Abstract
The bone remodelling relies on a dynamic balance between bone formation and resorption, mediated by osteoblasts and osteoclasts, respectively. Under certain stimuli, osteoprogenitor cells may differentiate into premature osteoblasts and further into mature osteoblasts. This process is marked by increased alkaline phosphatase (ALP) activity and mineralized nodule formation. In this study, we induced osteoblast differentiation in mouse osteoprogenitor MC3T3-E1 cells and divided the process into three stages. In the first stage (day 3), the MC3T3-E1 cell under osteoblast differentiation did not express ALP or deposit mineralized nodule. In the second stage, the MC3T3-E1 cell expressed ALP but did not form mineralized nodule. In the third stage, the MC3T3-E1 cell had ALP activity and formed mineralized nodule. In present study, we focused on morphological and proteomic changes of MC3T3-E1 cells in the early stage of osteoblast differentiation - a period when premature osteoblasts transform into mature osteoblasts. We found that mean cell area and mean stress fiber density were increased in this stage due to enhanced cell spreading and decreased cell proliferation. We further analyzed the proteins in the signaling pathway of regulation of cytoskeleton using proteomic approach and found upregulation of IQGAP1, gelsolin, moesin, radixin, and Cfl1. After analyzing the focal adhesion signaling pathway, we found the upregulation of FLNA, LAMA1, LAMA5, COL1A1, COL3A1, COL4A6, and COL5A2 as well as the downregulation of COL4A1, COL4A2, and COL4A4. In conclusion, the signaling pathway of regulation of cytoskeleton and focal adhesion play critical roles in regulating cell spreading and actin skeleton formation in the early stage of osteoblast differentiation.
Keywords: osteoblast differentiation, proteomics, cell shape, cytoskeleton, focal adhesions
Introduction
The bone remodelling relies on a dynamic balance between bone formation and resorption, mediated by osteoblasts and osteoclasts, respectively. The osteoblasts are derived from osteoprogenitor cells located in the periosteum or bone marrow. Several families of growth factors, such as bone morphogenetic proteins and transforming growth factor-β, have been shown to regulate osteoblast differentiation directly or indirectly through modulating the effects of other growth factors [1, 2]. In addition, the transcriptional regulators and the signaling pathways that mediate the osteoblast differentiation have been extensively characterized recently [3, 4]. Under certain stimuli, the osteoprogenitor cells may differentiate into premature osteoblasts and further into mature osteoblasts. This process is marked by increased alkaline phosphatase (ALP) expression and mineralized nodule formation. Quarles et al. divided the osteoblast differentiation process of mouse osteoprogenitor MC3T3-E1 cells into three stages. In the early stage (day 1–9), MC3T3-E1 cells actively replicate, maintain fusiform appearances, but do not express ALP and form mineralized nodules. In the second stage (day 9–16), the cells display cuboidal morphology and produce ALP. In the third phase (after day 16), the cells demonstrate both ALP activity and mineralized nodule formation [5].
The early stage is critical for osteoblast differentiation because premature osteoblasts transform into mature osteoblasts in this stage [5]. We performed the morphological analysis of MC3T3-E1 cells since the fate of osteoblast differentiation is also determined by cells shapes in vitro [6–10] and mechanical stimulation in vitro and in vivo [11–17]. Though how the shape of the osteoprogenitor is in vivo modified is not completely clear, the cell shapes in vivo may be regulated by both the mechanical stimulation and the differentiative status of the cells. For example, the spreading of adipogenic cells in vitro may inhibit adipogenesis since only a round, spherical cell shape allows for maximal lipid storage. Additionally, this process can be reversed by keeping cells round or by disrupting actin cytoskeleton [10]. On the contrary, cell spreading enhances the osteoblast differentiation in the osteoprogenitor cells and favours osteoblast matrix deposition during bone remodelling [7–9]. The mesenchymal stem cells are the main source of osteoprogenitor cells in vivo but may also differentiate into adipocytes or other cell lineages under certain conditions [18]. The cell shapes have been demonstrated to control the commitment of human mesenchymal stem cells to either osteogenic or adipogenic lineages [10]. As cell shapes are related to the expression of cytoskeleton proteins and integrins [19], a proper regulation of an intact cytoskeleton [20] may be required in the process of osteoblast differentiation. Mechanical stimulation has been shown to regulate osteoblast differentiation in vitro or in vivo [11]. The mechanical load on bone cells transiently and rapidly upregulates the expression of cyclooxygenase-2 gene [12–14], and activates the L-type voltage-sensitive Ca2+ channels, induces nitric oxide synthesis, and stimulates extracellular signal-regulated kinase [15]. In addition, the mechanical stress activates the p38 pathway through sensitization of the Ca2+ channels in osteoblastic-like cells [17].
In the present study, we cultured MC3T3-E1 cells in osteoblast differentiation medium for 7 days, and performed both morphological and mass spectrometry (MS)–based proteomic analysis to of MC3T3-E1 during initial differentiation by observing the changes of cell shapes and measuring protein expressions.
Materials and methods
Culture of MC3T3-E1 cells and induction of osteoblast differentiation
The MC3T3-E1 cell line was provided by Dr. Xin-hua Liu from Mount Sinai School of Medicine in New York, and the basic culture medium is Dulbecco’s modified Eagle’s medium-how glucose (DMEM-HG, Cell signal, Beverly, MA) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY) and 1% penicillin/streptomycin. The MC3T3-E1 cells were divided into osteoblast differentiation (OS) group and control (CON) group. The cells of OS group were grown in basic culture medium containing 10−7 M dexamethasone, 10−2 M β-glycerophosphate, and 50 µg/ml ascorbic acid in 5% CO2 at 37°C, while the cells of CON group were grown in only basic culture medium.
ALP activity assay
The MC3T3-E1 cells in OS and CON group were seeded in 6-well plates at the concentration of 1.0 × 104 cells/cm2 and cultured in the different media for 3, 7, 14, and 21 days before harvesting. The ALP activities in cell lysates were measured using a SensoLyte pNPP Alkaline Phosphatase Assay Kit (AnaSpec, San Jose, CA) following the manufacturer’s instruction. The values were normalized to the total protein content determined by the Bio-Rad Protein Assay reagent (BioRad, Hercules, CA) with bovine serum albumin used as the standard.
Cell proliferation assay
The MC3T3-E1 cells were separated into OS group and CON group and seeded at a 96-well plate at the concentration of 1.0×104 cells/cm2. After cultured in 5% CO2 at 37°C for 3, 7, and 14 days, the cell proliferat ion rate was measured by the reduction rate of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT, Sigma) into formazan. In brief, the cells were incubated with 0.5 mg/ml MTT for 4 h, and the formazan was then dissolved with 10% SDS in 0.01 M HCl at 37°C overnight. Absorbance at 590 nm was determined using a MCC 340 multi-scan microplate reader (Thermoelectron, MA, USA).
Alizarin red staining for mineralization
The MC3T3-E1 cells in OS and CON group were seeded in 6-well plates at the concentration of 1.0 × 104 cells/cm2 and cultured in the different media for 7, 14, and 21 days. The cells were then fixed with 4% paraformaldehyde in phosphate buffered saline (pH 7.4) at room temperature for 15 min, washed three times with phosphate buffered saline, and incubated with 40 mM Alizarin Red-S (pH 4.2, Sigma) for 10 min at room temperature. The cells were washed thoroughly with deionized water and the mineralization was observed.
