i.n. inoculated with serial 10-fold dilutions of virus to determine viral MID50 and 50% lethal dose (LD50) titers. Both H5N2 Pin/WA/40964 and H5N8 Gyr/WA/41088-6 viruses replicated in mouse lungs without prior adaptation but were lethal for mice only at high inoculation titers. Mice inoculated with ≥106 EID50 of Pin/WA/40964 virus exhibited substantial weight loss and succumbed to infection by day 6 p.i. (Fig. 1A and C). Virus titers in the lungs of these mice were 105.7 to 106.8 EID50/ml on day 3 p.i. (Fig. 1E). Mice inoculated with 105 EID50 of Pin/WA/40964 virus exhibited 20% mortality with lung virus titers ranging from 104.3 to 105.8 EID50/ml. The MID50 and MLD50 values for Pin/WA/40964 virus were calculated to be 102.0 and 105.0 EID50, respectively (Table 1). For the H5N8 Gyr/WA/41088-6 virus, severe morbidity and significant mortality were observed in mice inoculated with the highest dose (107 EID50) of virus (Fig. 1B and D). Virus titers in the lungs of these mice were 104.8 EID50/ml and 3 of 4 mice succumbed to infection. With the exception of one animal that was euthanized for left side paralysis on day 13, mice challenged with 106 EID50 did not exhibit signs of severe disease. Nonetheless, infectious virus could be detected in the lungs of mice inoculated with 104 to 107 EID50 of virus (Fig. 1F). The MID50 and MLD50 values for Gyr/WA/41088-6 virus were 104.3 EID50 and 106.4 EID50, respectively (Table 1). In general, systemic spread of infectious virus to the brain was not detected following either H5N8 or H5N2 virus infection. Only one mouse inoculated with 106 EID50 of Gyr/WA/41088-6 virus showed traces of infectious virus (102.25 EID50/ml) in brain tissue on day 6 p.i. Taken together, these results indicate that both H5Nx viruses have the ability to replicate in mice without prior adaptation but generally demonstrated a moderate-pathogenicity phenotype compared to other avian HPAI H5 viruses (13).
FIG 1.
Pathogenicity of H5N2 and H5N8 viruses in mice. Groups of five mice were intranasally inoculated with serial 10-fold dilutions, ranging from 103 to 107 EID50 of Pin/WA/40964 (H5N2) or Gyr/WA/41088-6 (H5N8) virus and observed for signs of morbidity and mortality for 14 days. Any mouse that lost ≥25% of initial body weight was euthanized. The percentage mean maximum weight loss (A and B) and percent survival (C and D) are shown. Additional groups of three mice infected with serial 10-fold dilutions ranging from 100 to 107 EID50 of H5N2 (Pin/WA/40964) (E) and H5N8 (Gyr/WA/41088-6) (F) were euthanized 3 days p.i., and groups of four mice infected with 103, 105, or 106 EID50 of H5Nx virus were euthanized 6 days p.i. The lung tissues were collected for virus titer determination. Virus titers are presented as the log10 EID50/ml. The limit of detection is 1.5 log10 EID50/ml. Mice infected with the highest dose of Pin/WA/40964 virus were euthanized before day 6 p.i. due to substantial weight loss, therefore lung titers are not represented for 106 EID50.
TABLE 1.
Virulence of H5N2 and H5N8 viruses in mice
| Virus | Stock titer (log10 EID50/ml)a | Wt loss (%)b | Mean viral titer (log10 EID50/ml) in lungs ± SDc |
Dose (log10 EID50)d |
||||
|---|---|---|---|---|---|---|---|---|
| Day 3 |
Day 6 |
|||||||
| 105 | 106 | 105 | 106 | MID50 | MLD50 | |||
| Pin/WA/40964 | 8.5 | 25.0 | 5.8 ± 0.1 | 5.7 ± 0.1 | 4.3 ± 0.4 | ND | 2.0 | 5.0 |
| Gyr/WA/41088-6 | 9.5 | 6.5 | 4.3 ± 0.4 | 4.8 ± 0.6 | 4.4 ± 1.2 | 5.4 ± 0.9 | 4.3 | 6.4 |
Titer of stock viruses prepared in 10-day-embryonated egg.
Percent mean maximum weight loss (five mice per group) after inoculation with 106 EID50 of virus.
Mean viral titers after inoculation with 106 or 105 EID50 of virus on day 3 (three mice per group) or day 6 (four mice per group) p.i. are expressed as the log10 EID50/ml. ND, not determined (mice were euthanized before day 6 p.i. due to severe morbidity).
MID50 and MLD50 are expressed as the log10 EID50 required to give one MLD50 or MLD50, respectively.
Pathogenesis and transmission of H5N2 and H5N8 viruses in ferrets.
