Abstract
Activation of the Keap1/Nrf2 pathway and consequent induction of phase 2 antioxidant enzymes is known to afford neuroprotection. Here, we present a series of novel electrophilic compounds that protect neurons via this pathway. Natural products, such as carnosic acid (CA), are present in high amounts in the herbs rosemary and sage as ortho-dihydroquinones, and have attracted particular attention because they are converted by oxidative stress to their active form (ortho-quinone species) that stimulate the Keap1/Nrf2 transcriptional pathway. Once activated, this pathway leads to the production of a series of antioxidant phase 2 enzymes. Thus, such dihydroquinones function as redox-activated “pro-electrophiles.” Here, we explored the concept that related para-dihydroquinones represent even more effective bioactive pro-electrophiles for the induction of phase 2 enzymes without producing toxic side effects. We synthesized several novel para-hydroquinone-type pro-electrophilic compounds (designated D1 and D2) in order to analyze their protective mechanism. DNA microarray, PCR, and Western blot analyses showed that compound D1 induced expression of heat-shock proteins (HSPs), including HSP70, HSP27 and DnaJ, in addition to phase 2 enzymes such as hemeoxygenase-1 (HO-1), NADP(H) quinine-oxidoreductase1, and the Na+-independent cystine/glutamate exchanger. Treatment with D1 resulted in activation of Nrf2 and HSF-1 transcriptional elements, thus inducing phase 2 enzymes and HSPs, respectively. In this manner, D1 protected neuronal cells from both oxidative and endoplasmic reticulum (ER)-related stress. Additionally, D1 suppressed induction of GRP78, an ER chaperone protein, and inhibited hyperoxidation of peroxiredoxin 2 (PRX2), a molecule that in it reduced state can protect from oxidative stress. These results suggest that D1 is a novel pro-electrophilic compound that activates both the Nrf2 and HSF-1 pathways, and may thus offer protection from oxidative and ER stress.
Introduction
Activation of the Nrf2/antioxidant response element (ARE) pathway
Electrophilic molecular species can induce the expression of a set of antioxidant enzymes, called ‘phase 2 enzymes,’ which include hemeoxygenase-1 (HO-1), NADPH quinone oxidoreductase 1 (NQO1), sulfiredoxin, glutamyl cysteine ligase modifier subunit (GCLM), and the Na+-independent-cystine/glutamate exchanger (xCT), all of which provide efficient cytoprotection by regulating the intracellular redox state (Talalay 2000; Itoh K et al. 2004; Satoh and Lipton 2007; Soriano et al. 2008; Satoh et al. 2000, 2001, 2003, 2006). A key cascade in activating the transcription of genes encoding phase 2 enzymes involves the Keap1/Nrf2 pathway, which is comprised of Keap1, a regulator protein, and Nrf2, a transcription factor that when released from Keap1 in the cytoplasm, enters the nucleus and binds to the antioxidant response element (AREs) (Talalay 2000; Itoh K et al. 2004; Padmanabhan et al. 2006; Satoh et al. 2006; Wang et al. 2010). Mechanistically, when electrophiles react with critical cysteine residues on Keap1 protein to form a covalent adduct, they perturb this system, thereby releasing Nrf2 and allowing it to be translocated from the cytoplasm into the nucleus, where it binds to AREs and stimulates transcription of phase 2 enzyme genes (Itoh K et al. 2004; Padmanabhan et al. 2006; Satoh et al. 2009b). Thus, Nrf2 has been considered a potential therapeutic target for the treatment of neurodegenerative diseases (Mattson and Cheng 2006; Satoh and Lipton 2007; Vargas and Johnson 2009).
Hyperoxidation of peroxiredoxin (PRX)
Aside from the glutathione system, the thioredoxin (TRX)-PRX pathway is arguably the most important antioxidant system that limits accumulation of intracellular peroxides via redox reactions at critical cysteine residues (Holmgren and Lu 2010; Winyard et al. 2005; Immenshuh and Baumgart-Vogt 2005). Expression of TRX-PRX is controlled by the Nrf2/ARE system (Soriano et al. 2008). In particular, TRX1-PRX2 is extremely abundant in the brain. The TRX1-PRX2 system detoxifies peroxides by transferring reducing equivalents from NADPH to peroxides via TRX reductase, TRX1, and finally PRX2 (Winyard et al. 2005; Immenshuh and Baumgart-Vogt 2005). The TRX1-PRX2 system can react with at least two types of oxidants, nitric oxide (NO) and peroxide (Fang et al. 2007). Through reaction with NO, PRX2 is S-nitrosylated at redox-active cysteine residues Cys-51 and Cys-172 to form PRX2-SNO, thus inhibiting the neuroprotective effect of PRX2 against oxidative stress (Fang et al. 2007). S-nitrosylation of PRX2, resulting in PRX2 dysfunction, therefore mechanistically links nitrosative and oxidative stress to neurodegeneration (Fang et al. 2007). In the absence of NO, PRX2 forms PRX2-SOH via reaction with peroxide, and can be further oxidized to a sulfinic (-SO2H) or sulfonic (-SO3H) acid derivative. This hyperoxidation of PRX2 causes inactivation of its peroxidase/neuroprotective activity (Rhee et al. 2007). Although PRX2-SO2/3H cannot be reduced by TRX, it can be reduced back to the catalytically active free thiol form in eukaryotic cells by the ATP-dependent reductase, sulfiredoxin 1(SRXN1; Rhee et al. 2007). The activity of SRXN1 restores inactive PRX2-SO2/3H back to the TRX cycle and prevents permanent oxidative inactivation of PRX2 by strong oxidative insults (Soriano et al. 2008). The induction of SRXN may represent a critical event for the neurprotective effect of thiol-containing electrophilic compounds (Soriano et al. 2008).
