Abstract
Purpose.
Poor vision may detrimentally impact functional status and affect allostatic load (AL), a measure of cumulative physiological wear and tear on the body's regulatory systems. We examined the direct effects of visual acuity (VA) on mortality and its indirect effect on mortality through its impact on functional status and AL in older adults.
Methods.
Data from 4981 participants (age ≥ 60 years) from the 1999–2004 National Health and Nutrition Examination Survey (NHANES) with mortality linkage through 2006 were analyzed. Functional status was assessed by activities of daily living (ADL) and instrumental activities of daily living (IADL). The AL index was composed of 10 biomarkers: systolic and diastolic blood pressures, body mass index (BMI), glycosylated hemoglobin, total cholesterol, triglycerides, albumin, C-reactive protein, homocysteine, and creatinine clearance. Visual acuity was categorized as no (20/20–20/25), mild (20/30–20/40), moderate (20/50–20/80), or severe (≥20/200) visual impairment. Structural equation modeling using three mediating variables representing ADL, IADL, and AL examined the effects of VA on all-cause and cardiovascular disease (CVD)-related mortality.
Results.
Adjusting for all covariates, a one-unit change in VA category increased mortality risk (hazard ratio [HR] = 1.17; 95% confidence interval [CI] 1.05, 1.32); IADL and AL predicted mortality (HR = 1.15; CI 1.10, 1.20 and HR = 1.13; CI 1.06, 1.20, respectively). Activities of daily living did not predict mortality (HR = 0.98; CI 0.91, 1.05). Worse VA was associated with increased AL (β = 0.11; P = 0.013) and worse IADL (β = 1.06; P < 0.001). Worse VA increased mortality risk indirectly through AL (HR = 1.01; CI 1.00, 1.03) and IADL (HR = 1.16; CI 1.09, 1.23). The total effect of VA on mortality including through IADL and AL was HR = 1.38 (CI 1.23, 1.54). Similar but slightly stronger patterns of association were found when examining CVD-related mortality, but not cancer-related mortality.
Conclusions.
Allostatic load and particularly IADL may function as mediators between VA impairment and mortality. Older adults with VA impairment could potentially benefit from interventions designed to prevent IADL functional status decline to reduce the risk of mortality.
Keywords: visual acuity, mortality, allostatic load, functional status, IADL
Analysis using structural equation modeling of data from 4981 participants aged 60 and older from NHANES 1999–2004 with mortality linkage through 2006 showed that visual acuity impairment increases mortality directly and indirectly through effects on allostatic load and IADL functional status.
Introduction
Visual impairment (VI) is associated with a broad range of psychosocial and functional consequences such as social isolation1–3; depression1–4; poor well-being4,5; frailty6; and functional decline,7,8 including declines in instrumental activities of daily living (IADL).9 Given these associations, it is not surprising that both self-reported and clinically assessed measures of VI are predictive of mortality even when controlling for other risk factors associated with longevity.10–13
Despite these consistent findings of increased mortality risk associated with VI, investigators have only recently begun to explore potential mechanisms that lead to this increased risk. Studies using structural equation modeling techniques indicate that VI increases mortality risk both directly and indirectly through its adverse impact on indicators of disability, health status, and mental well-being.13–15 Recently, using data collected over multiple time periods in a cohort of older adults, the impact of declining visual acuity (VA) on mortality was shown to operate indirectly through its adverse effect on IADL.16
Allostatic load (AL) is a biological construct thought to reflect cumulative bodily wear and tear in response to stress over the life course.17 High AL scores are associated with poorer cognitive and physical functioning and increased risk for incidence of cardiovascular disease (CVD).18 High AL score is also related to the risk of developing mobility limitations and frailty in older adults19,20; an increased risk of mortality, even after adjustment for other known risk factors of mortality20; and shorter telomeres, which are linked to premature mortality.17
To our knowledge, there has been no research examining associations between AL and VI. The broad psychosocial and functional consequences of VI signify that those who are visually impaired may experience increased stress in life, which in turn may lead to increases in AL levels. Also unknown is whether high AL levels can lead to increases in VI. Both factors are predictive of functional status indicators such as impaired mobility.20–22 Furthermore, given that increases in VI are associated with reduced mobility and IADL,9 possible complex feedback loops may exist that ultimately result in increased AL levels and increased risk of mortality. Therefore, the directionality between the relationship of AL and VI is unknown and possibly complex. For example, the complement pathway has been implicated in the development and progression of age-related macular degeneration,23 suggesting that inflammatory processes that are components of the AL index may play a role in the impairing ocular diseases. Using nationally representative data, this study explores possible direct and indirect associations between VA impairment, AL, activities of daily living (ADL), and IADL as they relate to mortality risk in older adults.