Rhodamine phalloidin staining and morphological measurements
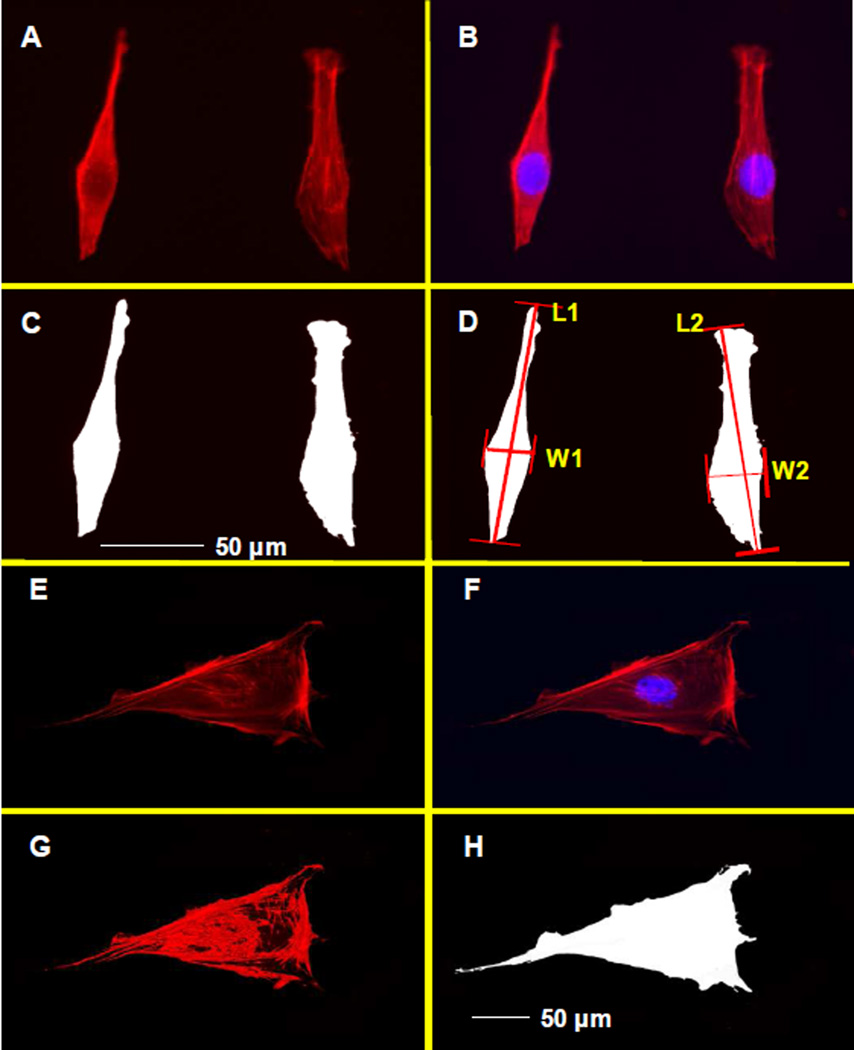
The MC3T3-E1 cells in OS and CON group were seeded in 12-well plates at the concentration of 1.0 × 104 cells/cm2 and allowed to grow in the different media for 3 and 7 days. The slides were gently washed with 37°C phosphate buffered saline, fixed with 4% formaldehyde in phosphate buffered saline for 10 min at room temperature, and permeabilized with 0.5% Triton X-100 in phosphate buffered saline for 5 min at room temperature. Then cells were incubated with 100 nM rhodamine phalloidin in dark for 30 min, and the nucleuses of cells were additionally counterstained with 100 nM DAPI in phosphate buffered saline. The slides were visualized using a fluorescent microscope, and the cell morphology was quantitatively analyzed using the software Image Pro Plus (Media Cybernetics, Silver Spring, MD). Briefly, the width and length were measured in the fusiform cells on day 3 (Fig 1 A, B, C, and D). In addition, the cell areas were quantitatively measured by selecting the full area of the cells based on the color differences, and the mean cell areas were divided by the number of nucleuses counterstained by DAPI. The F-actin stress fiber densities were measured by selecting the color of the stress fibers in the images, accumulatively counting the total density of the selected areas. The mean stress fiber density was calculated by dividing the total stress fiber density with the cell areas (Fig 1E, F, G and H).
Fig 1. Methods for morphological measurement of MC3T3-E1 cells.
A, B, C, and D: the measurement of the width (W), length (L), and area of the fusiform MC3T3-E1 cells. E: MC3T3-E1 cell stained with rhodamine phalloidin; F: Merge images show the number of nucleus; G: Selection the area of actin stress fibers; H: Selection for measurement of the cell area;
Protein extraction
The MC3T3-E1 cells in OS and CON group were seeded in 75 cm2 plastic flasks (Corning, New York, NY) at the concentration of 1.0 × 104 cells/cm2 and allowed to grow in the different media for 7 days. The cells were then trypsinized and washed with phosphate buffered saline for three times. The cells pellets were harvested by centrifugations at 1,500 ×g and homogenized in NP40 Cell Lysis Buffer (BioSource™, Camarillo, CA) complemented with 0.01m DTT and protease inhibitor. The homogenates were centrifuged at 14, 000 ×g for 25 min at 4 °C to collect the the supernatants, of which the protein concentration was measured as described previously.
Western blotting analysis
Twenty milligram protein samples were separated by a ten-well Novex® 4–12% Tris-Glycine Gel (Invitrogen, Carlsbad, CA) and transferred to a polyvinylidene difluoride membrane. After blocked using 1% bovine serum albumin, each membrane was incubated for 24 h at 4 °C with one of the following antibodies: rabbit polyclonal vinculin antibody (Santa Cruz, CA), rabbit polyclonal gelsolin (H-70) antibody (Santa Cruz, CA), rabbit polyclonal IQGAP1 antibody (Cell signaling, Beverly, MA), rabbit polyclonal Filamin A (FLNA) antibody (Cell signaling, Beverly, MA), goat polyclonal Filamin B (FLNB) antibody (Santa Cruz, CA), and rabbit polyclonal β-actin antibody (Santa Cruz, CA). The membranes were then incubated with HRP-conjugated anti-rabbit or anti-goat IgG (Santa Cruz, CA), and the proteins on the membranes were visualized using ECL™ western blotting detection reagents (GE Healthcare, Buckinghomshire, UK) with the signals detected by the Image Station 4000R (Kodak, New Haven, CT, USA).
SDS-PAGE and in-gel digestion
One hundred and fifty microgram of proteins extracted from each of OS or CON group were separated by a five-well Novex® 4–20% Tris-Glycine Gel (Invitrogen, Carlsbad, CA). The gel was fixed in 40% methanol/10% acetic acid for 15 min, stained with colloidal Coomassie Blue solution for 45 min, and destained in 40% methanol/10% acetic acid for 24 h. The symmetrical gel bands from OS and CON lanes were excised and minced into 1 mm × 1 mm smaller pieces. The gel pieces were then sufficiently destained with 50% acetonitrile/25 mM ammonium bicarbonate, dehydrated with 100% acetonitrile, and dried in a vacuum centrifuge. A standard trypsin digestion procedure was performed. In brief, the gel pieces were reduced in 20 mM DTT for 45 min at 55°C, carboxyamidomethylated in 55 mM iodoacetamide (IAA), and digested with trypsin (Promega, Madison, WI) solution (10 ng/µl dissolved in 25 mM ammonium bicarbonate, pH 8.0) overnight at 37°C. The peptides were extracted twice with 0.1% trifluoroacetic acid and 0.1% trifluoroacetic acid/50% acetonitrile, respectively, and dried in a vacuum centrifuge. The volumes of the extraction were adjusted to 20 µl with 0.1% trifluoroacetic acid, of which 5µl was loaded for LTQ-Orbitrap liquid chromatography-coupled tandem mass spectrometry (LC-MS/MS) analysis.
