Abstract
Background
Serious infections are a major concern for patients considering treatmentsfor rheumatoid arthritis (RA). Evidence is inconsistent on whether biologicsare associated with an increased risk of serious infection compared to traditional disease-modifying anti-rheumatic drugs (DMARDs).
Methods
A systematic literature search was undertaken using MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, and www.clinicaltrials.gov from inception through February 11, 2014. Search terms included biologics, rheumatoid arthritis and their synonyms. Trials were eligible for inclusion if they included any of the biologics and reported serious infections. The risk of bias was assessed using the Cochrane Risk of Bias Tool. We conducted a Bayesian network meta-analysis,using a binomial likelihood model, of published trials to assess the risk of serious infections of biologics in RA patients, compared to traditional DMARDs.
Findings
The systematic review identified 106 trials that included RA patients on biologic and reported on serious infections. Compared to traditional DMARDs, standard-dose biologic (odds ratio [OR],1.31; 95% credible interval [CrI], 1.09 to 1.58) andhigh-dose biologic (OR, 1.90; 95% Crl, 1.50 to 2.39) were associated with an increased risk of serious infections, while low-dose biologics (OR, 0.93; 95% CrI, 0.65 to 1.33) were not. The risk was lower in patients who are methotrexate naïve compared withtraditional DMARD- or anti-TNF-biologic-experienced. The absolute increase in the number of serious infectionsper 1000 patients treated each year compared to traditional DMARDs ranged from 6 for standard-dose biologic to 55 for combination biologic therapy.
Interpretation
Standard-dose and high-dose biologics (with/without traditional DMARDs) are associated with an increase in serious infections compared to traditional DMARDs in RA, while low-dose biologics are not.Clinicians should discuss the balance between benefit and harm with the individual RA patient before initiating biologic therapy.
Funding
Rheumatology division at the University of Alabama at Birmingham.
Keywords: rheumatoid arthritis, serious infection, harms, biologics, anti-TNF biologic, non-TNF biologic, methotrexate, DMARD, network meta-analysis, NMA, systematic review, meta analysis
INTRODUCTION
Biologics are a breakthrough new class of disease-modifying treatment options for rheumatoid arthritis (RA), with large clinical and radiographic improvements.12 Nine biologics are now approved for RA by the U.S. Food and Drug Administration and European Medicines Agency. Biologics are used to treat moderate to severe RA in patients who have not responded adequately to traditional DMARDs such as methotrexate (MTX).3,4 Infections, and in particular serious infections, are one of the greatest worries for patients considering biologics.
There is debate over whether biologic therapies are associated with serious infectious in patients with RA, the magnitude of this risk, and whether the risk varies among subpopulations of patients within RA.5 The clinical perception leans towards a belief that serious infection is an issue but this is not backed-up by consistent research evidence. The confusion lies in the four published systematic reviews with meta-analyses6-9 on the risk of serious infection with biologics in patients with RA. The firstmeta-analysis9, that included only three of the currently used biologics in only 9 trials, found an association, but the next three meta-analyses in RA including more biologics and a far greater sample size6-8 failed to find any association ofstandard-dose biologics with an increased risk of serious infections. Further, discordant results have also been reported for non-randomized studies assessing the risk of serious infection in RA,10-16 with some studies showing an association14-16 and others showing no association.10-13 Accordingly, there has been debate around the risk of serious infection with biologics in RA. Several-fold more trials are now available to perform a conclusive study to address this question. As well, all four meta-analyses in RA patients6-9 were limited in that they restricted the patient population (e.g., MTX-naïve patients),8 only considered a few biologics in their analyses,6-9 consisted primarily of studies which were more than a decade old,9 or failed to integrate findings across low, standard or high-dose biologics (i.e., conducted analyses separately).6-9 Availability of more robust evidence is critical for the development of RA treatment guidelines, which have been predominantly based on observational studies in the past.3
The objective of our study was to compare the risk of serious infections with biologics to non-biologic traditional DMARDs for the treatment of RA and subpopulations within RAusing network meta-analysis (NMA) to synthesize data from randomized controlled trials (RCTs).
METHODS
A systematic reviewthat included both a traditional meta-analysis and NMA was conducted to assess the risk of serious infection comparing biologics with each other, placebo or a control treatment (traditional DMARDs or their combinations) in RA. NMA considers direct and indirect evidence on the benefits and harms among multiple treatments simultaneously, whereas traditional meta-analysis only considers direct evidence between two treatment strategies.6 This systematic review, meta-analysis and NMA was performed according to the guidance specified in the Cochrane Handbook for Intervention Reviews,17 ISPOR NMA Guidance18,19 and the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement.20
We included RCTs in adults with RA treated with any of the nine biologics approved for the treatment of RA, alone or in combination as compared to each other, placebo or traditional DMARD (or DMARD combinations). Biologics included tumor necrosis factor blockers (etanercept, adalimumab, infliximab, golimumab, certolizumab pegol), interleukin (IL)-1 antagonist (anakinra), IL-6 antagonist (tocilizumab), anti-CD28 (abatacept), and anti-B cell (rituximab) biologic in any dose. The comparator was placebo, traditional DMARDs (including MTX, alone or in combination) or another biologic. We included tofacitinib doses as separate nodes in the network to improve precision of effect estimates for biologics (i.e. by borrowing strength from indirect evidence) and facilitate future updates of this review but do not report findings for tofacitinib at this time for many reasons (Appendix 1). Serious infection was the outcome of interest, defined as serious infection in each study (mostly included infections associated with death, hospitalization, or the use of intravenous antibiotics).