Methods
Study Population and Design
This study utilized participants age 60 years and older from the 1999–2004 National Health and Nutrition Examination Survey (NHANES). These are continuous surveys conducted by the National Center for Health Statistics. The NHANES sampled the US noninstitutionalized civilian population using stratified multistage probability design with planned oversampling of certain age and racial/ethnic groups. The survey examines a nationally representative sample of approximately 5000 persons located in counties across the United States each year.24 Participants were administered survey questionnaires and invited to mobile centers for examinations that included physical examination, vision testing, and laboratory blood tests. The NHANES 1999–2004 response rates for participants aged 60 and older was 72.5% for interviewed sample and 64.5% for examined sample.25 Data from NHANES adult participants were linked with the death certificate data of the National Death Index (NDI) with mortality follow-up through December 31, 2006.26 All participants with sufficient identifying data were linked with NDI data. The mortality linkage was completed for 4981 of 4984 participants who met the selection criteria for this study.
Assessment of Visual Acuity
Presenting VA was measured for each eye with the participant's usual distance vision correction using an autorefractor. Corrective lenses were then removed and an automated refraction was performed for each eye. For eyes with VA less than 20/25, VA was remeasured, aided by the autorefractor measurements.24 We used the VA of the participant's better-seeing eye as the VA measurement. For analysis purposes, VA measures were further categorized into a four-level VA impairment status variable: none (20/20–20/25), mild (20/30–20/40), moderate (20/50–20/80), severe (≥20/200).
Allostatic Load
Based on previous research and NHANES data availability, 10 biomarkers were included in the calculation of AL.27 These biomarkers exhibit the effects of hormones secreted in response to stress and measure the regulatory systems that are involved in the physiological response. The biomarkers included metabolic markers (body mass index [BMI], glycosylated hemoglobin), cardiovascular markers (systolic blood pressure, diastolic blood pressure, total cholesterol, triglycerides, homocysteine), inflammatory markers (albumin, C-reactive protein), and a marker of organ dysfunction (creatinine clearance). For each biomarker, we empirically determined the high-risk threshold on the basis of the distribution of that biomarker in the study sample. We assigned each participant 1 point for each biomarker reading beyond the cutoff threshold defined as below the 25th percentile for creatinine clearance and albumin and above the 75th percentile for all other biomarkers. The points were then summed to obtain the AL score, with a maximum possible score of 10. In this NHANES sample of older adults, high-risk thresholds were as follows: albumin, 40 g/L; BMI, 31.1; C-reactive protein, 0.58 mg/dL; creatinine clearance, 55.7 mg/dL; diastolic blood pressure, 77.3 mm Hg; systolic blood pressure, 154 mm Hg; glycosylated hemoglobin, 6.0%; homocysteine, 12.4 mol/L; total cholesterol, 234 mg/dL; triglycerides, 182 mg/dL.
Functional Status
Functional status was assessed by ADL and IADL. Activities of daily living assessments included four questions addressing difficulty getting in and out of bed; difficulty dressing oneself; difficulty walking between rooms on the same floor; and difficulty feeding oneself such as in using fork or knife or drinking from a glass. Instrumental activities of daily living assessments included three questions addressing difficulty doing household chores; difficulty preparing meals; and difficulty managing money. Each question started with the following: “By yourself and without using any special equipment, how much difficulty do you have . . . ?” Each question had one of the following possible answers: no difficulty, some difficulty, much difficulty, unable to do this.24 Coded answers to the questions were summed to create ADL and IADL scores, respectively.
Covariates
Several social demographic and health behavior variables that affect mortality were included in the model as controls: age in years (range, ≥60), sex (male reference), a four-category racial/ethnic identity variable (non-Hispanic white [reference], non-Hispanic black, Mexican American, other races), education (less than high school [reference], high school, above high school), marital status (married [reference], not married), smoking status at time of interview (nonsmoker [reference], current smoker, former smoker), health insurance status (covered by insurance [reference], not covered by insurance), and alcohol drinking status (nondrinker [reference], drinker). Participants were considered a drinker if they answered “Yes” to the question “Have you had at least 12 alcohol drinks in any one year?24
Cause of Death
Cause of death information was obtained from the National Death Index through linkage with NHANES. The underlying cause of death was determined by Underlying Cause of Death 113 Groups (ucod_113), which follows the ninth revision of the International Statistical Classification of Diseases, Injuries, and Causes of Death (ICD-9).28 Our study examined all-cause mortality, CVD-related mortality (ucod_113 code 053-075), and cancer-related mortality (ucod_113 code 019-043).
