Abstract
Background
Dihydroxyphenylacetaldehyde (DOPAL), a cytotoxic metabolite of dopamine, is the focus of the ‘catecholaldehyde hypothesis’ about the pathogenesis of Parkinson disease. This study explored whether DOPAL is detectable in human striatum – especially in the putamen (Pu), the main site of dopamine depletion in Parkinson disease – and is related to other neurochemical indices of catecholamine stores and metabolism in Parkinson disease.
Methods
Putamen, caudate (Cd), and frontal cortex (Ctx) catechols were measured in tissue from patients with pathologically proven end-stage Parkinson disease (N = 15) and control subjects (N = 14) of similar age with similar post-mortem intervals.
Results
Putamen DOPAL (3% of dopamine in controls) correlated with dopamine and dihydroxyphenylacetic acid both across all subjects and within the Parkinson disease and control groups. Pu dopamine was decreased by 93% and dihydroxyphenylacetic acid 95% in Parkinson disease vs. controls, with smaller decreases of DOPAL (83%) and norepinephrine (73%) in Pu and of dopamine (74%) and dihydroxyphenylacetic acid (82%) in Cd. In Parkinson disease, Pu DOPAL:dihydroxyphenylacetic acid averaged 3.4 times and DOPAL:dopamine 4.4 times control (P = 0.03 each). The main catecholamine in Ctx was norepinephrine, which was decreased by 51% in Parkinson disease patients.
Conclusions
Correlated decreases of DOPAL, dopamine, and dihydroxyphenylacetic acid in Parkinson disease reflect severe loss of Pu dopamine stores, which seems more extensive than loss of Pu norepinephrine or Cd dopamine stores. Increased Pu DOPAL:dihydroxyphenylacetic acid ratios in Parkinson disease suggest decreased detoxification of DOPAL by aldehyde dehydrogenase. Elevated levels of cytosolic DOPAL might contribute to loss of dopaminergic neurons in Parkinson disease.
Keywords: aldehyde dehydrogenase, dihydroxyphenylacetaldehyde, dihydroxyphenylglycol, dopamine, norepinephrine, Parkinson disease, putamen
Parkinson disease is one of the most common neurodegenerative diseases of the elderly, a major cause of morbidity and mortality, and a growing medical economic burden. The movement disorder results from depletion of the catecholamine dopamine in the nigrostriatal system, due to loss of nigral neurons and striatal terminals. The loss progresses over years, and by the time an individual develops symptoms of striatal dopamine deficiency most of the terminals are already gone. Identification of pathogenetic processes resulting in nigrostriatal dopaminergic denervation should foster development of approaches to retard progression of or even prevent the disease.
Dihydroxyphenylacetaldehyde (DOPAL) is a toxic catecholaldehyde produced continuously in dopaminergic neurons by the action of monoamine oxidase type A on cytosolic dopamine [1]. According to the ‘catecholaldehyde hypothesis’ increased DOPAL production or decreased detoxification by aldehyde dehydrogenase produces selective loss of nigrostriatal dopaminergic neurons in Parkinson disease [2–5]. Analogously, increased production or decreased detoxification of dihydroxyphenylglycolaldehyde, the deaminated metabolite of norepinephrine, by aldehyde/aldose reductase might produce selective loss of nor-adrenergic neurons (Fig. 1).
Figure 1.
The catecholaldehyde hypothesis. Dopamine (DA) that leaks from vesicles (V) into the cytoplasm (C) or that is taken up via the cell membrane dopamine transporter and escapes vesicular reuptake via the vesicular monoamine transporter is subject to oxidative deamination catalyzed by monoamine oxidase (MAO) to form the catecholaldehyde, dihydroxyphenylacetaldehyde (DOPAL), which is cytotoxic. DOPAL is detoxified by aldehyde dehydrogenase to form dihydroxyphenylacetic acid (DOPAC), the major metabolic route, or aldehyde/aldose reductase (AR) to form dihydroxyphenylethanol (DOPET), the minor metabolic route. In contrast, the catecholaldehyde formed from norepinephrine (NE), dihydroxyphenylglycolaldehyde (DOPEGAL), is detoxified mainly by AR to form dihydroxyphenylglycol (DHPG). Dihydroxymandelic acid (DHMA) is a minor NE metabolite. Other abbreviations: NET = cell membrane norepinephrine transporter.
The presence of DOPAL in human brain has not been clearly established. A previous study reported detecting DOPAL in post-mortem substantia nigra of Parkinson disease patients; however, concentrations of DOPAL and dopamine were not reported [6]. Another study quantified DOPAL (394 pg/mg wet weight, or 2592 fmol/mg) in substantia nigra from a single human subject. This concentration seemed high, because it exceeded the simultaneously measured dopamine concentration (275 pg/mg, or 1797 fmol/mg). To our knowledge DOPAL concentrations in putamen (Pu), caudate (Cd), and frontal cortex (Ctx) have not been reported previously in control subjects or in patients with Parkinson disease.