Evaluating the capacity for transmission of emerging influenza viruses is a key component of public health risk assessment. Ferrets are an excellent model for studying influenza transmission, as they exhibit clinical signs of disease that are similar to those seen during human influenza infection (21). Six ferrets per group were i.n. inoculated with 106 EID50 of Pin/WA/40964 or Gyr/WA/41088-6 virus, and three ferrets were observed for signs of infection. Nasal washes and rectal swabs were collected every 2 days to measure virus replication. The remaining three ferrets in each virus group were humanely euthanized on day 3 p.i. to assess viral spread in organs. Ferrets inoculated with Pin/WA/40964 (H5N2) virus exhibited mild lethargy during the peak of infection, minimal weight loss (1.2%), and no overt respiratory symptoms (Table 2). Mean maximum increase in body temperature was 1.4°C above baseline. Nasal wash titers of Pin/WA/40964 virus were in the range of 102.0 to104.8 EID50/ml on days 1, 3, and 5 p.i.; two of three ferrets had detectable virus on day 7 p.i., and virus was cleared from all animals by day 9 p.i. (Table 2 and Fig. 2A). Pin/WA/40964 virus was detected in nasal turbinates, trachea, and lungs of all inoculated ferrets at titers ranging from 103.5 to 105.5 EID50/g or ml of tissue (Fig. 3A). Virus was not detected in rectal swabs collected up to 9 days p.i. (data not shown) or in intestinal tissue (day 3 p.i.), indicating that the virus did not spread to or replicate in the gastrointestinal tract. Low virus titers (<103 EID50/g) were detected in the olfactory bulbs of all three ferrets; however, the virus was not otherwise detected in the brain, blood, or other tissues outside the respiratory tract.
TABLE 2.
Clinical signs and replication of H5N2 and H5N8 viruses in ferrets
| Virus | Wt lossa | Temp increase (°C)b | No. of animals exhibiting symptoms/total no. of animals examined |
Mean nasal titer (log10 EID50/ml) ± SDe | |
|---|---|---|---|---|---|
| Sneezingc | Lethargyd | ||||
| Pin/WA/40964 | 1.2 (2/3) | 1.4 | 0/3 | 1.1 (3/3) | 4.2 ± 0.8 (5) |
| Gyr/WA/41088-6 | 1.2 (1/3) | 1.3 | 1/3 | 1 (1/3) | 3.1 ± 1 (1) |
Percent mean maximum weight loss observed during the first 10 days p.i. The number of ferrets exhibiting weight loss is indicated in parentheses.
Mean maximum temperature increase over baseline (37.5 to 39.7°C) during the first 10 days p.i.
Number of ferrets that exhibited sneezing during the first 10 days p.i.
The relative inactivity index of ferrets during the first 10 days p.i. is presented. The number of ferrets displaying lethargy is indicated in parentheses.
The mean maximum nasal wash titer is expressed as log10 EID50/ml; the day postinoculation is indicated in parentheses. The limit of detection was 1.5 log10 EID50/ml.
FIG 2.
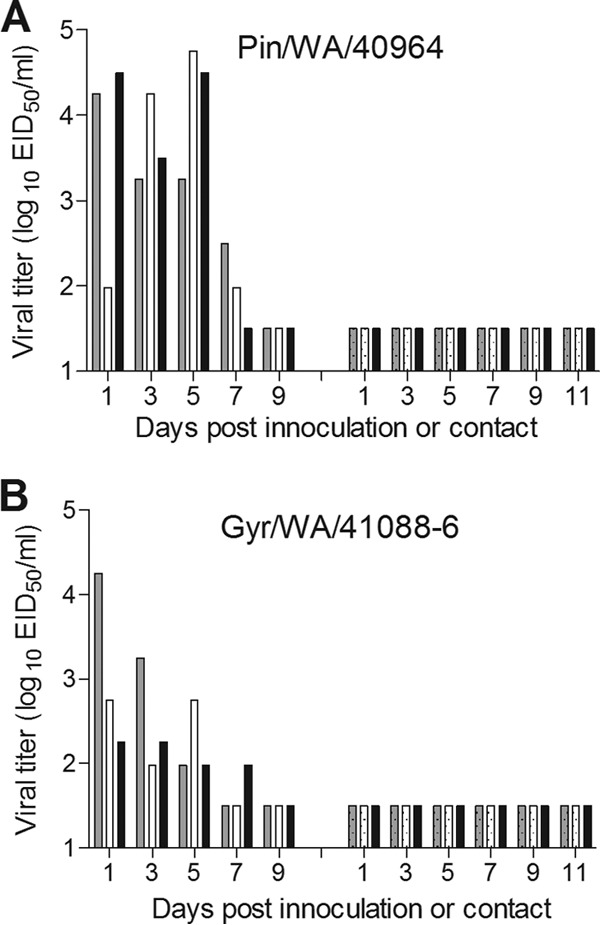
Transmissibility of H5N2 and H5N8 influenza viruses in ferrets. Groups of three ferrets were inoculated i.n. with 106 EID50 of H5N2 (Pin/WA/40964) (A) and H5N8 (Gyr/WA/41088-6) (B) virus. The following day, a serologically naive ferret was placed in the same cage with each inoculated ferret for the assessment of virus transmission between ferrets in direct contact. Nasal washes were collected from each ferret on the indicated days postinoculation or after contact. The results from individual ferrets are presented. The virus titers are presented as log10 EID50/ml. The limit of detection is 1.5 log10 EID50/ml.