LC-MS/MS analysis
For LC-MS/MS analysis, each digestion product was separated by a 60 min gradient elution with the Dionex capillary/nano-HPLC system (Dionex, Sunnyvale, CA) at a flow rate of 0.250 µl/min that is directly interfaced with the Thermo-Fisher LTQ-Obritrap mass spectrometer (Thermo Fisher, San Jose, CA) operated in a data-dependent scan mode. The analytical column was a fused silica capillary column (75 µm ID, 100 mm length, Upchurch, Oak Harbor, Wa) packed with C-18 resin (300 A, 5 µm, Varian, Palo Alto, CA). Mobile phase A was consisted of 0.1% formic acid and mobile phase B was consisted of 100% acetonitrile and 0.1% formic acid. The 60 min gradients with 250 nL/min flow rate for B solvent went from 0 to 55% in 34 min and then in 4 min to 80%. The B solvent stayed at 80% for another 8 min and then decreased to 5% in 8 min. Another 6 min was used for equilibration, loading and washing. The LTQ-Orbitrap mass spectrometer was operated in the data-dependent acquisition mode using the Xcalibur 2.0.7 software (Version 2.0.7, Thermo Fisher Scientific Inc.). The experiment consisted of a single full-scan mass spectrum in the Orbitrap (400–1800 m/z, resolution of 30,000), followed by 6 data-dependent MS/MS scans in the ion trap at 35% normalized collision energy. The dynamic exclusion parameters were as follows: repeat count = 1; repeat duration = 30; exclusion list = 100; and exclusion time = 90. The MS/MS scans from each LC-MS/MS run were converted from the .RAW file format to .DTA files using the Bioworks 3.3.1 software (Version 3.3.1 SP1, Thermo Fisher Scientific Inc.). DTA files were analyzed using the MASCOT software search algorithm.
Data processing and statistics
The peak lists were searched against the International Protein Index (IPI) mouse database using Mascot with the following parameters: monoisotopic masses of 20 ppm on MS, fully tryptic specificity, 1 missed cleavage sites allowed, cysteine carbamidomethylation as a fixed modification, and 1 Da and oxidation of methionine as variable modifications. We compared the spectral count matching for each protein between OS and CON groups. In addition, we classified that protein levels during the osteoblast differentiation as upregulated if OS spectral count/CON spectral count ≥ 2, as downregulated if OS spectral count/CON spectral count < 0.5, and as unchanged if 0.5 ≤ OS spectral count/CON spectral count < 2. The proteins data were also analyzed using an online analysis tool, Protein Interrogation of Gene Ontology and KEGG databases (PIGOK, http://pc4-133.ludwig.ucl.ac.uk/pigoksum.html), by submitting IPI access number of all identified proteins [21]. Data are expressed as the mean ± SEM and statistically analyzed by software GraphPad Prism 3.0 (GraphPad software Inc, California, USA). Student’s t-tests were used to compare the differences between two groups. ANOVA was used to determine the differences between three of more groups. Post-hoc analyses were performed with Newman-Keuls tests. Differences were regarded as significant if P < 0.05.
Results
Identification of osteoblast differentiation
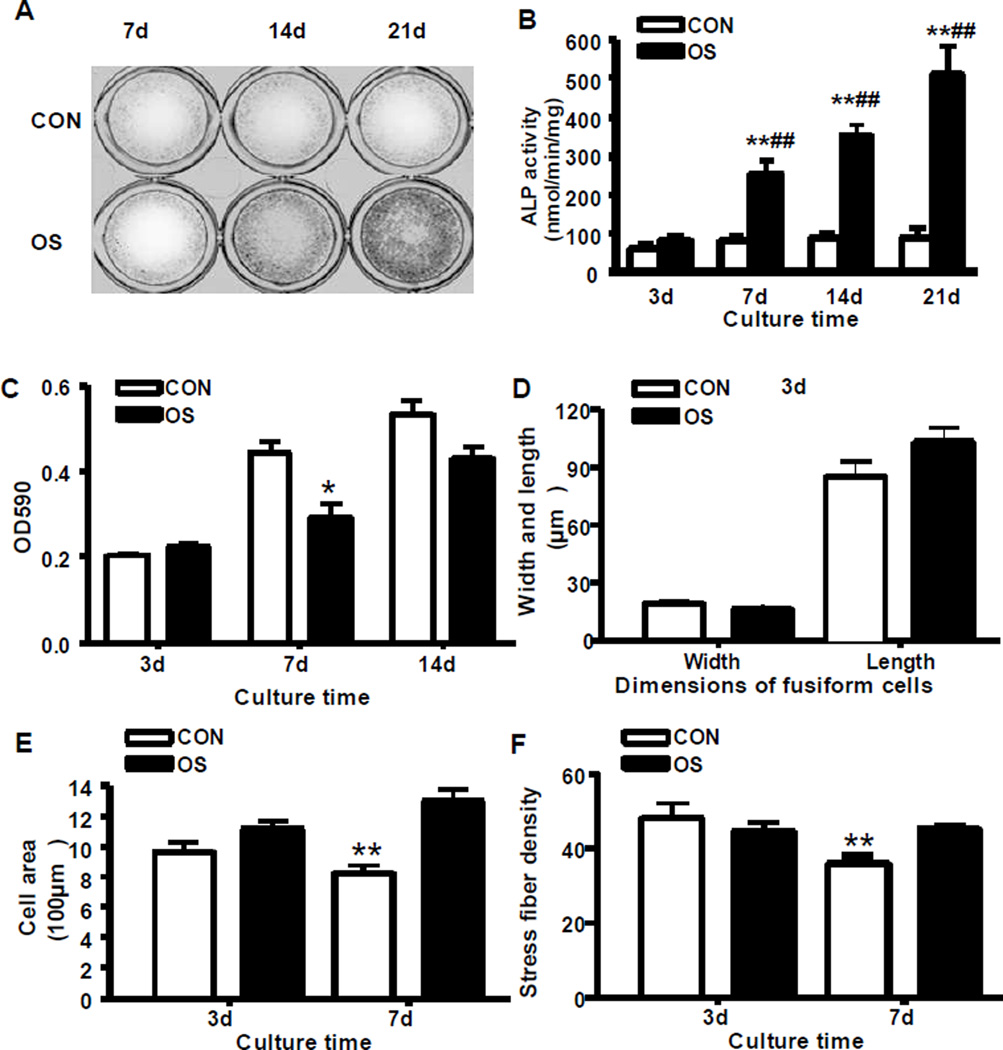
The formation of mineral nodules was observed in OS group by Alizarin red staining on day 14 and 21 (Fig 2A), and the ALP activity of MC3T3-E1 cells in OS group were significantly increased on day 7, 14 and 21 (P < 0.01) (Fig 2B). On the contrary, no mineral nodules were formed and the ALP activity remained unchanged in CON group from day 3 to day 21 (P > 0.05) (Fig 2A and 2B). The OD 590 in OS group was significantly lower than that in OS group on day 7 (P < 0.05) (Fig 2C), suggesting a decreased cell proliferation at the early stage of osteoblast differentiation. We did not found changes in cell proliferation among two groups on day 14 (P > 0.05) (Fig 2C), which may be explained by growth inhibition by over-confluent cells in the 96-well-plate.
Fig 2. Functional and morphological analysis of osteoblast differentiation in MC3T3-E1 cells.