Search and Systematic Review Methods
A Cochrane librarian (TR) performed a literature search (Appendix 2) and retrieved published trials of biologics or tofacitinib based on the above criteria in: a] the Cochrane Central Register of Controlled Trials (CENTRAL, via the Cochrane Library), Medline (from 1946), and EMBASE databases (from 1947) up to February 11, 2014; b] data from the two previously published Cochrane systematic reviews of biologics21,22; c] data from two reviews comparing traditional DMARD monotherapy with traditional DMARD combination therapies,23,24 and d] through a search of the www.clinicaltrials.gov website. The search protocols for both Cochrane reviews are accessible online.21,22 Search terms included biologics, rheumatoid arthritis and their synonyms (Appendix 2). Studies were eligible for inclusion if they included any of the biologics and reported serious infections; no restrictions were applied by the length of follow-up. Two reviewers assessed titles and abstracts (SN, MT), full text articles (SN, TC) and extracted the data (SN, MT) independently; any disagreements were resolved by consensus and when needed, by a third reviewer (JS). Dataon serious infections and the total number of patients in each treatment arm and key patient and study characteristics (Appendix 3) were extracted using a standardized data abstraction sheet. The risk of bias was assessed using the Cochrane Risk of Bias Tool.25
Statistical Methods
The odds ratio (OR) of serious infection was the primary measure of treatment effect. Absolute risk differences per 1,000 patients treated were also calculated using the mean annualized baseline risk of serious infection in traditional DMARD arms of included studies greater than 6 months in duration. We conducted traditional meta-analyses, cumulative meta-analyses (meta-analyses over time), and Bayesian NMA. Traditional and cumulative meta-analyses (comparing standard-dose (approved) biologic versus traditional DMARD) were conducted using Comprehensive Meta-analysis (BioStat, Englewood, US). The Mantel Haenszel method using a fixed effects model and an adjusted continuity correction factor centered around 0.5 to handle zero cells.26
Bayesian NMA were conducted using WinBUGS software (MRC Biostatistics Unit, Cambridge, UK).27 A binomial likelihood model,28 which allows for the use of multi-arm trials, was used for Bayesian NMA because many studies included multi-arms trials. Both fixed and random-effects NMA were conducted, although the random-effects model with an informative prior29 on the between study variance was used for the primary analysis. Point estimates and 95% credible intervals (CrI) for ORs were derived using Markov Chain Monte Carlo methods. We assessed model fit and inconsistency30 using standard approaches (Appendix 3).
For traditional and cumulative meta-analyses, we used the standard-doses of the biologics, provided in Appendix 3. For the NMA, we included all doses (low, standard, high) of biologics. Pre-specified study and patient characteristics were assessed to ensure similarity and to investigate the potential impact of heterogeneity on effect estimates (Appendix 3). In particular, we stratified results by the following pre-defined populations: MTX-naïve, MTX-experienced, and anti-TNF-biologic-experienced. We also conducted numerous sensitivity analyses related to methods for handling zero events.26
Role of the funding source
This research was funded by the rheumatology division at the University of Alabama at Birmingham. The funders played no role in the data collection, analysis, interpretation, writing of the manuscript and the decision to submit the manuscript for publication.
