Abstract
Constitutive activation of the PI3K pathway has been implicated in glioblastoma (GBM) pathogenesis. Pharmacologic inhibition can both inhibit tumor survival and downregulate expression of programmed death ligand-1, a protein highly expressed on glioma cells that strongly contributes to cancer immunosuppression. In that manner, PI3K pathway inhibitors can help optimize GBM vaccine immunotherapy. In this review, we describe and assess the potential integration of various classes of PI3K pathway inhibitors into GBM immunotherapy. While early-generation inhibitors have a wide range of immunosuppressive effects that could negate their antitumor potency, further work should better characterize how contemporary inhibitors affect the immune response. This will help determine if these inhibitors are truly a therapeutic avenue with a strong future in GBM immunotherapy.
Keywords: cancer, glioblastoma, immunotherapy, PI3K pathway inhibitor, treatment, vaccine
Augmenting vaccine immunotherapy with PI3K pathway inhibition
Standard of care for glioblastoma (GBM) has not notably progressed since the seminal Stupp protocol was established in 2005 [1]. In the hopes of achieving a more durable antitumor response, much work has been invested towards elucidating the potential of immunotherapy, particularly vaccines, as adjunctive treatments. Vaccine immunotherapy revolves around the induction of a robust tumor and patient-specific immune response. Functional T lymphocytes are therefore paramount to therapeutic success. In GBM, investigated modalities for vaccine delivery have included dendritic cells (DCs), tumor antigen peptides, heat shock proteins, autologous tumor cells and in situ genetic transfer with viral vectors. Promising outcome data from clinical trials have shown minimal side effects, thus underscoring their promise for inclusion into GBM therapy [2–4].
However, vaccines offer only temporary respite from GBM virulence, largely due to tumor-induced suppression of T cell-mediated immunity. By way of MHC downregulation, expansion of Tregs and other modulations of the immune response, GBM tumors perpetuate an ‘immune escape’ phenotype that derails efforts to prolong overall (OS) and progression-free survival (PFS) [5]. Within the wide repertoire of mechanisms for immune evasion, programmed death ligand-1 (PD-L1), a cell surface protein that can be highly expressed on gliomas, has been increasingly validated as a key player in immunosuppression [6]. Regulated by the PI3K pathway [7], PD-L1 binds to programmed death-1 (PD-1) receptors on T lymphocytes and induces T-cell apoptosis [6]. Correspondingly, monocytes and macrophages exposed to PD-L1+ gliomas also upregulate PD-L1 on their cell surfaces and induce T-cell apoptosis [8].
Targeted inhibition of PD-L1 should enhance GBM vaccine immunotherapy by reversing an important aspect of tumor and monocyte-mediated immune evasion. Inhibitors of the PI3K pathway offer the means to achieve this as they can downregulate PD-L1 to preserve the immune response in glioma [7,9]. Initially, these pharmaceuticals rose to prominence for their potency against tumors bearing PI3K mutations [10]. In that manner, PI3K pathway inhibitors form an enticing therapeutic proposition because they can contest tumor growth on two fronts; concurrently inhibiting tumor survival while mitigating mechanisms for immunosuppression.
Given the complex pathobiology of immunosuppression in GBM, it is highly unlikely that PI3K pathway inhibitors will provide a magic bullet solution. Nevertheless, few studies in GBM, if any, have evaluated the prospect of combining PI3K pathway inhibitors with vaccine immunotherapy. Therefore, the primary objective of this review is to bridge this gap in our understanding. The first part of this review will analyze how PI3K pathway inhibitors may potentiate or mollify various immune effector arms that are critical to vaccine therapy. This will be followed by a discussion on optimizing strategies for integrating these agents into the treatment algorithm for GBM.
The PI3K/AKT/mTOR pathway in GBM
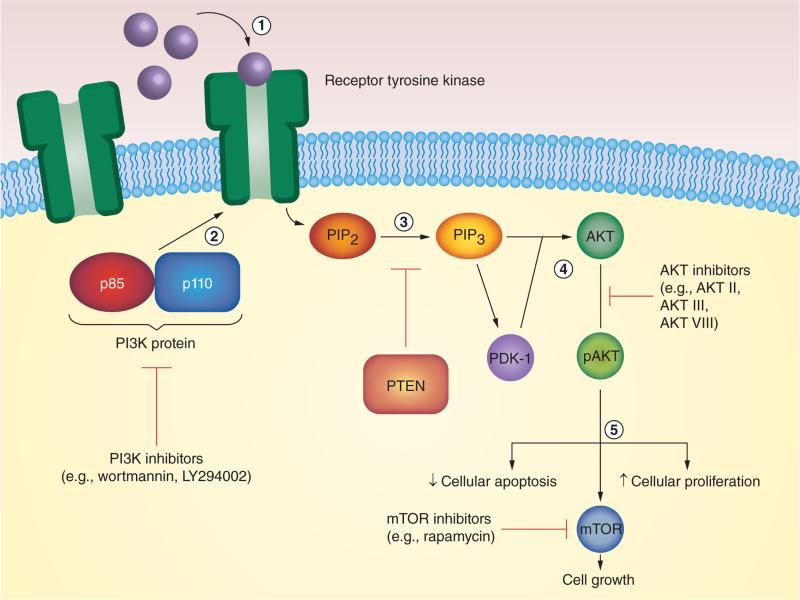
The PI3K/AKT/mTOR pathway is a well-described signaling cascade of intracellular phosphorylation reactions that regulates cell-cycle progression, proliferation and survival [11]. Pathway initiation commences with ligand–receptor interactions that promote phosphorylation of receptor tyrosine kinases or G-protein-coupled receptors. Receptor phosphorylation activates the PI3K proteins, which catalyze the conversion of phosphatidylinositol-4,5-bisphosphate to phosphatidylinositol-3,4,5-trisphosphate. PTEN, a tumor suppressor, serves as a regulatory checkpoint [11]. Subsequently, phosphatidylinositol-3,4,5-trisphosphate recruits phosphoinositide-dependent protein kinase-1 and AKT to the plasma membrane, the former of which phosphorylates the latter. AKT exercises control over the cell cycle by neutralizing mechanisms for apoptosis while stimulating proliferation. AKT further regulates mTOR, a downstream protein that additionally regulates cell growth (Figure 1) [12].
Figure 1. The progression of the PI3K pathway.
(1) First, ligands bind to receptors to promote receptor phosphorylation. (2) The PI3K protein attaches to the receptor, and (3) facilitates the conversion of PIP2 to PIP3. PTEN inhibits this step. (4) PIP3 goes to activate both PDK-1 and AKT, the former of which is required to activate the latter. (5) Phosphorylated AKT subsequently is involved in cell apoptosis and proliferation, and also activates mTOR. PI3K pathway inhibitors act on various steps within this pathway to inhibit pathway signaling. PDK-1: Phosphoinositide-dependent protein kinase-1; PIP2: Phosphatidylinositol-4,5-bisphosphate; PIP3: Phosphatidylinostiol-3,4,5-trisphosphate.
PI3K pathway dysregulation, as caused by mutations in proteins such as PTEN, drastically alters cellular mechanics for replication, thereby facilitating uncontrolled cell growth and proliferation. Head and neck, skin, breast, ovaries, colon and the brain are but a few organ systems that can trace their malignant ontogeny to PI3K mutations [10]. In light of this fact, approximately 30 PI3K pathway inhibitors are under active investigation in clinical cancer trials as of 2012 [10]. Most of these extant inhibitors target at least one out of three constituent proteins of the PI3K pathway: PI3K, AKT or mTOR (Figure 1) [10].
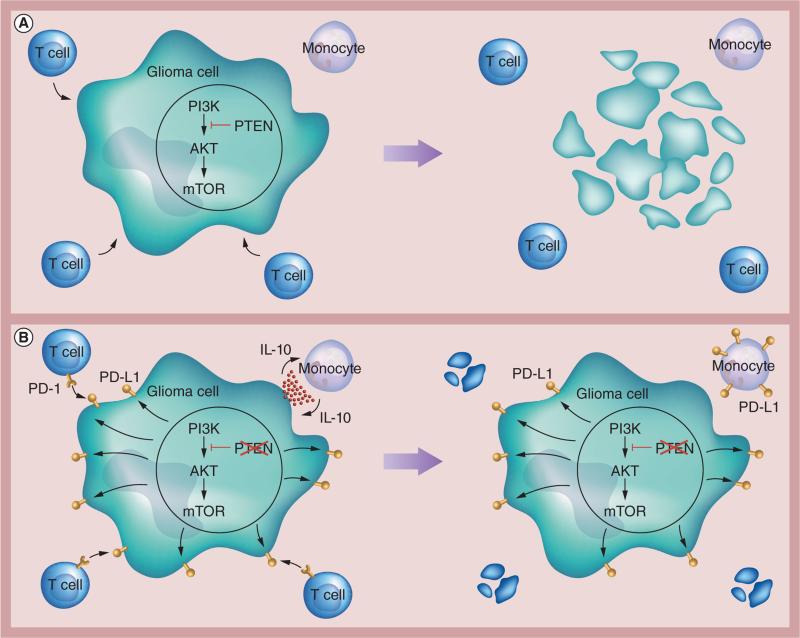
In GBM, the ramifications of aberrant PI3K signaling are far-reaching, as this not only initiates oncogenesis, but also upregulates PD-L1 expression. Increased PD-L1 expression has been characterized in gliomas and their respective monocytes, brain tumor stem-like cells and tumor-infiltrating lymphocytes (TILs) [7,8,13,14], and the degree of PD-L1 expression correlates with tumor malignancy [13]. Consequently, when expressed on gliomas, PD-L1 induces cytotoxicity in lymphocytes and inhibits the secretion of proinflammatory cytokines such as IFN-γ [7,15]. In turn, PD-L1+ gliomas secrete soluble factors that induce monocytes to release IL-10 in autocrine fashion, which in turn stimulates monocytic expression of PD-L1 (Figure 2) [8].
