Abstract
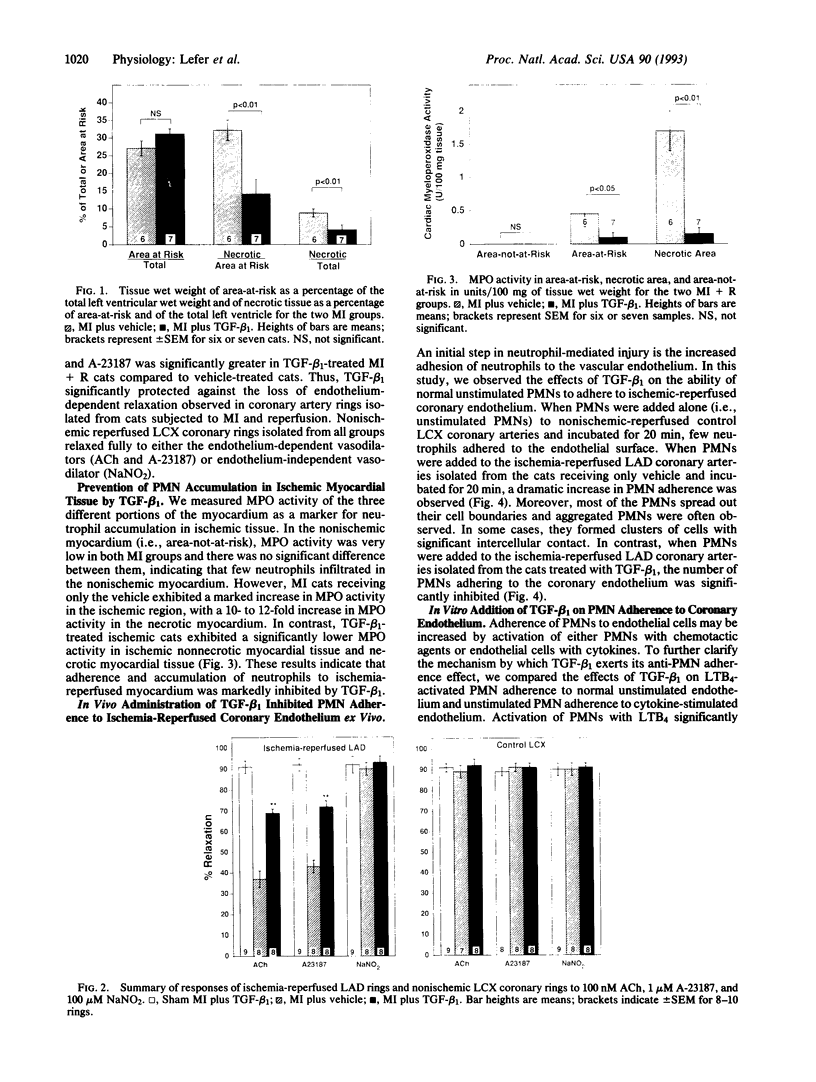
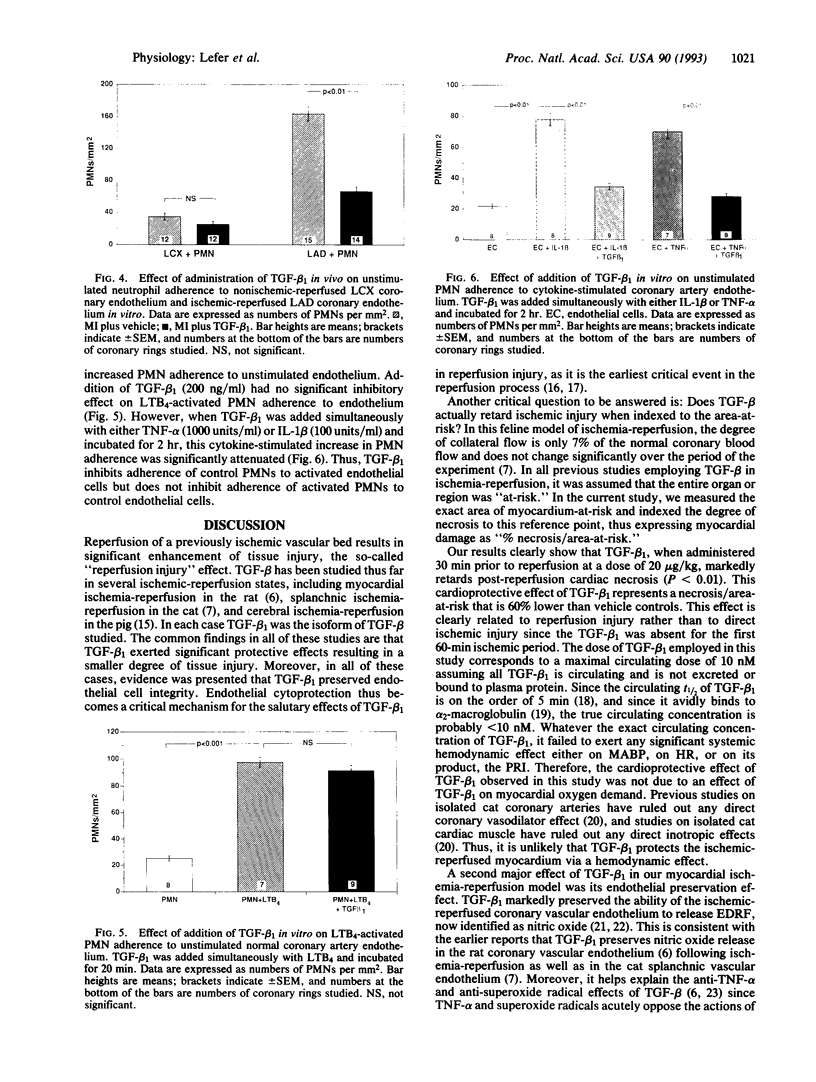
We studied the effects of transforming growth factor beta 1 (TGF-beta 1) in a feline model of myocardial ischemia (1.5 hr) and reperfusion (4.5 hr). Myocardial ischemia followed by reperfusion resulted in severe myocardial injury, endothelial dysfunction, high cardiac myeloperoxidase activity indicative of neutrophil accumulation in the ischemic myocardium, and significant neutrophil adherence to the ischemic coronary endothelium. In contrast, intravenous administration of TGF-beta 1 (20 micrograms/kg) 30 min prior to reperfusion significantly attenuated myocardial necrosis (13.8% +/- 3.5% vs. 32.2% +/- 2.9% of area-at-risk, P < 0.01) and attenuated endothelial dysfunction (P < 0.01) associated with ischemia-reperfusion. Moreover, myeloperoxidase activity in the ischemic myocardium was significantly lower than vehicle controls (0.2 +/- 0.1 vs. 1.7 +/- 0.3 units/100 mg of tissue, P < 0.01) and neutrophil adherence to ischemic coronary endothelium was significantly (P < 0.01) attenuated in TGF-beta 1-treated cats. These results demonstrate that TGF-beta 1 exerts a significant cardioprotective effect in a feline model of myocardial ischemia and reperfusion. The mechanism of this protective effect appears to relate to endothelial preservation by TGF-beta 1 inhibiting circulating neutrophils from adhering to the endothelium, a critical step in neutrophil-induced reperfusion injury.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki N., Siegfried M., Lefer A. M. Anti-EDRF effect of tumor necrosis factor in isolated, perfused cat carotid arteries. Am J Physiol. 1989 May;256(5 Pt 2):H1509–H1512. doi: 10.1152/ajpheart.1989.256.5.H1509. [DOI] [PubMed] [Google Scholar]
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley P. P., Priebat D. A., Christensen R. D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982 Mar;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Kost L. J., Lyons R. M., Moses H. L., LaRusso N. F. Hepatic processing of transforming growth factor beta in the rat. Uptake, metabolism, and biliary excretion. J Clin Invest. 1987 Sep;80(3):750–757. doi: 10.1172/JCI113130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espevik T., Figari I. S., Ranges G. E., Palladino M. A., Jr Transforming growth factor-beta 1 (TGF-beta 1) and recombinant human tumor necrosis factor-alpha reciprocally regulate the generation of lymphokine-activated killer cell activity. Comparison between natural porcine platelet-derived TGF-beta 1 and TGF-beta 2, and recombinant human TGF-beta 1. J Immunol. 1988 Apr 1;140(7):2312–2316. [PubMed] [Google Scholar]