RESULTS
Study characteristics of included trials
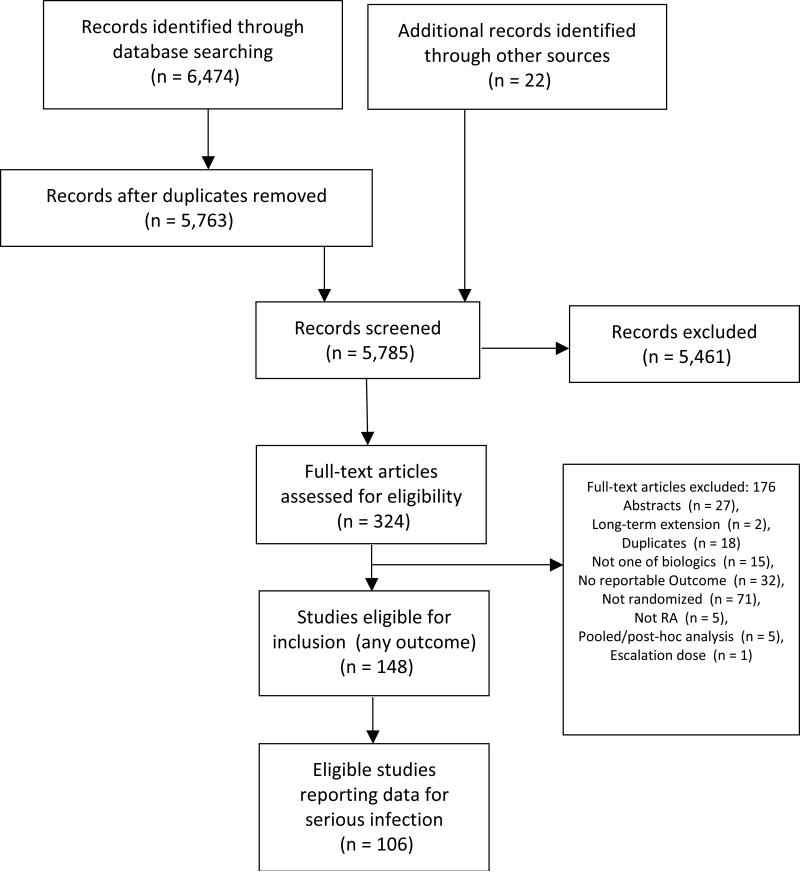
We identified 106 randomized trials published between 1992 and 2014 involving 42,330 RA patients (Figure 1, Appendix 4). There were 24 (23%), 71 (67%), 11 (10%) studies conducted in patients who were MTX-naïve, traditional DMARD-experienced, and anti-TNF-biologic-experienced, respectively (Table 1; Figure 2). Study and patient characteristics, overall and for each of these three populations, are summarized in Table 1. Treatment duration ranged from 2-36 months, and the mean RA duration ranged from 0.1-13.5 years (Table 1). RCTs reported serious infection on an intention-to-treat (ITT; 70%) or modified ITT (30%) basis. Detailed characteristics of included studies are summarized in Appendix 4 and the risk of bias assessment in Appendix 5.
Figure 1.
PRISMA Diagram of selection of studies
Table 1.
Summary of patient and study characteristics among populations of patients with rheumatoid arthritis
| All Populations | Traditional DMARD naïve | Traditional DMARD experienced | TNF Experienced | |
|---|---|---|---|---|
| Number of trials | 106 (100%) | 24 (22.6%) | 71 (67%) | 11 (10.4%) |
| No. of patients in trials | 42,330 (100%) | 8,375 (19.8%) | 29,167 (68.9%) | 4,788 (11.3%) |
| No. of patients with serious infection | 965 (100%) | 227 (23.5%) | 646 (66.9%) | 92 (9.5%) |
| Median year of Publication (range) | 2008 (1992-2013) | 2006 (1992-2013) | 2008 (1994-2013) | 2008 (2005-2013) |
| No. of treatment nodes | 10 | 5 | 10 | 6 |
| No. of 2-arm trials | 63 | 19 | 38 | 6 |
| No. of multi-arm trials | 43 | 5 | 33 | 5 |
| Mean follow-up duration, months (range) | 9 (1,60) | 13.1 (3,24) | 8 (1,60) | 6.3 (2,12) |
| Trials with duration ≥12 months | 33 (31.1%) | 17 (70.8%) | 18 (25.4%) | 2 (18.2%) |
| Mean RA duration, years (range) | 6.9 (0.1,13.5) | 0.7 (0.1,3.5) | 8.5 (2.2,13.5) | 10.8 (6.4,12.9) |
| Mean annualized baseline risk of serious infection in traditional DMARDs | 2% (0%, 9.2%) | 2% (0%, 9.2%) | 2% (0%, 8%) | 2.4% (0%, 4.5%) |
DMARD= disease-modifying anti-rheumatic drugs; MTX=methotrexate; RCT=randomized controlled trial; TNF= Tumor necrosis factor
* Only included trials greater than 6 months in duration for calculation
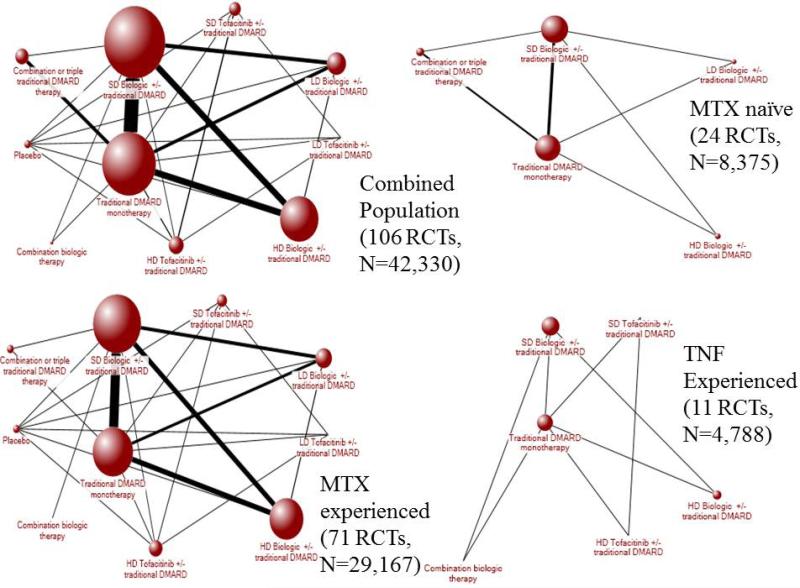
Figure 2.