Analysis
Descriptive and model-based analyses were completed with adjustments for sample weights and design effects using SAS 9.229 and Mplus 6 statistical packages.30
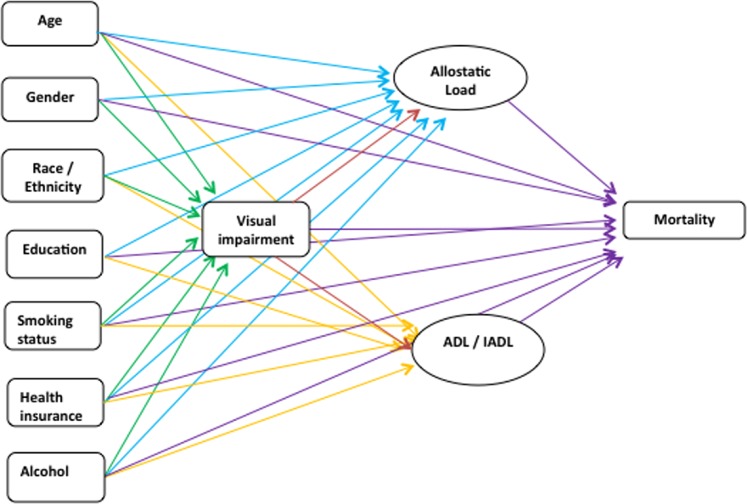
The Figure depicts the relationships that were examined in the structural equation model. Five regression models were evaluated simultaneously: (1) an ordinal regression model of the VA impairment variable on the demographic variables; (2) a multiple linear regression model of the AL variable on demographic variables and VA; (3) a multiple linear regression model of ADL variable on the same variables as in (2); (4) a multiple linear regression model of IADL variable on the same variables as in (2); and (5) Cox proportional hazard regression of all above-mentioned variables on mortality.
Figure.
Path diagram.
Visual acuity was hypothesized to directly affect mortality, as well as to affect mortality indirectly through AL, ADL, and IADL. All models were estimated simultaneously using a pseudo-maximum likelihood estimator31 with robust standard errors.32 Regression coefficients from the Cox proportional hazard regression were exponentiated to obtain the hazard ratios (HRs), while coefficients from the logistic models were similarly exponentiated to obtain odds ratios. The NHANES complex survey design was accounted for in all model estimations.
Indirect effects were calculated by multiplying the two parameters involved in the mediation relationship.33,34 For example, the effect of VA on AL was multiplied by the effect of AL on mortality. The new parameter was exponentiated to obtain the HR for the indirect effect. Total HR effects were calculated by taking the exponentiation of the summed direct and indirect coefficients. Standard errors for both indirect and total effects were obtained using the delta method.35
Results
The study sample of 4981 adults aged 60 and older represents an estimated 45 million older adults in the noninstitutionalized US population. The average age of this population was 71.8 years (standard deviation [SD] 8 years). The population was 49% male, 57% non-Hispanic white, 21% Mexican American, 16% non-Hispanic black, and 6% “other” race. Twenty-three percent of participants had a high school diploma, and 34% had above high school education. At the time of interview, 93% of participants had health insurance coverage, 12% were smokers, and 41% were former smokers. With respect to VA impairment, 3813 (84.5%) had none, 364 (8.1%) had mild VI, 243 (5.4%) had moderate VI, and 75 (1.7%) had severe VI. The mean AL score was 2.5 with SD 1.7. The mean IADL and ADL scores were 4.7 (SD 1.7) and 4.2 (SD 2.3), respectively. Mortality linkage with the National Death Index with follow-up through December 2006 identified 894 (18%) observed deaths in the study sample, of which 351 were CVD-related deaths and 214 were cancer-related deaths. The demographic characteristics of those in the study who died are listed in Table 1.
Table 1.
Demographic Characteristics of Study Participants, NHANES 1999–2004: Age ≥ 60
|
Characteristic |
All Participants |
Those Who Died |
||
|
N |
% |
N |
% |
|
| Sex | ||||
| Male | 2452 | 49 | 526 | 59 |
| Female | 2529 | 51 | 368 | 41 |
| Race | ||||
| Non-Hispanic white | 2844 | 57 | 559 | 63 |
| Mexican American | 1036 | 21 | 137 | 15 |
| Non-Hispanic black | 810 | 16 | 156 | 17 |
| Other race | 291 | 6 | 42 | 5 |
| Education | ||||
| Less than high school | 2137 | 43 | 424 | 47 |
| High school diploma | 1148 | 23 | 208 | 23 |
| Above high school | 1675 | 34 | 255 | 29 |
| Marital status | ||||
| Married | 2786 | 56 | 424 | 49 |
| All other marital status | 2044 | 44 | 470 | 51 |
| Smoking status | ||||
| Never smoker | 2327 | 47 | 358 | 40 |
| Current smoker | 611 | 12 | 141 | 16 |
| Former smoker | 2032 | 41 | 394 | 44 |
| Health insurance | ||||
| Covered | 4617 | 93 | 865 | 97 |
| Not covered | 302 | 7 | 23 | 3 |
| Alcohol drinking | ||||
| Nondrinker | 1831 | 39 | 325 | 40 |
| Alcohol drinker | 2844 | 61 | 490 | 60 |
| Mean | SD | Mean | SD | |
| Average age, y | 71.8 | 8.0 | 76.7 | 7.5 |
| Allostatic load score | 2.5 | 1.6 | 2.6 | 1.7 |
| ADL | 4.6 | 1.7 | 5.5 | 2.4 |
| IADL | 4.2 | 2.3 | 5.4 | 3.1 |
Odds ratio estimates from the ordinal regression of VA impairment on demographic variables are displayed in Table 2. Results of three multiple linear regressions for AL, IADL, and ADL are summarized in Table 3. Worse VA was associated with increased AL and increased ADL and IADL. A one-unit change of VA impairment status, for example, from mild to moderate VI, was associated with a 0.112-unit increase in AL, a 1.059-unit increase in IADL score, and a 0.68-unit increase in ADL score. Higher AL was found in women compared to men (β = 0.31, P < 0.001) and in non-Hispanic blacks compared to non-Hispanic whites (β = 0.43, P < 0.001). Higher education and being married were associated with lower AL and better ADL or IADL status, that is, fewer difficulties.