Figure 2. PI3K pathway and programmed death ligand-1 expression.
(A) In a normal glioma cell with an intact PI3K pathway, T cells are able to bind antigenically and induce cellular apoptosis. (B) In a glioma cell with aberrant PI3K pathway signaling, PD-L1 expression is upregulated. This has two consequences: T cells bind through the PD-1 receptor and undergo apoptosis; and glioma cells secrete soluble factors (in red), which are taken up by monocytes. Monocytes secrete IL-10, which then upregulates PD-L1 expression on their cell surface. PD-1: Programmed death-1; PD-L1: Programmed death ligand-1.
Clinical trials for five PI3K pathway inhibitors – BKM120 (pan-PI3K), XL765 (dual PI3K/ mTOR), GDC-0084 (dual PI3K/mTOR), temsirolimus (mTOR), and everolimus (mTOR) – are currently ongoing for GBM, and results have only been published for temsirolimus and everolimus [10]. Treatment outcomes have been largely equivocal (Tables 1 & 2) [16–24]. In two Phase II studies in which recurrent GBM patients were treated with temsirolimus, rates of 6-month PFS were just 2.4 and 7.8%, respectively [16,17]. In a pilot study for everolimus combined with gefitinib, an inhibitor of the EGF receptor, 6-month PFS was approximately 5% [18]. Comparatively, these rates of 6-month PFS are worse than that of the antiangiogenic agent bevacizumab (Avastin®; Genetech Inc., CA, USA), which was approved in May 2009 for use in recurrent GBM [25]. One Phase II study by Hainsworth et al. did demonstrate a relatively high rate of 6-month PFS (73%) with everolimus, but this was probably due to the fact that all the patients in their cohort had additionally been treated with bevacizumab [20].
Table 1.
Select clinical trials of temsirolimus (CCI-779) in glioblastoma.
| Study (year) | Phase | Dose (n) | PR (%) | OS | PFS | Ref. |
|---|---|---|---|---|---|---|
| Chang et al. (2005) | II | 170 mg/week iv. (n = 16); 250 mg/week iv. (n = 27) |
4.9 | Median: N/A; 6-month: N/A |
Median: 9 weeks; 6-month: 2.4% |
[16] |
| Galanis et al. (2005) | II | 250 mg/week iv. (n = 65) | 0 | Median: 4.4 months; 6-month: 7.8% |
Median: 2.3 months; 6-month: 7.8% |
[17] |
| Lassen et al. (2013) | II | 25 mg/week iv. (n = 13) | 0 | Median: 15 weeks; 6-month: N/A |
Median: 8 weeks; 6-month: N/A |
[23] |
| Lee et al. (2012) | I/II | 25 mg/week iv. (n = 18) | 11.7 | Median: N /A; 6-month: N/A |
Median: 8 weeks; 6-month: 0% |
[24] |
iv.: Intravenous; N/A: Not assessed; OS: Overall survival; PFS: Progression-free survival; PR: Partial response.
Table 2.
Select clinical trials of everolimus (RAD-001) in glioblastoma.
| Study (year) | Phase | Dose (n) | PR (%) | OS | PFS | Ref. |
|---|---|---|---|---|---|---|
| Kreisl et al. (2009) | Pilot | DE PO (n = 2); 70 mg/week PO (n = 20) |
14 | Median: 5.8 months; 6-month: N/A |
Median: 2.6 months; 6-month: 4.5% |
[18] |
| Chinnaiyan et al. (2013) | I | 2.5 mg/day PO (n = 8); 5 mg /day PO (n = 9); 10 mg/day PO (n = 8) |
N/A | Median: N/A; 6-month: 100% |
Median: N/A; 6-month: N/A |
[19] |
| Sarkaria et al. (2011) | I | 30 mg/week PO (n = 6); 50 mg/week PO (n = 6); 70 mg/week PO (n = 6) |
11 | Median: N/A; 6-month: N/A |
Median: N/A; 6-month: N/A |
[22] |
| Mason et al. (2012) | I | 2.5 mg/day PO (n = 5); 5 mg/day PO (n = 15); 10 mg/day PO (n = 12) |
11 | Median: N/A; 6-month: N/A |
Median: N/A; 6-month: N/A |
[21] |
| Hainsworth et al. (2012) | II | 10 mg/day PO (n = 57) | 61 | Median: 13.9 months; 6-month: N/A |
Median: 11.3 months; 6-month: 73% |
[20] |
DE: Dose escalation; N/A: Not assessed; OS: Overall survival; PFS: Progression-free survival; PO: Per oral; PR: Partial response.
While these results may appear to discourage the application of PI3K pathway inhibitors in GBM, such conclusions cannot readily be drawn based on temsirolimus and everolimus alone for several reasons. From a broad perspective, treatment with PI3K pathway inhibitors poses many challenges including difficul-ties in optimizing clinical trial design, in determining appropriate therapeutic indices and in achieving sufficient drug delivery through the blood–brain barrier into the tumor microenvironment [26]. Perhaps more importantly, temsirolimus and everolimus are mTOR (not PI3K or AKT) inhibitors, and resistance to mTOR inhibition has been partially attributed to increased PI3K activation secondary to loss of negative feedback loops [27,28]. Furthermore, the PI3K pathway regulates many intracellular processes exclusive of mTOR activation such as the expression of hypoxia-inducible factor, forkhead box-o transcription factors, glycogen synthase kinase 3 and many others, some of which may become protumorigenic with dysregulation [27–32]. In reflection of these factors, combination PI3K and mTOR inhibition has sometimes been proposed as a more efficacious therapeutic strategy [27,33].
However, an additional potential explanation for the failure to achieve desired end points with temsirolimus and everolimus may be the inhibitory effects of PI3K pathway inhibitors on multiple facets of normal T-cell function. Naturally, this would erect considerable barriers towards the use of these pharmaceuticals with vaccine immunotherapy. For reference in the following sections, wortmannin and LY294002 are the earliest and most extensively investigated iterations of PI3K-specific inhibitors [11]. AKT inhibitors either compete for the catalytic ATP binding site of AKT or block phosphorylation of AKT in allosteric fashion [12]. Rapamycin, an antibiotic isolated from Streptomyces hygroscopicus, is the prototypical mTOR inhibitor that gave rise to analogs such as RAD001 (everolimus), CCI-779 (temsirolimus), and AP23574 (deforolimus) [34].
PI3K pathway inhibitors & interleukins
Interleukins are a broad family of lymphokines that plays an important role in the differentiation of naive CD4+ and CD8+ T cells. When antigen-presenting cells (APCs) bind and stimulate naive T cells with the appropriate MHC, the resulting interactive release of specific interleukins and other lymphokines determine the ultimate fate of the cell. CD4+ T cells can undergo lineage differentiation into a Th1 or Th2 phenotype. The former is critical for fighting against intracellular pathogens; the latter specifically targets extracellular pathogens. By contrast, naive CD8+ T cells differentiate into mature cytotoxic T lymphocytes (CTLs) that trigger tumor cell death. The Th1 and Th2 phenotypes each carry different implications for GBM immunotherapy. Th1 favors CD8+ T cell-mediated targeting and cytotoxic destruction of tumor cells. On the other hand, Th2 orchestrates humoral immunity [35]. Importantly, GBM tumors tend to skew patient cell-mediated immunity to a Th2 phenotype, thereby exhausting the immunological reserve of functional CD8+ cells [36].
IL-2
Both Th1 and Th2 differentiation rely on IL-2, which upregulates lymphocyte expression of receptors for IL-12 and IL-4, respectively. In addition, IL-2 stimulates the differentiation, proliferation, activation-induced cell death and cytolytic activity of CTLs [35]. Cellular processes mediated by IL-2, however, are inhibited in GBM. Early studies revealed that the T lymphocytes of GBM patients are unable to produce sufficient quantities of IL-2 or one of its receptor subunits (IL-2Rα) compared with healthy controls [37]. Furthermore, PD-L1 expression on gliomas is a known inhibitor of IL-2 production, thereby imposing an additional layer of immunosuppression apart from induction of T-cell apoptosis [38].
In the context of vaccine therapy, preservation of the IL-2 response leads to improved antitumor outcomes. Jie and colleagues recently published data from a clinical trial demonstrating that treatment with autologous DC vaccine increases the circulating pool of T lymphocytes and the release of cytokines (IL-2, IL-12 and IFN-γ), thus suggesting that vaccines can cultivate an immune profile favorable to combating tumor growth [39]. Notably, patients receiving vaccine also had improved tumor control rates, higher quality of life and prolonged survival [39]. More direct efforts to treat with IL-2 supplementation have further highlighted the importance of IL-2 in mounting an immune response against glioma [40].
PI3K pathway inhibitors impair either IL-2 production or cellular responsiveness to IL-2. Wortmannin and LY294002 both elicit dose-dependent reductions in IL-2 by inhibiting signaling cascades responsible for IL-2 production in addition to conferring resistance to IL-2 stimulation [41–43]. Treatment of T cells with rapamycin has been shown to cause post-transcriptional inhibition of cyclin production in response to IL-2, thereby resulting in cell-cycle arrest [43]. With PI3K pathway inhibitors of all classes, impairment of IL-2-mediated reactions ultimately leads to inhibition of T-cell proliferation as well as promotion of T-cell apoptosis [9].