Evidence networks for serious infection among populations. The width of the lines is proportional to the number of randomized controlled trials comparing each pair of treatments, and the size of each treatment node is proportional to the number of randomized participants (sample size).
DMARD= disease-modifying anti-rheumatic drugs; MTX=methotrexate; RCT= randomized controlled trial
Traditional Meta-Analysis – Standard-dose biologics
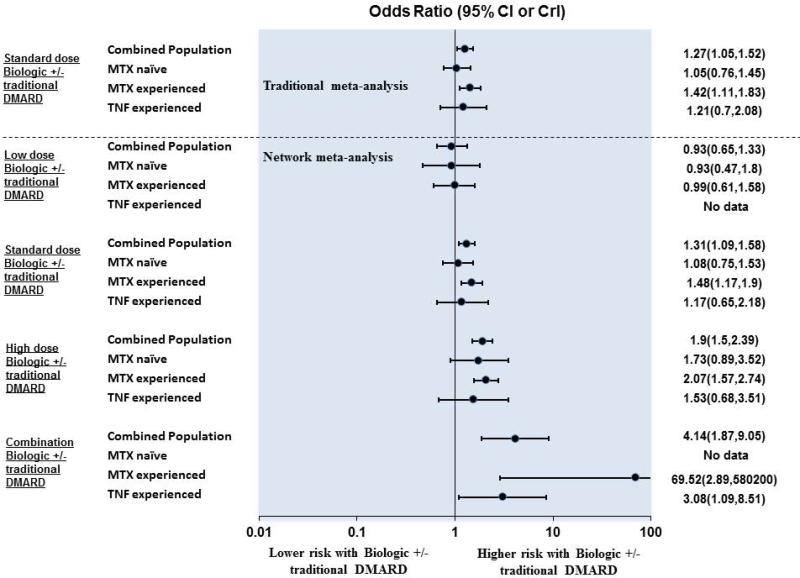
There were 59 trials comparing standard-dose biologic +/−traditional DMARD. Of the 59 trials, 53 (89%) reported at least one serious infection in the study. There were a total of 525 serious infections among the 59 trials, involving 68 comparisons of standard-dose biologic +/− traditional DMARD (342 events) with traditional DMARD monotherapy(183 events). A significant increase in serious infections with biologics was found (OR, 1.27; 95% CI, 1.05 to 1.52; p=0.012) (Figure 3). The risk of serious infections with biologics varied depending on previous treatment experience, being statistically significantly higher in MTX-experienced, but not statistically significant in patients who were MTX-naïveor anti-TNF-biologic-experienced (Figure 3).
Figure 3.
Summary of findings from traditional meta-analysis and network meta-analysis for serious infection among populations compared with traditional DMARD monotherapy.
CI= confidence interval; CrI= Credible interval; DMARD= disease-modifying anti-rheumatic drugs; RCT= randomized controlled trial
Stratified analyses adjusting for differences in other patient and study-level characteristics were conducted and are presented in Appendix 6. Aclinically important and statistically significantly higher risk of serious infections with biologic compared to traditional DMARDs was also seen in: duration of follow-up 6-12 months; biologic when used in combination with traditional DMARDs; established RA (2 to 10 years of disease duration); studies published between 2000 and 2004; studies with a low risk of bias; and when the comparator was traditional DMARD plus placebo (Appendix 6). The results did not vary substantively when different statistical models were used (Appendix 7). Detailed findings from the traditional meta-analysis are reported in Appendix 5 and 5a.
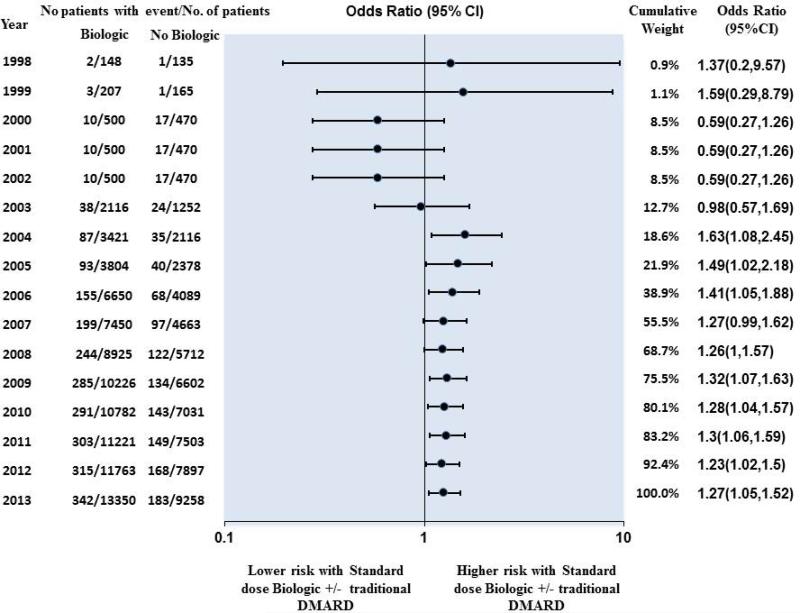
Cumulative Meta-Analysis – Standard-dose biologics
Cumulative meta-analysis (Figure 4) showed that an increased risk of serious infection associated with using standard-dose biologic became evident in 2004, when 5,537 patients had been randomized and 129 events had occurred (OR, 1.63; 95% CI, 1.08 to 2.45; p=0.02). Subsequent trials increased the number of patients to 22,608 and the number of events to 525 for this treatment comparison. This resulted in a reduction in the odds ratio with a narrowing of CI (OR, 1.27; 95% CI, 1.05 to 1.52; p=0.012), although the point estimate remained above 1 for years following 2004 and very similar from 2007 onwards.