FIG 3.
Detection of H5N2 and H5N8 viruses in ferret tissues. Groups of three ferrets each were inoculated i.n. with 106 EID50 of H5N2 (Pin/WA/40964) (A) and H5N8 (Gyr/WA/41088-6) (B) virus. Tissues were collected on day 3 p.i. to assess virus titers. Blood and nasal turbinate virus titers are presented as the log10 EID50/ml, and titers for the kidney, spleen, liver, intestines (pooled duodenum, jejuno-ileal loop, and descending colon), olfactory bulb (BnOB), brain (pooled anterior and posterior brain), lungs, and trachea are presented as the log10 EID50/g of tissue. The limit of detection is 1.5 log10 EID50/ml.
The Gyr/WA/41088-6 (H5N8) virus caused mild lethargy, a moderate increase in body temperature (1.3°C above baseline), minimal weight loss (1.2%), and no overt respiratory symptoms (Table 2). The amount of Gyr/WA/41088-6 virus detected in nasal washes was generally lower than that of Pin/WA/40964-infected ferrets, since titers on days 1, 3, and 5 p.i. were in the range of 102.0 to 104.3 EID50/ml. One of the three ferrets had detectable virus in nasal wash at day 7 p.i. but was cleared by day 9 p.i. (Fig. 2B). Infectious virus was detected in nasal turbinates of all three ferrets infected with Gyr/WA/41088-6 virus with a mean titer of 103.0 EID50/ml. Two of three ferrets in this group had virus present in trachea and lung tissues at titers ranging from 102.2 to 104.7 EID50/g and in the olfactory bulb at low titers (≤103 EID50/g). Although virus was not detected in rectal swabs from any of the ferrets (data not shown), low titers of infectious virus were detected in intestinal tissues of two animals (Fig. 3B).
To evaluate the ability of Pin/WA/40964 and Gyr/WA/41088-6 viruses to transmit in the ferret model, a serologically naive ferret was cohoused with each of three inoculated ferrets. Contact animals were observed for signs of infection, while nasal wash and rectal swab samples were collected every 2 days after exposure to infected animals to test for virus shedding. None of the contact ferrets exhibited signs of infection, and no virus was detected in nasal wash or rectal swab samples among contact animals. Furthermore, convalescent-phase sera collected from contact ferrets and tested by hemagglutination inhibition assay confirmed that Pin/WA/40964 (Fig. 2A) and Gyr/WA/41088-6 viruses (Fig. 2B) were not transmitted between the ferret pairs (data not shown). Taken together, these two novel HPAI viruses showed relatively mild virulence characteristics in ferrets and no transmission in the direct contact model.
Replication kinetics of H5N2 and H5N8 viruses in human Calu-3 cells.
We next investigated the replication efficiency of the H5Nx viruses in a relevant cell line, Calu-3, derived from human bronchial epithelium. Calu-3 cells were chosen because, when grown on transwell inserts, they form tight, polar monolayers that share some similarities with human airway epithelium, the primary site of replication of influenza viruses in humans (16). Avian influenza viruses are adapted for growth at 40 to 41°C, the temperature of the avian enteric tract, and a proposed mammalian adaptation marker for avian influenza viruses is the ability to replicate at lower temperatures (33°C) found in the mammalian upper airway (22, 23). The replication kinetics of Pin/WA/40964 (H5N2) and Gyr/WA/41088-6 (H5N8) viruses were assessed in Calu-3 cells at 33 and 37°C and compared to the replication kinetics of the seasonal H1N1 virus, A/Brisbane/59/2007 (Bris/59) and HPAI H5N1 virus A/Thailand/16/2004 H5N1 (Th/16), which is highly virulent for ferrets and mice (13). In general, all viruses tested resulted in a productive infection in Calu-3 cells at both 37°C and 33°C; all viruses reached high titers, ca. 107 to 108 PFU/ml, by 72 h p.i. (Fig. 4). At 37°C, the Pin/WA/40964 and Gyr/WA/41088-6 viruses replicated with efficiency equal to that of the seasonal Bris/59 virus, but significantly lower (P < 0.05) than titers recovered from H5N1 (Th/16) virus-infected cultures (Fig. 4A). At 33°C, in general, the avian virus (H5N1, H5N2, and H5N8) replication was delayed and less efficient at 33°C than at 37°C, especially during the early replication cycles. Only the seasonal Bris/59 virus replicated equally well at both temperatures and reached similar titers at the time points examined (Fig. 4A versus B). Similar to the data at 37°C, at the lower 33°C temperature, the H5Nx viruses replicated to significantly lower (P < 0.001) titers compared to H5N1 virus. In addition, the H5Nx virus titers were significantly lower than that observed for Bris/59 virus at 16, 24, and 48 h p.i. (P < 0.01) Taken together, these results demonstrate that in human airway cells H5Nx viruses do not replicate as well as the virulent H5N1 virus at both 37 and 33°C temperatures and the seasonal influenza virus at the lower temperatures (33°C) found in the upper airways of mammals.