Methods
Patient material
Post-mortem brain tissue was obtained from 15 neuropathologically confirmed cases of end-stage idiopathic Parkinson disease and 14 control subjects, most of whom were autopsied at the University of Miami/National Parkinson Foundation Brain Endowment Bank. The study was conducted with approval of the Human Subjects Research Office (M809) of the University of Miami. Post-mortem intervals (duration between death and brain freezing) were recorded. In all subjects the post-mortem interval was ≤20 h. The control subjects were selected to have similar mean age and post-mortem interval as the Parkinson disease group. Causes of death are listed in Table 1.
Table 1.
Clinical characteristics of Parkinson disease patients and control subjects
| Age (years) |
PMI (hours) |
Cause of death | |
|---|---|---|---|
| Patients | |||
| 1 | 76 | 6 | Advanced coronary arteriosclerosis |
| 2 | 83 | 15 | Parkinson disease |
| 3 | 66 | 6 | Cardiorespiratory arrest |
| 4 | 74 | 4 | Septicemia |
| 5 | 69 | 10 | Parkinson disease |
| 6 | 78 | 8 | Lung cancer |
| 7 | 77 | 3 | Parkinson disease |
| 8 | 75 | 15 | Coronary artery disease |
| 9 | 77 | 4 | Parkinson disease |
| 10 | 76 | 5 | System shut down |
| 11 | 74 | 6 | Parkinson disease |
| 12 | 79 | 4 | Respiratory failure |
| 13 | 72 | 5 | Acute aspiration |
| 14 | 85 | 5 | Multiple diseases of the aged |
| 15 | 83 | 20 | Parkinson disease |
| Mean (±SEM) | 76 ± 1 | 8 ± 1 | |
| Control subjects | |||
| 1 | 76 | 5 | Respiratory failure |
| 2 | 76 | 9 | Myocardial infarction |
| 3 | 65 | 11 | Cardiac arrest |
| 4 | 66 | 15 | Cardiac arrest |
| 5 | 67 | 19 | Cardiopulmonary arrest |
| 6 | 67 | 22 | Cardiac infarction |
| 7 | 74 | 4 | Lung cancer |
| 8 | 76 | 16 | Cardiopulmonary arrest |
| 9 | 79 | 9 | Atherosclerotic coronaryartery disease |
| 10 | 79 | 15 | Cardiac arrest |
| 11 | 91 | 8 | Congestive heart gailure |
| 12 | 82 | 8 | Gall bladder cancer |
| 13 | 67 | 19 | Blunt force trauma |
| 14 | 68 | 10 | Coronary atheriosclerosis and thrombosis |
| Mean ± SEM | 74 ± 2 | 12 ± 2 |
PMI, post-mortem interval.
Tissue assays
The assayed Pu samples were matched at the same posterior level and subregional localization, since variations in tissue concentrations across the striatum can be substantial. The samples were stored at −70°C or colder until thawed for assay in the Clinical Neurochemistry Laboratory at the NIH.
Samples from Parkinson disease patients and control subjects were assayed for catechol contents in simultaneous runs. After obtaining the wet weight of the tissue, 500 µl/100 mg tissue of a solution was added that contained 9.2 ml glacial acetic acid and 460 µl phosphoric acid per liter of water. The tissue was sonicated, and after centrifugation the supernate was frozen and stored at −70°C or colder until assayed for catechols by liquid chromatography with electrochemical detection after batch alumina extraction [7]. Briefly, mobile phase containing octanesulfonic acid as an ion pairing agent was pumped isocratically through a reverse phase liquid chromatographic column. Catechols were quantified by the current produced upon exposure of the eluate to a flow-through electrodes set to oxidizing and then reducing potentials in series, with recording from the last electrode reflecting reversibly oxidized species.
Dihydroxyphenylacetaldehyde standard was synthesized in the laboratory of and provided by Dr. Kenneth L. Kirk (NIDDK). Identity of the standard was confirmed by mass spectroscopy, nuclear magnetic resonance, and liquid chromatography with time-offlight mass spectroscopy.