Figure 4.
Cumulative meta-analysis – Risk of serious infection among patients using standard dose biologics +/− traditional DMARD compared with traditional DMARD monotherapy
DMARDs= disease-modifying anti-rheumatic drugs; RCT= randomized controlled trial
Network meta-analysis
Standard-dose biologics +/− traditional DMARD (OR, 1.31; 95% CrI, 1.09 to 1.58) were associated with an increased risk of serious infection (Figure 3, Appendix 8, 9 and 9a). High-dose biologics +/− traditional DMARD (OR, 1.90; 95% CrI, 1.50 to 2.39) and combination biologic therapy (OR, 4.14; 95% CrI, 1.87 to 9.05) were associated with an increased risk of serious infection while low-dose biologics +/− traditional DMARD (OR, 0.93; 95% CrI, 0.65 to1.33]) were not. These findings aligned with traditional meta-analyses (Appendix 10).
There were differences observed among the a priori defined RA populations. In patients who are MTX-naïve, standard-dose biologics +/− traditional DMARD (OR, 1.08; 95% CrI, 0.75 to 1.53) and high-dose biologics +/− traditional DMARD (OR, 1.73; 95% CrI 0.89 to 3.52) were not associated with a statistically significant increase in risk of serious infection (Figure 3). In contrast, in MTX-experienced patients, standard-dose biologics +/− traditional DMARD (OR,1.48; 95% CrI, 1.17 to 1.90) and high-dose biologics +/− traditional DMARD (OR, 2.07; 95% CrI, 1.57 to 2.74]) were associated with an increased risk of serious infections. Information on combination biologic therapy was only available for MTX-experienced and anti-TNF-biologic-experienced patients and was associated with a significant increase in serious infections in both patient groups (Figure 3).
Absolute risk of serious infection
In patients using traditional DMARDs, the median absolute annual risk of a serious infection reported was approximately 2% or 20 per 1000 patients treated each year. The absolute increase in the number of serious infections compared to traditional DMARDs was: 6 per 1000 for standard-dose biologic therapy +/− traditional DMARD, 17 per 1000 for high-dose biologic therapy +/− traditional DMARD, and 55 per 1000 for combination biologic therapy.
DISCUSSION
There is uncertainty around the risk of serious infection of biologic therapiesin RA and the magnitude of effect. Although the first meta-analysis showed an association, when more trials were completed, three subsequent meta-analysesfound that standard-dose biologics were not associated with an increased risk of serious infection compared with traditional DMARDs. Now that there is evidence from 42,330 patients with RA from 106 RCTs, this increased sample size provides a more precise estimate of an increased risk of serious infection. To the best of our knowledge, this is the most comprehensive meta-analysis of RCTs on the risk of serious infections in RA, adhering to recommended PRISMA reporting standards.20 Our analysis greatly exceeds the sample size in the largest meta-analysis of 18 RCTs conducted in RA to date (N=8,808)7 by >5 times and includes 88 more RCTs (Appendix 11). We included data from nine biologics; reported detailed stratified analyses; integrated findings for all doses of biologics; presented findings on both the relative and absolute scale, and tested the robustness of findings with sensitivity analyses (see appendices).
We found standard-dose, high-dose, and combination biologics (with/without DMARDs) are associated with more serious infections compared to traditional DMARDs. Our comprehensive study investigated biologic dose in RA in more detail than previous studies (Appendix 11). Bongartz et al.9 found that two of the three biologics studied (infliximab, adalimumab) were associated with significantly increased odds of serious infections (OR, 2.0; 95% CI, 1.3 to 3.1), compared to placebo in 9 trials up to 2005 including 5,005 patients. In contrast, several recent meta-analyses including more biologics and more RCTs reported different findings.6-8 Salliot et al7 examined 12 RCTs up to 2007 (N=6,879) and reported that the risk of serious infections with rituximab and abatacept did not differ from placebo, but was significantly higher with high-doses of anakinra versus low-dose anakinra (OR, 9.63; 95% CI, 1.31 to 70.91) and versus placebo (OR, 3.40; 95% CI 1.11 to 10.46, respectively). Leombruno et al6 analyzed 18 RCTs of three anti-TNF biologics up to 2007 (N=8,808) and found no significant increase in serious infections (OR, 1.21; 95% CI, 0.89 to 1.63), but found higher risk in patients receiving 2-3 times higher than recommended doses of anti-TNF biologic in unadjusted and pooled meta-analysis, but not in exposure-adjusted analyses. Thompson et al.8 included 6 RCTs of five anti-TNF biologics in early RA up to 2009 (N=3,419) and found no significant increase in odds of serious infections with biologics compared to MTX (OR, 1.28; 95% CI, 0.82 to 2.00).