FIG 4.
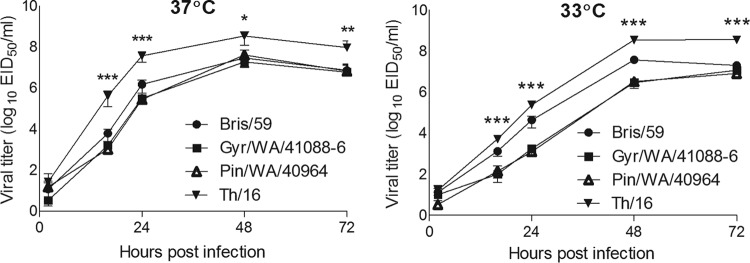
Replication kinetics of influenza viruses in polarized human airway epithelial cells. Calu-3 cells grown on transwells were infected apically in triplicate at an MOI of 0.01 with Pin/WA/40964 (H5N2), Gyr/WA/41088-6 (H5N8), Bris/59 (H1N1), or Th/16 (H5N1). The cells were incubated at 37°C (A) or 33°C (B), and culture supernatants were collected at 2, 16, 24, 48, and 72 h p.i. for virus titer determination by standard plaque assay. Asterisks indicate the statistical significance between Th/16 and other tested H5Nx viruses (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
DISCUSSION
Since their first appearance in Asia in 1997, HPAI H5N1 influenza viruses have spread to multiple countries on several continents and caused considerable damage to the poultry industry and loss of human life (24). More than 800 confirmed human cases of avian influenza (H5N1) have been reported to the World Health Organization since 2003, almost 60% of which were fatal (25). Since 1997, the HA genes of H5N1 have diversified into multiple clades, including the novel clade 2.3.4.4 viruses (6, 7, 26, 27). There has been an increased prevalence of clade 2.3.4.4 viruses in domestic and wild birds since 2010, and now the virus has reached North America (8). The H5N8 virus isolated in the United States showed 99% nucleotide identity with viruses isolated in South Korea (8). Since its first appearance in North America, the H5N8 virus reassorted in the Pacific flyway with North American avian influenza viruses resulting in novel HPAI viruses, H5N2 and H5N1 (8). These highly pathogenic H5Nx viruses have caused several outbreaks in domestic poultry in multiple states in the United States, therefore raising significant concerns in the poultry industry and general public due to the potential risk of human infection. In this study, we investigated the replication kinetics, pathogenesis, and transmission of Pin/WA/40964 (H5N2) and Gyr/WA/41088-6 (H5N8) viruses isolated from birds in Washington State in December 2014.
Using this mouse model, we have demonstrated that viruses with an MLD50 of >106.5 are considered of low virulence, while viruses with an MLD50 of <103 are considered to be highly virulent (28). Based on these criteria the novel H5N2 virus (MLD50 = 105.0 EID50) and H5N8 virus (MLD50 = 106.4 EID50) exhibited moderate pathogenicity in mice (13). Similarly, a low- to moderate-pathogenicity phenotype was observed in the ferret model. Both viruses were shed in respiratory tract secretions for up to 7 days p.i., but all animals recovered fully from the infection. Of note, infection with both H5Nx viruses led to detectable virus in the ferret lungs. Influenza virus replication in lung tissue is thought to be a pathogenic trait that may contribute to the severity of H5Nx infection, if humans became infected by close contact with H5Nx-infected poultry. Overall, our results are consistent with previous pathogenicity studies with clade 2.3.4.4 H5N8 viruses isolated from South Korea and the Netherlands (29), viruses which also exhibited low to moderate virulence in mammalian models (4, 30). Furthermore, the virus titers we observed in ferret respiratory tract tissues were comparable to those observed with other avian influenza virus strains that exhibited moderate pathogenicity in ferrets (13).
In this study, neither of the H5Nx viruses had the ability to efficiently spread systemically in ferrets, although virus was detected in the gastrointestinal tract of some ferrets. Virus detected in the ferret gastrointestinal tract likely originates from virus swallowed during inoculation and not from systemic spread of the H5N1 virus (31). Conversely, a previous study reported low levels of H5N8 virus in spleen, liver, and brains of ferrets infected with A/mallard duck/Korea/W452/2014 virus (4), indicating that some of the currently circulating H5N8 strains have the ability to disseminate to multiple extrapulmonary tissues in mammals. Virus detection in the olfactory bulb, but not the anterior or posterior brain, of H5N2 and H5N8 viruses in ferrets day 3 p.i. is typical among both human and avian influenza viruses, which replicate to high titers in the nasal turbinates, and likely does not represent neurotropism (32). Virulence and the ability to replicate outside the respiratory tract have been, in part, linked with the presence of a multibasic amino acid cleavage site in the HA (33). The HA of the novel H5Nx viruses possess multibasic residues at the HA cleavage site, albeit fewer basic residues (PLRERRRKR/GLF) compared to the highly virulent H5N1 Thai/16 virus, which has one additional arginine basic residue (PQRERRRKKR/GLF) upstream of the furin recognition motif (RXXR). It is currently not clear whether reduced number of basic residues at the HA cleavage site contributes to the reduced virulence of H5Nx virus in the mammalian models.