IL-12
Stimulated APCs such as monocytes, macrophages and DCs endogenously produce IL-12 to drive maturation of naive CD4+ cells into Th1 cells. Among its many roles, IL-12 also activates immune cell subsets including NK cells, lymphokine-activated killer cells and CTLs [44]. In contrast to IL-2, IL-12 is a proinflammatory cytokine that is upregulated by PI3K pathway inhibition. In PI3K-knockout mice, stimulated splenic DCs produce dramatically higher concentrations of IL-12. Interestingly, when these mice were challenged immunologically with a parasitic infection (Leishmaniasis major), knock-out mice exhibited a cytokine skew towards a Th1 phenotype, with functionally enhanced resistance against parasitic infection [45]. In a similar vein, inhibitor treatment with wortmannin, LY294002, AKT II and rapamycin have all been shown to amplify IL-12 production [45–47].
In glial neoplasms, levels of circulating IL-12 are significantly lower compared with matched controls [48]. Furthermore, IL-12 administration has shown potent antitumor effects, which raises the possibility of utilizing PI3K pathway inhibitors to boost this response. Preclinical studies combining IL-12 with DC vaccines initially highlighted their therapeutic promise, with treated mice surviving longer than their control counterparts and having significantly higher CTL activity [49]. In a subsequent clinical trial conducted by Kikuchi et al., 22 patients received DC vaccines with IL-12. Five patients had some clinical response to the tumor, two had stable disease post-treatment and specimens stained highly positive for CD8+ T cells [50]. Furthermore, patients receiving DC vaccine therapy alone demonstrate elevations in serum IL-12, with translational benefits of improved tumor control and survival [39]. However, the cytolytic activity of autologous peripheral blood lymphocytes against glioma cells varied greatly between patients, and some patients had worse CTL function after vaccination [50]. In addition, Akasaki et al. showed that in mice with CD8+ T-cell depletion, the antitumor effects of DC vaccines plus IL-12 were insignificant, implying that the CD8+ population is critical for DC vaccines and IL-12 to function properly [49].
IL-4, IL-10 & IL-13
IL-4 is a lymphokine primarily involved in driving Th2 differentiation, and these Th2 cells subsequently generate IL-4 and IL-13 via positive feedback [35]. Bias towards Th2 is often evident in GBM patients, as secretion of cytokines such as IL-4 and IL-10 by peripheral blood mononuclear cells (PBMCs) are significantly higher compared with controls. By contrast, Th1 cytokine secretion is reduced [48,51]. Furthermore, IL-4 production levels are even higher in TILs compared with autologous PBMCs or TILs from patients with other brain tumors [52]. However, while IL-4 production certainly diverts resources away from Th1-mediated cytotoxicity against tumor cells, the functional significance is not entirely clear, as evidence suggests that IL-4 secretion is not intrinsically detrimental. Studies have shown that certain polymorphisms of the IL-4 receptor (rs1805016) may actually have a protective effect and lead to better long-term survival in glioma [53]. One possible reason for this may be that such patients have tumors that are resistant to cellular processes mediated by IL-4.
While IL-4 supplementation has been administered in conjunction with GBM vaccine immuno-therapy, the long-term benefits of this approach are ambiguous. Okada et al. conducted a clinical trial in which glioma patients were divided into two separate cohorts [54]. In the first cohort, patients received either surgery alone or surgery followed by vaccination with autologous fibroblasts that were virally transfected to express IL-4. Although vaccinated patients demonstrated a positive response in IFN-γ levels, only two patients received this therapy. In the second cohort, patients received either surgery with radiation alone versus with adjuvant DC vaccines loaded with tumor lysates and IL-4-transfected fibroblasts. Patients failed to elicit a positive IFN-γ response and treatment failed to prolong PFS [54].
Much like IL-4, IL-13 can be overexpressed in GBM. More specifically, both tumor cells and glioma stem-like cancer-initiating cells upregulate expression of the IL-13 receptor α2 (IL13Rα2), which is not typically found in normal brain tissue. Brown et al. genetically modified a T-cell line to express IL13-zetakine, a receptor that binds to IL13Rα2 and initiates cytolytic destruction [55]. Importantly, IL-13-zetakine+ T cells predisposed glioma stem-like cancer-initiating cells to CTL-mediated tumor cell death in vitro and arrested their ability to initiate tumor growth in vivo [55].
In porcine endothelial cells, both IL-4 and IL-13 were found to regulate AKT, and cytokine activation protected these cells from complement-induced cell death. This protective effect was abrogated upon treatment with LY294002 or when cells were engineered to have functional knockouts of the AKT protein [56]. In colorectal cancer cell lines, LY294002 inhibited the migratory capacity of cells and their survival despite IL-13-mediated stimulation of IL13Rα2 [57]. Although these results were derived from nonimmune cells, analogous studies performed on immune cells have corroborated PI3K regulation of IL-4, IL-10 and IL-13. For example, CAL-101, an isoform-specific PI3K inhibitor, inhibited the ability of normal NK and T cells to secrete IL-4. Interestingly, cell survival was unaffected [58]. LY294002 acts as a downstream antagonist of IL-4 activation in murine microglial cells [59] and reduces IL-4-promoting activation in Th2 murine cells [60]. When LY294002 was administered in a murine model for asthma, treatment reduced immunologic production of IL-13 on broncheoalveolar lavage [61]. Similarly, when DCs are incubated with PI3K pathway inhibitors, IL-10 secretion is considerably lower [62].
Summary: interleukins
While IL-2 mediates both Th1 and Th2 differentiation, IL-12 and IL-4/IL-10/IL-13 primarily assist with Th1 and Th2 differentiation, respectively. The influence of PI3K pathway inhibitors on each of the discussed inter-leukins is summarized in Table 3. On the one hand, their inhibition of IL-4-, IL-10- and IL-13-mediated Th2 reactions strongly advocate for their use. Given the natural tendency of GBM to adopt a Th2 phenotype [36], PI3K pathway inhibitors may be capable of shifting the homeostatic balance in favor of cytolytic tumor cell destruction. On the other hand, IL-2 inhibition merits equal consideration, as PI3K pathway inhibitors may aggravate baseline tumor-mediated inhibition of IL-2 in addition to depleting the reserve of healthy CD8+ T cells required for vaccine therapy, ultimately contributing to worse clinical outcomes.
Table 3.
Impact of PI3K inhibitors on interleukins.
| Interleukin | Physiologic function | Th skew | Phenotype in GBM | Phenotype with PI3K inhibition | Overall effect of PI3K inhibitors | Ref. |
|---|---|---|---|---|---|---|
| IL-2 | Proinflammatory | Th1, Th2 | ↓ IL-2; ↓ IL-2Rα | ↓ IL-2; ↓ Response to IL-2; ↓ T-cell proliferation; ↓ T-cell apoptosis | ↓ Antitumor response | [9,27,29-35] |
| IL-4 | Proinflammatory | Th2 | ↑ IL-4 | ↓ IL-4; ↓ Response to IL-4 | ↑ Antitumor response | [27,43-46,48,50-52] |
| IL-10 | Prinflammatory | Th2 | ↑ IL-10 | ↓ IL-10 | ↑ Antitumor response | [40,43,54] |
| IL-12 | Proinflammatory | Th1 | ↓ IL-12 | ↑ IL-12 | ↑ Antitumor response | [31,36-42] |
| IL-13 | Proinflammatory | Th2 | ↑ IL-13; ↑ Mutated receptor (IL3Rα2) | ↓ IL-13 | ↑ Antitumor response | [47-49,53] |
GBM: Glioblastoma; IL13R2: IL-13 receptor α2; IL-2Rα: IL-2 receptor α.
An interesting point could be made, however, regarding IL-12 secretion. Despite IL-2's regulatory capacity over IL-12, PI3K pathway inhibitors actually favor IL-12 release. In theory, PI3K pathway inhibitors should therefore boost patient immunity by enhancing APC and CTL function, which could lead to synergistic effects with DC vaccines. However, it remains unclear whether inhibitor-induced cytotoxicity secondary to IL-2 inhibition supersedes the benefits incurred from IL-12 hyper-secretion. Furthermore, the primary work demonstrating enhanced IL-12 secretion was conducted using murine models of melanoma, colon and lung cancer [62]. Similar studies have yet to be conducted using murine gliomas and it may be that GBMs may present with different results. Functional assays of T-cell survival, proliferation and apoptosis using in vivo glioma models treated with DCs and/or IL-12 with a panel of different PI3K pathway inhibitors must be performed to: validate potency in GBM; and select the optimum PI3K pathway inhibitor with marginal effects on IL-2. Such PI3K pathway inhibitors would represent the best chances for becoming incorporated as adjuncts to GBM immunotherapy.
PI3K pathway inhibitors & interferons
Interferons are immunologic proteins that can be subdivided into three classes: type I (IFN-α and IFN-β); type II (IFN-γ); and type III (IFN-γ). Overall, interferons adopt a proinflammatory role by opposing viral replication and tumor growth, and by mediating cellular chemotaxis. All cells, to some extent, secrete type I interferons in response to a foreign body. Type II interferons are predominantly generated and secreted by lymphocytes and immune cells [63].
Type I interferons
IFN-α is responsible for both Th1 and Th2 differentiation, and works by upregulating the transcript and protein expression of TGF- and IL-4 [64]. By suppressing IFN-γ production while upregulating that of IL-4 and IL-10, IFN-β generally favors Th2 differentiation [65]. Gliomas are known to bear mutational deletions of the IFN-α gene, and high-grade gliomas trend towards less IFN-α gene expression [66,67]. This perhaps offers an explanation as to why attempts to administer IFN-α so far have failed to prompt improvements in outcomes [68]. By contrast, IFN-β treatment has been met with slightly greater success. In a study published by Motomura et al., 39 patients received adjuvant IFN-β following maximal safe resection with concurrent temozolomide [69]. Median OS of the IFN-β and standard treatment groups were 19.9 and 12.7 months, respectively. Importantly, OS was also prolonged among the patient cohort with unmethylated MGMT promoter genes, which normally confers resistance to temozolomide. Median OS for IFN-β with temozolomide was 17.2 versus 12.5 months in the group receiving temozolomide alone [69].