HSF-1 system
Oxidative stress can also lead to endoplasmic reticulum (ER) stress, which may play a key role in chronic neurodegeneration; in this case, accumulation of misfolded proteins in the ER induces ER dysfunction (Bukau et al. 2006; Bredesen 2008; Morimoto 2008; Kim et al. 2008; Nakamura and Lipton 2009). To limit such stress, a powerful endogenous protective mechanism is represented by the induction of molecular chaperones, including heat-shock proteins (HSPs) such as HSP70 and DnaJ, as well as heat-shock factor-binding protein (HSBP) and heat-shock 105-kDa/110-kDa protein 1 (HSPH1). These molecular chaperones are known to suppress protein misfolding (Bukau et al. 2006; Morimoto 2008; Kim et al. 2008; Nakamura and Lipton 2009). The expression of molecular chaperones after exposure to various types of cell stress is known to be regulated by heat-shock transcription factor 1 (HSF-1). Under unstressed conditions, HSF-1 is localized in the cytosol and is inactivated in a protein complex including HSP90. Upon exposure to stress-inducing compounds, HSF-1 dissociates from the HSP90 protein complex, translocates into the nucleus, and binds to the HSH response element (HSE) in the promoter region of various molecular chaperone genes to induce their expression. For this reason, HSF-1 has been considered to be a potential target for the treatment of neurodegenerative diseases (Fujimoto et al. 2005; Morimoto 2008; Trott et al. 2008; Fujikake et al. 2008). In fact, several HSF-1 activators reportedly protect neurons and the brain in vivo (Sano 2001; Lu et al. 2004; Trott et al. 2008; Fujikake et al. 2008).
Novel electrophiles
Hydroquinone-type compounds, such as carnosic acid (CA) and tert-butyl hydroquinone (TBHQ), are not themselves electrophilic but become electrophilic via oxidative conversion to the quinone, which occurs when they encounter free radicals generated by cellular oxidative stress (Satoh et al. 2006, 2008a, 2008b, 2009a; Lipton 2007; Tamaki et al. 2010; Lee et al. 2003; Kraft et al. 2004; Shih et al. 2005). Thus, these hydroquinone-type compounds act as pro-electrophilic drugs, which require conversion from hydroquinone to quinone in order to exert their neuroprotective effect (Satoh et al. 2008a and 2009a). In the present study, we synthesize new parahydroquinone-type molecular compounds in order to generate improved neuroprotective electrophiles. We prepared two novel pro-electrophilic molecules (D1 and D2, Fig. 1C) for this purpose and investigated their protective actions against oxidative and ER stress-induced neuronal cell death. We found that the HSF-1/HSE pathway may be activated along with the Nrf2/ARE pathway in the neuroprotective action of such electrophilic drugs.
Fig. 1. Induction of phase-2 enzymes and HSPs by D1.
(A) PCR analysis of phase 2 and HSP genes induced by 20 μM D1. Total RNAs were extracted from ARPE cells after treatment with 20 μM D1 or D2 for 24 h in serum-free medium. RT-PCR was performed with the specific primers listed in the Materials and Methods. (B) Western blot analysis to show dose-dependent induction of HO-1 and HSP70 proteins by D1. Lysates were prepared from cells after treatment with various concentrations of D1; 10 μg protein samples were loaded per lane and subjected to SDS-PAGE prior to immunoblotting. The experiments were repeated three times with similar results. Lower panel: Cell lysates (10 μg protein/lane) were subjected to SDS-PAGE and immunoblotted for HO-1 and HSP70. Upper panel: Analysis of HO-1 and HSP70 normalized to β-actin by taking the ratio of their densitometric values on immunoblots. * p < 0.05 from control cells.
Materials and methods
Materials
Antibodies and their sources were as follows: anti-Nrf2 and anti-78 kDa glucose-regulated protein (GRP78) polyclonal rabbit IgGs (H300 and H129, respectively, Santa Cruz Biotech, Santa Cruz, CA), anti-HSF-1 polyclonal rabbit IgG (#4356, Cell Signaling Tech, Danvers, MA), anti-HO-1 polyclonal rabbit IgG (OSA-150, Assay design, Plymouth Meeting, PA), anti-HSP70 monoclonal mouse IgG (200-301-A27, Rockland Immunological Inc., Gilbertsville, PA), anti-actin monoclonal mouse IgM (#MAB1501, Millipore, Billerica, MA), anti-microtubule-associated protein2 (MAP2) monoclonal mouse IgG (HM2, Sigma-Aldrich, St. Louis, MO), anti-NeuN monoclonal IgG (A60, Millipore, Billerica, MA), anti-PRX2 rabbit polyclonal IgG (LF-PA0007, Lab Frontier, Clear Lake, IA), anti-PRX2-SO3H rabbit polyclonal antibody (ab16830, Abcam, Cambridge, MA), peroxidase-conjugated anti-mouse IgM (Calbiochem, Darmstadt, Germany), peroxidase-conjugated anti-rabbit IgG, FITC-conjugated anti-rabbit IgG, and rhodamine-conjugated anti-mouse IgG, (Jackson Immuno Research Laboratories, West Grove, PA). Other chemicals including dimethylsulfoxide (DMSO), sodium glutamate, fluorescein diacetate (FDA), hydrogen peroxide (H2O2) and Hoechst 33,258 were obtained from Sigma-Aldrich (St. Louis, MO). Rabbit polyclonal antibody against human Srxn1 is courtesy of Dr. Sue Goo Rhee, Ewha Womans University, Seoul, South Korea (Chang et al. 2004).
Culture of human retinal pigmented epithelial cells 19 (ARPE-19) cells
The outermost layer of human retina is the retinal pigment epithelium (RPE). This RPE represents a primary target of injury in age-related macular degeneration (AMD), which is thought to be induced in part by oxidative stress. ARPE-19 cells are a primary human cell line derived from human RPE. To study the biochemistry and molecular biology of oxidative stress in ARPE-19 cells, several investigators have used these cells for in vitro culture. For example, exposure of ARPE-19 cells to H2O2, is known to induce apoptosis and has thus been used as an in vitro model of retinal degeneration triggered by oxidative stress. ARPE-19 cells were obtained from ATCC and maintained in 10-cm dishes containing Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FCS (Zareba et al. 2006). The cells were introduced into wells of a 24-well plate at a density of 105 cells/cm2 and incubated for 24 h. The medium was exchanged for serum-free medium containing the compounds to be tested (D1 or D2; 20 μM), and the cultures were incubated for 24 h. Then, H2O2 or tunicamycin (TM) was added and the cells incubated for an additional 4 h or 24 h, respectively. Finally, the cells were stained with FDA (1 μM) and Hoechst dye 33,258 (5 μg/ml) and then observed under epifluorescence microscopy.