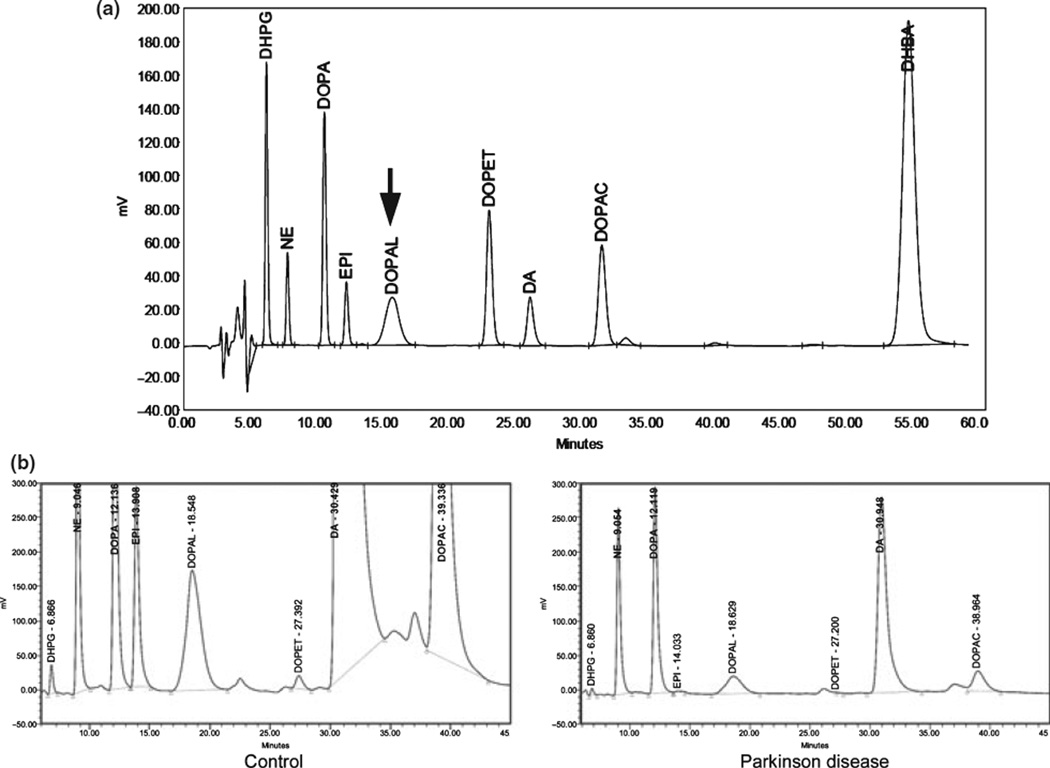
The chromatographic peak corresponding to DOPAL had an unusual short, broad shape compared to subsequent peaks (Fig. 2a). This characteristic helped identify DOPAL in patient samples (Fig. 2b).
Figure 2.
(a) Chromatograph of catechol standards (250 pg each for norepinephrine (NE), epinephrine (EPI), and Dopamine; 1000 pg each for dihydroxyphenylglycol (DHPG), dihydroxyphenylalanine (DOPA), Dihydroxyphenylacetaldehyde (DOPAL), dihydroxyphenylethanol (DOPET), and dihydroxyphenylacetic acid (DOPAC). (b) Chromatographs of putamen catechols in a control subject and a patient with Parkinson disease.
Data analysis and statistics
Tissue catechol concentrations were expressed as fmol/mg wet weight. For statistical tests individual neurochemical data were log transformed. This is a commonly used and appropriate approach when compared groups differ substantially not only in mean values but also in standard deviations and the standard deviations vary directly with the mean values. Catechol concentrations in Parkinson disease and control groups were compared by independent means t-tests and concentrations in different areas of the same subjects by dependent means t-tests. Correlation coefficients relating neurochemical values across subjects were calculated by linear regression using Kaleidagraph 4.0 (Synergy Software, Reading, PA, USA).
Results
Clinical characteristics
The Parkinson disease and control groups had similar mean ages and post-mortem intervals (Table 1). In the Parkinson disease patients the time since last levodopa dose was not available. In one Parkinson disease patient the 3,4-dihydroxyphenylalanine concentration in the Ctx was more than 2 standard deviations above the concentrations in the rest of the group. In this patient, however, striatal dopamine, DOPAL, and dihydroxyphenylacetic acid concentrations were not noticeably different from concentrations in the remaining patients, and so except as noted, data from this patient were included in the analysis.
Putamen
Dihydroxyphenylacetaldehyde was detected in Pu from all control subjects, at a mean concentration about 3% that of dopamine (Table 2). Across all subjects the logs of Pu DOPAL, dopamine, and dihydroxyphenylacetic acid concentrations were positively correlated with post-mortem intervals (r = 0.69, P < 0.0001; r = 0.42, P = 0.02; r = 0.45, P = 0.02). The slopes of the lines of best fit were similar for the three catechols (0.082, 0.071, and 0.072 fmol/mg per hour). Tissue catechol concentrations were unrelated to subject age.
Table 2.