- Frolik C. A., Dart L. L., Meyers C. A., Smith D. M., Sporn M. B. Purification and initial characterization of a type beta transforming growth factor from human placenta. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3676–3680. doi: 10.1073/pnas.80.12.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Gamble J. R., Vadas M. A. Endothelial adhesiveness for blood neutrophils is inhibited by transforming growth factor-beta. Science. 1988 Oct 7;242(4875):97–99. doi: 10.1126/science.3175638. [DOI] [PubMed] [Google Scholar]
- Johnson G., 3rd, Furlan L. E., Aoki N., Lefer A. M. Endothelium and myocardial protecting actions of taprostene, a stable prostacyclin analogue, after acute myocardial ischemia and reperfusion in cats. Circ Res. 1990 May;66(5):1362–1370. doi: 10.1161/01.res.66.5.1362. [DOI] [PubMed] [Google Scholar]
- Johnson G., 3rd, Tsao P. S., Mulloy D., Lefer A. M. Cardioprotective effects of acidified sodium nitrite in myocardial ischemia with reperfusion. J Pharmacol Exp Ther. 1990 Jan;252(1):35–41. [PubMed] [Google Scholar]
- Karasawa A., Guo J. P., Ma X. L., Lefer A. M. Beneficial effects of transforming growth factor-beta and tissue plasminogen activator in splanchnic artery occlusion and reperfusion in cats. J Cardiovasc Pharmacol. 1991 Jul;18(1):95–105. doi: 10.1097/00005344-199107000-00013. [DOI] [PubMed] [Google Scholar]
- Lafrado L. J., Olsen R. G. Demonstration of depressed polymorphonuclear leukocyte function in nonviremic FeLV-infected cats. Cancer Invest. 1986;4(4):297–300. doi: 10.3109/07357908609017509. [DOI] [PubMed] [Google Scholar]
- Lefer A. M. Mechanisms of the protective effects of transforming growth factor-beta in reperfusion injury. Biochem Pharmacol. 1991 Sep 12;42(7):1323–1327. doi: 10.1016/0006-2952(91)90441-7. [DOI] [PubMed] [Google Scholar]
- Lefer A. M., Tsao P. S., Lefer D. J., Ma X. L. Role of endothelial dysfunction in the pathogenesis of reperfusion injury after myocardial ischemia. FASEB J. 1991 Apr;5(7):2029–2034. doi: 10.1096/fasebj.5.7.2010056. [DOI] [PubMed] [Google Scholar]
- Lefer A. M., Tsao P., Aoki N., Palladino M. A., Jr Mediation of cardioprotection by transforming growth factor-beta. Science. 1990 Jul 6;249(4964):61–64. doi: 10.1126/science.2164258. [DOI] [PubMed] [Google Scholar]
- Lefer D. J., Nakanishi K., Vinten-Johansen J., Ma X. L., Lefer A. M. Cardiac venous endothelial dysfunction after myocardial ischemia and reperfusion in dogs. Am J Physiol. 1992 Sep;263(3 Pt 2):H850–H856. doi: 10.1152/ajpheart.1992.263.3.H850. [DOI] [PubMed] [Google Scholar]
- Ma X. L., Johnson G., 3rd, Lefer A. M. Low doses of superoxide dismutase and a stable prostacyclin analogue protect in myocardial ischemia and reperfusion. J Am Coll Cardiol. 1992 Jan;19(1):197–204. doi: 10.1016/0735-1097(92)90073-v. [DOI] [PubMed] [Google Scholar]