Statistical analysis
Results are presented as mean ± standard deviation (SD). An analysis of variance was performed for multiple comparisons and a Student’s t-test for single comparisons.
Other Methods are mentioned in the Supporting Document.
Results
Induction of phase 2 enzymes and HSPs by the novel pro-electrophilic drug, D1
We hypothesized that the biological activity of D1 and D2 should be closely related to their transcriptional activation of the Nrf2/ARE pathway. In order to examine this possibility, we initially performed a microarray analysis using ARPE-19 cells exposed to these compounds vs. control diluent (Table S1). The top 20 genes induced by 20 μM D1 or D2 are listed in Table S1. D1 induced several phase 2 enzymes such as HO-1 and xCT, as well as HSPs. In contrast, induction of these genes by D2 was very small or completely negative. In order to confirm that D1 indeed induced the genes encoding phase 2 enzymes and HSPs, we performed an RT-PCR analysis using primers for HO-1, xCT, GCLM, and NQO-1 (phase 2 enzymes) as well as for HSP70, DnaJ, HSBP, and HSPH1 (HSPs) (Fig. 1A). All of these were significantly induced by D1, although the magnitude of induction varied from gene to gene. In contrast, these same genes were only very weakly induced by D2 (Fig 1A). HO-1, xCT, and HSP70 were strongly induced by D1, whereas GCLM, NQO1, DNAJ, HSBP, and HSPH1 manifested weaker, yet significant induction. Next, we confirmed the induction of HO-1 and HSP70 at the protein level by performing Western blot analysis (Fig 1B). D1 induced HO-1 and HSP70 proteins in a dose-dependent manner. Taken together, these data suggested that D1 potently induced both phase 2 enzymes and HSPs.
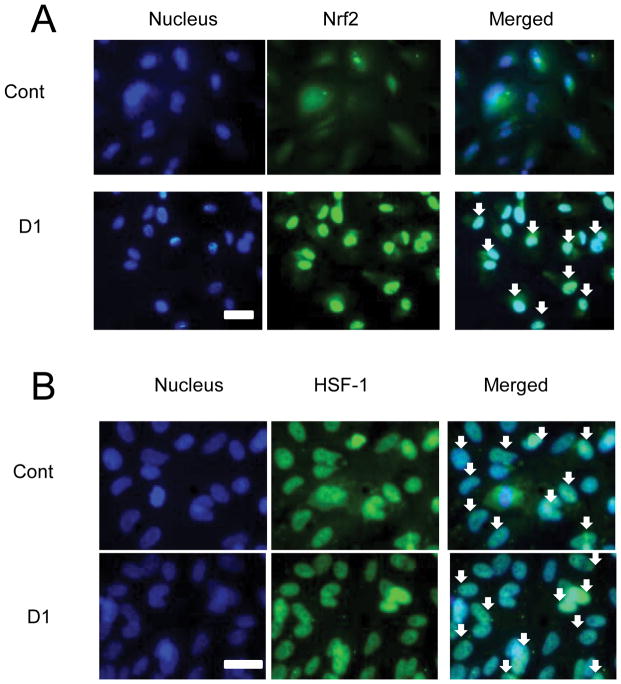
Translocation of Nrf2 after treatment with D1
Induction of phase 2 enzymes and HSPs is largely regulated by the transcription factors Nrf2 and HSF-1, respectively. For transcriptional activation, both factors must be in the nucleus. Thus, we determined the distribution of Nrf2 and HSF-1 in cells in the presence and absence of D1 (20 μM). In an analogous fashion to NF-κB and IκB, Nrf2 translocates from the cytoplasm into the nucleus after dissociation from Keap1. In the absence of D1, we observed that Nrf2 was predominantly localized in the cytoplasm (Fig. 2A). When D1 was added, we found a dramatic translocation of Nrf2 into the nucleus (Fig. 2A). In contrast, in some but not all cases, HSF-1 has been found in the nucleus even under basal conditions (Nakai et al. 1995; Mercier et al. 1999). Here, we found that HSF-1 was present in the nucleus under control conditions even in the absence of D1 (Fig. 2B).
Fig. 2. Nuclear translocation of Nrf2 and HSF-1 after treatment with D1.
ARPE-19 cells were plated at 105 cells/cm2 and incubated for 24 h. The medium was then exchanged to serum-free medium containing vehicle (DMSO) or D1 (20 μM), and the cells were incubated for an additional 24 h. Cells were fixed and stained (green) with anti-Nrf2 (A) or anti-HSF-1 antibody (B) as well as with Hoechst dye 33,258 (5 μM, blue). Scale bar, 50 μm. Double-positive cells are marked by arrows, indicating nuclear localization. Each experiment was repeated at least twice.
Activation of ARE and HSE after treatment with D1
Next, we investigated whether D1 would activate both Nrf2 and HSF-1. To test this notion, we performed reporter gene assays on ARPE-19 cells transfected with the cDNAs under the transcriptional control of the ARE (Fig. 3A) and HSE (Fig. 3B). We found that the response to D2 was completely negative, whereas D1 significantly activated both transcriptional elements, indicating that D1 activated both the Nrf2/ARE and HSF-1/HSE systems.
Fig. 3. Transcriptional activation of ARE and HSE after treatment with D1.
ARPE cells were plated at 105 cells/cm2, incubated for 24 h, and then transfected with cDNA reporter constructs (ARE- or HSE-luciferase). After 5 h in serum-containing medium, the medium was exchanged for serum-free medium containing vehicle (DMSO) or D1 (20 μM). Cell lysates were obtained after 24 h later and subjected to reporter gene assays. Values are mean ± SD; *p < 0.01. Results represent two independent experiments.