Table 2.
Logistic Regression of Visual Acuity on Demographic Variables
|
Variables |
Odds Ratio |
95% Confidence Interval |
PValues |
| Age, y | 1.15 | 1.13, 1.17 | <0.01 |
| Male | 1.00 | ||
| Female | 0.89 | 0.69, 1.13 | 0.33 |
| Non-Hispanic white | 1.00 | ||
| Mexican American | 1.75 | 1.28, 2.39 | <0.01 |
| Non-Hispanic black | 1.54 | 1.23, 1.92 | <0.01 |
| Other race | 3.63 | 2.68, 4.91 | <0.01 |
| Less than high school | 1.00 | ||
| High school diploma | 0.77 | 0.56, 1.06 | 0.11 |
| Above high school | 0.55 | 0.42, 0.74 | <0.01 |
| Married | 1.00 | ||
| All other marital status | 1.13 | 0.89, 1.42 | 0.32 |
| Nonsmoker | |||
| Current smoker | 0.87 | 0.61, 1.25 | 0.45 |
| Former smoker | 1.14 | 0.93, 1.39 | 0.21 |
| Covered by health insurance | 1.00 | ||
| Not covered by health insurance | 1.89 | 1.01, 3.52 | 0.05 |
| Nondrinker | 1.00 | ||
| Alcohol drinker | 1.04 | 0.84, 1.29 | 0.73 |
Table 3.
Multiple Regressions of Allostatic Load, ADL, and IADL on Demographic Variables and Visual Acuity
|
Independent Variables |
Allostatic Load as Outcome |
IADL as Outcome |
ADL as Outcome |
|||
|
β |
PValue |
β |
PValue |
β |
PValue |
|
| Visual acuity | 0.11 | 0.01 | 1.06 | <0.01 | 0.68 | <0.01 |
| Age, y | 0.01 | 0.001 | 0.03 | <0.01 | 0.01 | 0.074 |
| Female | 0.31 | <0.01 | 0.02 | 0.74 | 0.06 | 0.28 |
| Mexican American | 0.12 | 0.13 | 0.34 | 0.02 | 0.26 | 0.01 |
| Non-Hispanic black | 0.43 | <0.01 | 0.13 | 0.11 | 0.11 | 0.17 |
| Other race | −0.01 | 0.91 | 0.24 | 0.13 | 0.07 | 0.51 |
| High school diploma | −0.12 | 0.1 | −0.30 | 0.004 | −0.20 | 0.002 |
| Above high school | −0.25 | <0.01 | −0.30 | 0.001 | −0.23 | <0.01 |
| Not married | 0.20 | <0.01 | 0.13 | 0.053 | 0.09 | 0.06 |
| Current smoker | −0.01 | 0.9 | 0.06 | 0.62 | 0.20 | 0.03 |
| Former smoker | 0.10 | 0.03 | 0.03 | 0.75 | 0.09 | 0.05 |
| Not covered by health insurance | 0.08 | 0.57 | −0.24 | 0.08 | −0.17 | 0.06 |
| Alcohol drinker | 0.23 | <0.01 | 0.14 | 0.05 | 0.08 | 0.08 |
The Cox proportional hazard model regression results for all variables on all-cause mortality are given in Table 4. Worse VA was significant in predicting mortality; a one-unit change in VA impairment status was associated with a 17% increase in mortality hazard (HR = 1.17; 95% confidence interval [CI] 1.04, 1.32, P = 0.01). The AL and IADL variables were also significantly associated with mortality. A one-unit increase in AL was associated with a 13% increase in mortality (HR = 1.13; CI 1.06, 1.20, P < 0.001). A one-unit change in IADL was associated with a 15% increase in mortality (HR = 1.15; CI 1.10, 1.20, P < 0.001). Activities of daily living, on the other hand, did not predict mortality after controlling for IADL, AL, and other covariates (HR = 0.98; CI 0.91, 1.05, P = 0.54). As expected, age predicted mortality; a 1-year increase in age increased the hazard of death by a factor of 1.09 (P < 0.001). Females had a lower mortality hazard compared to males (HR = 0.56, P < 0.001). Both current and former smokers at the time of interview had much higher risk of mortality compared to nonsmokers (HR = 2.97; CI 2.12, 4.17, P < 0.01 and HR = 1.40; CI 1.17, 1.68, P < 0.01, respectively).
Table 4.