PI3K pathway inhibitors inhibit the production of both type I interferons. Recent data from Guiducci et al. showed that PI3K inhibition in mouse DCs and macrophages decreases IFN-α production in a dose-dependent fashion [70]. In turn, LY294002 reduces the activation of IFN-β reporter genes within murine macrophages, subsequently inhibiting its production [71]. Of interest, Li et al. reported a link between the PI3K pathway and IFN-β-mediated autophagy of established glioma cell lines [72]. Mechanistically, IFN-β decreased the phosphorylation activation of AKT and mTOR, leading the authors to conclude that: the normal PI3K pathway inhibits autophagy; and IFN-β increases autophagy via PI3K pathway inhibition [72].
Type II interferons
IFN-γ is a proinflammatory cytokine generated by NK cells in innate immunity and by T lymphocytes in adaptive immunity, and is responsible for conferring cytotoxic activity to these cells [63]. IFN-γ production is influenced by other lymphokines such as IL-12, and upregulates production of the IL-12 receptor as well [44,73]. Compared with age-matched controls, T cells in GBM patients are impaired in their ability to secrete IFN-γ, regardless of whether stimulatory factors are present [36,51,74]. This impairment is profound among patients with recurrent GBM as well [36]. Importantly for immunotherapy, PD-L1 further exacerbates lymphocytic expression of IFN-γ [38]. Paradoxically, however, treatment of GBM cells with IFN-γ leads to even higher expression levels of PD-L1 on the cell surface [15,75]. This perhaps offers a mechanistic explanation behind why patients treated with IFN-γ in clinical trials have failed to consistently demonstrate a clear benefit in survival [76].
Expression of IFN-γ is regulated in part by the PI3K pathway. In genetically engineered PI3K-knockout mice, NK cells cannot adequately generate cytokines or chemokines. However, their intrinsic cytotoxic functions against tumor cells are preserved [77]. Treatment with PI3K pathway inhibitors replicates these findings. Herman et al. treated leukemic cells with CAL-101, an isoform-specific PI3K inhibitor, and found that the drug induced apoptosis of cancer cells [58]. Survival of normal NK and T cells remained intact, although both populations were significantly inhibited in their ability to produce and secrete IFN-γ and other cytokines [58]. Similarly, treating human peripheral blood NK cells with LY294002 and treating human lung and colon cancer lines with P2281, an mTOR inhibitor, decreased IFN-γ production [78,79]. Human PBMCs also reduce IFN-γ secretion in response to wortmannin, LY294002 and rapamycin [9].
Summary: interferons
In response to PI3K pathway inhibitors, immune cells thus decrease production of type I and type II interferons, which stymies the immune response against tumors (Table 4). PI3K-mediated inhibition of glioma autophagy certainly raises an interesting point, as PI3K pathway inhibition should then enhance tumor cell death. However, given that these drugs also concurrently reduce IFN-β production, GBM patients may not have adequate levels of circulating IFN-β to induce sufficient autophagy. Nevertheless, prospective clinical trials designed to treat patients simultaneously with PI3K pathway inhibitors and incremental doses of IFN-β may reveal a tipping point beyond which IFN-β supplementation can override the lack of inherent production.
Table 4.
Overall impact of PI3K inhibitors on interferons.
| Interferons | Physiologic function | Th skew | Phenotype in GBM | Phenotype with PI3K inhibition | Overall effect of PI3K inhibitors | Ref. |
|---|---|---|---|---|---|---|
| IFN-α | Proinflammatory | Th1, Th2 | ↓ IFN-α | ↓ IFN-α | ↓ Antitumor response | [56,58,59,62] |
| IFN-β | Proinflammatory | Th2 | ↓ IFN-β | ↓ IFN-β; ↑ Glioma autophagy |
↓ Antitumor response | [57,61,63,64] |
| IFN-γ | Proinflammatory | Th1 | ↓ IFN-γ | ↓ IFN-γ | ↓ Antitumor response | [9,15,28,30,36,43,50,55,65-71] |
GBM: Glioblastoma.
Encouraging results from vaccine studies have revealed that patients are able to dramatically increase their production of IFN-γ, and these patients clearly demonstrate prolonged median survival [80]. However, utilization of PI3K pathway inhibitors in conjunction with vaccines could represent a double-edged sword. By reducing the ability of immune cells to generate IFN-γ, PI3K pathway inhibitors repress their cytotoxic activity, which is critical to immunologic identification and targeting of tumor cells. Conversely, this inhibition could prove a blessing in disguise by precluding super-induction of PD-L1 expression.
PI3K pathway inhibitors & other immune effectors
Tregs
Tregs constitute a subset of anti-inflammatory CD4+ T cells that physiologically suppress the immune response. Most immune cells, including B/T lymphocytes, NK cells and APCs, are ubiquitously affected by Tregs, which systemically dampen the secretion of proinflammatory cytokines and lend towards promulgating the Th2 phenotype [81]. In GBM tumors, Tregs can be widespread in immune cell infiltrates and their prevalence increases in proportion to tumor malignancy [14,81]. As a result, Tregs represent one of the major obstacles to attaining long-term remission with GBM immuno-therapy. While Tregs themselves are not inherently detrimental, they pose problems when they gain selective survival advantage over other T cells, which allows them to induce immunosuppression without impunity.
Preclinical findings derived from in vivo antagonization of Tregs have highlighted the potential impact of assimilating this treatment strategy into GBM immunotherapy. Fecci et al. utilized a systemic anti-CD25 (PC61) antibody to block the function of Tregs in a murine model [82]. Other T-cell populations were left relatively untouched and, importantly, these cells retained their proliferative capacity and ability to secrete IFN-γ [82]. Using the same PC61 antibody, Curtin et al. additionally prolonged the survival of mice with GBM [83]. Others have alternatively targeted Treg development and maturation, which is contingent on cytokines such as IL-2 and TGF-β [35,84]. Recently, a small pilot study evaluated the benefits of infusing a monoclonal antibody against the IL-2Rα receptor (daclizumab) in GBM patients, and the authors concluded that daclizumab favors Treg depletion [85].
PI3K pathway inhibitors do not directly impact Treg proliferation. However, they increase the relative proportion of Tregs among circulating lymphocytes, specifically by inhibiting survival of other T cells. When Sauer et al. treated murine T cells with LY294002, Akti-1/2 or rapamycin, they observed a significant increase in Foxp3 expression, a specific marker for functional Tregs [86]. Interestingly, they also discovered that the PI3K pathway may play a more pivotal role in Treg proliferation than TGF-β does [86]. In another study, Qu et al. treated mice with rapamycin and revealed diminishment of CD4+/CD8+ T cells, with Treg preservation within the spleen and thymus [87]. When rapamycin-treated Tregs were transplanted into a diabetic murine model of pancreatic islet transplantation, the majority of mice did not undergo immune rejection postoperatively versus none of the control mice [88].
TGF-β
The overarching function of TGF-β is to inhibit both the innate and adaptive arms of the immune response. In innate immunity, TGF-β subdues the transcriptional capacity of NK cells to produce IFN-γ and subsequently instigate Th1 differentiation, impairs most basic functions of DCs while enhancing their stimula-tory effects on Tregs, converts macrophages/monocytes to the immunosuppressive M2 phenotype and nullifies neutrophilic cytotoxicity. In adaptive immunity, TGF-β impedes CTL survival and cytoxicity, trends CD4+ T-cell homeostasis towards Th2, and induces Treg formation [84]. Elevated levels of TGF-β – believed to derive from the glioma itself or by microglia – have been well described within the GBM literature, as has the extensive immunosuppression that ensues. Beyond its effects on the immune system, however, TGF-β directly promotes GBM tumor growth through multimodal mechanisms, running the gamut from stimulating angiogenesis to facilitating invasion. Pursuant to its eminent involvement in GBM pathogenicity, several clinical trials have attempted pharmacologic inhibition of TGF-β, to varying degrees of success [89].
Like most other cytokines, TGF-β expression is mediated by the PI3K pathway. Utilizing phosphorylation of Smad2 as a marker for TGF-β signaling, Sauer et al. found that pSmad2 expression levels decrease when T cells are treated with LY294002 or rapamycin [86]. When Marshall and colleagues extracted pulsed DCs and incubated them with an assortment of PI3K pathway inhibitors, they showed that treated DCs were significantly impaired in their ability to secrete TGF-β and IL-10 [62]. By contrast, the T-cell response was robust for IFN-γ and IL-2 secretion, thereby creating an immune profile favorable for antitumor immunity. Improvements in functional end points were seen as well, with prolonged survival, reductions in tumor growth rates and resistance to tumor rechallenge observed in a murine model of melanoma [62].
Summary: other immune effectors
In brief, PI3K pathway inhibitors asymmetrically skew the immune microenvironment towards selective propagation of Tregs at the expense of other immune cell subsets (Table 5). On the other hand, their inhibitory effect on TGF-β secretion may not only counteract Treg expansion, but also hinder other facets of GBM pathogenicity. While in theory, PI3K pathway inhibitors may thus enhance the antitumor response by inhibiting TGF-β-mediated survival of Tregs, this is unlikely to be the case, as an intact PI3K pathway is more important than TGF-β with respect to Treg regulation [86]. As a result, PI3K pathway inhibitors could pose considerable risk to patient safety by imposing a ‘double hit’ through inhibition of normal T-cell function and bolstering Treg activity.
Table 5.
Overall impact of PI3K inhibitors on other immune effectors.
| Immune effectors | Physiologic function | Th skew | Phenotype in GBM | Phenotype with PI3K inhibition | Overall effect of PI3K inhibitors | Ref. |
|---|---|---|---|---|---|---|
| Tregs | Anti-inflammatory | Th2 | ↑ Survival advantage | ↑ Survival advantage | ↓ Antitumor response | [14,27,73-80] |
| TGF-β | Anti-inflammatory | Th2 | ↑ TGF-β | ↓ TGF-β | ↑ Antitumor response | [54,76,78,81] |
GBM: Glioblastoma.