Reduction of GRP78 by D1
We next studied whether D1 could ameliorate ER stress through induction of HSPs. In particular, HSP70 is engaged in a number of folding events, including folding newly synthesized proteins, transporting proteins across membranes, refolding of misfolded or aggregated proteins, and controlling the activity of regulatory proteins (Morimoto 2008). To monitor the effect of D1 on ER stress, we performed Western blotting using anti-GRP78, a marker of ER stress (Fig. 4). GRP78 is rapidly induced during ER stress in the typical unfolded protein response (UPR) (Bukau et al. 2006). Thus, if D1 could ameliorate ER stress by induction of HSPs, we would expect that the level of GRP78 would be decreased. We found that D1 markedly increased the levels of HO- 1 and HSP70 both in the absence and presence of 1μM TM (Fig. 4B), suggesting that ER stress did not affect the induction of these proteins. However, the addition of 1 μM TM markedly increased the level of GRP78 protein, indicating that TM induced ER stress, but this increase was markedly attenuated by D1 (Fig. 4A). These results suggest that D1 could relieve ER stress, possibly through induction of HSPs.
Fig. 4. Reduction in GRP78 levels after treatment with D1.
ARPE-19 cells were plated at 105 cells/cm2 and incubated for 24 h. Then, the medium was exchanged for serum-free medium containing vehicle (DMSO) or D1 (10 or 20 μM), and incubated for an additional 24 h; following this, TM 1 (μM) or vehicle was added to the cells and incubated for 8 h. Lower panel: Cell lysates (10 μg protein/lane) were subjected to SDS-PAGE and immunoblotted for GRP78 (A), or HO-1 and HSP70 (B). Upper panel: Analysis of GRP78 (A), or HO-1 and HSP70 (B), normalized to β-actin by taking the ratio of their densitometric values on immunoblots. * p < 0.05. Each experiment was replicated at least twice.
Inhibition of PRX2 hyperoxidation by D1
One of the most effective protective mechanisms against peroxide is the TRX1-PRX2 anti-oxidant system in the brain (Winyard et al. 2005; Immenschuh and Baumgart-Vogt 2005). When there is a peroxide overload, thiol groups on PRX2 are oxidized to –SOH (sulfenic acid), which is reversible by TRX1, but intense insult results in hyperoxidation to –SO2H (sulfinic acid) or –SO3H (sulfonic acid) that is not enzymatically reversed by TRX1. However, SRXN1 can reduce –SO2/3H to -SH in the presence of TRX, ATP, and NADPH (Rhee et al. 2007). Thus, we hypothesized that this defense system might play a role in the protective effect afforded by D1 against oxidative stress. To test this idea, we examined the ratio of PRX2-SO2/3H/PRX2 at the protein level in ARPE-19 cells exposed to 1 mM H2O2. The ratio was very low under control conditions but increased after exposure to H2O2 (Fig. 5A). Pretreatment with D1 significantly inhibited the ratio, suggesting that D1 led to chemical reduction of PRX2-SO2/3H to PRX2-SH. Since the Nrf2/ARE pathway mediates transcriptional activation of SRXN1 (Soriano et al. 2008) and we had shown that D1 activates the ARE, we monitored SRXN1 expression by RT-PCR (Fig. 5B) and western blot (Fig. 5C) after treatment with D1. Indeed, we found that D1 increased SRXN1 message and protein, consistent with transcriptional induction (Fig. 5B and C).
Fig. 5. Effect of D1 on PRX2 hyperoxidation and SRXN1 Induction.
(A) Inhibition of PRX2 hyperoxidation. ARPE-19 cells were plated at 105 cells/cm2 and incubated for 24 h. Then, the medium was exchanged for serum-free medium containing vehicle (DMSO) or D1 (20 μM), and the cells were incubated for an additional 24 h, after which H2O2 (1 mM) or vehicle was added to the cells for 4 h. Lower panel: Cell lysates (10 μg protein/lane) were subjected to SDS-PAGE and immunoblotted for PRX2-SO2/3H. Upper panel: Analysis of PRX2-SO2/3H normalized to total PRX2 by taking the ratio of their densitometric values on the immunoblots. *p < 0.05. Each experiment was repeated at least twice. Samples loaded onto the gel in each experiment were from duplicate wells of a 6-well plate. Membranes were probed with β-actin to insure equal loading. (B) Induction of SRXN1. For mRNA levels, total RNA was extracted from ARPE-19 cells after treatment for 24 h with vehicle or D1 (20 μM) in serum-free medium and subjected to RT-PCR for SRXN1 and β-actin. For protein levels, lysates were prepared from cells after treatment with vehicle or D1 (20 μM); 10 μg protein samples were loaded per lane and subjected to SDS-PAGE prior to immunoblotting. The experiments were repeated two times with similar results. Analysis of SRXN1 normalized to total β-actin by taking the ratio of their densitometric values on the immunoblots. *p < 0.05.
Protective effect of D1 against oxidative and ER stress in APRE-19 cells and cortical neurons
Importantly, our previous work had shown that pro-electrophilic drugs afford protection against oxidative stress (Satoh et al. 2006, 2008a, 2008b, 2009a, 2009b; Satoh and Lipton 2007); thus, we tested D1 in this manner. Initially, we examined whether D1 could protect ARPE-19 cells from H2O2. This retinal-derived cell lines is often used as an experimental model of oxidative stress in age-related macular degeneration (Wada et al. 2001; Kim et al. 2003; Zareba et al. 2006; Mandel et al. 2009). We found that exposure to 1 mM H2O2 for 4 h in serum-free medium induced extensive cell death (Fig. 6), but D1 significantly protected the cells. Similarly, D1 also protected primary cortical neurons in culture from H2O2 (Fig. S2).
Fig. 6. (A) Protective effects of D1 on ARPE-19 cells.