Cox Proportional Hazard Model: Direct Effect on All-Cause and CVD-Related Mortality
|
Variables |
HR for All-Cause Mortality |
95% CI for All-Cause Mortality |
HR for CVD-Related Mortality |
95% CI for CVD-Related Mortality |
| Age, y | 1.09* | 1.08, 1.11 | 1.11* | 1.08, 1.14 |
| Female | 0.56* | 0.45, 0.71 | 0.56* | 0.39, 0.81 |
| Mexican American | 0.77 | 0.59, 1.01 | 0.68† | 0.47, 0.99 |
| Non-Hispanic black | 1.24 | 0.91, 1.68 | 1.12 | 0.73, 1.71 |
| Other race | 0.76 | 0.56, 1.02 | 0.59 | 0.28, 1.22 |
| High school diploma | 1.14 | 0.93, 1.40 | 0.97 | 0.65, 1.45 |
| Above high school | 0.93 | 0.78, 1.11 | 0.82 | 0.62, 1.08 |
| All other marital status | 1.13 | 0.89, 1.44 | 1.16 | 0.80, 1.67 |
| Current smoker | 2.97* | 2.12, 4.17 | 3.62* | 2.10, 6.25 |
| Former smoker | 1.40* | 1.17, 1.68 | 1.30 | 0.97, 1.74 |
| Not covered by health insurance | 1.26 | 0.76, 2.09 | 0.85 | 0.35, 2.04 |
| Alcohol drinker | 1.18 | 0.94, 1.48 | 1.22 | 0.87, 1.70 |
| Visual acuity | 1.17† | 1.04, 1.32 | 1.21† | 1.01, 1.44 |
| Allostatic load | 1.13* | 1.06, 1.20 | 1.19* | 1.09, 1.31 |
| IADL | 1.15* | 1.10, 1.20 | 1.18* | 1.11, 1.26 |
| ADL | 0.98 | 0.91, 1.05 | 0.96 | 0.88, 1.04 |
P < 0.01.
P < 0.05.
Allostatic load was significantly associated with both VA and mortality and therefore served as a mediator between the VA and mortality relationship. The path from VA to AL, the presumed mediator, was significant (β = 0.112, P = 0.013). Secondly, the path from AL to mortality, the outcome, was also significant (HR = 1.13, P < 0.001).
Similar results were found for IADL: The path from VA to IADL was significant (β = 1.06, P < 0.01), and the path from IADL to mortality was also significant (HR = 1.15; P < 0.01). Therefore, IADL also served as a mediator between the relationship of VA and mortality. Because the relationship between ADL and all-cause mortality was not significant, ADL did not serve as a mediator between the relationship of VA and mortality. The direct effect of VA on mortality remained significant (HR = 1.17; P = 0.01) after including AL and IADL in the regression. Therefore, the mediation effects of AL and IADL on VA and all-cause mortality were partial mediation effects.36
The indirect effect of VA on all-cause mortality through IADL was HR = 1.16; CI 1.09, 1.23, P < 0.05 (Table 5). The indirect effect of VA and mortality through AL was HR = 1.01; CI 1.00, 1.03, P < 0.05. The total effect of VA on all-cause mortality, which combines the direct effect and the indirect effects through AL and IADL, was HR = 1.38; CI 1.23, 1.54, P < 0.05.
Table 5.
Total and Indirect Effect of Visual Acuity on Risk of All-Cause Mortality and Cardiovascular Disease Mortality Through Allostatic Load and Instrumental Activities of IADL Mediators
|
HR for All-Cause Mortality |
96% CI for All-Cause Mortality |
HR for CVD-Related Mortality |
95% CI for CVD-Related Mortality |
|
| Direct effect | 1.17* | 1.04, 1.32 | 1.21* | 1.01, 1.44 |
| Indirect effect through allostatic load | 1.01* | 1.00, 1.03 | 1.02* | 1.00, 1.04 |
| Indirect effect through IADL | 1.16* | 1.09, 1.23 | 1.20* | 1.12, 1.28 |
| Total effect, direct plus both indirect pathways | 1.38* | 1.23, 1.54 | 1.47* | 1.26, 1.71 |
P < 0.05.
The analysis was repeated for cause-specific mortality. Visual acuity was significant in predicting CVD-related mortality (HR = 1.21; CI 1.01, 1.44, P = 0.04) (Table 4). Both AL and IADL were also significant in predicting CVD-related mortality (HR = 1.19; CI 1.09, 1.31, P < 0.001 and HR = 1.18; CI 1.11, 1.26, P < 0.001, respectively). Activities of daily living were not significant in predicting CVD-related mortality (HR = 0.96; CI 0.88, 1.04, P = 0.54).
Visual acuity was also significantly associated with AL and IADL; VA affected CVD-related mortality not only directly, but also indirectly through the effect on AL and IADL. The indirect effect of VA on CVD-related mortality through AL and IADL was HR = 1.02; CI 1.00, 1.04, P < 0.05 and HR = 1.20; CI 1.12, 1.28, P < 0.05, respectively. The total effect of VA on CVD-related mortality was HR = 1.47; CI 1.26, 1.71, P < 0.05 (Table 5). This pattern of association was similar to the relationship between VA and all-cause mortality but was slightly stronger. No associations were found between VA, AL, or IADL and cancer-related mortality.