Future perspective
Concerns of pronounced immunosuppression with the use of PI3K pathway inhibitors significantly cautions against their use not only as specific adjuncts to immuno-therapy, but also as broad therapeutics for glioma. Their anti-inflammatory effects on the secretion of interleukins and interferons as well as their augmentation of Treg activity call into question the utility of continuing the scientific and clinical pursuit of PI3K pathway inhibitors. The future of GBM immunotherapy will be predicated on the development of innovative strategies for counteracting mechanisms of GBM-mediated immunosuppression, and the evidence in favor of utilizing PI3K pathway inhibitors has not been auspicious. Considering that the PI3K pathway closely regulates essential functions of immune cells [90], the observed anti-inflammatory effects are perhaps unsurprising. Ironically, these drugs may thus exacerbate the very problem they are expected to address, as PI3K pathway inhibitors collectively induce tumor cell toxicity but this toxicity extends to immune cells as well, and this poses a significant drawback to their use in immunotherapy. The ideal inhibitor must promote tumor cell apoptosis while preserving the immune response, and few inhibitors successfully strike this balance. Nevertheless, several mitigating factors and alternative approaches must be considered prior to ruling out any potential involvement that these drugs may have in the treatment of GBM.
Modern PI3K pathway inhibitors
Although evidence overwhelmingly substantiates the immunosuppressive capacity of PI3K pathway inhibitors, the literature is somewhat outdated. Several of the agents described, such as wortmannin and LY294002, are considered nonspecific with far-reaching effects extending beyond PI3K inhibition [10], which limits the conclusions that can be drawn from these drugs. The majority of the PI3K pathway inhibitors detailed throughout this review have been supplanted by more novel and selective agents, and only these contemporary drugs have established the credibility for investigation in clinical trials [10]. Characterization of their immunosuppressive effects, if any, remains largely unexplored. However, existing findings from the few studies addressing this issue indicate that the degree of immunosuppression may vary according to the inhibitor. The dual PI3K/mTOR inhibitor PI-103, for example, unexpectedly facilitated melanoma growth in vivo by depleting the viable thymocyte population by 70% [91]. By contrast, the dual mTORC1/mTORC2 inhibitor AZD8055 preserved the CTL response in an in vivo model of metastatic renal carcinoma to the liver. Treated mice exhibited marked infiltration of CD8+ T cells, macrophages and DCs within the liver and additionally possessed the highest proportion of activated CD8+ T cells and NK cells, as evidenced by adequate secretion of Th1 cytokines such as IL-12 and IFN-γ [92]. In that regard, delineating the unique interactions between each PI3K pathway inhibitor and the immune system, specifically in preclinical models of glioma, will better equip us to identify and select those with minimal immunologic toxicity.
Immuno-preservative inhibitors
Honokiol, a bioactive herbal compound isolated from Magnolia officinalis [93], induces tumor cell apoptosis [94], inhibits angiogenesis [95] and even downregulates tumor adhesion and invasion [96]. Although its precise biochemical activity remains elusive, a growing body of evidence suggests that it functions as a PI3K pathway inhibitor [9]. Honokiol manifests both pro- and anti-inflammatory properties, depending on the dose. At lower doses, honokiol exerts low cytotoxicity against murine macrophage and human promonocytic cells; at higher doses, greater cytotoxicity is detected. However, honokiol induces baseline immunosuppression regardless of dose, as it inhibits the proliferation of murine lymphocytes and CD8+ cells while reducing the production of proinflammatory cytokines such as TNF-α, PGE2 and NO [97].
Despite this, honokiol-induced immunosuppression may be tolerable. Although lymphocyte proliferation and survival are somewhat impaired, they do not appear to be exhausted to such a degree that an immune response cannot be mounted. We have previously shown that honokiol, compared with other PI3K pathway inhibitors including wortmannin, LY294002, AKT III and rapamycin, exerts the least inhibitory influence over T-cell proliferation and apoptosis [9]. At low doses (20 μmol/l), honokiol treatment of lymphocytes extracted from GBM patients did not induce significant cytotoxicity, although proliferation and survival were not as vigorous as that in the untreated control group. In contrast to TNF-α, PGE2 and NO, which are involved in innate immunity, treated cells also maintained production of cytokines such as IFN-γ and IL-17, which are involved in adaptive immunity [9].
Harnessing the proinflammatory properties of this drug can thus provide an option for augmenting vaccine therapy. However, it must be noted that the level of evidence for utilizing honokiol is preliminary and research must be further conducted to better delineate the specific mechanism of action of this drug, as its antitumor and immunopreservative effects may potentially be attributable to other intracellular pathways that the drug may target. The efficacy, therapeutic index, effect specificity and safety of this drug also remains to be determined. In vivo studies so far have shown that honokiol sufficiently crosses the blood–brain barrier in addition to inhibiting tumor angiogenesis. No significant toxicities – as quantified by changes in bodyweight, blood work, serum electrolytes, liver function panels, eating habits and tissue pathology – were noted [98]. Nevertheless, determining the optimal delivery, proper dosing and possible combinatorial effects with other therapeutics warrants further investigation [99–101].
Isoform selectivity
Exploiting isoform selectivity can offer another avenue through which we may bypass inhibitor-induced immunosuppression. As an illustrative example, the PI3K protein belongs to three broad classes that can be further subdivided based on isoforms. Class I PI3Ks, the only class known to activate AKT, consists of the catalytic p110 and regulatory p85 domains [11,12]. Class IA and IB PI3Ks are activated by receptor tyrosine kinases and G-protein-coupled receptors, respectively [11,12]. Four p110 isoforms (class IA: α, β and δ, and class IB: γ) and several of p85 have been described [11]. Within lymphocytes, the p110δ or p110γ subunits predominate [90].
Isoform distribution and activity is dependent on cell type. Although the p110α or p110β isoforms are widely expressed throughout, p110δ and p110γ are predominantly expressed within lymphocytes and have been extensively linked to the regulation of physiologic immune function. Much of the seminal work elucidating the importance of isoforms has been published since the turn of the century. Robust evidence derived from genetically engineered p110δ- or p110γ-null mice as well as isoform-selective inhibition have consistently shown significant impairment in inflammation and, important to immunotherapy, the development, activation, signaling, trafficking and differentiation of lymphocytes [90,102–105]. Specific to the nervous system, p110γ has additionally shown expression in microglia, which act as the resident macrophages within the CNS [106,107].
Consequently, selective p110α or p110β inhibition could minimize drug-induced suppression of immune cells, and this therapeutic approach has demonstrated some promise. Berenjeno et al. explored the overall benefits conferred by p110α and p110β inhibition in mice heterozygous for PTEN mutations [108]. A cohort of such mice underwent additional knockouts of p110α, while another was treated with p110β-specific inhibitors. The former group showed delayed development of thyroid cancer, while the latter showed delayed development of prostate cancer. Neither, however, showed delayed onset of immune-based tumors such as lymphoma [108]. Their findings importantly conclude that isoform-specific inhibition can attenuate tumor formation in the context of PTEN (i.e., PI3K pathway) mutations. Furthermore, So et al. selectively inhibited p110α, p110β and p110δ by using the inhibitors MLN1117, TGX-221 and IC87114, respectively. Among them, p110δ inhibition significantly reduced lymphocytic proliferation and survival, while selective p110α inhibition at doses known to induce an antitumor response preserved lymphocytic function in vivo [109].
Conversely, Maxwell et al. utilized a mouse model for systemic lupus erythematous and induced partial knockdown of p110δ function in a cohort of mice [110]. In comparison to their wild-type counterparts, haploinsufficient mice demonstrated reduced production of autoimmune antibody titers, decreased activation of T cells and attenuation of inflammation, thereby providing further evidence for the importance of preserving p110δ for immune function [110].
Interestingly, however, Schmid et al. have also presented evidence to the contrary by demonstrating that inhibition of p110γ-mediated signaling pathways reduces the recruitment of myeloid-derived suppressor cells and other mediators of chronic inflammation, ultimately impeding tumor growth and progression [111,112]. This implies that even selective blockade of p110 subunits involved in the immune response may prove beneficial to antitumor therapy by mitigating inflammatory processes that favor tumor growth and survival.
While the explanation for this discrepancy is not entirely clear, one potential factor could be that p110γ and p110δ appear to adopt distinct roles within the immune response and in different immune cell types, the specifics of which have yet to be fully understood [102]. In that manner, p110δ and p110γ inhibition may induce different effects, with the former leading to the inhibition of proinflammatory antitumor responses (e.g., a robust T-cell response), while p110γ inhibition may repress immune functions that actually favor inflammation-associated tumor progression (e.g., propagation of anti-inflammatory myeloid suppressor cells). In addition, the strength of the antitumor response may outweigh potential deleterious effects on immune cells. For example, Tzenaki et al. demonstrated a link between p110δ and PTEN activity in tumors, with inhibition of p110δ in breast and prostate cancer cells leading to increased PTEN activity and subsequent tumor growth suppression [113]. As such, selective inhibition of p110δ or p110γ may present a valid therapeutic strategy, but further research must be conducted to better understand their cumulative effects on tumor and immune cell viability, specifically in T cells.
Currently, several isoform-selective inhibitors are under active investigation in clinical cancer trials, including p110α (BYL-719 and MLN1117) and p110δ inhibitors (GS-1101 and AMG-319) [10,103]. However, these agents have yet to be tested extensively in the context of GBM, and determining appropriate bio-availability as well as blood–brain barrier and tumor penetration will pose significant challenges. Evaluating this or similar agents in GBM, however, could prove worthwhile if these drugs retain their antitumor capacity while conserving the immune response.