One of the factors that may limit avian influenza virus infection in humans is the inability to efficiently replicate at temperatures found in the upper human respiratory tract (23). Unlike human influenza viruses, which replicate efficiently in respiratory tracts where the temperatures range from 32°C in the upper respiratory tract to 37°C in lower respiratory tract (34, 35), avian influenza viruses have adapted to replicate at the higher temperatures, 40 to 41°C, of the avian enteric tract (36). The upper respiratory tract provides a large surface area of susceptible cells and is usually the initial site of infection and likely the predominant site of influenza virus replication in mammalian species (37). Therefore, in order to replicate efficiently and spread among humans, it is believed that avian influenza viruses need to acquire the ability to replicate efficiently at lower temperatures. Replication kinetics of the H5N2 and H5N8 viruses in polarized Calu-3 cells at 37°C were similar to that of the human seasonal virus Bris/59; however, these viruses exhibited significantly delayed replication kinetics at 33°C, suggesting that that H5Nx viruses have not yet acquired all of the features required for mammalian host adaptation. Efficiency of replication of avian influenza viruses at lower temperatures was previously linked to an amino acid substitution Glu627Lys in PB2 (38). These results were subsequently supported by a study showing increased replication of H5N1 viruses possessing Lys at position 627 in PB2 in the upper respiratory tract of mice (39). Both tested H5Nx viruses possess Glu at the position 627 in PB2 which could contribute to inefficient replication at lower temperatures. HPAI H5N1 Thai/16 virus, which has Lys at the 627 position of PB2, replicated to higher titers at 33°C than Pin/WA/40964 and Gyr/WA/41088-6, but all three avian viruses exhibited delayed replication kinetics at 33°C compared to 37°C. Scull et al. demonstrated that amino acid substitution Glu627Lys in PB2 does not entirely account for conferring temperature dependency in mammalian cells. It was suggested that viruses bearing avian or avian-like surface glycoproteins have a reduced capacity to establish productive infection at the temperature of the human proximal airways (36).
Avian-adapted influenza viruses bind preferentially to the α-2,3-linked sialic acid receptors abundant in the gastrointestinal tract of birds, while human influenza viruses preferentially bind to α-2,6-linked sialic acid receptors on cells found in the upper respiratory tract (40). The distribution of influenza virus receptors in the ferret respiratory tract resembles the airways of humans which may contribute to the utility of this animal model (41, 42). However, several differences between the ferret and human respiratory tract have been identified which could influence the outcome of infection. For example, the presence of Sda epitopes that carry α-2,3-NeuAc in the ferret respiratory tract is hypothesized to reduce potential binding sites for avian influenza viruses (43, 44). The HA of H5Nx viruses possess the key residues Gln226 and Gly228 (H3 numbering) required for 2,3-linked sialic acid binding, indicating the virus has not adapted toward human-type receptor specificity. Interestingly, the novel H5N8 and H5N2 viruses circulating in United States have a Thr-to-Ala substitution in HA at position 160, which results in a loss of a glycosylation motif at asparagine residue 158. The removal of this glycosylation site has previously been shown to be critical for H5 subtype influenza viruses to gain enhanced receptor binding affinity to human-like receptors and transmission in mammalian hosts (45, 46). The weak binding of the H5Nx viruses to human-like receptors (30, 47) along with reduced replication efficiency at temperatures found in the upper respiratory tract of humans likely contribute to the poor transmission of the H5Nx viruses. Others have reported similar transmission results for clade 2.3.4.4 HPAI H5N8 viruses in ferrets (4) and guinea pigs (47). However, an H5N2 virus isolated in China (A/duck/Eastern China/1112/2011) that had high nucleotide identity with H5N8 viruses circulating in South Korea was able to efficiently transmit between cohoused guinea pigs (47).
Overall, despite being highly pathogenic in chickens and turkeys, the novel H5Nx viruses exhibited low to moderate virulence in mammals. However, as these and other H5 viruses continue to circulate in wild birds and cause widespread outbreaks in commercial poultry operations and backyard flocks, the likelihood of human exposure increases. Each exposure event serves as an opportunity for these viruses to evolve and adapt to humans increasing the potential for an H5 pandemic. Moreover, the ability of these viruses to reassort with other circulating influenza A viruses raises the concern that they may reassort with a human influenza lineage virus and increase their adaptation to mammalian hosts. Influenza virus surveillance and timely risk assessment of newly emerging strains is critical to pandemic preparedness and assessing the threat to public health.