Our findings focus solely on results reported in RCTs. These studies are often limited in that elderly and high-risk patients are often underrepresented, and that treatments are often compared with placebo as opposed to active treatments. Indeed, the RCTs included in our analysis were largely compared with placebo. Accordingly, our risk estimates mostly represent biologics + DMARD versus DMARD comparisons. However, “no treatment” may not be considered a realistic comparator in clinical practice. As such, we conducted several analyses where we compared biologics with combination or triple DMARD therapy. For these analyses, the odds ratio was slightly higher (Appendix 6, 9 and 10) but more uncertain because this comparison was only based on data from 4 recent RCTs comparing biologics plus DMARD with combination or triple DMARD therapy. However, the majority of these trials for this comparison did indeed report a higher number of serious infections among the biologic group. Complementary evidence to meta-analyses of RCTs is provided by non-randomized studies. A recent review has summarized the range of effect estimates reported in non-randomized studies, where effect estimates for biologics versus DMARDs have ranged from 1.0 to 2.2.31 While there have been differences among non-randomized studies, these studies have reported that there is an association with infection that is higher early in the course of treatment, but that declines with time.31,32 However, the latter finding should be interpreted with caution – studies investigating the long-term use of DMARD treatment are limited to highly selected populations who are adherent and responding well to DMARDs.
These findings have practical implications. The benefits of biologic therapy for patients with RA are well known, and now these patients, at time of decision-making regarding treatment with biologics, can consider these benefits alongside the absolute risk increase of serious infections with biologic therapy (6 per 1000 for standard-dose biologic and 17 per 1000 for high-dose biologic therapy). Clinical guidelines should also reflect this finding that this risk differs by several patient characteristics, such as previous DMARD exposure, concurrent use of traditional DMARD or not, established vs. early RA, is important information that should also be discussed.
Our study findings must be interpreted considering the following limitations. Our analysis includes studies, which span a 15-year period. Patients enrolled in early studies may differ from those included in more recent studies. We conducted a sensitivity analysis investigating this issue. We found that the point estimate for the odds ratio remained above unity over the 15-year period (Appendix 6) but decreased from 1995-1999 (OR, 1.59; 95% CrI, 0.29 to 8.79) to 2010-2014 (OR, 1.11; 95% CrI, 0.76 to 1.62). It is unclear whether the decrease in relative effect is evidence that the risk of biologics causing serious infections is declining over time or attributable to changes in regions where recent trials were performed or duration of placebo application among included studies (i.e., increased use of rescue medications for placebo arm); slight change in the inclusion/exclusion criteria of included RCTs may have occurred over time including that greater proportion of RCTs in recent years excluded patients with positive TB tests. Future research is needed in this area.
There are a number of other limitations, which warrant consideration. We observed variability across studies in terms of duration of RA, duration of follow-up and other covariates (Appendix 12). Therefore, we report findings for a number of sub-groups of patients to allow comparisons across patient groups (Figure 3; Appendices 6 and 8). Second, meta-analyses and NMA of less frequent outcomes are more challenging due to the inherent difficulties in handling of zero cells. To manage this issue, we conducted a number of analyses using different statistical models and assumptions.26 Results were consistent using alternative approaches (Appendix 7). Most studies presented the data using ITT or modified ITT, rather than as-treated analyses, which may underestimate the serious infection risk. In addition, withdrawals were labeled due to adverse events, but not serious infections and some patients may have discontinued biologic before these qualified for serious infections. However the magnitude is likely small, given thelow number of withdrawals and crossovers reported. Data on compliance with drugs was not reported in most RCTs; however, these expensive drugs are usually dispensed and adherence recorded as part of the RCT conduct. Finally, our analyses only incorporate published data. Future work should focus on integrating more unpublished data5 if it becomes available. The lack of detailed patient level data, particularly on steroid use, also limits interpretation of these analyses.
CONCLUSIONS
Standard-dose and high-dose biologics (with/without DMARDs) are associated with an increase in serious infections compared to traditional DMARDs in RA, while low-dose biologics are not. This new knowledge, when balanced against the demonstrated clinically important benefits of biologics, will help patients and their physicians make evidence-based decisions that align with their values, preferences and tolerance of risks of harm and benefits.