PD-L1/PD-1 antibody
In place of modulating PD-L1 expression through the PI3K pathway, two recent Phase I clinical trials blocked the binding interaction between PD-L1 and its receptor PD-1. In a report published by Topalian et al., patients with solid tumors were treated with BMS-936558, a blocking antibody against PD-1. Objective overall clinical responses ranged from 18 to 28%, depending on the tumor type, but more importantly, in a representative sample of patients with proven PD-L1+ tumors, the response rate was higher at 36%. Rates of adverse events with immunological etiologies such as vitiligo, pneumonitis, hepatitis and colitis were relatively low [114]. Similarly, Brahmer et al. treated 207 patients with BMS-936559, a blocking antibody against PD-L1 [115]. Clinical response rates were lower, ranging from 6 to 17%. Immune-related adverse effects were observed in 39% of patients and rates for individual complications ranged from 1 to 10% [115].
Both of these studies illustrate the efficacy of blocking antibodies while accomplishing relative preservation of the immune response. However, while this approach is steadily gaining momentum, blocking antibodies have yet to undergo rigorous examination through clinical trials in GBM and, as was the case with PI3K pathway inhibitors, appropriate penetration of the blood–brain barrier and adequate drug delivery to tumor will need to be ascertained. Furthermore, unlike the PI3K pathway inhibitors, one primary limitation is that antibodies alone cannot induce tumor cell death. However, while the direct antitumor response may not be as robust, these antibodies may confer greater long-term advantage by maintaining the systemic antitumor immune response.
Conclusion
Immunosuppression should be foremost amongst our concerns when considering treatment options for GBM, and we must carefully select modalities that maintain or bolster the optimum immune environment for combating tumor growth. PI3K pathway inhibitors bear promise due to their toxicity to tumor cells and capacity to mitigate mechanisms of immune evasion. However, their immunosuppressive effects raise significant concerns over their potential use in GBM immunotherapy. On the other hand, with supporting evidence from further experimental research, isoform-selective PI3K inhibitors or less immunosuppressive PI3K pathway inhibitors such as AZD-8005 may eventually rise to the forefront as adjuvants to vaccine immunotherapy.
Executive summary.
Augmenting vaccine immunotherapy with PI3K pathway inhibition
Glioblastoma (GBM) tumors develop multimodal mechanisms by which they can evade immune surveillance, among which the programmed death ligand-1 (PD-L1) protein bears significance.
PI3K pathway inhibitors can induce tumor cell death while reversing PD-L1-mediated mechanisms for immunosuppression.
Few studies have investigated the viability of utilizing PI3K pathway inhibitors in conjunction with vaccine immunotherapy.
The PI3K/AKT/mTOR pathway & GBM
PI3K/AKT/mTOR pathway mutations induce GBM tumorigenesis and PD-L1 upregulation.
PD-L1 causes T-cell apoptosis.
Five pathway inhibitors are currently in clinical trials for GBM.
PI3K pathway inhibitors & interleukins
GBM tumors cause Th2 skew by increasing production of Th2 cytokines (IL-4, IL-10 and IL-13)m while reducing Th1 cytokines (IL-2 and IL-12).
PI3K pathway inhibitors impair cytotoxic T lymphocyte activity and cellular reactions mediated by IL-2, and increase IL-12 secretion.
PI3K pathway inhibitors decrease Th2 cytokine production, which may potentiate the antitumor response.
PI3K pathway inhibitors & interferons
Interferons are proinflammatory cytokines downregulated in GBM.
Inhibitors may exacerbate the antitumor response by decreasing interferon production.
PI3K pathway inhibitors & other immune effectors
Tregs are immunosuppressive T cells that present significant therapeutic challenges.
PI3K pathway inhibitors increase survival advantage of Tregs.
TGF-β is an immunosuppressive cytokine that is highly present in GBM.
PI3K pathway inhibitors reduce TGF-β production, which may favor the antitumor response.
Although both the PI3K pathway and TGF-β are involved in Treg proliferation, the former takes precedence.
Selective Treg expansion induced by inhibitors must be avoided if they are to become integrated into GBM therapeutics.
Conclusion
Current evidence suggests that PI3K pathway inhibitor use in GBM immunotherapy may pose therapeutic limitations by impairing the immune response.
Although these inhibitors are broadly immunosuppressive, select agents such as isoform-selective inhibitors warrant further investigation.
Antibodies against PD-L1 and/or PD-1 may provide an alternate solution to mitigating the downstream effects of PD-L1 expression.
Acknowledgments
ME Ivan is funded by the National Research Education Foundation through the American Association of Neurological Surgeons. MZ Sun and ET Sayegh are Howard Hughes Medical Institute Medical Research Fellows. M Safaee was supported by a grant from the Doris Duke Charitable Foundation. AT Parsa is partially funded by the Michael J Marchese Chair in Neurosurgery.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest;
•• of considerable interest
- 1.Stupp R, Mason WP, Van Den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar LK, Arvizu M, Aguilar-Cordova E, Chiocca EA. The spectrum of vaccine therapies for patients with glioblastoma multiforme. Curr. Treat. Options Oncol. 2012;13(4):437–450. doi: 10.1007/s11864-012-0208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3••.Jackson C, Ruzevick J, Brem H, Lim M. Vaccine strategies for glioblastoma: progress and future directions. Immunotherapy. 2013;5(2):155–167. doi: 10.2217/imt.12.155. [Discusses all the major strategies that have been utilized in vaccine immunotherapy for glioblastoma.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka S, Louis DN, Curry WT, Batchelor TT, Dietrich J. Diagnostic and therapeutic avenues for glioblastoma: no longer a dead end? Nat. Rev. Clin. Oncol. 2013;10(1):14–26. doi: 10.1038/nrclinonc.2012.204. [DOI] [PubMed] [Google Scholar]
- 5.Dunn GP, Fecci PE, Curry WT. Cancer immunoediting in malignant glioma. Neurosurgery. 2012;71(2):201–222. doi: 10.1227/NEU.0b013e31824f840d. [DOI] [PubMed] [Google Scholar]
- 6.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 7••.Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007;13(1):84–88. doi: 10.1038/nm1517. [Links aberrant PI3K pathway signalling to high expression of programmed death ligand-1 (PD-L1; also known as B7-H1) in glioma cells.] [DOI] [PubMed] [Google Scholar]
- 8••.Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin. Cancer Res. 2013;19(12):3165–3175. doi: 10.1158/1078-0432.CCR-12-3314. [Links aberrant PI3K pathway signalling to high expression of PD-L1 (also known as B7-H1) in glioma-associated monocytes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Crane C, Panner A, Pieper RO, Arbiser J, Parsa AT. Honokiol-mediated inhibition of PI3K/mTOR pathway: a potential strategy to overcome immunoresistance in glioma, breast, and prostate carcinoma without impacting T cell function. J. Immunother. 2009;32(6):585–592. doi: 10.1097/CJI.0b013e3181a8efe6. [Discusses an alternative PI3K inhibitor that may preserve the immune response.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Wen PY, Lee EQ, Reardon DA, Ligon KL, Alfred Yung WK. Current clinical development of PI3K pathway inhibitors in glioblastoma. Neurooncology. 2012;14(7):819–829. doi: 10.1093/neuonc/nos117. [Details all of the clinical trials for PI3K pathway inhibitors as of 2012.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crabbe T, Welham MJ, Ward SG. The PI3K inhibitor arsenal: choose your weapon! Trends Biochem. Sci. 2007;32(10):450–456. doi: 10.1016/j.tibs.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat. Rev. Clin. Oncol. 2013;10(3):143–153. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- 13.Yao Y, Tao R, Wang X, Wang Y, Mao Y, Zhou LF. B7-H1 is correlated with malignancy-grade gliomas but is not expressed exclusively on tumor stem-like cells. Neurooncology. 2009;11(6):757–766. doi: 10.1215/15228517-2009-014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs JF, Idema AJ, Bol KF, et al. Regulatory T cells and the PD-L1/PD-1 pathway mediate immune suppression in malignant human brain tumors. Neurooncology. 2009;11(4):394–402. doi: 10.1215/15228517-2008-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avril T, Saikali S, Vauleon E, et al. Distinct effects of human glioblastoma immunoregulatory molecules programmed cell death ligand-1 (PDL-1) and indoleamine 2,3-dioxygenase (IDO) on tumour-specific T cell functions. J. Neuroimmunol. 2010;225(1–2):22–33. doi: 10.1016/j.jneuroim.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Chang SM, Wen P, Cloughesy T, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest. New Drugs. 2005;23(4):357–361. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 17.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J. Clin. Oncol. 2005;23(23):5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 18.Kreisl TN, Lassman AB, Mischel PS, et al. A pilot study of everolimus and gefitinib in the treatment of recurrent glioblastoma (GBM). J. Neurooncol. 2009;92(1):99–105. doi: 10.1007/s11060-008-9741-z. [DOI] [PubMed] [Google Scholar]