ACKNOWLEDGMENTS
We thank Mia Kim-Torchetti at the National Veterinary Services Laboratories for facilitating access to viruses.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
J.A.P.-P., X.S., and H.M.C. are supported by the Oak Ridge Institute for Science and Education.
REFERENCES
- 1.Jhung MA, Nelson DI, Centers for Disease Control and Prevention. 2015. Outbreaks of avian influenza A (H5N2), (H5N8), and (H5N1) among birds–United States, December 2014-January 2015. MMWR Morb Mortal Wkly Rep 64:111. [PMC free article] [PubMed] [Google Scholar]
- 2.Lee CW, Swayne DE, Linares JA, Senne DA, Suarez DL. 2005. H5N2 avian influenza outbreak in Texas in 2004: the first highly pathogenic strain in the United States in 20 years? J Virol 79:11412–11421. doi: 10.1128/JVI.79.17.11412-11421.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee YJ, Kang HM, Lee EK, Song BM, Jeong J, Kwon YK, Kim HR, Lee KJ, Hong MS, Jang I, Choi KS, Kim JY, Lee HJ, Kang MS, Jeong OM, Baek JH, Joo YS, Park YH, Lee HS. 2014. Novel reassortant influenza A(H5N8) viruses, South Korea, 2014. Emerg Infect Dis 20:1087–1089. doi: 10.3201/eid2102.141268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HM, Kim CK, Lee NJ, Chu H, Kang C, Kim K, Lee JY. 2015. Pathogenesis of novel reassortant avian influenza virus A (H5N8) isolates in the ferret. Virology 481:136–141. doi: 10.1016/j.virol.2015.02.042. [DOI] [PubMed] [Google Scholar]
- 5.Kang HM, Lee EK, Song BM, Jeong J, Choi JG, Jeong J, Moon OK, Yoon H, Cho Y, Kang YM, Lee HS, Lee YJ. 2015. Novel reassortant influenza A(H5N8) viruses among inoculated domestic and wild ducks, South Korea, 2014. Emerg Infect Dis 21:298–304. doi: 10.3201/eid2102.141268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao K, Gu M, Zhong L, Duan Z, Zhang Y, Zhu Y, Zhao G, Zhao M, Chen Z, Hu S, Liu W, Liu X, Peng D, Liu X. 2013. Characterization of three H5N5 and one H5N8 highly pathogenic avian influenza viruses in China. Vet Microbiol 163:351–357. doi: 10.1016/j.vetmic.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 7.Donis RO, Smith GJ, World Health Organization/World Organization for Animal Health/Agriculture Organization H4 Evolution Working Group. 2015. Nomenclature updates resulting from the evolution of avian influenza A(H5) virus clades 2.1.3.2a, 2.2.1, and 2.3.4 during 2013-2014. Influenza Other Respir Viruses 9:271–276. doi: 10.1111/irv.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hon S. Ip MKT, Rocio Crespo, Paul Kohrs, Paul DeBruyn, Kristin G.Mansfield, Timothy Baszler, Lyndon Badcoe, Barbara Bodenstein, Valerie Shearn-Bochsler, Mary Lea Killian, Janice C.Pedersen, Nichole Hines, Thomas Gidlewski, DeLiberto T, Sleeman JM. 2015. Novel Eurasian highly pathogenic avian influenza A H5 viruses in wild birds, Washington, USA, 2014. Emerg Infect Dis 21:886–90. doi: 10.3201/eid2105.142020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong J, Kang HM, Lee EK, Song BM, Kwon YK, Kim HR, Choi KS, Kim JY, Lee HJ, Moon OK, Jeong W, Choi J, Baek JH, Joo YS, Park YH, Lee HS, Lee YJ. 2014. Highly pathogenic avian influenza virus (H5N8) in domestic poultry and its relationship with migratory birds in South Korea during 2014. Vet Microbiol 173:249–257. doi: 10.1016/j.vetmic.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Liu Q, Liu DY, Yang ZQ. 2013. Characteristics of human infection with avian influenza viruses and development of new antiviral agents. Acta Pharmacol Sinica 34:1257–1269. doi: 10.1038/aps.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arzey GG, Kirkland PD, Arzey KE, Frost M, Maywood P, Conaty S, Hurt AC, Deng YM, Iannello P, Barr I, Dwyer DE, Ratnamohan M, McPhie K, Selleck P. 2012. Influenza virus A (H10N7) in chickens and poultry abattoir workers, Australia. Emerg Infect Dis 18:814–816. doi: 10.3201/eid1805.111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, de Vries RP, Tzarum N, Zhu X, Yu W, McBride R, Paulson JC, Wilson IA. 2015. A human-infecting H10N8 influenza virus retains a strong preference for avian-type receptors. Cell Host Microbe 17:377–384. doi: 10.1016/j.chom.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maines TR, Lu XH, Erb SM, Edwards L, Guarner J, Greer PW, Nguyen DC, Szretter KJ, Chen LM, Thawatsupha P, Chittaganpitch M, Waicharoen S, Nguyen DT, Nguyen T, Nguyen HH, Kim JH, Hoang LT, Kang C, Phuong LS, Lim W, Zaki S, Donis RO, Cox NJ, Katz JM, Tumpey TM. 2005. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J Virol 79:11788–11800. doi: 10.1128/JVI.79.18.11788-11800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maines TR, Jayaraman A, Belser JA, Wadford DA, Pappas C, Zeng H, Gustin KM, Pearce MB, Viswanathan K, Shriver ZH, Raman R, Cox NJ, Sasisekharan R, Katz JM, Tumpey TM. 2009. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science 325:484–487. doi: 10.1126/science.1177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed L, Muench HA. 1938. A simple method of estimating fifty per cent endpoints. Am J Hyg 27:493–497. [Google Scholar]