A: The mineral nodule formation by Alizarin red staining, B: Alkaline phosphatase (ALP) activity of the MC3T3-E1 cells in 21-day- culture; C: Cell proliferation assay. D: The width and length of the MC3T3-E1 cells on day 3; E: The cell area of the MC3T3-E1 cells on day 3 and 7; F: The actin stress fiber density of MC3T3-E1 cells on day 3 and 7. *: P < 0.5, **: P < 0.01, comparison between CON and OS; ##: P < 0.01, value comparison of day 7, 14, and 21 to day 3.
Morphological measurement of osteoprogenitor cells during the early stage of differentiation
The MC3T3-E1 cells in both OS and CON groups on day 3 demonstrated fusiform shapes and showed no significant differences in width, length, and area of the cells (P > 0.05) (Fig 2D and E). However, more cells in OS group showed cuboidal shapes than cells in CON group, which was proven by the significantly increased mean cell areas in OS group (P < 0.01) (Fig 2E).
We also observed that on day 3 there was no difference in the mean stress fiber density between the OS and CON groups. However, on day 7, the cells in OS group have significantly higher mean stress fiber density than the cells in CON group (P < 0.01). We found that this difference was due to the reduction of mean stress fiber density in CON group rather than the amplification of mean stress fiber density in OS group (Fig 2F).
Proteomic profile of osteoprogenitor cells during the early stage of differentiation
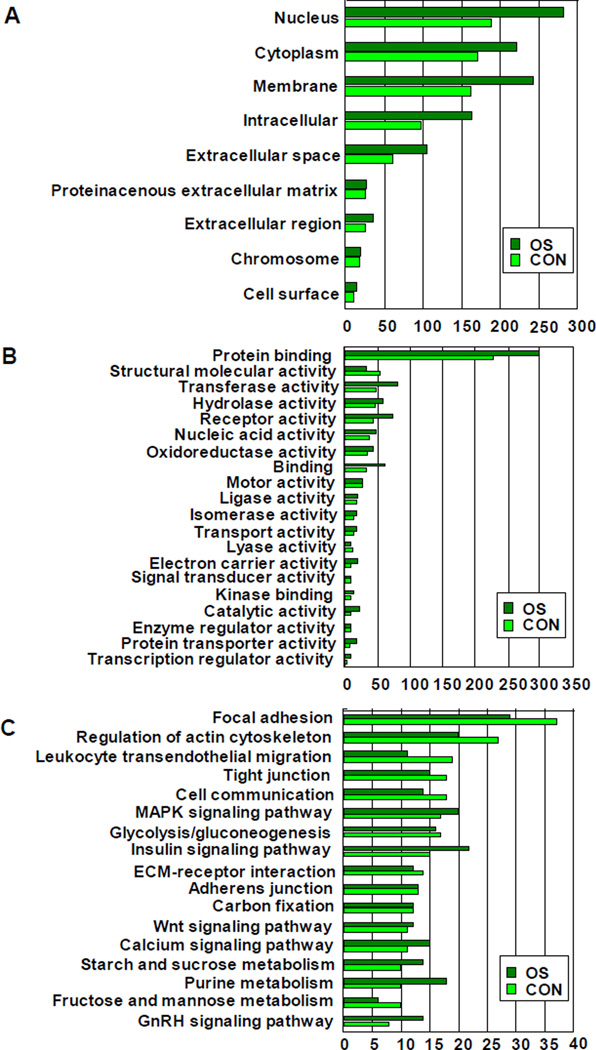
A total of 2642 proteins were identified by LC-MS/MS and their spectral counts were compared between OS and CON groups. According to the mentioned comparison criteria, 1109 (41.98%) proteins were classified as upregulated, 1385 (52.42%) as downregulated, and 148 (5.60%) as unchanged. The data were further analyzed using PIGOK. First, in the categorization of cellular components of the identified proteins, the most matched proteins are nucleus proteins, cytoplasm proteins, and membrane proteins (Fig. 3A). Secondly, the most matched proteins according to the molecular function classification were categorized into mainly protein binding, the transferase and receptor activity. In this category, the number of matched proteins was increased in OS group (Fig. 3B). Finally, PIGOK matched 46 KEGG signaling pathways from the identified proteins, and the most matched 17 KEGG signaling pathways were shown (Fig. 3C). In this study, the signaling pathways of the regulation of actin cytoskeleton and focal adhesion were further investigated as follows.
Fig 3. Comparison of the number of matched proteins according to classification of PIGOK in OS and CON group.
A: Celluar components classification; B: The molecular function classification; C: The KEGG signaling pathway.
Analysis of the signaling pathway of the regulation of actin cytoskeleton
The regulation of actin cytoskeleton is vital for the formation and maintenance of specialized structure, cell division, and cell motility [22, 23]. Nevertheless, how the signaling pathway of the regulation of actin cytoskeleton affects cell shape and osteoblast differentiation remains obscure. The PIGOK matched 35 proteins of this signaling pathway. According to a more strict criterion (at least two unique peptides and score > 40), 19 proteins with detail proteomic data were listed [Table 1].
Table 1.
The protein matched in signaling pathways of regulation of actin cytoskeleton and focal adhesion
| IPI number | Protein names | Gene | SCM |
Seq. Coverage |
Regu. | ||
|---|---|---|---|---|---|---|---|
| names | CON | OS | CON | OS | |||
| Regulation of actin cytoskeleton | |||||||
| IPI00467447 | Ras GTPase-activating-like protein IQGAP1 | Iqgap1 | 49 | 189 | 30% | 52% | ↑ |
| IPI00117167 | Isoform 1 of Gelsolin precursor | Gsn | 51 | 123 | 49% | 41% | ↑ |
| IPI00110588 | Moesin | Msn | 13 | 58 | 29% | 44% | ↑ |
| IPI00308324 | Radixin | Rdx | 0 | 27 | - | 31% | ↑ |
| IPI00338604 | Myosin, heavy polypeptide 10, non-muscle | Myh10 | 71 | 193 | 27% | 44% | ↑ |
| IPI00114593 | Actin, alpha cardiac muscle 1 | Actc1 | 0 | 403 | - | 87% | ↑ |
| IPI00407543 | Cofilin 1, non-muscle homolog | Cfl1 | 0 | 70 | - | 37% | ↑ |
| IPI00138691 | Actin-related protein 2/3 complex subunit 4 | Arpc4 | 8 | 0 | 23% | - | ↓ |
| IPI00676416 | Similar to KIAA1694 protein isoform 5 | Arhgef1 | 181 | 0 | 48% | - | ↓ |
| IPI00224740 | Profilin-1 | Pfn1 | 157 | 245 | 85% | 90% | - |