Brain tissue mean (±SEM) concentrations (fmol/mg wet weight) of catechols in control subjects and patients with end-stage Parkinson disease
| Patients | Controls | P | |
|---|---|---|---|
| Putamen | |||
| DA | 1130 ± 397 | 15488 ± 2086 | <0.0001 |
| DOPAC | 183 ± 62 | 3460 ± 653 | <0.0001 |
| DOPA | 647 ± 135 | 1756 ± 592 | 0.04 |
| DOPAL | 86 ± 43 | 496 ± 164 | 0.0001 |
| NE | 61 ± 23 | 220 ± 34 | <0.0001 |
| DOPET | 6 ± 3 | 42 ± 13 | 0.02 |
| DHPG | 4 ± 1 | 19 ± 3 | 0.0006 |
| Caudate | |||
| DA | 2969 ± 803 | 10352 ± 1381 | 0.009 |
| DOPAC | 411 ± 104 | 2847 ± 708 | 0.009 |
| DOPA | 552 ± 89 | 950 ± 315 | n.s. |
| DOPAL | 187 ± 94 | 355 ± 66 | n.s. |
| NE | 43 ± 18 | 107 ± 27 | 0.05 |
| DOPET | 8 ± 2 | 37 ± 10 | 0.03 |
| DHPG | 6 ± 2 | 17 ± 5 | n.s. |
| Cortex | |||
| DA | 83 ± 63 | 15 ± 6 | n.s. |
| DOPAC | 7 ± 2 | 6 ± 2 | n.s. |
| DOPA | 379 ± 184 | 158 ± 18 | n.s. |
| DOPAL | 10 ± 3 | 7 ± 2 | n.s. |
| NE | 22 ± 15 | 44 ± 7 | 0.0004 |
| DOPET | 1 ± 1 | 2 ± 1 | n.s. |
| DHPG | 3 ± 1 | 9 ± 2 | 0.007 |
DA, dopamine; DOPAC, dihydroxyphenylacetic acid; DOPA, dihydroxyphenylalanine; DOPAL, dihydroxyphenylacetaldehyde; NE, norepinephrine; DOPET, dihydroxyphenylethanol; DHPG, dihydroxyphenylglycol.
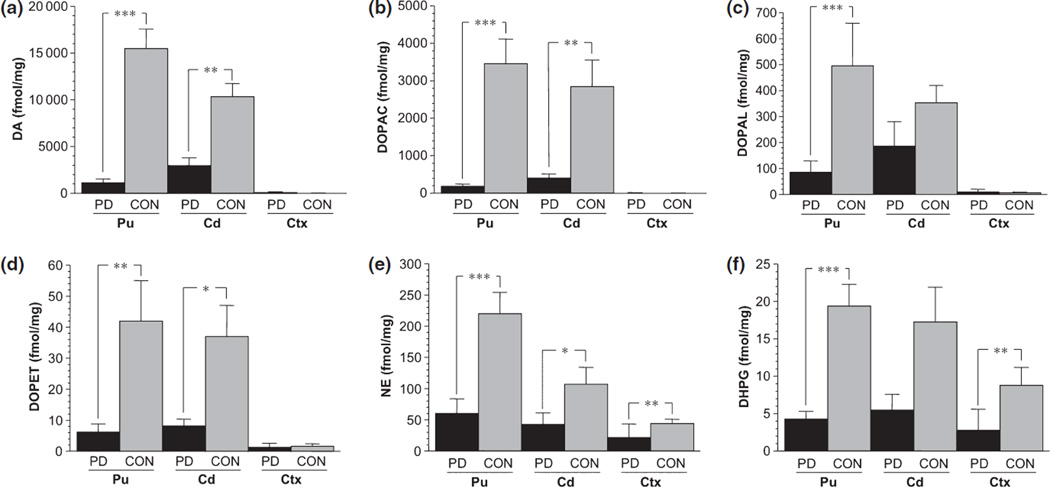
Putamen dopamine was decreased by 93% and dihydroxyphenylacetic acid by 95% in Parkinson disease patients (P < 10−6 each; Figs 3 and 4). Levels of DOPAL and dihydroxyphenylethanol (DOPET) were also decreased, to 17% (P < 0.0001) and 15% (P = 0.002) of control.
Figure 3.
Mean (±SEM) tissue concentrations of (a) DA, (b) DOPAC, (c) DOPAL, (d) DOPET, (e) NE, and (f) DHPG in patients with Parkinson disease (black) or control subjects (gray) in putamen (Pu), caudate (Cd), and frontal cortex (Ctx). Significant group difference, *P < 0.05; **P < 0.01; ***P < 0.001.
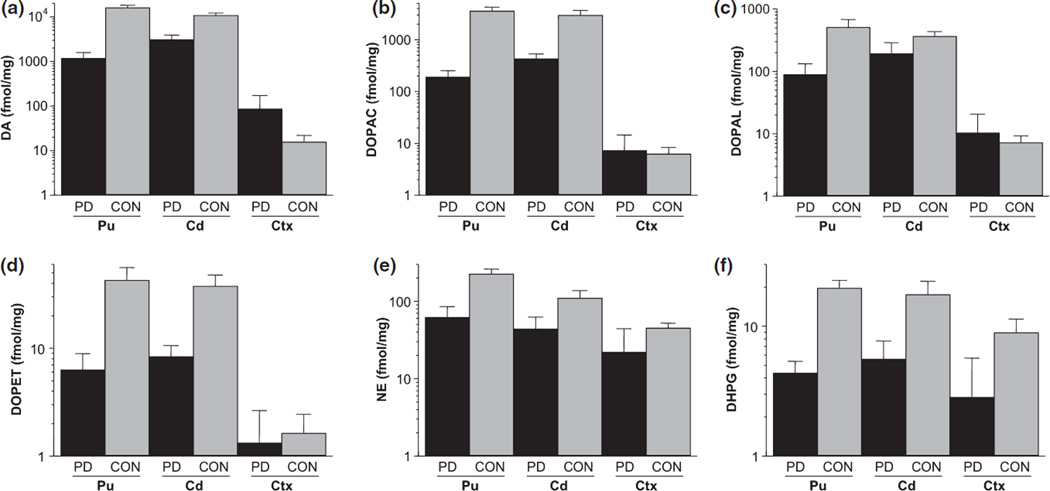
Figure 4.