ARPE-19 cells were plated at 105 cells/cm2 and incubated for 24 h. Then, the medium was exchanged for serum-free medium containing vehicle (DMSO) or D1 20 μM, and the cells were incubated for an additional 24 h. Thereafter, the cells were incubated with 1 mM H2O2, 3 μM TM, or vehicle for 4 h and then stained with FDA (green) and Hoechst 33,258 (blue). Note that cells labeled only with blue represent dead cells while blue/green cells are living. Scale bar, 100 μm. (B) Statistical analysis of protective effects of D1. Number of surviving cells is scored on the abscissa. *p < 0.01. Each experiment was performed at least three times. Cells labeled only with blue represent dead cells, while blue/green cells were scored as alive.
Next, we considered the possible effect of D1 on ER stress because many reports have suggested that induction of HSPs can increase cell resistance to ER stress, and we had found that D1 increased HSPs. Indeed, we observed that D1 decreased susceptibility to ER stress elicited by TM in both ARPE-19 cells and primary cortical neurons (Figs. 6 and S2).
Protective effect of D1 against oxidative glutamate toxicity in HT22 cells
Finally, we examined the protective effects of D1 against oxidative glutamate toxicity in mouse hippocampal HT22 cells, representing another type of oxidative stress. In these cells, a high concentration of glutamate induces cell death through depletion of glutathione, which is induced by inhibition of the glutamate-cystine antiporter (Tan et al. 1998; Sagara et al. 2000). We found that the addition of D1 significantly protected the cells against oxidative glutamate toxicity (IC50 = 8.6 μM), whereas D2 was ineffective (Fig. S3). The protective effect of D1 required preincubation prior to the addition of the glutamate. In fact, when D1 was added simultaneously with glutamate, the cells were not protected (data not shown). This finding is consistent with the mechanism show above that transcriptional events are essential for the protective effects of D1.
Discussion
Involvement of both the HSF/HSE and Nrf2/ARE pathways in protection by pro-electrophilic drugs
An important point of the present study is the discovery that the HSF-1/HSE pathway is a contributing neuroprotective mechanism that is activated by the novel electrophile, D1. In previous studies, we and others had found that activation of the Nrf2/ARE pathway is an important neuroprotective mechanism for electrophilic compounds (Satoh and Lipton 2007; Vargas and Johnson 2009). This pathway represents an endogenous protective mechanism against oxidative stress because it stimulates transcription of several phase 2 enzymes that are involved in redox regulation. Additionally, it has been previously reported that activation of Nrf2 in turn stimulates HSP70 production (Renaldi et al. 2011). Accordingly in the present study, we show that D1 also activates the HSF-1 transcriptional pathway to decrease the level of hyperoxidized PRX2. By microarray analysis and RT-PCR, we show that D1 induces the phase 2 enzymes xCT and GCLM, which are involved in the regulation of glutathione metabolism. This finding is particularly important for the potential clinical tolerability for D1 since electrophiles that have proven to be toxic actually react with GSH to lower the cell content of reduced glutathione (Satoh and Lipton 2007). Additionally, we found that D1 induces SRXN1, which subsequently reduces hyperoxidized PRX2 to native PRX2 (Fig. 5).
Here, we show that the HSF-1/HSE pathway also contributes to protection by at least some types of electrophilic compounds, including D1. The HSF-1/HSE pathway has been considered an endogenous protective mechanism, primarily from ER stress (Morimoto 2008). We show that our novel compound D1 can not only protect from oxidative stress by activating the Nrf2/ARE pathway, but also attenuates ER stress by activating the HSF-1/HSE pathway (Fig. 7).
Fig. 7. Proposed protective mechanism of D1.
D1 transcriptionally activates both the Nrf2/ARE system and the HSF-1/HSE system, inducing phase 2 enzymes and HSPs, respectively, thus protecting neurons against oxidative stress and ER stress. Some of the phase 2 enzymes induced by D1 are capable of reversing the hyperoxidation of PRX2, thus affording neurons significant resistance to oxidative stress. By also inducing HSPs, D1 attenuates protein misfolding, thus ameliorating ER stress.
The HSF-1/HSE system
The ER is a reticulated organelle in which proteins are synthesized and modified for proper folding. Approximately 30% of newly synthesized proteins in normal cells are misfolded, and some of these proteins are refolded to achieve their correct structures. This process of refolding is facilitated by ER chaperones. However, other proteins remain misfolded, accumulate in the ER, and induce ER stress (Bukau et al. 2006; Morimoto 2008). Transcriptional induction of molecular chaperones is governed by the stress-inducible heat-shock transcription factor known as HSF1, which plays a key regulatory role in the response to environmental stress (Bukau et al. 2006; Morimoto 2008). HSF-1-modulating compounds such as HSP90 inhibitors have protective effects against various types of stress including ER stress (Fujimoto et al. 2005; Fujikakake et al. 2008; Trott et al. 2008). Interestingly, radicicol and geladanamycin, HSF-1 activators, protect neurons against oxidative stress (Sano 2001; Xiao et al. 2002). Thus, this HSF-1/HSE system may be protective against oxidative stress in addition to ER stress. In this background, we found in the present study that D1-mediated induction of HSPs plays a role in neuroprotection against ER stress as well as against oxidative stress. While electrophiles have been shown to affect HSPs (Groeger and Freeman 2010), to our knowledge, this is the first report of a pro-electrophilic drug inducing transcription via the HSF-HSE pathway in addition to the Nrf2/ARE pathway.