- 19.Chinnaiyan P, Won M, Wen PY, et al. RTOG 0913: a Phase 1 study of daily everolimus (RAD001) in combination with radiation therapy and temozolomide in patients with newly diagnosed glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2013;86(5):880–884. doi: 10.1016/j.ijrobp.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hainsworth JD, Shih KC, Shepard GC, Tillinghast GW, Brinker BT, Spigel DR. Phase II study of concurrent radiation therapy, temozolomide, and bevacizumab followed by bevacizumab/everolimus as first-line treatment for patients with glioblastoma. Clin. Adv. Hematol. Oncol. 2012;10(4):240–246. [PubMed] [Google Scholar]
- 21.Mason WP, Macneil M, Kavan P, et al. A Phase I study of temozolomide and everolimus (RAD001) in patients with newly diagnosed and progressive glioblastoma either receiving or not receiving enzyme-inducing anticonvulsants: an NCIC CTG study. Invest. New Drugs. 2012;30(6):2344–2351. doi: 10.1007/s10637-011-9775-5. [DOI] [PubMed] [Google Scholar]
- 22.Sarkaria JN, Galanis E, Wu W, et al. North Central Cancer Treatment Group Phase I trial N057K of everolimus (R and temozolomide in combination with radiation therapy in patients with newly diagnosed glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 2011;81(2):468–475. doi: 10.1016/j.ijrobp.2010.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lassen U, Sorensen M, Gaziel TB, Hasselbalch B. Poulsen Phase II study of bevacizumab and temsirolimus combination therapy for recurrent glioblastoma multiforme. Anticancer Res. 2013;33(4):1657–1660. [PubMed] [Google Scholar]
- 24.Lee EQ, Kuhn J, Lamborn KR, et al. Phase I/II study of sorafenib in combination with temsirolimus for recurrent glioblastoma or gliosarcoma: North American Brain Tumor Consortium study 05–02. Neurooncology. 2012;14(12):1511–1518. doi: 10.1093/neuonc/nos264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 26.Granville CA, Memmott RM, Gills JJ, Dennis PA. Handicapping the race to develop inhibitors of the phosphoinositide 3-kinase/Akt/mammalian target of rapamycin pathway. Clin. Cancer Res. 2006;12(3 Pt 1):679–689. doi: 10.1158/1078-0432.CCR-05-1654. [DOI] [PubMed] [Google Scholar]
- 27.Figlin RA, Kaufmann I, Brechbiel J. Targeting PI3K and mTORC2 in metastatic renal cell carcinoma: new strategies for overcoming resistance to VEGFR and mTORC1 inhibitors. Int. J. Cancer. 2013;133(4):788–796. doi: 10.1002/ijc.28023. [DOI] [PubMed] [Google Scholar]
- 28.Santoni M, Pantano F, Amantini C, et al. Emerging strategies to overcome the resistance to current mTOR inhibitors in renal cell carcinoma. Biochim. Biophys. Acta. 2014;1845(2):221–231. doi: 10.1016/j.bbcan.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Zhang QL, Cui BR, Li HY, et al. MAPK and PI3K pathways regulate hypoxia-induced atrial natriuretic peptide secretion by controlling HIF-1 alpha expression in beating rabbit atria. Biochem. Biophys. Res. Comm. 2013;438(3):507–512. doi: 10.1016/j.bbrc.2013.07.106. [DOI] [PubMed] [Google Scholar]
- 30.Lin A, Piao HL, Zhuang L, Sarbassov DD, Ma L, Gan B. FoxO transcription factors promote AKT Ser473 phosphorylation and renal tumor growth in response to pharmacological inhibition of the PI3K-AKT pathway. Cancer Res. 2014;74(6):1682–1693. doi: 10.1158/0008-5472.CAN-13-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Y, Gao C, Luo M, et al. Aspidin PB, a phloroglucinol derivative, induces apoptosis in human hepatocarcinoma HepG2 cells by modulating PI3K/Akt/GSK3beta pathway. Chem. Biol. Interact. 2013;201(1–3):1–8. doi: 10.1016/j.cbi.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat. Rev. Mol. Cell Biol. 2012;13(3):195–203. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Zhao M, Hao M, et al. Dual inhibition of PI3K and mTOR mitigates compensatory AKT activation and improves tamoxifen response in breast cancer. Mol. Cancer Res. 2013;11(10):1269–1278. doi: 10.1158/1541-7786.MCR-13-0212. [DOI] [PubMed] [Google Scholar]
- 34.Baldo P, Cecco S, Giacomin E, Lazzarini R, Ros B, Marastoni S. mTOR pathway and mTOR inhibitors as agents for cancer therapy. Curr. Cancer Drug Targets. 2008;8(8):647–665. doi: 10.2174/156800908786733513. [DOI] [PubMed] [Google Scholar]
- 35.Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr. Opin. Immunol. 2011;23(5):598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimato S, Maier LM, Maier R, Bruce JN, Anderson RC, Anderson DE. Profound tumor-specific Th2 bias in patients with malignant glioma. BMC Cancer. 2012;12:561. doi: 10.1186/1471-2407-12-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashkenazi E, Deutsch M, Tirosh R, Weinreb A, Tsukerman A, Brodie C. A selective impairment of the IL-2 system in lymphocytes of patients with glioblastomas: increased level of soluble IL-2R and reduced protein tyrosine phosphorylation. Neuroimmunomodulation. 1997;4(1):49–56. doi: 10.1159/000097315. [DOI] [PubMed] [Google Scholar]
- 38.Wintterle S, Schreiner B, Mitsdoerffer M, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63(21):7462–7467. [PubMed] [Google Scholar]
- 39.Jie X, Hua L, Jiang W, Feng F, Feng G, Hua Z. Clinical application of a dendritic cell vaccine raised against heat-shocked glioblastoma. Cell Biochem. Biophys. 2012;62(1):91–99. doi: 10.1007/s12013-011-9265-6. [DOI] [PubMed] [Google Scholar]
- 40.Colombo F, Barzon L, Franchin E, et al. Combined HSV-TK/ IL-2 gene therapy in patients with recurrent glioblastoma multiforme: biological and clinical results. Cancer Gene Ther. 2005;12(10):835–848. doi: 10.1038/sj.cgt.7700851. [DOI] [PubMed] [Google Scholar]
- 41.Eder AM, Dominguez L, Franke TF, Ashwell JD. Phosphoinositide 3-kinase regulation of T cell receptor-mediated interleukin-2 gene expression in normal T cells. J. Biol. Chem. 1998;273(43):28025–28031. doi: 10.1074/jbc.273.43.28025. [DOI] [PubMed] [Google Scholar]
- 42.Karnitz LM, Burns LA, Sutor SL, Blenis J, Abraham RT. Interleukin-2 triggers a novel phosphatidylinositol 3-kinase-dependent MEK activation pathway. Mol. Cell. Biol. 1995;15(6):3049–3057. doi: 10.1128/mcb.15.6.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breslin EM, White PC, Shore AM, Clement M, Brennan P. LY294002 and rapamycin co-operate to inhibit T-cell proliferation. Br. J. Pharm. 2005;144(6):791–800. doi: 10.1038/sj.bjp.0706061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3(2):133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 45.Fukao T, Tanabe M, Terauchi Y, et al. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat. Immunol. 2002;3(9):875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 46.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 2005;6(8):777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohtani M, Nagai S, Kondo S, et al. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112(3):635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar R, Kamdar D, Madden L, et al. Th1/Th2 cytokine imbalance in meningioma, anaplastic astrocytoma and glioblastoma multiforme patients. Oncol. Rep. 2006;15(6):1513–1516. doi: 10.3892/or.15.6.1513. [DOI] [PubMed] [Google Scholar]
- 49.Akasaki Y, Kikuchi T, Homma S, Abe T, Kofe D, Ohno T. Antitumor effect of immunizations with fusions of dendritic and glioma cells in a mouse brain tumor model. J. Immunother. 2001;24(2):106–113. [PubMed] [Google Scholar]
- 50.Kikuchi T, Akasaki Y, Abe T, et al. Vaccination of glioma patients with fusions of dendritic and glioma cells and recombinant human interleukin 12. J. Immunother. 2004;27(6):452–459. doi: 10.1097/00002371-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Zisakis A, Piperi C, Themistocleous MS, et al. Comparative analysis of peripheral and localised cytokine secretion in glioblastoma patients. Cytokine. 2007;39(2):99–105. doi: 10.1016/j.cyto.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 52.Waziri A, Killory B, Ogden AT, 3rd, et al. Preferential in situ CD4+CD56+ T cell activation and expansion within human glioblastoma. J. Immunol. 2008;180(11):7673–7680. doi: 10.4049/jimmunol.180.11.7673. [DOI] [PubMed] [Google Scholar]
- 53.Scheurer ME, Amirian E, Cao Y, et al. Polymorphisms in the interleukin-4 receptor gene are associated with better survival in patients with glioblastoma. Clin. Cancer Res. 2008;14(20):6640–6646. doi: 10.1158/1078-0432.CCR-07-4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okada H, Lieberman FS, Walter KA, et al. Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of patients with malignant gliomas. J. Transl. Med. 2007;5:67. doi: 10.1186/1479-5876-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown CE, Starr R, Aguilar B, et al. Stem-like tumor-initiating cells isolated from IL13Ralpha2 expressing gliomas are targeted and killed by IL13-zetakine-redirected T Cells. Clin. Cancer Res. 2012;18(8):2199–2209. doi: 10.1158/1078-0432.CCR-11-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grehan JF, Levay-Young BK, Fogelson JL, Francois-Bongarcon V, Benson BA, Dalmasso AP. IL-4 and IL-13 induce protection of porcine endothelial cells from killing by human complement and from apoptosis through activation of a phosphatidylinositide 3-kinase/Akt pathway. J. Immunol. 2005;175(3):1903–1910. doi: 10.4049/jimmunol.175.3.1903. [DOI] [PubMed] [Google Scholar]
- 57.Barderas R, Bartolome RA, Fernandez-Acenero MJ, Torres S, Casal JI. High expression of IL-13 receptor alpha2 in colorectal cancer is associated with invasion, liver metastasis, and poor prognosis. Cancer Res. 2012;72(11):2780–2790. doi: 10.1158/0008-5472.CAN-11-4090. [DOI] [PubMed] [Google Scholar]