Same data as in Fig. 3, but with concentrations displayed on log scales, to facilitate comparison of striatum with cortex. (a) DA, (b) DOPAC, (c) DOPAL, (d) DOPET, (e) NE, and (f) DHPG.
Across all subjects, individual values for DOPAL, dopamine, and dihydroxyphenylacetic acid were positively inter-correlated (Table 3). Within the Parkinson disease and control groups considered separately, Pu DOPAL also correlated positively with Pu dihydroxyphenylacetic acid and dopamine.
Table 3.
Inter-correlations among putamen tissue concentrations of catechols in (a) control subjects and patients with end-stage Parkinson disease, (b) patients with end-stage Parkinson disease, and (c) control subjects
| DA | DOPAC | DOPET | DOPA | NE | DHPG | |
|---|---|---|---|---|---|---|
| (a) | ||||||
| DOPAL | 0.83* | 0.87* | 0.65* | 0.48** | 0.80* | 0.65* |
| DA | 0.93* | 0.69* | 0.53** | 0.81* | 0.46** | |
| DOPAC | 0.76* | 0.58* | 0.81* | 0.58* | ||
| DOPET | 0.52** | 0.50*** | 0.69* | |||
| DOPA | 0.25 | 0.13 | ||||
| NE | 0.67* | |||||
| (b) | ||||||
| DOPAL | 0.63*** | 0.80* | 0.30 | 0.31 | 0.70** | 0.33 |
| DA | 0.86* | 0.26 | 0.39 | 0.57*** | −0.13 | |
| DOPAC | 0.35 | 0.48 | 0.63** | 0.06 | ||
| DOPET | −0.40 | 0.30 | 0.58 | |||
| DOPA | −0.02 | −0.30 | ||||
| NE | 0.41 | |||||
| (c) | ||||||
| DOPAL | 0.68** | 0.55*** | 0.57*** | 0.41 | 0.42 | 0.47 |
| DA | 0.36 | 0.57*** | 0.58*** | 0.29 | 0.03 | |
| DOPAC | 0.78* | 0.65** | −0.10 | 0.65** | ||
| DOPET | 0.77** | 0.03 | 0.35 | |||
| DOPA | −0.17 | 0.31 | ||||
| NE | 0.16 |
P ≤ 0.001;
P ≤ 0.01;
P < 0.05.
DOPAL, dihydroxyphenylacetaldehyde; DOPET, dihydroxyphenylethanol; DA, dopamine; DHPG, dihydroxyphenylglycol; NE, norepinephrine; DOPAC, dihydroxyphenylacetic acid; DOPA, dihydroxyphenylalanine.
Mean Pu dihydroxyphenylacetic acid:dopamine ratios did not differ between the Parkinson disease and control groups (0.42 ± 0.17 vs. 0.29 ± 0.08).
Norepinephrine and dihydroxyphenylglycol were detected in all Pu samples from control subjects. The concentration of dopamine averaged more than 100 times that of norepinephrine and the dihydroxyphenylacetic acid concentration about 300 times that of dihydroxyphenylglycol. In Parkinson disease patients Pu concentrations of norepinephrine and dihydroxyphenylglycol were decreased compared to controls (27% of control, P < 0.0001 and 22% of control, P = 0.0006; Fig. 3), but to lesser extents than were concentrations of dopamine and dihydroxyphenylacetic acid in the same samples. Among Parkinson disease patients, norepinephrine concentrations were positively correlated with dopamine concentrations (r = 0.52, P = 0.05).
Putamen dihydroxyphenylalanine in Parkinson disease was decreased to 37% of control (P = 0.04) – i.e. to a much smaller extent than Pu dopamine. After exclusion of data from a Parkinson disease patient with high frontal cortical DOPA, Pu DOPA in Parkinson disease patients was decreased to 32% of control (P = 0.02). Across all subjects, values for Pu DOPA correlated positively with dopamine and dihydroxyphenylacetic acid but weakly with DOPAL (Table 2).
Putamen DOPAL:dihydroxyphenylacetic acid ratios in Parkinson disease patients averaged 3.4 times those in controls (P = 0.03; Fig. 5). In two Parkinsonian patients, the DOPAL:dihydroxyphenylacetic acid ratio was more than 1.0, far above the range of controls (Fig. 2). For given Pu dopamine concentrations, an index of surviving dopaminergic neurons, DOPAL concentrations in Parkinson disease patients averaged 4.4 times those in controls (P = 0.03).
Figure 5.