| IPI00453996 | Isoform 1 of Myosin-14 | Myh14 | 110 | 159 | 28% | 32% | - |
| IPI00266188 | Cofilin-2 | Cfl2 | 27 | 35 | 33% | 23% | - |
| Focal adhesion signaling pathway | |||||||
| IPI00664643 | Filamin, alpha | Flna | 127 | 323 | 42% | 52% | ↑ |
| IPI00113726 | Laminin subunit alpha-1 precursor | Lama1 | 0 | 143 | - | 29% | ↑ |
| IPI00116913 | Laminin subunit alpha-5 precursor | Lama5 | 0 | 175 | - | 31% | ↑ |
| IPI00623191 | Isoform 2 of Collagen alpha-1(I) chain precursor | Col1a1 | 66 | 147 | 45% | 64% | ↑ |
| IPI00129571 | Collagen alpha-1(III) chain precursor | Col3a1 | 73 | 228 | 54% | 73% | ↑ |
| IPI00113475 | Collagen, type IV, alpha 6 | Col4a6 | 0 | 151 | - | 56% | ↑ |
| IPI00121120 | Collagen alpha-2(V) chain precursor | Col5a2 | 82 | 263 | 45% | 65% | ↑ |
| IPI00228360 | Zyxin | Zyx | 0 | 33 | - | 38% | ↑ |
| IPI00109588 | Collagen alpha-1(IV) chain precursor | Col4a1 | 88 | 0 | 46% | - | ↓ |
| IPI00338452 | Collagen alpha-2(IV) chain precursor | Col4a2 | 83 | 0 | 51% | - | ↓ |
| IPI00626353 | Isoform 1 of Collagen alpha-4(IV) chain precursor | Col4a4 | 105 | 0 | 47% | - | ↓ |
| IPI00338785 | laminin B1 subunit 1 | Lamb1-1 | 34 | 0 | 24% | - | ↓ |
| IPI00222801 | Neuronal proto-oncogene tyrosine-protein kinase Src | Src | 17 | 0 | 35% | - | ↓ |
| IPI00663627 | Filamin-B | Flnb | 128 | 234 | 37% | 50% | - |
| IPI00128689 | Collagen alpha-1(V) chain precursor | Col5a1 | 122 | 179 | 36% | 58% | - |
| Crosstalks between signaling pathways of regulation of actin cytoskeleton and focal adhesion | |||||||
| IPI00121827 | Platelet derived growth factor receptor, beta polypeptide | Pdgfrb | 0 | 92 | - | 42% | ↑ |
| IPI00405227 | Vinculin | Vcl | 327 | 154 | 58% | 59% | ↓ |
| IPI00315100 | Transforming protein RhoA precursor | Rhoa | 11 | 14 | 44% | 28% | - |
| IPI00311873 | Serine/threonine-protein phosphatase PP1-beta catalytic subunit | Ppp1cb | 11 | 14 | 45% | 42% | - |
| IPI00387557 | Actn2 actinin alpha 2 | Actn2 | 43 | 42 | 34% | 43% | - |
| IPI00136701 | Alpha-actinin-3 | Actn3 | 40 | 54 | 38% | 43% | - |
| IPI00110827 | Actin, alpha skeletal muscle | Acta1 | 398 | 416 | 76% | 81% | - |
IPI: International protein index; MW: molecular weight; ↑: upregulation; ↓: downregulation.
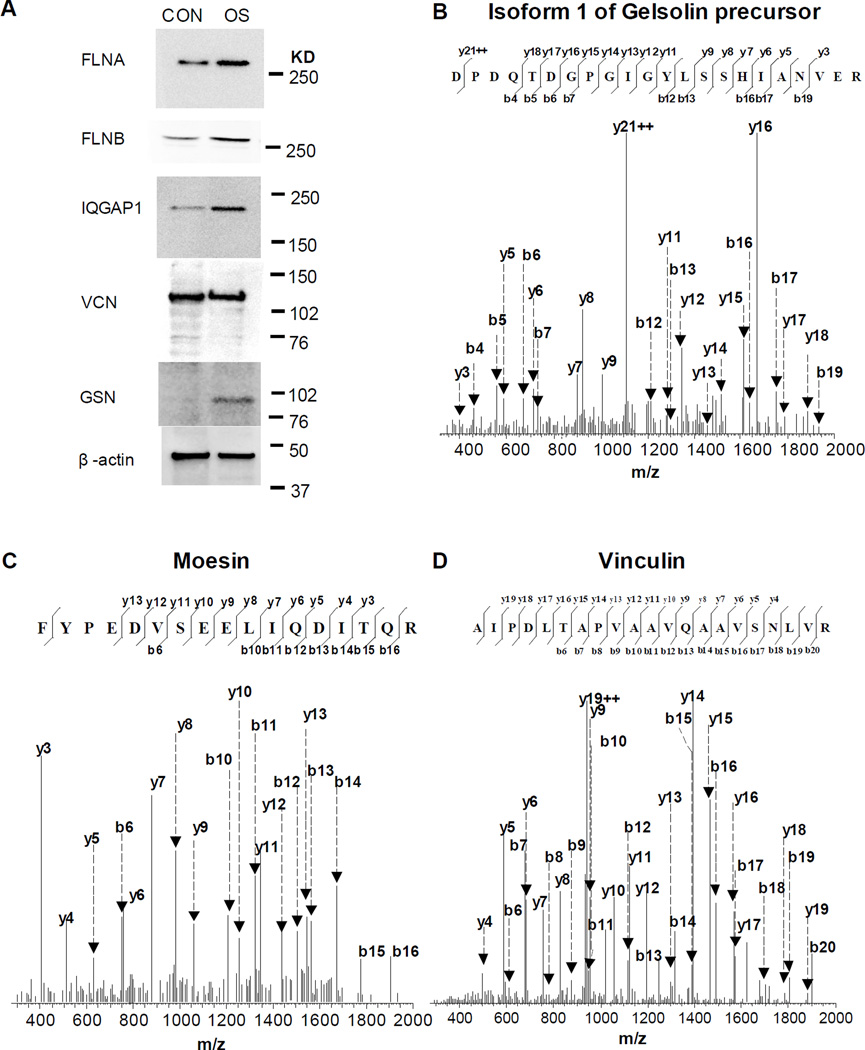
Interestingly, we first identified a significant upregulation of GTPase-activating-like protein 1 (IQGAP1) in OS group on day 7, which is proven by the Western Blot analysis (Fig 4A). We next found that the protein level of isoform 1 of gelsolin (GSN) precursor was upregulated in OS group on day 7 in Table 1, which is supported by the Western Blot analysis (Fig 4A). The expression of isoform 1 of GSN precursor in OS group was also confirmed by MS/MS spectra (Fig 4B). The moesin and radixin were elevated in the OS group, and the elevation of moesin was shown in the OS group by MS/MS spectra (Fig 4C). In this study, the level of cofilin 1 (CFL1) but not cofilin-2 (CFL2) was significantly increased during the early stage of osteoblast differentiation [Table 1].
Fig 4. The regulation of proteins in the signaling pathway of regulation of actin cytoskeleton and focal adhesion on day 7.
A: Western Blot analysis of proteins. Molecular weight: FLNA (280 K), FLNB (280 K), IQGAP1 (195 K), Vinculin (117 K), Gelsolin (90 K), and β-actin (45 k); B, C, D: The MS/MS spectra of the peptides.
Analysis of focal adhesion signaling pathway
Focal adhesion is specialized structures formed at the cell-extracellular matrix (ECM) contact points that connect the cell cytoskeleton to the ECM, and they play roles in cell motility, cell proliferation, and cell differentiation [24, 25]. The PIGOK matched 53 proteins in focal adhesion signaling pathway. Filtered by a stricter criterion (at least two unique peptides and score > 40), 23 proteins of focal adhesion signaling pathway were listed [Table 1]. We found that FLNA is upregulated in OS group on day 7, which is also confirmed by the Western Blot analysis (Fig. 4A). Though SCM-OS/SCM-CON of FLNB was marginally smaller than 2 (1.83), Western Blot analysis showed that the expression of FLAB was also upregulated (Fig. 4A). In this study, several laminins (LAMs), such as LAMA1 and LAMA5, but not LAMB1, were upregulated. We additionally found the protein changes of collagens (COLs). Present data showed that the levels of COL1A1, COL3A1, COL4A6, and COL5A2 were upregulated, while COL4A1, COL4A2 and COL4A4 were downregulated [Table 1].