The Nrf2/ARE system
Several groups, including our own, have reported that the Nrf2/ARE system is the primary target of electrophilic neuroprotective compounds such as NEPP11, CA, TBHQ, celastrol, plumbagin, ceftriaxone, and 3H-1,-dithiole-3-thione (Kraft et al. 2004; Satoh et al. 2006; Satoh and Lipton 2007; Soriano et al. 2008; Trott et al. 2008; Lewerenz et al. 2009; Vargas and Johnson 2009; Son et al. 2010; Wang et al. 2010). Para-hydroquinone type electrophiles, such as TBHQ, can produce oxidative stress themselves via conversion to a quinone, which in turn may be responsible for activation of the Keap1/Nrf2 pathway (Imhoff and Hansen 2010). Because D1 is also a para-hydroquinone-type electrophile, it is possible that D1 might activate the Nrf2 pathway by this mechanism. Prior microarray analyses using CA and TBHQ have suggested that many of the genes induced by electrophiles are phase 2 enzymes (Takahashi et al. 2009; Tamaki et al. 2009; Lee et al. 2003). One of the enzymatic pathways so induced results in an increase in glutathione synthesis (Satoh et al. 2008a, 2008b; Shih et al. 2005). Accordingly, in the present study we show that D1 induced GCLM and xCT, both of which are involved in the regulation of glutathione metabolism. Several groups, including our own, have proposed that prolonged activation of this pathway by electrophilic compounds may be an effective pharmacological approach to help combat neurodegenerative diseases. This proposal was based on the hypothesis that oxidative stress mediates, at least in part, the neuronal damage caused by various types of stress (Satoh and Lipton 2007; Hara and Snyder 2007; Nakamura and Lipton 2007). Several reports, however, have suggested that oxidative stress is not the initial event leading to neurodegeneration. Instead, ER stress, precipitated by the accumulation of specific misfolded proteins, such as β-amyloid, α-synuclein or polyQ-containing peptides, is often considered to be the inciting event (Hara and Snyder 2007; Kim et al. 2008; Bredesen 2008; Nakamura and Lipton 2009). Thus, in order for an electrophilic compound to be maximally effective for neurodegenerative diseases, mechanistic action against ER stress in addition to oxidative stress would be most helpful. Our present discovery with the novel pro-electrophilic drug D1 appears to meet this requirement, and thus further investigation of this and related compounds is warranted.
Supplementary Material
Acknowledgments
The authors thank Prof. Akira Nakai (Yamaguchi University) for providing us with p-tK-hHSP70-luc, Dr. Larry D. Frye for editorial help with the manuscript. This work was supported in part by a grant from the JSPS, Joint Project of Japan–U.S. (2008–2010) and by grants to T.S. from the MEXT Japan, from Grants-in-aid for Scientific Research (No.19500261, 2007–2009; No. 22500282; 2011-2013), and from Grants-in-aid for Scientific Research on Innovative Areas (No. 2011701; 2010-2014). The work was also supported in part from NIH grants R01 EY05477, R01 EY09024, P01 ES016738, P01 HD29587, and P30 NS057096 to S.A.L.
Abbreviations
- ARE
antioxidant response element
- ARPE
apical retinal pigment epithelium
- CA
carnosic acid
- DIV
days in vitro
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethyl sulfoxide
- E17
embryonic day 17
- ER
endoplasmic reticulum
- FCS
fetal calf serum
- FDA
fluorescein diacetate
- GCLM
glutamyl cysteine ligase modifier subunit
- GRP78
78 kDa glucose-regulated protein
- H2O2
hydrogen peroxide
- HO-1
heme oxygenase-1
- HSE
heat-shock factor response element
- HSF-1
heat-shock factor-1
- HSP
heat-shock protein
- HSPH1
heat-shock 105kDa/110kDa protein 1
- HSBP
heat-shock factor-binding protein
- HSE
heat-responsive element
- MAP2
microtubule-associated protein 2
- NQO1
NADPH quinone oxidoreductase 1
- Nrf2
nuclear factor erythroid 2-related factor 2
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NEPP
NEurite outgrowth-Promoting Prostaglandin
- NO
nitric oxide
- PBS (−)
Ca2+, Mg2+ (−)-phosphate-buffered saline
- PRX2
peroxiredoxin2
- RT-PCR
reverse transcription-polymerase chain reaction
- SD
standard deviation
- SDS-PAGE
sodium dodecylsulfate-polyacrylamide gel electrophoresis
- SRXN1
sulfiredoxin1
- TBHQ
tert-butyl hydroquinone
- TRX
thioredoxin
- TM
tunicamycin
- UPR
unfolded protein response
- xCT
Na+-independent cystine-glutamate exchanger
Footnotes
The authors do not have financial or personal conflicts of interest associated with this work.
References
- Bredesen DE. Programmed cell death mechanism in neurological diseases. Curr Mol Med. 2008;8:173–186. doi: 10.2174/156652408784221315. [DOI] [PubMed] [Google Scholar]
- Bukau B, Weisman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Chang TS, Jeong W, Woo HA, Lee SM, Park S, Rhee SG. Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine. J Biol Chem. 2004;279:50994–51001. doi: 10.1074/jbc.M409482200. [DOI] [PubMed] [Google Scholar]
- Fang J, Nakamura T, Cho DH, Gu Z, Lipton SA. S-nitrosylation of peroxiredoxin 2 promotes oxidative stress-induced neuronal cell death in Parkinson’s disease. Proc Natl Acad Sci USA. 2007;104:18742–18747. doi: 10.1073/pnas.0705904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikake N, Nagai Y, Popiel HA, Okamoto Y, Yamaguchi M, Toda T. Heat shock transcription factor 1-activating compounds suppress polyglutamine-induced neurodegeneration through induction of multiple molecular chaperones. J Biol Chem. 2008;283:26188–26197. doi: 10.1074/jbc.M710521200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Takaki E, Hayashi T, Kitaura Y, Tanaka Y, Inouye S, Nakai A. Active HSH-1 significantly suppresses polyglutamine aggregate formation in cellular and mouse models. J Biol Chem. 2005;280:34908–34916. doi: 10.1074/jbc.M506288200. [DOI] [PubMed] [Google Scholar]