(a) Mean (±SEM) putamen tissue ratios of DOPAL:DA and DOPAL:DOPAC in patients with Parkinson disease (black) or control subjects (gray) in putamen (Pu) and caudate (Cd). Significant group difference, *P < 0.05. DOPAL, Dihydroxyphenylacetaldehyde; DA, Dopamine; DOPAC, dihydroxyphenylacetic acid.
Caudate
Abnormalities of tissue catechols and catechol ratios in Parkinson disease were generally less severe in the Cd than Pu. Cd concentrations of dopamine, dihydroxyphenylacetic acid, and DOPET were decreased in Parkinson disease patients, to 29%, (P = 0.009), 14% (P = 0.008), and 22% (P = 0.03) of controls, whereas DOPAL was not significantly decreased (Figs 3 and 4). Among controls, Pu dopamine and norepinephrine concentrations were higher than Cd dopamine and norepinephrine concentrations (P = 0.009, P = 0.018 by dependent means t-tests), whereas DOPAL:dihydroxyphenylacetic acid ratios did not differ. Among Parkinson disease patients, dopamine and dihydroxyphenylacetic acid tended to be higher in Cd than Pu (P = 0.09 each).
Caudate DOPAL:dihydroxyphenylacetic acid ratios in Parkinson disease patients averaged more than twice those in controls (P = 0.04). For given Cd dopamine concentrations, DOPAL concentrations in Parkinson disease patients averaged 2.6 times those in controls (P = 0.08).
Mean Cd dihydroxyphenylacetic acid:dopamine ratios did not differ between the Parkinson disease and control groups (0.30 ± 0.11 vs. 0.09 ± 0.04).
In Parkinson disease patients, Cd concentrations of norepinephrine were decreased to 40% (P = 0.05) and dihydroxyphenylglycol to 32% (P = 0.12) of controls (Figs 3 and 4). Cd DOPA in Parkinson disease was decreased non-significantly, to 58% of control (55% of control after exclusion of data from the Parkinson disease patient with high Ctx DOPA).
Frontal cortex
Cortex concentrations of DOPAL, dopamine, dihydroxyphenylacetic acid, and DOPET were normal in Parkinson disease (Figs 3 and 4), whereas norepinephrine concentrations in Parkinson disease were decreased to 49% (P = 0.0004) and dihydroxyphenylglycol to 32% (P = 0.007) of control. After exclusion of data from the Parkinson disease patient with high Ctx DOPA, the Parkinson disease group still did not differ from controls in terms of Ctx DOPA, DOPAL, dopamine, or dihydroxyphenylacetic acid. DOPAL was below the detection limit in some and DOPET in most Ctx samples from Parkinson disease patients and control subjects.
Mean Ctx dihydroxyphenylacetic acid:dopamine ratios did not differ between the Parkinson disease and control groups (0.75 ± 0.15 vs. 0.64 ± 0.15).
Across all subjects, norepinephrine concentrations in Ctx were positively correlated with norepinephrine concentrations in Pu (r = 0.67, P < 0.0001) and Cd (r = 0.55, P = 0.004).
Discussion
This study shows that human post-mortem striatum contains DOPAL, that Pu concentrations of DOPAL are correlated with those of dopamine and dihydroxyphenylacetic acid, and that in Parkinson disease, Pu DOPAL:dihydroxyphenylacetic acid and DOPAL:dopamine ratios are higher than corresponding ratios in control subjects. As explained below, these results fit with the view that DOPAL may participate in the striatal dopaminergic denervation that characterizes Parkinson disease.
One mechanism of DOPAL toxicity is protein reactivity [8]. DOPAL potently oligomerizes alpha-synuclein [9], aggregation of which is a hallmark of Parkinson disease. The technical challenge of measuring tissue DOPAL concentrations, which in the Pu average only about 3% of dopamine, obviated until now direct assessment of this potential mechanism of dopaminergic autotoxicity in the striatum of patients with Parkinson disease.
Strongly positive intercorrelations among Pu contents of DOPAL, dopamine, and dihydroxyphenylacetic acid are consistent with an intermediate position of DOPAL in dopamine metabolism, in which cytosolic dopamine is converted to DOPAL by monoamine oxidase-A and DOPAL is converted to dihydroxyphenylacetic acid by aldehyde dehydrogenase. A straightforward explanation for low tissue levels of all three compounds, as well as low levels of DOPET, a minor DOPAL metabolite formed from enzymatic action of aldehyde/aldose reductase, is decreased vesicular stores of dopamine from loss of dopaminergic terminals in Parkinson disease.
It is unlikely that low tissue DOPAL with respect to dopamine and dihydroxyphenylacetic acid reflected disproportionate loss of the aldehyde after death, because the post-mortem rates of increase (perhaps related to tissue water loss) were quite similar for DOPAL, dopamine, and dihydroxyphenylacetic acid. Also, in mice from which the brains have been removed and striata dissected out and frozen immediately after sacrifice, the pattern of striatal catechols (dopamine > dihydroxyphenylacetic acid > DOPAL > DOPET) resembles closely that in humans (unpublished observations). This order of concentrations probably reflects rapid conversion of cytosolic dopamine to DOPAL via monoamine oxidase-A followed by rapid conversion of DOPAL to dihydroxyphenylacetic acid via aldehyde dehydrogenase and slower conversion of DOPAL to DOPET via aldehyde/aldose reductase.