Crosstalks between signaling pathways of regulation of actin cytoskeleton and focal adhesion
There are seven proteins listed in formed crosstalks between the signaling pathways of regulation of actin cytoskeleton and focal adhesion [Table 1]. The protein levels of platelet derived growth factor receptor, β polypeptide (PDGFRB) was increased, but vinculin (VCL) was decreased slightly in OS group on day 7. The western blot also showed a reduction of VCL in the OS group (Fig. 4A), and the MS/MS spectrum of the peptide is displayed (Fig 4D).
Discussion
In the present study, we classified the different stages of osteoprogenitor cell maturation in the MC3T3-E1 cells. In the first stage (day 4), the MC3T3-E1 cells did not show increased ALP activity and form mineralized nodules. In the second stage (day 7), the MC3T3-E1 cells showed increased ALP activity but did not form mineralized nodules. In the third stage (day 14 and 21), the MC3T3-E1 cells demonstrated increased ALP activities and the formation of mineralized nodules. Our study confirmed Quarles et al.’s classification in which the second stage of osteoblast differentiation is a transformation period that premature osteoblasts turn into mature osteoblasts [5]. We further defined the second stage as the early stage of osteoblast differentiation, which took place on day 7 in our experiment, earlier than day 9 to 16 in Quarles et al.’s study [5]. This may be the result of dissimilarities in cell lines and experimental conditions. Kalajzic et al marked different subpopulations or stages of mouse calvarial osteoblast cells and marrow stromal cells during osteoblast differentiation by Col1a1GFP transgenes [26] and also found that transgenic cells are ALP-positive 5 to 7 days before they form mineral nodules [26]. In the present study, MC3T3-E1 cells cultured in osteoblast differentiation medium for 3 days were still in an undifferentiated status and cells treated for more than 14 days were considered as mature osteoblasts. Therefore, these osteoprogenitor cells under osteoblast differentiation for 7 days were in the early stage of osteoblast differentiation – a critical stage of transformation from premature osteoblasts to mature osteoblasts.
There were no differences in the mean cell area, cell width and length between two groups when the fusiform-shape-cells were still in the undifferentiated stage. However, the cells showed significantly increased mean cell area in the early stage of osteoblast differentiation, which may be the result of by both enhanced cell spreading and decreased cell proliferation. We did not observe the differences in main stress fiber density in undifferentiated cells between OS and CON groups. However, the cells in the early stage of osteoblast differentiation showed significantly higher main stress fiber density than undifferentiated cells, suggesting the main stress fiber density is not reduced in the stage when cells are spreading. The cell shapes of osteoblasts were proposed to be determined by the matrix deposition activity of the cells [27, 28]. However, our study demonstrated that the cell shapes, determined by cell area and actin fiber density, have already been changed in the early stage of osteoblast differentiation with solely increased ALP activity.
In the proteomic profile of osteoprogenitor cells during the early stage of osteoblast differentiation, the number of upregulated proteins was similar to that of dowregulated proteins and only 5.60% of proteins were unchanged, indicating there is a significant change of protein expression in this stage. In addition, the cells in OS group matched more proteins of protein binding and transferase and receptor activity. We further analyzed the the signaling pathway of the regulation of actin cytoskeleton and focal adhesion signaling pathway because a comparatively larger number of matched proteins was found in this two KEGG pathways.
After analyzing the signaling pathway of the regulation of actin cytoskeleton by proteomics, we first found that IQGAP1 was significantly upregulated in the early stage of osteoblast differentiation. IQGAP1 may modulate the cytoskeleton indirectly through the Rho GTPases, members of small GTPases that are critical for cell proliferation and differentiation [29–32]. Mammalian IQGAP1 may bind to F-actin with calponin homology domain and enhance actin polymerization in vitro [33–35]. Though the IQGAP has a Ras GTPase-activating protein-related domain, IQGAP1 appears to inhibit the GTPase activity [36, 37]. In this study, IQGAP1 may enhance the actin polymerization via regulation of GTPase activity during the early stage of rhBMP-2-induced osteoblast differentiation. In addition, we found that GSN was significantly upregulated in the early stage of osteoblast differentiation. GSN is one of the most potent members of the actin-severing gelsolin/villin superfamily [38], suggesting that the actin filament assembly and disassembly were amplified during the early stage of osteoblast differentiation, indicating the enhanced expression of ezrin–radixin–moesin and the upregulation of actin skeleton, membrane dynamics, membrane trafficking, and interaction with the plasma membrane [39, 40]. CFL and the related protein actin-depolymerizing factor are involved in depolymerizing actin filaments [41]. The remodelling of filament networks occurs rapidly, on a time scale of seconds, and depolymerization of filaments is crucial to provide an adequate supply of actin monomers for the assembly of new actin-filament structures [42]. The upregulation of CFL1 and unchanging of main stress fiber density in this study demonstrated that both disassembly and assembly of actin-filament are enhanced during the early stage of osteoblast differentiation.
In analysis of focal adhesion signalling pathway by proteomics and Western blotting analysis, we confirmed the upregulation of FlNA and FLNB FLNs are actin-binding proteins that organize actin filaments into parallel arrays or three-dimensional webs, linking them to cellular membrane [43]. As a result, the upregulated FLNA and FLNB may account for the formation of actin stress fibers during the cell spreading process. The proteomic data also showed regulation of LAMs and COLs (Table 1). LAMs are biologically active parts of the basal lamina with marked effects on cell differentiation, migration, and adhesion [44]. The LAMA1 and LAMB1 are both components of LAM1 [45], and the LAM1 is able to recruit osteoprogenitor cells in vitro through a cell attachment effect in early fetal rat calvaria cell populations [46]. The diverse regulation of LAMA1 and LAMB-1 in this study suggested a dynamic assembly of LAM1 during the cell spreading. Analysis of RNA expression showed that Lama5 is widely expressed in adult mouse tissues, such as heart, lung, kidney, brain, muscle, and testis, indicating that LAMA5 is a major LAM chain of adult basal lamina [47]. LAMA5 has been shown to play roles in embryonic development, maintain kidney structure and function, and promote skin regeneration [48–50]. However, the role of LAMA5 in osteoblast differentiation is currently unknown. COLs are the most abundant proteins ECM of most animals. The inhibitors of COL synthesis suppress cell proliferation and differentiation in various kinds of cells [51, 52]. But the COL family comprises many types of molecules and it is not known what type of collagen is responsible for cell spreading and osteoblast differentiation. The mutations of COL1A1 and COLlA2 may cause osteogenesis imperfecta [53], and the production of COL1 happens in the earlier event during the osteoblast differentiation [54]. Our data also supported that the upregulation of COL1A1 is required in the early stage of osteoblast differentiation. The ascorbic acid 2-phosphate upregulates the expression of mRNA for COL3 but not affect the expression of COL1 while stimulates the cell growth in human osteoblast-like MG-63 cells, normal human osteoblasts, and human bone marrow MSCs [55]. As the ascorbic acid is also a major component of our osteoblast differentiation medium, upregulation of COL3A1 in MC3T3 cells may due to the stimulus of ascorbic acid. COL4 is a major component of basement membranes and COL4A1 encodes the α1 chain of COL4. Microarray confirmed that COL4A1 mRNA is higher expressed in osteoporotic human bone than in normal human bone [56]. It is well known that the osteoblast differentiation is hindered in osteoporotic patients. Therefore, the downregulation of COL4A1 in present study may be a critical marker for early stage of osteoblast differentiation. Present data also showed diverse regulation of COL4A6, COL5A2, COL4A2, and COL4A4 but the underlying mechanism is not clear.