Discussion
As has been found in previous studies, we found that lower VA levels were predictive of mortality in a nationally representative sample of adults aged 60 years and older. We report, for the first time, a cross-sectional association between AL levels and VA. In addition, we found modest but significant indirect mortality effects of VA operating through higher AL levels and increased IADL impairments. The total effect of VA on mortality including its indirect influence through IADL and AL was substantial, indicating the importance of maintaining good ocular health (HR = 1.38; CI 1.23, 1.54). These findings add to the small but growing literature on the identification of potential mechanisms by which poor ocular health impacts overall health and mortality risk.14–16
Similar patterns of association were found when analyses were repeated for CVD-related mortality. Visual impairment not only directly increased the risk of CVD-related mortality; it also indirectly increased the risk of mortality through its effect on AL and IADL. The total effect of VI on CVD mortality was HR = 1.47 (CI 1.26, 1.71) (Table 5). However, no association was found on cancer mortality and the parameters of interest. These CVD and cancer mortality findings were consistent with previous research.37–39
Mediators such as AL and IADL appear to be involved in a cascade of effects resulting from declining VA leading to increased mortality risk. Increasingly adverse health behavior profiles may result from increases in social isolation that may be a consequence of vision loss leading to higher AL levels40 and worsened IADL. Reduced activity levels associated with declining vision may lead to increased AL levels, which are shown to be correlated with VA in the present analysis. Activity restriction among those with a variety of medical conditions is strongly correlated with risk of depression (r = 0.39),41 which itself is associated with increased AL.42 Similar activity restriction associated with declining vision may be one mechanism by which depression risk, which is itself an independent predictor of mortality,43 is elevated. Maintaining physical activity levels may be one strategy to lower AL levels,44 though research in older adults is lacking.
Exercise has potent protective effects and has been shown to rival more traditional approaches to manage chronic conditions and to lower risk of mortality.45 Furthermore, exercise interventions may be effective in improving functional status in community-dwelling frail older individuals.46 For those experiencing declining vision, participation in vision rehabilitation programs may be associated with maintaining social networks among friends.47 However, the effects of rehabilitation on measures of affect, including depressive symptomatology, have been mixed.48 Nevertheless, vision rehabilitation programs that add interventions designed to maintain or increase physical activity levels are warranted.
It is worth noting that we found IADL to be the stronger mediator relative to the AL findings. The indirect effect of VA on mortality through IADL is HR = 1.16; CI 1.09, 1.23, whereas the indirect effect through AL is HR = 1.01; CI 1.00, 1.03. This strong mediation effect of IADL is consistent with our previous research.16 This finding reinforces the importance of maintaining IADL for older adults who are experiencing declining vision. Older adults with VA impairment could potentially benefit from interventions designed to prevent IADL functional status decline to reduce the risk of mortality.
As to study limitations, one limitation was that relationships among changes in VA, ADL, IADL, and AL and their impact on mortality risk could not be examined since these indicators were measured only cross-sectionally in our analysis. We specified in our models that VA impacted ADL, IADL, and AL, but given the cross-sectional design we cannot confirm that these functional and biological factors can adversely impact VA over time. Longitudinal studies that measure all parameters at multiple time points are needed to determine directionality of effects. A life course perspective for these future studies is important given that the presence of chronic conditions in middle age is associated with the development of frailty in elders.49 Careful measurement of a wide range of chronic conditions is necessary to isolate the effects of declining vision on health.
Our models did not include other potentially important measures of mental and social functioning. We also lacked more complete information on other important measures of visual functioning (e.g., contrast sensitivity). Our measure of AL was patterned after those employed by other investigators utilizing NHANES data.27 However, this dataset does not include some parameters used in other AL studies (e.g., cortisol, serum dehydroepiandrosterone sulfate, waist-to-hip ratio). Thus our findings are not directly comparable to those obtained with other AL indices. We attempted to model AL as a latent variable but had model convergence issues. This lack of convergence may have been due to the restricted age range of our analysis (≥60 years); other investigators have reported fit issues when modeling AL as a latent variable in older adults.50
To summarize, this nationally representative study of adults aged 60 years and older confirmed the direct effects of reduced VA on mortality risks and identified potential biological and functional mechanisms by which VA also indirectly influences risk. The findings reinforce the importance of improving poor vision as a target for public health interventions to maximize population health. Additional longitudinal studies are needed to fully determine underlying mechanisms by which VI influences mortality risk.
Acknowledgments
Supported by Centers for Disease Control and Prevention (Grants 5U58DP002651, 1U58DP002652, 5U58DP002653, 1U58DP002655). Grantees include the University of Alabama at Birmingham, Johns Hopkins University, Wills Eye Hospital, and the University of Miami.