Since aldehyde dehydrogenase detoxifies DOPAL by converting it to dihydroxyphenylacetic acid, the finding of increased DOPAL:dihydroxyphenylacetic acid ratios in Parkinson disease suggests decreased aldehyde dehydrogenase activity. In two Parkinson disease patients, the Pu DOPAL concentration exceeded the dihydroxyphenylacetic acid concentration, a finding not seen in any control subjects. To our knowledge aldehyde dehydrogenase activity has not been assessed directly in Parkinson disease. Post-mortem substantia nigra tissue from Parkinson disease patients has been reported to have decreased aldehyde dehydrogenase 1A1 gene expression [10,11] and protein content [12], but these abnormalities could be the result rather than cause of decreased numbers of dopaminergic neurons. In Parkinson disease, Pu DOPAL concentrations averaged about 13% of dopamine – i.e. more than four times those expected from simultaneously assayed dopamine concentrations. Assuming that tissue dopamine provides an estimate of dopaminergic terminal density, high Pu DOPAL:dopamine ratios are consistent with increased DOPAL in the remaining terminals.
Putamen concentrations of norepinephrine and of dihydroxyphenylglycol, the main neuronal metabolite of norepinephrine, were only modestly decreased in Parkinson disease, in contrast with markedly decreased concentrations of dopamine and of dihydroxyphenylacetic acid. A previous study also found more extensive loss of dopamine than of norepinephrine in Pu of Parkinson disease patients [13]. Literature searches failed to reveal prior post-mortem human studies reporting Pu dihydroxyphenylglycol. Since dihydroxyphenylglycol production mainly reflects net leakage of norepinephrine from vesicular stores [14], low Pu concentrations of both norepinephrine and dihydroxyphenylglycol in Parkinson disease probably reflect loss of noradrenergic terminals. The finding of correlated norepinephrine levels in Ctx and Pu suggests a common source, such as the locus ceruleus [15], the main site of origin of noradrenergic innervation in brain.
Two potential explanations may apply to smaller losses of Pu norepinephrine and dihydroxyphenylglycol than of dopamine and dihydroxyphenylacetic acid in Parkinson disease. Both explanations fit with the catecholaldehyde toxicity concept. The first is that norepinephrine is a poorer substrate for monoamine oxidase- A than is dopamine, so that there is less formation of dihydroxyphenylglycolaldehyde in noradrenergic neurons than of DOPAL in dopaminergic neurons. We recently found that dopamine is much more readily metabolized by monoamine oxidase-A than is norepinephrine (unpublished observations). Second, whereas DOPAL is detoxified mainly by aldehyde dehydrogenase to form dihydroxyphenylacetic acid, dihydroxyphenylglycolaldehyde is detoxified mainly by aldehyde/aldose reductase to form dihydroxyphenylglycol. If Pu catecholaminergic terminals had decreased aldehyde dehydrogenase activity, there would be more accumulation of DOPAL than of dihydroxyphenylglycolaldehyde and therefore greater loss of dopaminergic than of noradrenergic neurons.
Association does not imply causation, and the present results do not necessarily mean that buildup of endogenous DOPAL actually causes degeneration of striatal dopaminergic terminals in Parkinson disease. Any of a variety of insults could lead to lipid peroxidation of dopaminergic terminal membranes, and lipid peroxidation products potently inhibit dihydroxyphenylacetic acid production from DOPAL in catecholaminergic cells [16].
Because DOPAL oligomerizes alpha-synuclein, one can envision a pathogenetic positive feedback loop in which DOPAL produces synucleinopathy and synucleinopathy increases DOPAL generation. Analogously, since vesicular sequestration is energy dependent, mitochondrial dysfunctions decreasing ATP availability for the vesicular monoamine transporter would be expected to augment DOPAL production due to increased net leakage of dopamine from vesicular stores into the cytosol. Finally, the pesticide and Complex I inhibitor rotenone, which produces neurotoxic effects mimicking Parkinson disease in animals [17], augments DOPAL formation in rat pheochromocytoma PC-12 cells [18], and DOPAL potentiates rotenone-induced cytotoxicity [19]. The monoamine aldehyde hypothesis therefore can account for relatively selective loss of nigrostriatal dopaminergic innervation, more severe dopamine than norepinephrine depletion in Pu, widespread but modest loss of noradrenergic terminals, and increased Pu DOPAL:dihydroxyphenylacetic acid and DOPAL:dopamine ratios in Parkinson disease.
Acknowledgement
The research reported here was supported by the intramural research program of the NINDS.