PANEL: RESEARCH IN CONTEXT
Systematic review
We searched Cochrane Central Register of Controlled Trials, Medline, and EMBASE databases up to February 11, 2014. We also did a search of ClinicalTrials.gov to identify relevant studies. We included randomized controlled trialsin adults with rheumatoid arthritis treated with any of the nine biologics approved for the treatment of rheumatoid arthritis, used alone or in combination as compared to each other, placebo or traditional DMARD (or DMARD combinations). We extracted data on the risk of serious infection from included studies. We assessed the quality of identified studies using the Cochrane Risk of Bias Tool.
Interpretation
This meta-analysis is the first to include all nine biologics and integrate findings for all doses of biologics. Our analysis includes 88 more studies than the most recent meta-analysis on this topic, thereby improving the power to find a difference in the risk of serious infections compared to traditional DMARDs. We also stratify results by the various rheumatoid arthritis populations. We show that standard-dose, high-dose and combination biologics (with/without DMARDs) are associated with an increase in serious infections compared to traditional DMARDs in rheumatoid arthritis, while low-dose biologics are not. Our findings offer clinicians a more comprehensive picture of the risk of serious infection among biologics and will help patients and their physicians make evidence-based decisions that align with their values, preferences and tolerance of risks of harm and benefits when using biologics.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Tamara Rader, Cochrane librarian, for performing the literature searches.
Funding information: This research was supported through grants from Rheumatology division at the University of Alabama at Birmingham.
JAS is supported by grants from the Agency for Health Quality and Research Center for Education and Research on Therapeutics (AHRQ CERTs) U19 HS021110, National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS) P50 AR060772 and U34 AR062891, National Institute of Aging (NIA) U01 AG018947, National Cancer Institute (NCI) U10 CA149950, and research contract CE-1304-6631 from the Patient Centered Outcomes Research Institute (PCORI). JAS is also supported by the resources and the use of facilities at the VA Medical Center at Birmingham, Alabama, USA. CC receives funding from the Canadian Institute of Health Research (CIHR) Canada Vanier Graduate Scholarship (Funding reference number – CGV 121171). CC also receives support from the Canadian Network and Centre for Trials Internationally and is a trainee on a team grant through the Canadian Institute of Health Research (CIHR) Drug Safety and Effectiveness Network (Funding reference number – 116573). The Musculoskeletal Statistics Unit at the Parker Institute (RC) is supported by grants from the Oak Foundation.
Role of Funding Agencies: The funding agencies played no role in study design, collection, analysis, interpretation of data, writing of the manuscript, or in the decision to submit the paper for publication. They accept no responsibility for the contents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: JS conceived the study design, performed some of the data extraction, and drafted the manuscript. CC conceived the study design, performed some of the data extraction, conducted all of the analyses, and drafted the manuscript. GW conceived the study design and made major revisions to the manuscript. PT/RC helped with the data analysis, and reviewed the manuscript for important intellectual content. SN/MT/EG performed the systematic review, data extraction, and reviewed the manuscript for important intellectual content. TC/DC conceived the study and reviewed the manuscript for important intellectual content. All authors have read and approved the final version of the manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support for this work from any organization/s associated with any of the medications discussed in the submitted work.
JAS has received other research grants from Takeda and Savient and consultant fees from Savient, Takeda, Regeneron and Allergan (none manufacture the medications discussed in this paper). JAS is a member of the executive of OMERACT, an organization that develops outcome measures in rheumatology and receives arms-length funding from 36 companies; a member of the American College of Rheumatology's Guidelines Subcommittee of the Quality of Care Committee; and a member of the Veterans Affairs Rheumatology Field Advisory Committee. CC is a recipient of a Vanier Canada Graduate Scholarship from the Canadian Institutes of Health Research and has received funding from Canadian Network and Centre for Trials Internationally (CANNeCTIN) and is a trainee on the CIHR Drug Safety and Effectiveness Network team grant. PT has received grants/honoraria from Bristol Myers, Chiltern International, and UCB. GAW has received research grant and consultant fee from Bristol-Myers Squibb; consultant fees from Abbott, Amgen, UCB; Data Safety Monitoring for Novartis. RC has received consulting fees, honoraria, research or institutional support, educational grants, equipment, services or expenses from: Abbott, Astellas Pharma, Axellus, Bristol-Myers Squibb, Cambridge Nutritional Foods, Centocor, DSM Nutritional Products, HypoSafe, MSD, MundiPharma, NorPharma, Pharmavie, Pfizer, Roche, Sanofi-Aventis and Scandinavian Clinical Nutrition. Other authors declare no financial conflicts.