- 16.Zeng H, Goldsmith C, Thawatsupha P, Chittaganpitch M, Waicharoen S, Zaki S, Tumpey TM, Katz JM. 2007. Highly pathogenic avian influenza H5N1 viruses elicit an attenuated type I interferon response in polarized human bronchial epithelial cells. J Virol 81:12439–12449. doi: 10.1128/JVI.01134-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chosewood LC, Wilson DE, Centers for Disease Control and Prevention. 2009. Biosafety in microbiological and biomedical laboratories, 5th ed Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institutes of Health, Washington, DC. [Google Scholar]
- 18.Maines TR, Chen LM, Matsuoka Y, Chen H, Rowe T, Ortin J, Falcon A, Nguyen TH, Mai le Q, Sedyaningsih ER, Harun S, Tumpey TM, Donis RO, Cox NJ, Subbarao K, Katz JM. 2006. Lack of transmission of H5N1 avian-human reassortant influenza viruses in a ferret model. Proc Natl Acad Sci U S A 103:12121–12126. doi: 10.1073/pnas.0605134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng H, Pappas C, Katz JM, Tumpey TM. 2011. The 2009 pandemic H1N1 and triple-reassortant swine H1N1 influenza viruses replicate efficiently but elicit an attenuated inflammatory response in polarized human bronchial epithelial cells. J Virol 85:686–696. doi: 10.1128/JVI.01568-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belser JA, Tumpey TM. 2013. H5N1 pathogenesis studies in mammalian models. Virus Res 178:168–185. doi: 10.1016/j.virusres.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belser JA, Katz JM, Tumpey TM. 2011. The ferret as a model organism to study influenza A virus infection. Dis Models Mech 4:575–579. doi: 10.1242/dmm.007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng H, Goldsmith CS, Maines TR, Belser JA, Gustin KM, Pekosz A, Zaki SR, Katz JM, Tumpey TM. 2013. Tropism and infectivity of influenza virus, including highly pathogenic avian H5N1 virus, in ferret tracheal differentiated primary epithelial cell cultures. J Virol 87:2597–2607. doi: 10.1128/JVI.02885-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Hoeven N, Pappas C, Belser JA, Maines TR, Zeng H, Garcia-Sastre A, Sasisekharan R, Katz JM, Tumpey TM. 2009. Human HA and polymerase subunit PB2 proteins confer transmission of an avian influenza virus through the air. Proc Natl Acad Sci U S A 106:3366–3371. doi: 10.1073/pnas.0813172106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webster RG, Govorkova EA. 2006. H5N1 influenza: continuing evolution and spread. N Engl J Med 355:2174–2177. doi: 10.1056/NEJMp068205. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. 2015. Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003-2015. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 26.Gu M, Liu W, Cao Y, Peng D, Wang X, Wan H, Zhao G, Xu Q, Zhang W, Song Q, Li Y, Liu X. 2011. Novel reassortant highly pathogenic avian influenza (H5N5) viruses in domestic ducks, China. Emerg Infect Dis 17:1060–1063. doi: 10.3201/eid/1706.101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao G, Gu X, Lu X, Pan J, Duan Z, Zhao K, Gu M, Liu Q, He L, Chen J, Ge S, Wang Y, Chen S, Wang X, Peng D, Wan H, Liu X. 2012. Novel reassortant highly pathogenic H5N2 avian influenza viruses in poultry in China. PLoS One 7:e46183. doi: 10.1371/journal.pone.0046183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz JM, Lu X, Tumpey TM, Smith CB, Shaw MW, Subbarao K. 2000. Molecular correlates of influenza A H5N1 virus pathogenesis in mice. J Virol 74:10807–10810. doi: 10.1128/JVI.74.22.10807-10810.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richard M, Herfst S, van den Brand JM, Lexmond P, Bestebroer TM, Rimmelzwaan GF, Koopmans M, Kuiken T, Fouchier RA. 2015. Low virulence and lack of airborne transmission of the Dutch highly pathogenic avian influenza virus H5N8 in ferrets. PLoS One 10:e0129827. doi: 10.1371/journal.pone.0129827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YI, Pascua PN, Kwon HI, Lim GJ, Kim EA, Yoon SW. 2014. Pathobiological features of a novel, highly pathogenic avian influenza A (H5N8) virus. Emerg Microbes Infect 3:e75. doi: 10.1038/emi.