References
- 1.Blaschko H. Amine oxidase and amine metabolism. Pharmacol Rev. 1952;4:415–458. [PubMed] [Google Scholar]
- 2.Mattammal MB, Haring JH, Chung HD, Raghu G, Strong R. An endogenous dopaminergic neurotoxin: implication for Parkinson’s disease. Neurodegeneration. 1995;4:271–281. doi: 10.1016/1055-8330(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 3.Burke WJ, Li SW, Williams EA, Nonneman R, Zahm DS. 3,4-Dihydroxyphenylacetaldehyde is the toxic dopamine metabolite in vivo: implications for Parkinson’s disease pathogenesis. Brain Res. 2003;989:205–213. doi: 10.1016/s0006-8993(03)03354-7. [DOI] [PubMed] [Google Scholar]
- 4.Marchitti SA, Deitrich RA, Vasiliou V. Neurotoxicity and metabolism of the catecholamine-derived 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde: the role of aldehyde dehydrogenase. Pharmacol Rev. 2007;59:125–150. doi: 10.1124/pr.59.2.1. [DOI] [PubMed] [Google Scholar]
- 5.Galvin JE. Interaction of alpha-synuclein and dopamine metabolites in the pathogenesis of Parkinson’s disease: a case for the selective vulnerability of the substantia nigra. Acta Neuropathol. 2006;112:115–126. doi: 10.1007/s00401-006-0096-2. [DOI] [PubMed] [Google Scholar]
- 6.Mattammal MB, Chung HD, Strong R, Hsu FF. Confirmation of a dopamine metabolite in parkinsonian brain tissue by gas chromatography-mass spectrometry. J Chromatogr. 1993;614:205–212. doi: 10.1016/0378-4347(93)80310-z. [DOI] [PubMed] [Google Scholar]
- 7.Holmes C, Eisenhofer G, Goldstein DS. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl. 1994;653:131–138. doi: 10.1016/0378-4347(93)e0430-x. [DOI] [PubMed] [Google Scholar]
- 8.Rees JN, Florang VR, Eckert LL, Doorn JA. Protein reactivity of 3,4-dihydroxyphenylacetaldehyde, a toxic dopamine metabolite, is dependent on both the aldehyde and the catechol. Chem Res Toxicol. 2009;22:1256–1263. doi: 10.1021/tx9000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke WJ, Kumar VB, Pandey N, et al. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008;115:193–203. doi: 10.1007/s00401-007-0303-9. [DOI] [PubMed] [Google Scholar]
- 10.Mandel S, Grunblatt E, Riederer P, et al. Gene expression profiling of sporadic Parkinson’s disease substantia nigra pars compacta reveals impairment of ubiquitin-proteasome subunits, SKP1A, aldehyde dehydrogenase, and chaperone HSC-70. Ann N Y Acad Sci. 2005;1053:356–375. doi: 10.1196/annals.1344.031. [DOI] [PubMed] [Google Scholar]
- 11.Galter D, Buervenich S, Carmine A, Anvret M, Olson L. ALDH1 mRNA: presence in human dopamine neurons and decreases in substantia nigra in Parkinson’s disease and in the ventral tegmental area in schizophrenia. Neurobiol Dis. 2003;14:637–647. doi: 10.1016/j.nbd.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Werner CJ, Heyny-von Haussen R, Mall G, Wolf S. Proteome analysis of human substantia nigra in Parkinson’s disease. Proteome Sci. 2008;6:8. doi: 10.1186/1477-5956-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong J, Hornykiewicz O, Kish SJ. Inverse relationship between brain noradrenaline level and dopamine loss in Parkinson disease: a possible neuroprotective role for noradrenaline. Arch Neurol. 2006;63:1724–1728. doi: 10.1001/archneur.63.12.1724. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56:331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- 15.Porrino LJ, Goldman-Rakic PS. Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. J Comp Neurol. 1982;205:63–76. doi: 10.1002/cne.902050107. [DOI] [PubMed] [Google Scholar]
- 16.Florang VR, Rees JN, Brogden NK, Anderson DG, Hurley TD, Doorn JA. Inhibition of the oxidative metabolism of 3,4-dihydroxyphenylacetaldehyde, a reactive intermediate of dopamine metabolism, by 4-hydroxy-2-nonenal. Neurotoxicology. 2007;28:76–82. doi: 10.1016/j.neuro.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 18.Lamensdorf I, Eisenhofer G, Harvey-White J, Hayakawa Y, Kirk K, Kopin IJ. Metabolic stress in PC12 cells induces the formation of the endogenous dopaminergic neurotoxin, 3,4-dihydroxyphenylacetaldehyde. J Neurosci Res. 2000;60:552–558. doi: 10.1002/(SICI)1097-4547(20000515)60:4<552::AID-JNR14>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 19.Lamensdorf I, Eisenhofer G, Harvey-White J, Nechustan A, Kirk K, Kopin IJ. 3,4-Dihydroxyphenylacetaldehyde potentiates the toxic effects of metabolic stress in PC12 cells. Brain Res. 2000;868:191–201. doi: 10.1016/s0006-8993(00)02309-x. [DOI] [PubMed] [Google Scholar]