After analyzing cross-talks between the two signaling pathways, we found the downregulation of VCL. In mammalian cells, vinculin may transfer the mechanical stresses for cytoskeletal remodelling [57], and our result suggests that VCL is critical for both actin skeleton regulation and focal adhesion. The strong expression of VCL in both groups indicates that VCL plays an important role during the cell proliferation and osteoblast differentiation, and the VCL may be more abundantly expressed in the cell proliferation.
Conclusions
In the present study, the osteoblast differentiation process of MC3T3-E1 cells is divided into three stages: initial stage, early stage, and final stage. In morphological and proteomic analysis, we demonstrated that enhanced cell spreading and actin fiber formation in the early stage of osteoblast differentiation and the signaling pathway of regulation of cytoskeleton and focal adhesion play critical roles in regulating cell spreading and actin skeleton formation in this stage.
Acknowlegements
This work is supported by the NIH (Grant DE 018385), the National Natural Science Foundation of China (30950019), and the Medicine and Health Research Fund of Zhejiang Province, China (2009B166). We thank Chantal M. Sottas for the technical supports.
Abbreviations
- DAPI
4’,6-diamidino-2-phenylindole
- ALP
alkaline phosphatase
- ECM
extracellular matrix
- CFL
Cofilin
- COL
Collagen
- DMEM-HG
Dulbecco’s modified Eagle’s medium-how glucose
- FLN
filamin
- GSN
gelsolin
- IQGAP1
GTPase-activating-like protein 1
- IAA
iodoacetamide
- IPI
International protein index
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LAM
Laminin
- LC-MS/MS
liquid chromatography-coupled tandem mass spectrometry
- MS
mass spectrometry
- PIGOK
Protein Interrogation of Gene Ontology and KEGG databases
- SDS-PAGE
sodium dodecylsulfate – polyacrylamide gel electrophoresis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sampath TK, Reddi AH. Homology of bone-inductive proteins from human, monkey, bovine, and rat extracellular matrix. Proc Natl Acad Sci U S A. 1983;80:6591–6595. doi: 10.1073/pnas.80.21.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sampath TK, Reddi AH. Importance of geometry of the extracellular matrix in endochondral bone differentiation. J Cell Biol. 1984;98:2192–2197. doi: 10.1083/jcb.98.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komori T. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem. 2006;99:1233–1239. doi: 10.1002/jcb.20958. [DOI] [PubMed] [Google Scholar]
- 4.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Quarles LD, Yohay DA, Lever LW, Caton R, Wenstrup RJ. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J Bone Miner Res. 1992;7:683–692. doi: 10.1002/jbmr.5650070613. [DOI] [PubMed] [Google Scholar]
- 6.Papachroni KK, Karatzas DN, Papavassiliou KA, Basdra EK, Papavassiliou AG. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol Med. 2009;15:208–216. doi: 10.1016/j.molmed.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Parfitt AM. Age-related structural changes in trabecular and cortical bone: cellular mechanisms and biomechanical consequences. Calcif Tissue Int. 1984;36(Suppl 1):S123–S128. doi: 10.1007/BF02406145. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho RS, Schaffer JL, Gerstenfeld LC. Osteoblasts induce osteopontin expression in response to attachment on fibronectin: demonstration of a common role for integrin receptors in the signal transduction processes of cell attachment and mechanical stimulation. J Cell Biochem. 1998;70:376–390. doi: 10.1002/(sici)1097-4644(19980901)70:3<376::aid-jcb11>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Thomas CH, Collier JH, Sfeir CS, Healy KE. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc Natl Acad Sci U S A. 2002;99:1972–1977. doi: 10.1073/pnas.032668799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 11.Papachristou DJ, Papachroni KK, Basdra EK, Papavassiliou AG. Signaling networks and transcription factors regulating mechanotransduction in bone. Bioessays. 2009;31:794–804. doi: 10.1002/bies.200800223. [DOI] [PubMed] [Google Scholar]
- 12.Kawata A, Mikuni-Takagaki Y. Mechanotransduction in stretched osteocytes--temporal expression of immediate early and other genes. Biochem Biophys Res Commun. 1998;246:404–408. doi: 10.1006/bbrc.1998.8632. [DOI] [PubMed] [Google Scholar]
- 13.Bakker AD, Klein-Nulend J, Burger EH, Mechanotransduction in bone cells proceeds via activation of COX-2. but not COX-1. Biochem Biophys Res Commun. 2003;305:677–683. doi: 10.1016/s0006-291x(03)00831-3. [DOI] [PubMed] [Google Scholar]
- 14.Forwood MR. Inducible cyclo-oxygenase (COX-2) mediates the induction of bone formation by mechanical loading in vivo. J Bone Miner Res. 1996;11:1688–1693. doi: 10.1002/jbmr.5650111112. [DOI] [PubMed] [Google Scholar]
- 15.Loza J, Stephan E, Dolce C, Dziak R, Simasko S. Calcium currents in osteoblastic cells: dependence upon cellular growth stage. Calcif Tissue Int. 1994;55:128–133. doi: 10.1007/BF00297188. [DOI] [PubMed] [Google Scholar]
- 16.Rubin J, Murphy TC, Zhu L, Roy E, Nanes MS, Fan X. Mechanical strain differentially regulates endothelial nitric-oxide synthase and receptor activator of nuclear kappa B ligand expression via ERK1/2 MAPK. J Biol Chem. 2003;278:34018–34025. doi: 10.1074/jbc.M302822200. [DOI] [PubMed] [Google Scholar]
- 17.Ng AF, Yang YO, Wong RW, Hagg EU, Rabie AB. Factors regulating condylar cartilage growth under repeated load application. Front Biosci. 2006;11:949–954. doi: 10.2741/1851. [DOI] [PubMed] [Google Scholar]
- 18.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 19.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 20.Pavalko FM, Chen NX, Turner CH, Burr DB, Atkinson S, Hsieh YF, Qiu J, Duncan RL. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeleton-integrin interactions. Am J Physiol. 1998;275:C1591–C1601. [PubMed] [Google Scholar]
- 21.Jacob RJ, Cramer R. PIGOK: Linking protein identity to gene ontology and function. J Proteome Res. 2006;5:3429–3432. doi: 10.1021/pr0601537. [DOI] [PubMed] [Google Scholar]
- 22.Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 23.Welch MD, Mallavarapu A, Rosenblatt J, Mitchison TJ. Actin dynamics in vivo. Curr Opin Cell Biol. 1997;9:54–61. doi: 10.1016/s0955-0674(97)80152-4. [DOI] [PubMed] [Google Scholar]
- 24.Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol. 2007;213:565–573. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- 25.Damsky CH. Extracellular matrix-integrin interactions in osteoblast function and tissue remodeling. Bone. 1999;25:95–96. doi: 10.1016/s8756-3282(99)00106-4. [DOI] [PubMed] [Google Scholar]
- 26.Kalajzic I, Kalajzic Z, Kaliterna M, Gronowicz G, Clark SH, Lichtler AC, Rowe D. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J Bone Miner Res. 2002;17:15–25. doi: 10.1359/jbmr.2002.17.1.15. [DOI] [PubMed] [Google Scholar]