- Groeger AL, Freeman BA. Signaling actions of electrophiles: Anti-inflammatory therapeutic candidates. Mol Interven. 2010;10:39–50. doi: 10.1124/mi.10.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara MR, Snyder SH. Cell signaling and neuronal death. Annu Rev Pharmacol Toxicol. 2007;47:117–141. doi: 10.1146/annurev.pharmtox.47.120505.105311. [DOI] [PubMed] [Google Scholar]
- Holmgren A, Lu J. Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem Biophys Res Commun. 2010;396:120–124. doi: 10.1016/j.bbrc.2010.03.083. [DOI] [PubMed] [Google Scholar]
- Imhoff BR, Hansen JM. Tert-butylhydroquinone induces mitochondrial oxidative stress causing Nrf2 activation. Cell Biol Toxicol. 2010;26:541–551. doi: 10.1007/s10565-010-9162-6. [DOI] [PubMed] [Google Scholar]
- Immenschuh S, Baumgart-Vogt E. Peroxiredoxin, oxidative stress and cell proliferation. Antioxid Redox Signal. 2005;7:768–777. doi: 10.1089/ars.2005.7.768. [DOI] [PubMed] [Google Scholar]
- Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- Kim MH, Chung Y, Yang JW, Chung SM, Kwag NH, Yoo JS. Hydrogen peroxide-induced cell death in a human retinal pigment epithelial cell line, ARPE-19. Korean J Opthalmol. 2003;17:19–28. doi: 10.3341/kjo.2003.17.1.19. [DOI] [PubMed] [Google Scholar]
- Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Calkins MJ, Chan K, Kan YW, Johonson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Albrecht P, Tien ML, Henke N, Karumbayaram S, Komblum HI, Wiedau-Pazos M, Schuber D, Maher P, Methner A. Induction of Nrf2 and xCT are involved in the action of neuroprotective antibiotic ceftiaxone in vitro. J Neurochem. 2009;111:332–343. doi: 10.1111/j.1471-4159.2009.06347.x. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Pathologically-activated therapeutics. Nature Rev Neurosci. 2007;8:803–808. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- Lu A, Ran R, Parmentier-Batteur S, Nee SA, Sharp FR. Geldanamycin induces heat shock proteins in brain and protects against focal cerebral ischemia. J Neucem. 2004;81:355–364. doi: 10.1046/j.1471-4159.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- Mandel MN, Patlolla JM, Zheng L, Agbaga MP, Tran JT, Wicker L, Kasus-Jacobi A, Elliott MH, Rao CV, Anderson RE. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic Biol Biol Med. 2009;46:672–679. doi: 10.1016/j.freeradbiomed.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Cheng A. Neurohormetic phytochemicals: low-dose toxins that induce adaptive neuronal stress response. Trends Neurosci. 2006;29:632–639. doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Mercier PA, Winegarden NA, Westwood JT. Human heat shock factor 1 is predominantly a nuclear protein before and after heat stress. J Cell Sci. 1999;112:2765–2774. doi: 10.1242/jcs.112.16.2765. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22:1427–1438. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, Schnaar RL, Coyle JT. Immature cortical neurons are uniquely sensitive to glutamate toxicity by inhibition of cysteine uptake. FASEB J. 1990;4:1624–1633. [PubMed] [Google Scholar]
- Nakai A, Kawazoe Y, Tanabe N, Nagata K, Morimoto RI. The DNA-binding properties of two heat shock factors, HSF1 and HSF3, are induced in the avian erythroblast cell line HD6. Mol Cell Biol. 1995;15:5268–5278. doi: 10.1128/mcb.15.10.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamuara T, Lipton SA. Cell death: protein misfolding and neurodegenerative diseases. Apoptosis. 2009;14:455–468. doi: 10.1007/s10495-008-0301-y. [DOI] [PubMed] [Google Scholar]
- Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, Kang MI, Kobayashi A, Yokoyama S, Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21:689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Jeong W, Chang TS, Woo HA. Sulfiredoxin, the cysteine sulfinic acid reductase specific to 2-Cys peroxiredoxin: its discovery, mechanism of action, and biological significance. Kidney Int Suppl. 2007;106:S3–S8. doi: 10.1038/sj.ki.5002380. [DOI] [PubMed] [Google Scholar]
- Renaldi ME, Boncangra V, Manucha W, Lorenzo AG, Valles PG. The Nrf2-Keap1 cellular defense system pathway and heat shock protein 70 (Hsp70) response. Role in protection against oxidative stress in early neonatal unilateral ureteral obstruction (UUO) Cell Stress Chap. 2011;16:57–68. doi: 10.1007/s12192-010-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara Y, Ishige K, Tsai C, Maher P. Tyrphostins protect neuronal cells from oxidative stress. J Biol Chem. 2002;277:36204–36215. doi: 10.1074/jbc.M203895200. [DOI] [PubMed] [Google Scholar]
- Sakai S, Tomomura Y, Yoshida H, Kawagishi H. Orirbenone D to G phenones from the Tricholoma orirubens mushroom. Biosci Biotechnol Biochem. 2005;69:1630–1632. doi: 10.1271/bbb.69.1630. [DOI] [PubMed] [Google Scholar]
- Sano M. Radicicol and geladanamycin prevent neurotoxic effects of anti-cancer drugs on cultured embryonic sensory neurons. Neuropharmacol. 2001;40:947–953. doi: 10.1016/s0028-3908(01)00018-1. [DOI] [PubMed] [Google Scholar]
- Satoh T, Furuta K, Tomokiyo K, Nakatsuka D, Tanikawa M, Nakanishi M, Miura M, Tanaka S, Koike T, Hatanaka H, Ikuta K, Suzuki M, Watanabe Y. Facilitatory roles of novel compounds designed from cyclopentenone prostaglandins on neurite outgrowth-promoting activities of nerve growth factor. J Neurochem. 2000;75:1092–1102. doi: 10.1046/j.1471-4159.2000.0751092.x. [DOI] [PubMed] [Google Scholar]
- Satoh T, Furuta K, Tomokiyo K, Namura S, Nakatsuka D, Sugie Y, Ishikawa Y, Hatanaka H, Suzuki M, Watanabe Y. Neurotrophic actions of novel compounds designed from cyclopentenone prostaglandins. J Neurochem. 2001;77:50–62. doi: 10.1046/j.1471-4159.2001.t01-1-00229.x. [DOI] [PubMed] [Google Scholar]