Disclosure: D.D. Zheng, None; S.L. Christ, None; B.L. Lam, None; S.L. Tannenbaum, None; C.L. Bokman, None; K.L. Arheart, None; L.A. McClure, None; C.A. Fernandez, None; D.J. Lee, None
References
- 1. Carabellese C, Appollonio I, Rozzini R, et al. Sensory impairment and quality of life in a community elderly population. J Am Geriatr Soc. 1993; 41: 401–407. [DOI] [PubMed] [Google Scholar]
- 2. Bookwala J, Lawson B. Poor vision, functioning, and depressive symptoms: a test of the activity restriction model. Gerontologist. 2011; 51: 798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wallhagen MI, Strawbridge WJ, Shema SJ, Kurata J, Kaplan GA. Comparative impact of hearing and vision impairment on subsequent functioning. J Am Geriatr Soc. 2001; 49: 1086–1092. [DOI] [PubMed] [Google Scholar]
- 4. Burmedi D, Becker S, Heyl V, Wahl H, Himmelsbach I. Emotional and social consequences of age-related low vision: a narrative review. Vis Impair Res. 2002; 4: 47–71. [Google Scholar]
- 5. Pinquart M, Pfeiffer JP. Psychological well-being in visually impaired and unimpaired individuals. Br J Vis Impair. 2011; 29: 27–45. [Google Scholar]
- 6. Klein BE, Klein R, Knudtson MD, Lee KE. Relationship of measures of frailty to visual function: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2003; 101: 191–196, discussion 196–199. [PMC free article] [PubMed] [Google Scholar]
- 7. Burmedi D, Becker S, Heyl V, Wahl H, Himmelsbach I. Behavioral consequences of age-related low vision: a narrative review. Vis Impair Res. 2002; 4: 15–45. [Google Scholar]
- 8. Tinetti ME, Allore H, Araujo KL, Seeman T. Modifiable impairments predict progressive disability among older persons. J Aging Health. 2005; 17: 239–256. [DOI] [PubMed] [Google Scholar]
- 9. Lam BL, Christ SL, Zheng DD, et al. Longitudinal relationships among visual acuity and tasks of everyday life: the Salisbury Eye Evaluation study. Invest Ophthalmol Vis Sci. 2013; 54: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thompson JR, Gibson JM, Jagger C. The association between visual impairment and mortality in elderly people. Age Ageing. 1989; 18: 83–88. [DOI] [PubMed] [Google Scholar]
- 11. McCarty CA, Nanjan MB, Taylor HR. Vision impairment predicts 5 year mortality. Br J Ophthalmol. 2001; 85: 322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee DJ, Gomez-Marin O, Lam BL, Zheng DD. Visual acuity impairment and mortality in US adults. Arch Ophthalmol. 2002; 120: 1544–1550. [DOI] [PubMed] [Google Scholar]
- 13. Huang CY, Zheng CM, Wu CC, Lo L, Lu KC, Chu P. Effects of pamidronate and calcitriol on the set point of the parathyroid gland in postmenopausal hemodialysis patients with secondary hyperparathyroidism. Nephron Clin Pract. 2012; 122: 93–101. [DOI] [PubMed] [Google Scholar]
- 14. Christ SL, Lee DJ, Lam BL, Zheng DD, Arheart KL. Assessment of the effect of visual impairment on mortality through multiple health pathways: structural equation modeling. Invest Ophthalmol Vis Sci. 2008; 49: 3318–3323. [DOI] [PubMed] [Google Scholar]
- 15. Karpa MJ, Mitchell P, Beath K, Rochtchina E, Cumming RG, Wang JJ. Direct and indirect effects of visual impairment on mortality risk in older persons. Arch Ophthalmol. 2009; 127: 1347–1353. [DOI] [PubMed] [Google Scholar]
- 16. Christ SL, Zheng DD, Swenor BK, et al. Longitudinal relationships among visual acuity, functioning, and mortality: the Salisbury Eye Evaluation Study. JAMA Ophthalmol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010; 35: 2–16. [DOI] [PubMed] [Google Scholar]
- 18. Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation—allostatic load and its health consequences: MacArthur studies of successful aging. Arch Intern Med. 1997; 157: 2259–2268. [PubMed] [Google Scholar]
- 19. Gruenewald TL, Seeman TE, Karlamangla AS, Sarkisian CA. Allostatic load and frailty in older adults. J Am Geriatr Soc. 2009; 57: 1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goldman N, Turra CM, Glei DA, Lin YH, Weinstein M. Physiological dysregulation and changes in health in an older population. Exp Gerontol. 2006; 41: 862–870. [DOI] [PubMed] [Google Scholar]
- 21. Cacciatore F, Abete P, Maggi S, et al. Disability and 6-year mortality in elderly population. Role of visual impairment. Aging Clin Exp Res. 2004; 16: 382–388. [DOI] [PubMed] [Google Scholar]
- 22. Salive ME, Guralnik J, Glynn RJ, Christen W, Wallace RB, Ostfeld AM. Association of visual impairment with mobility and physical function. J Am Geriatr Soc. 1994; 42: 287–292. [DOI] [PubMed] [Google Scholar]