- 27.Grigoriadis AE, Heersche JN, Aubin JE. Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell population: effect of dexamethasone. J Cell Biol. 1988;106:2139–2151. doi: 10.1083/jcb.106.6.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sikavitsas VI, Temenoff JS, Mikos AG. Biomaterials and bone mechanotransduction. Biomaterials. 2001;22:2581–2593. doi: 10.1016/s0142-9612(01)00002-3. [DOI] [PubMed] [Google Scholar]
- 29.Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 30.Welsh CF, Roovers K, Villanueva J, Liu Y, Schwartz MA, Assoian RK. Timing of cyclin D1 expression within G1 phase is controlled by Rho. Nat Cell Biol. 2001;3:950–957. doi: 10.1038/ncb1101-950. [DOI] [PubMed] [Google Scholar]
- 31.Takano H, Komuro I, Oka T, Shiojima I, Hiroi Y, Mizuno T, Yazaki Y. The Rho family G proteins play a critical role in muscle differentiation. Mol Cell Biol. 1998;18:1580–1589. doi: 10.1128/mcb.18.3.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sordella R, Jiang W, Chen GC, Curto M, Settleman J. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell. 2003;113:147–158. doi: 10.1016/s0092-8674(03)00271-x. [DOI] [PubMed] [Google Scholar]
- 33.Bashour AM, Fullerton AT, Hart MJ, Bloom GS. IQGAP1, a Rac- and Cdc42-binding protein, directly binds and cross-links microfilaments. J Cell Biol. 1997;137:1555–1566. doi: 10.1083/jcb.137.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erickson JW, Cerione RA, Hart MJ. Identification of an actin cytoskeletal complex that includes IQGAP and the Cdc42 GTPase. J Biol Chem. 1997;272:24443–24447. doi: 10.1074/jbc.272.39.24443. [DOI] [PubMed] [Google Scholar]
- 35.Fukata M, Kuroda S, Fujii K, Nakamura T, Shoji I, Matsuura Y, Okawa K, Iwamatsu A, Kikuchi A, Kaibuchi K, Regulation of cross-linking of actin filament by IQGAP1. a target for Cdc42. J Biol Chem. 1997;272:29579–29583. doi: 10.1074/jbc.272.47.29579. [DOI] [PubMed] [Google Scholar]
- 36.Briggs MW, Sacks DB. IQGAP1 as signal integrator: Ca2+, calmodulin, Cdc42 and the cytoskeleton. FEBS Lett. 2003;542:7–11. doi: 10.1016/s0014-5793(03)00333-8. [DOI] [PubMed] [Google Scholar]
- 37.Mateer SC, Wang N, Bloom GS. IQGAPs: integrators of the cytoskeleton, cell adhesion machinery, and signaling networks. Cell Motil Cytoskeleton. 2003;55:147–155. doi: 10.1002/cm.10118. [DOI] [PubMed] [Google Scholar]
- 38.Sun HQ, Yamamoto M, Mejillano M, Yin HL, Gelsolin a multifunctional actin regulatory protein. J Biol Chem. 1999;274:33179–33182. doi: 10.1074/jbc.274.47.33179. [DOI] [PubMed] [Google Scholar]
- 39.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 40.Tsukita S, Yonemura S. Cortical actin organization: lessons from ERM (ezrin/radixin/moesin) proteins. J Biol Chem. 1999;274:34507–34510. doi: 10.1074/jbc.274.49.34507. [DOI] [PubMed] [Google Scholar]
- 41.Ono S. Mechanism of depolymerization and severing of actin filaments and its significance in cytoskeletal dynamics. Int Rev Cytol. 2007;258:1–82. doi: 10.1016/S0074-7696(07)58001-0. [DOI] [PubMed] [Google Scholar]
- 42.Lin MC, Galletta BJ, Sept D, Cooper JA. Overlapping and distinct functions for cofilin, coronin and Aip1 in actin dynamics in vivo. J Cell Sci. 2010 doi: 10.1242/jcs.065698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorlin JB, Yamin R, Egan S, Stewart M, Stossel TP, Kwiatkowski DJ, Hartwig JH. Human endothelial actin-binding protein (ABP-280, nonmuscle filamin): a molecular leaf spring. J Cell Biol. 1990;111:1089–1105. doi: 10.1083/jcb.111.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979;254:9933–9937. [PubMed] [Google Scholar]
- 45.Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR. The laminin alpha chains: expression, developmental transitions, and chromosomal locations of alpha1-5, identification of heterotrimeric laminins 8–11, and cloning of a novel alpha3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roche P, Goldberg HA, Delmas PD, Malaval L. Selective attachment of osteoprogenitors to laminin. Bone. 1999;24:329–336. doi: 10.1016/s8756-3282(98)00194-x. [DOI] [PubMed] [Google Scholar]
- 47.Miner JH, Lewis RM, Sanes JR. Molecular cloning of a novel laminin chain alpha 5 and widespread expression in adult mouse tissues. J Biol Chem. 1995;270:28523–28526. doi: 10.1074/jbc.270.48.28523. [DOI] [PubMed] [Google Scholar]
- 48.Paquet-Fifield S, Schluter H, Li A, Aitken T, Gangatirkar P, Blashki D, Koelmeyer R, Pouliot N, Palatsides M, Ellis S, Brouard N, Zannettino A, Saunders N, Thompson N, Li J, Kaur P. A role for pericytes as microenvironmental regulators of human skin tissue regeneration. J Clin Invest. 2009;119:2795–2806. doi: 10.1172/JCI38535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shannon MB, Patton BL, Harvey SJ, Miner JH. A hypomorphic mutation in the mouse laminin alpha5 gene causes polycystic kidney disease. J Am Soc Nephrol. 2006;17:1913–1922. doi: 10.1681/ASN.2005121298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kikkawa Y, Miner JH. Molecular dissection of laminin alpha 5 in vivo reveals separable domain-specific roles in embryonic development and kidney function. Dev Biol. 2006;296:265–277. doi: 10.1016/j.ydbio.2006.04.463. [DOI] [PubMed] [Google Scholar]
- 51.Reddi AH. Cell biology and biochemistry of endochondral bone development. Coll Relat Res. 1981;1:209–226. doi: 10.1016/s0174-173x(81)80021-0. [DOI] [PubMed] [Google Scholar]
- 52.Royce PM, Barnes MJ. Interaction of embryonic chick calvarial bone cells with collagen substrata; attachment characteristics and growth behaviour. Connect Tissue Res. 1988;17:55–70. doi: 10.3109/03008208808992794. [DOI] [PubMed] [Google Scholar]
- 53.Colige A, Sokolov BP, Nugent P, Baserga R, Prockop DJ. Use of an antisense oligonucleotide to inhibit expression of a mutated human procollagen gene (COL1A1) in transfected mouse 3T3 cells. Biochemistry. 1993;32:7–11. doi: 10.1021/bi00052a002. [DOI] [PubMed] [Google Scholar]
- 54.Stein GS, Lian JB. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr Rev. 1993;14:424–442. doi: 10.1210/edrv-14-4-424. [DOI] [PubMed] [Google Scholar]
- 55.Maehata Y, Takamizawa S, Ozawa S, Izukuri K, Kato Y, Sato S, Lee MC, Kimura A, Hata R. Type III collagen is essential for growth acceleration of human osteoblastic cells by ascorbic acid 2-phosphate, a long-acting vitamin C derivative. Matrix Biol. 2007;26:371–381. doi: 10.1016/j.matbio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 56.Hopwood B, Tsykin A, Findlay DM, Fazzalari NL. Gene expression profile of the bone microenvironment in human fragility fracture bone. Bone. 2009;44:87–101. doi: 10.1016/j.bone.2008.08.120. [DOI] [PubMed] [Google Scholar]
- 57.Ezzell RM, Goldmann WH, Wang N, Parashurama N, Ingber DE. Vinculin promotes cell spreading by mechanically coupling integrins to the cytoskeleton. Exp Cell Res. 1997;231:14–26. doi: 10.1006/excr.1996.3451. [DOI] [PubMed] [Google Scholar]