- Satoh T, Baba M, Nakatsuka D, Ishikawa Y, Aburatani H, Furuta K, Ishikawa T, Hatanaka H, Suzuki M, Watanabe Y. Role of heme oxygenase-1 protein in the neuroprotective effects by cyclopentenone prostaglandin derivatives as a sustained phase of neuronal survival promoting mechanism under oxidative stress. Eur J Neurosci. 2003;17:2249–2255. doi: 10.1046/j.1460-9568.2003.02688.x. [DOI] [PubMed] [Google Scholar]
- Satoh T, Okamoto S, Cui J, Watanabe Y, Furuta K, Suzuki M, Tohyama K, Lipton SA. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic phase II inducers. Proc Nat Acad Sci, USA. 2006;103:768–773. doi: 10.1073/pnas.0505723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Lipton SA. Redox regulation of neuronal survival by electrophilic compounds. Trends Neurosci. 2007;30:38–45. doi: 10.1016/j.tins.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y, Kitajima C, Cui J, Kamins J, Okamoto S, Shirasawa T, Lipton SA. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of specific cysteines. J Neurochem. 2008a;104:1116–1131. doi: 10.1111/j.1471-4159.2007.05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Izumi M, Inukai Y, Tsutumi Y, Nakayama N, Kosaka K, Kitajima C, Itoh K, Yokoi T, Shirasawa T. Carnosic acid protects neuronal HT22 cells through activation of the antioxidant-responsive element in free carboxylic acid- and catechol hydroxyl moieties-dependent manners. Neurosci Lett. 2008b;434:260–265. doi: 10.1016/j.neulet.2008.01.079. [DOI] [PubMed] [Google Scholar]
- Satoh T, Saitoh S, Hosaka H, Kosaka K. Simple ortho- and para-hydroquinones as neuroprotective compounds against oxidative stress associated with a specific transcriptional activation. Biochem Biophys Res Comm. 2009a;379:537–541. doi: 10.1016/j.bbrc.2008.12.106. [DOI] [PubMed] [Google Scholar]
- Satoh T, Harada N, Hosoya T, Tohyama K, Yamamoto M, Itoh K. Keap1/Nrf2 system regulates neuronal survival as revealed through study of keap1 gene knockout mice. Biochem Biophys Res Comm. 2009b;380:298–302. doi: 10.1016/j.bbrc.2009.01.063. [DOI] [PubMed] [Google Scholar]
- Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci. 2005;25:10321–10335. doi: 10.1523/JNEUROSCI.4014-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son TG, Camandola S, Arumugam TV, Cutler RG, Telljohann RS, Mughal MR, Moore TA, Luo W, Yu QS, Johnson DA, Johnson JA, Greig NH, Mattson MP. Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ishchemia. J Neurochem. 2010;112:1316–1326. doi: 10.1111/j.1471-4159.2009.06552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano FX, Leveille F, Papadia S, Higgins LG, Varley J, Baxter P, Hayes JD, Hardingham GE. Induction of sulfiredoxin expression and reduction of peroxiredoxin hyperoxidtion by neuroprotective Nrf2 activator 3H-1,2-dithiol-3-thione. J Neurochem. 2008;107:533–543. doi: 10.1111/j.1471-4159.2008.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Tabuchi T, Tamaki Y, Kosaka K, Takikawa Y, Satoh T. Carnosic acid and carnosol inhibit adipocyte differentiation in mouse 3T3-L1 cells through induction of phase 2 enzymes and activation of glutathione metabolism. Bioche Biophys Res Commun. 2009;382:549–554. doi: 10.1016/j.bbrc.2009.03.059. [DOI] [PubMed] [Google Scholar]
- Takii R, Inouye S, Fujimoto M, Nakamura T, Shinkawa T, Prakasam R, Tan K, Hayashida N, Ichikawa H, Hai T, Nakai A. Heat shock transcription factor 1 inhibits induction of IL-6 through inducing activation transcription factor 3. J Immunol. 2010;184:1041–1048. doi: 10.4049/jimmunol.0902579. [DOI] [PubMed] [Google Scholar]
- Talalay P. Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors. 2000;12:5–11. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- Tamaki Y, Tabuchi T, Takahashi T, Kosaka K, Satoh T. Activated glutathione metabolism participates in protective effects of carnosic acid against oxidative stress in neuronal HT22 cells. Planta Med. 2010;76:683–688. doi: 10.1055/s-0029-1240622. [DOI] [PubMed] [Google Scholar]
- Tan S, Sagara Y, Liu Y, Maher P, Schubert D. The regulation of reactive oxygen species during programmed cell death. J Cell Biol. 1998;15:1423–1432. doi: 10.1083/jcb.141.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott A, West JD, Klai L, Westerheide SD, Silverman RB, Morimoto RI, Morano KA. Activation of heat shock and antioxidant responses by the natural product celastrol: Transcriptional signatures of a thiol-targeted molecule. Mol Biol Cell. 2008;19:1104–1112. doi: 10.1091/mbc.E07-10-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MR, Johnson JA. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev Mol Med. 2009;11:e17. doi: 10.1017/S1462399409001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada AM, Gelfman CM, Handa JT, Hjelmeland LM. Downregulation of differentiation-specific gene expression by oxidative stress in ARPE-19 cells. Invest Ophthamol Vis Sci. 2001;42:2706–2713. [PubMed] [Google Scholar]
- Wang XJ, Hayes JD, Higgins LJ, Wolf CR, Dinkova-Kostova AT. Activation of the NRF2 signaling pathway by copper-mediated redox cycling of para- and ortho-hydroquinones. Chem Biol. 2010;17:75–85. doi: 10.1016/j.chembiol.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Winyard PG, Moody CJ, Jacob C. Oxidative activation of antioxidant defence. Trends Biochem. 2005;30:453–461. doi: 10.1016/j.tibs.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Xiao N, Callaway CW, Lipinski CA, Hicks SD, DeFranco DB. Geldanamycin provides posttreatment protection against glutamate-induced oxidative toxicity in a mouse hippocampal cell line. J Neurochem. 2002;72:95–101. doi: 10.1046/j.1471-4159.1999.0720095.x. [DOI] [PubMed] [Google Scholar]
- Zareba M, Raciti M, Henry MM, Sarna T, Burke JM. Oxidative stress in ARPE-19 cultures: Do melanosomes cofer cytoprotection? Free Radic Biol Med. 2006;40:87–100. doi: 10.1016/j.freeradbiomed.2005.08.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.