- Ma X. L., Lefer D. J., Lefer A. M., Rothlein R. Coronary endothelial and cardiac protective effects of a monoclonal antibody to intercellular adhesion molecule-1 in myocardial ischemia and reperfusion. Circulation. 1992 Sep;86(3):937–946. doi: 10.1161/01.cir.86.3.937. [DOI] [PubMed] [Google Scholar]
- Ma X. L., Tsao P. S., Lefer A. M. Antibody to CD-18 exerts endothelial and cardiac protective effects in myocardial ischemia and reperfusion. J Clin Invest. 1991 Oct;88(4):1237–1243. doi: 10.1172/JCI115427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X. L., Tsao P. S., Viehman G. E., Lefer A. M. Neutrophil-mediated vasoconstriction and endothelial dysfunction in low-flow perfusion-reperfused cat coronary artery. Circ Res. 1991 Jul;69(1):95–106. doi: 10.1161/01.res.69.1.95. [DOI] [PubMed] [Google Scholar]
- Massagué J., Like B. Cellular receptors for type beta transforming growth factor. Ligand binding and affinity labeling in human and rodent cell lines. J Biol Chem. 1985 Mar 10;260(5):2636–2645. [PubMed] [Google Scholar]
- McEver R. P. GMP-140: a receptor for neutrophils and monocytes on activated platelets and endothelium. J Cell Biochem. 1991 Feb;45(2):156–161. doi: 10.1002/jcb.240450206. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989 Jun 1;38(11):1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- Mullane K. M., Kraemer R., Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985 Nov;14(3):157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- O'Connor-McCourt M. D., Wakefield L. M. Latent transforming growth factor-beta in serum. A specific complex with alpha 2-macroglobulin. J Biol Chem. 1987 Oct 15;262(29):14090–14099. [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987 Sep;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Meyers C. A., Wideman J., Blacher R., Pan Y. C., Stein S., Lehrman S. R., Smith J. M., Lamb L. C. Purification and properties of a type beta transforming growth factor from bovine kidney. Biochemistry. 1983 Dec 6;22(25):5692–5698. doi: 10.1021/bi00294a002. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986 May;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao P. S., Aoki N., Lefer D. J., Johnson G., 3rd, Lefer A. M. Time course of endothelial dysfunction and myocardial injury during myocardial ischemia and reperfusion in the cat. Circulation. 1990 Oct;82(4):1402–1412. doi: 10.1161/01.cir.82.4.1402. [DOI] [PubMed] [Google Scholar]
- Weissmann G. The role of neutrophils in vascular injury: a summary of signal transduction mechanisms in cell/cell interactions. Springer Semin Immunopathol. 1989;11(3):235–258. doi: 10.1007/BF00197305. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Fleming B. P. A method for isolation and fluorescent labeling of rat neutrophils for intravital microvascular studies. Microvasc Res. 1990 Sep;40(2):218–229. doi: 10.1016/0026-2862(90)90021-i. [DOI] [PubMed] [Google Scholar]