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shinya K, Makino A, Tanaka H, Hatta M, Watanabe T, Le MQ, Imai H, Kawaoka Y. 2011. Systemic dissemination of H5N1 influenza A viruses in ferrets and hamsters after direct intragastric inoculation. J Virol 85:4673–4678. doi: 10.1128/JVI.00148-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shinya K, Makino A, Hatta M, Watanabe S, Kim JH, Hatta Y, Gao P, Ozawa M, Le QM, Kawaoka Y. 2011. Subclinical brain injury caused by H5N1 influenza virus infection. J Virol 85:5202–5207. doi: 10.1128/JVI.00239-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schrauwen EJ, Herfst S, Leijten LM, van Run P, Bestebroer TM, Linster M, Bodewes R, Kreijtz JH, Rimmelzwaan GF, Osterhaus AD, Fouchier RA, Kuiken T, van Riel D. 2012. The multibasic cleavage site in H5N1 virus is critical for systemic spread along the olfactory and hematogenous routes in ferrets. J Virol 86:3975–3984. doi: 10.1128/JVI.06828-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McFadden ER Jr, Pichurko BM, Bowman HF, Ingenito E, Burns S, Dowling N, Solway J. 1985. Thermal mapping of the airways in humans. J Appl Physiol 58:564–570. doi: 10.1063/1.335663. [DOI] [PubMed] [Google Scholar]
- 35.Lindemann J, Leiacker R, Rettinger G, Keck T. 2002. Nasal mucosal temperature during respiration. Clin Otolaryngol Allied Sci 27:135–139. doi: 10.1046/j.1365-2273.2002.00544.x. [DOI] [PubMed] [Google Scholar]
- 36.Scull MA, Gillim-Ross L, Santos C, Roberts KL, Bordonali E, Subbarao K, Barclay WS, Pickles RJ. 2009. Avian Influenza virus glycoproteins restrict virus replication and spread through human airway epithelium at temperatures of the proximal airways. PLoS Pathog 5:e1000424. doi: 10.1371/journal.ppat.1000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayden F, Croisier A. 2005. Transmission of avian influenza viruses to and between humans. J Infect Dis 192:1311–1314. doi: 10.1086/444399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massin P, van der Werf S, Naffakh N. 2001. Residue 627 of PB2 is a determinant of cold sensitivity in RNA replication of avian influenza viruses. J Virol 75:5398–5404. doi: 10.1128/JVI.75.11.5398-5404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatta M, Hatta Y, Kim JH, Watanabe S, Shinya K, Nguyen T, Lien PS, Le QM, Kawaoka Y. 2007. Growth of H5N1 influenza A viruses in the upper respiratory tracts of mice. PLoS Pathog 3:1374–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. 2006. Avian flu: influenza virus receptors in the human airway. Nature 440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- 41.van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, Kuiken T. 2007. Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. Am J Pathol 171:1215–1223. doi: 10.2353/ajpath.2007.070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ng PS, Bohm R, Hartley-Tassell LE, Steen JA, Wang H, Lukowski SW, Hawthorne PL, Trezise AE, Coloe PJ, Grimmond SM, Haselhorst T, von Itzstein M, Paton AW, Paton JC, Jennings MP. 2014. Ferrets exclusively synthesize Neu5Ac and express naturally humanized influenza A virus receptors. Nat Commun 5:5750. doi: 10.1038/ncomms6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jayaraman A, Chandrasekaran A, Viswanathan K, Raman R, Fox JG, Sasisekharan R. 2012. Decoding the distribution of glycan receptors for human-adapted influenza A viruses in ferret respiratory tract. PLoS One 7:e27517. doi: 10.1371/journal.pone.0027517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jia N, Barclay WS, Roberts K, Yen HL, Chan RW, Lam AK, Air G, Peiris JS, Dell A, Nicholls JM, Haslam SM. 2014. Glycomic characterization of respiratory tract tissues of ferrets: implications for its use in influenza virus infection studies. J Biol Chem 289:28489–28504. doi: 10.1074/jbc.M114.588541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. 2012. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336:1534–1541. doi: 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yen HL, Aldridge JR, Boon AC, Ilyushina NA, Salomon R, Hulse-Post DJ, Marjuki H, Franks J, Boltz DA, Bush D, Lipatov AS, Webby RJ, Rehg JE, Webster RG. 2009. Changes in H5N1 influenza virus hemagglutinin receptor binding domain affect systemic spread. Proc Natl Acad Sci U S A 106:286–291. doi: 10.1073/pnas.0811052106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Q, Wang X, Gu M, Zhu J, Hao X, Gao Z, Sun Z, Hu J, Hu S, Wang X, Liu X, Liu X. 2014. Novel H5 clade 2.3.4.6 viruses with both alpha-2,3 and alpha-2,6 receptor binding properties may pose a pandemic threat. Vet Res 45:127. doi: 10.1186/s13567-014-0127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]