- 23. Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005; 308: 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Health and Nutrition Examination Survey Linked Mortality Files, Public-Use File. http://www.cdc.gov/nchs/data_access/data_linkage/mortality/nhanes_99_04_linkage.htm. Accessed August 10, 2014. [Google Scholar]
- 25. Statistics NCHS. National Health and Nutrition Examination Survey, NHANES Response Rates and Population Totals. Available at: http://www.cdc.gov/nchs/nhanes/response_rates_cps.htm. Accessed May 13, 2014. [Google Scholar]
- 26. National Health and Nutrition Examination Survey Linked Mortality Files, Public-Use File http://www.cdc.gov/nchs/data_access/data_linkage/mortality/nhanes_99_04_linkage.htm. Accessed August 10, 2014. [Google Scholar]
- 27. Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006; 96: 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. NCHS. National Health and Nutrition Examination Survey (NHANES 1999–2004) Linked Mortality Files, Public-Use File 2010. Available at: http://www.cdc.gov/nchs/data_access/data_linkage/mortality/nhanes_99_04_linkage.htm. Accessed June 22, 2014. [Google Scholar]
- 29. SAS. SAS Version 9.2. Cary NC: SAS Institute, Inc; 2009. [Google Scholar]
- 30. Muthén LK, Muthén BO. Mplus User's Guide. Los Angeles, CA: Muthén & Muthén; 1998–2007. [Google Scholar]
- 31. Skinner CJ. Domain means, regression and multivariate analysis. In: Skinner CJ, Holt D, Smith TMF. eds Analysis of Complex Surveys. New York: Wiley; 1989: 59–87. [Google Scholar]
- 32. Binder D. On the variance of asymptotically normal estimators from complex surveys. Int Stat Rev. 1983; 51: 279–292. [Google Scholar]
- 33. Bollen KA. Total, direct, and indirect effects in structural equation models. In: Clogg C. ed Sociological Methodology 1987. Washington, DC: American Sociological Association; 1987: 37–69. [Google Scholar]
- 34. MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002; 7: 83–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davison AC. Statistical Models. Cambridge, UK: Cambridge University Press; 2003: 33–35. [Google Scholar]
- 36. Baron MKD. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986; 51: 1173–1182. [DOI] [PubMed] [Google Scholar]
- 37. Lee DJ, Gomez-Marin O, Lam BL, Zheng DD. Glaucoma and survival: the National Health Interview Survey 1986–1994. Ophthalmology. 2003; 110: 1476–1483. [DOI] [PubMed] [Google Scholar]
- 38. Knudtson MD, Klein BE, Klein R. Age-related eye disease, visual impairment, and survival: the Beaver Dam Eye Study. Arch Ophthalmol. 2006; 124: 243–249. [DOI] [PubMed] [Google Scholar]
- 39. Zheng DD, Christ SL, Lam BL, Arheart KL, Galor A, Lee DJ. Increased mortality risk among the visually impaired: the roles of mental well-being and preventive care practices. Invest Ophthalmol Vis Sci. 2012; 53: 2685–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McEwen BS, Tucker P. Critical biological pathways for chronic psychosocial stress and research opportunities to advance the consideration of stress in chemical risk assessment. Am J Public Health. 2011; 101 (suppl 1): S131–S139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mausbach BT, Chattillion EA, Moore RC, Roepke SK, Depp CA, Roesch S. Activity restriction and depression in medical patients and their caregivers: a meta-analysis. Clin Psychol Rev. 2011; 31: 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003; 54: 200–207. [DOI] [PubMed] [Google Scholar]
- 43. Cuijpers P, Smit F. Excess mortality in depression: a meta-analysis of community studies. J Affect Disord. 2002; 72: 227–236. [DOI] [PubMed] [Google Scholar]
- 44. Panagiotakos DB, Pitsavos C, Chrysohoou C, Kavouras S, Stefanadis C. The associations between leisure-time physical activity and inflammatory and coagulation markers related to cardiovascular disease: the ATTICA Study. Prev Med. 2005; 40: 432–437. [DOI] [PubMed] [Google Scholar]
- 45. Naci H, Ioannidis JPA. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ. 2013; 347: f5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002; 50: 1921–1928. [DOI] [PubMed] [Google Scholar]
- 47. Reinhardt JP, Boerner K, Benn D. Predicting individual change in support over time among chronically impaired older adults. Psychol Aging. 2003; 18: 770–779. [DOI] [PubMed] [Google Scholar]
- 48. Binns AM, Bunce C, Dickinson C, et al. How effective is low vision service provision? A systematic review. Surv Ophthalmol. 2012; 57: 34–65. [DOI] [PubMed] [Google Scholar]
- 49. Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci. 1998; 53: S9–S16. [DOI] [PubMed] [Google Scholar]
- 50. Booth T, Starr JM, Deary I. Modeling multisystem biological risk in later life: allostatic load in the Lothian birth cohort study 1936. Am J Hum Biol. 2013; 25: 538–543. [DOI] [PubMed] [Google Scholar]