Ethical approval: Not required
References
- 1.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 2.Tugwell P, Singh JA, Wells GA. Biologicals for rheumatoid arthritis. BMJ. 2011;343:d4027. doi: 10.1136/bmj.d4027. [DOI] [PubMed] [Google Scholar]
- 3.Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64(5):625–39. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509. doi: 10.1136/annrheumdis-2013-204573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ioannidis JP, Karassa FB, Druyts E, Thorlund K, Mills EJ. Biologic agents in rheumatology: unmet issues after 200 trials and $200 billion sales. Nature reviews Rheumatology. 2013;9(11):665–73. doi: 10.1038/nrrheum.2013.134. [DOI] [PubMed] [Google Scholar]
- 6.Leombruno JP, Einarson TR, Keystone EC. The safety of anti-tumour necrosis factor treatments in rheumatoid arthritis: meta and exposure-adjusted pooled analyses of serious adverse events. Ann Rheum Dis. 2009;68(7):1136–45. doi: 10.1136/ard.2008.091025. [DOI] [PubMed] [Google Scholar]
- 7.Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann Rheum Dis. 2009;68(1):25–32. doi: 10.1136/ard.2007.083188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson AE, Rieder SW, Pope JE. Tumor necrosis factor therapy and the risk of serious infection and malignancy in patients with early rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum. 2011;63(6):1479–85. doi: 10.1002/art.30310. [DOI] [PubMed] [Google Scholar]
- 9.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA : the journal of the American Medical Association. 2006;295(19):2275–85. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 10.Dixon WG, Symmons DP, Lunt M, Watson KD, Hyrich KL, Silman AJ. Serious infection following anti-tumor necrosis factor alpha therapy in patients with rheumatoid arthritis: lessons from interpreting data from observational studies. Arthritis Rheum. 2007;56(9):2896–904. doi: 10.1002/art.22808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneeweiss S, Setoguchi S, Weinblatt ME, et al. Anti-tumor necrosis factor alpha therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheum. 2007;56(6):1754–64. doi: 10.1002/art.22600. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum. 2006;54(2):628–34. doi: 10.1002/art.21568. [DOI] [PubMed] [Google Scholar]
- 13.Grijalva CG, Chen L, Delzell E, et al. Initiation of tumor necrosis factor-alpha antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA : the journal of the American Medical Association. 2011;306(21):2331–9. doi: 10.1001/jama.2011.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Askling J, Fored CM, Brandt L, et al. Time-dependent increase in risk of hospitalisation with infection among Swedish RA patients treated with TNF antagonists. Ann Rheum Dis. 2007;66(10):1339–44. doi: 10.1136/ard.2006.062760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strangfeld A, Eveslage M, Schneider M, et al. Treatment benefit or survival of the fittest: what drives the time-dependent decrease in serious infection rates under TNF inhibition and what does this imply for the individual patient? Ann Rheum Dis. 2011;70(11):1914–20. doi: 10.1136/ard.2011.151043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galloway JB, Hyrich KL, Mercer LK, et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 2011;50(1):124–31. doi: 10.1093/rheumatology/keq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Cochrane Collaboration Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] 2011 Available at http://handbook.cochrane.org.
- 18.Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2011;14(4):429–37. doi: 10.1016/j.jval.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2011;14(4):417–28. doi: 10.1016/j.jval.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;(2):CD008794. doi: 10.1002/14651858.CD008794.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh JA, Christensen R, Wells GA, et al. Biologics for rheumatoid arthritis: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2009;(4):CD007848. doi: 10.1002/14651858.CD007848.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaujoux-Viala C, Nam J, Ramiro S, et al. Efficacy of conventional synthetic disease-modifying antirheumatic drugs, glucocorticoids and tofacitinib: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katchamart W, Trudeau J, Phumethum V, Bombardier C. Methotrexate monotherapy versus methotrexate combination therapy with non-biologic disease modifying anti-rheumatic drugs for rheumatoid arthritis. Cochrane Database Syst Rev. 2010;(4):CD008495. doi: 10.1002/14651858.CD008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JP, Altman DG, Sterne JA, The Cochrane Collaboration . Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) 2011. Chapter 8 Available from http://www.cochrane-handbook.org. In: Higgins JP, Green S, eds.; 2011. [Google Scholar]
- 26.Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Statistics in medicine. 2007;26(1):53–77. doi: 10.1002/sim.2528. [DOI] [PubMed] [Google Scholar]
- 27.Spiegelhalter D, Thomas A, Best N. WinBUGS user manual. Version 1.4. MRC Biostat; Jan, 2003. Unit 2. https://faculty.washington.edu/jmiyamot/p548/spiegelhalter%20winbugs%20user%20manual.pdf. 2003. [Google Scholar]
- 28.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Medical decision making : an international journal of the Society for Medical Decision Making. 2013;33(5):607–17. doi: 10.1177/0272989X12458724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. International journal of epidemiology. 2012;41(3):818–27. doi: 10.1093/ije/dys041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Medical decision making : an international journal of the Society for Medical Decision Making. 2013;33(5):641–56. doi: 10.1177/0272989X12455847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernatsky S, Habel Y, Rahme E. Observational studies of infections in rheumatoid arthritis: a metaanalysis of tumor necrosis factor antagonists. J Rheumatol. 2010;37(5):928–31. doi: 10.3899/jrheum.091107. [DOI] [PubMed] [Google Scholar]
- 32.Novosad SA, Winthrop KL. Beyond tumor necrosis factor inhibition: the expanding pipeline of biologic therapies for inflammatory diseases and their associated infectious sequelae. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;58(11):1587–98. doi: 10.1093/cid/ciu104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.