- 58.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116(12):2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iribarren P, Cui YH, Le Y, et al. IL-4 down-regulates lipopolysaccharide-induced formyl peptide receptor 2 in murine microglial cells by inhibiting the activation of mitogen-activated protein kinases. J. Immunol. 2003;171(10):5482–5488. doi: 10.4049/jimmunol.171.10.5482. [DOI] [PubMed] [Google Scholar]
- 60.Nirula A, Ho M, Phee H, Roose J, Weiss A. Phosphoinositide-dependent kinase 1 targets protein kinase A in a pathway that regulates interleukin 4. J. Exp. Med. 2006;203(7):1733–1744. doi: 10.1084/jem.20051715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duan W, Aguinaldo Datiles AM, Leung BP, Vlahos CJ, Wong WS. An anti-inflammatory role for a phosphoinositide 3-kinase inhibitor LY294002 in a mouse asthma model. Int. Immunopharmacol. 2005;5(3):495–502. doi: 10.1016/j.intimp.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 62.Marshall NA, Galvin KC, Corcoran AM, Boon L, Higgs R, Mills KH. Immunotherapy with PI3K inhibitor and Toll-like receptor agonist induces IFN-gamma+IL-17+ polyfunctional T cells that mediate rejection of murine tumors. Cancer Res. 2012;72(3):581–591. doi: 10.1158/0008-5472.CAN-11-0307. [DOI] [PubMed] [Google Scholar]
- 63.Rauch I, Muller M, Decker T. The regulation of inflammation by interferons and their STATs. Jak-Stat. 2013;2(1):e23820. doi: 10.4161/jkst.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shibuya H, Hirohata S. Differential effects of IFN-alpha on the expression of various TH2 cytokines in human CD4+ T cells. J. Allergy Clin. Immunol. 2005;116(1):205–212. doi: 10.1016/j.jaci.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 65.Sega S, Wraber B, Mesec A, Horvat A, Ihan A. IFN-beta1a and IFN-beta1b have different patterns of influence on cytokines. Clin. Neurol. Neurosurg. 2004;106(3):255–258. doi: 10.1016/j.clineuro.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 66.Sgorbissa A, Tomasella A, Potu H, Manini I, Brancolini C. Type I IFNs signaling and apoptosis resistance in glioblastoma cells. Apoptosis. 2011;16(12):1229–1244. doi: 10.1007/s10495-011-0639-4. [DOI] [PubMed] [Google Scholar]
- 67.Olson JJ, James CD, Lawson D, Hunter S, Tang G, Billingsley J. Correlation of the response of recurrent malignant gliomas treated with interferon alpha with tumor interferon alpha gene content. Int. J. Oncol. 2004;25(2):419–427. [PubMed] [Google Scholar]
- 68.Buckner JC, Schomberg PJ, Mcginnis WL, et al. A Phase III study of radiation therapy plus carmustine with or without recombinant interferon-alpha in the treatment of patients with newly diagnosed high-grade glioma. Cancer. 2001;92(2):420–433. doi: 10.1002/1097-0142(20010715)92:2<420::aid-cncr1338>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 69.Motomura K, Natsume A, Kishida Y, et al. Benefits of interferon-beta and temozolomide combination therapy for newly diagnosed primary glioblastoma with the unmethylated MGMT promoter: a multicenter study. Cancer. 2011;117(8):1721–1730. doi: 10.1002/cncr.25637. [DOI] [PubMed] [Google Scholar]
- 70.Guiducci C, Ghirelli C, Marloie-Provost MA, et al. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J. Exp. Med. 2008;205(2):315–322. doi: 10.1084/jem.20070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dong LW, Kong XN, Yan HX, et al. Signal regulatory protein alpha negatively regulates both TLR3 and cytoplasmic pathways in type I interferon induction. Mol. Immunol. 2008;45(11):3025–3035. doi: 10.1016/j.molimm.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 72.Li Y, Zhu H, Zeng X, et al. Suppression of autophagy enhanced growth inhibition and apoptosis of interferon-beta in human glioma cells. Mol. Neurobiol. 2013;47(3):1000–1010. doi: 10.1007/s12035-013-8403-0. [DOI] [PubMed] [Google Scholar]
- 73••.Smeltz RB, Chen J, Ehrhardt R, Shevach EM. Role of IFN-gamma in Th1 differentiation: IFN-gamma regulates IL-18R alpha expression by preventing the negative effects of IL-4 and by inducing/maintaining IL-12 receptor beta 2 expression. J. Immunol. 2002;168(12):6165–6172. doi: 10.4049/jimmunol.168.12.6165. [Details the importance of Tregs and how they present barriers to achieving success in immunotherapy.] [DOI] [PubMed] [Google Scholar]
- 74.Raychaudhuri B, Rayman P, Ireland J, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neurooncology. 2011;13(6):591–599. doi: 10.1093/neuonc/nor042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han SJ, Ahn BJ, Waldron JS, et al. Gamma interferon-mediated superinduction of B7-H1 in PTEN-deficient glioblastoma: a paradoxical mechanism of immune evasion. Neuroreport. 2009;20(18):1597–1602. doi: 10.1097/WNR.0b013e32833188f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wolff JE, Wagner S, Reinert C, et al. Maintenance treatment with interferon-gamma and low-dose cyclophosphamide for pediatric high-grade glioma. J. Neurooncol. 2006;79(3):315–321. doi: 10.1007/s11060-006-9147-8. [DOI] [PubMed] [Google Scholar]
- 77.Guo H, Samarakoon A, Vanhaesebroeck B, Malarkannan S. The p110 delta of PI3K plays a critical role in NK cell terminal maturation and cytokine/chemokine generation. J. Exp. Med. 2008;205(10):2419–2435. doi: 10.1084/jem.20072327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Girart MV, Fuertes MB, Domaica CI, Rossi LE, Zwirner NW. Engagement of TLR3, TLR7, and NKG2D regulate IFN-gamma secretion but not NKG2D-mediated cytotoxicity by human NK cells stimulated with suboptimal doses of IL-12. J. Immunol. 2007;179(6):3472–3479. doi: 10.4049/jimmunol.179.6.3472. [DOI] [PubMed] [Google Scholar]
- 79.Bhonde MR, Gupte RD, Dadarkar SD, et al. A novel mTOR inhibitor is efficacious in a murine model of colitis. Am. J. Phys. 2008;295(6):G1237–1245. doi: 10.1152/ajpgi.90537.2008. [DOI] [PubMed] [Google Scholar]
- 80.Crane CA, Han SJ, Ahn B, et al. Individual patient-specific immunity against high-grade glioma after vaccination with autologous tumor derived peptides bound to the 96 KD chaperone protein. Clin. Cancer Res. 2013;19(1):205–214. doi: 10.1158/1078-0432.CCR-11-3358. [DOI] [PubMed] [Google Scholar]
- 81.Humphries W, Wei J, Sampson JH, Heimberger AB. The role of Tregs in glioma-mediated immunosuppression: potential target for intervention. Neurosurg. Clinics North Am. 2010;21(1):125–137. doi: 10.1016/j.nec.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fecci PE, Sweeney AE, Grossi PM, et al. Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin. Cancer Res. 2006;12(14 Pt 1):4294–4305. doi: 10.1158/1078-0432.CCR-06-0053. [DOI] [PubMed] [Google Scholar]
- 83.Curtin JF, Candolfi M, Fakhouri TM, et al. Treg depletion inhibits efficacy of cancer immunotherapy: implications for clinical trials. PLoS ONE. 2008;3(4):e1983. doi: 10.1371/journal.pone.0001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat. Rev. Immunol. 2010;10(8):554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sampson JH, Schmittling RJ, Archer GE, et al. A pilot study of IL-2Ralpha blockade during lymphopenia depletes regulatory T-cells and correlates with enhanced immunity in patients with glioblastoma. PLoS ONE. 2012;7(2):e31046. doi: 10.1371/journal.pone.0031046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sauer S, Bruno L, Hertweck A, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl Acad. Sci. USA. 2008;105(22):7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qu Y, Zhang B, Zhao L, et al. The effect of immunosuppressive drug rapamycin on regulatory CD4+CD25+Foxp3+ T cells in mice. Transpl. Immunol. 2007;17(3):153–161. doi: 10.1016/j.trim.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 88.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105(12):4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 89.Joseph JV, Balasubramaniyan V, Walenkamp A, Kruyt FA. TGF-beta as a therapeutic target in high grade gliomas – promises and challenges. Biochem. Pharmacol. 2013;85(4):478–485. doi: 10.1016/j.bcp.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 90••.Fung-Leung WP. Phosphoinositide 3-kinase delta (PI3Kdelta) in leukocyte signaling and function. Cell. Signal. 2011;23(4):603–608. doi: 10.1016/j.cellsig.2010.10.002. [Details the importance of the PI3K pathway in normal immune cell function.] [DOI] [PubMed] [Google Scholar]
- 91.Lopez-Fauqued M, Gil R, Grueso J, et al. The dual PI3K/mTOR inhibitor PI-103 promotes immunosuppression, in vivo tumor growth and increases survival of sorafenib-treated melanoma cells. Int. J. Cancer. 2010;126(7):1549–1561. doi: 10.1002/ijc.24926. [DOI] [PubMed] [Google Scholar]
- 92.Jiang Q, Weiss JM, Back T, et al. mTOR kinase inhibitor AZD8055 enhances the immunotherapeutic activity of an agonist CD40 antibody in cancer treatment. Cancer Res. 2011;71(12):4074–4084. doi: 10.1158/0008-5472.CAN-10-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Watanabe K, Watanabe HY, Goto Y, Yamamoto N, Yoshizaki M. Studies on the active principles of magnolia bark. Centrally acting muscle relaxant activity of magnolol and honokiol. Jap. J. Pharmacol. 1975;25(5):605–607. doi: 10.1254/jjp.25.605. [DOI] [PubMed] [Google Scholar]
- 94.Xu HL, Tang W, Du GH, Kokudo N. Targeting apoptosis pathways in cancer with magnolol and honokiol, bioactive constituents of the bark of Magnolia officinalis. Drug Discov. Ther. 2011;5(5):202–210. doi: 10.5582/ddt.2011.v5.5.202. [DOI] [PubMed] [Google Scholar]
- 95.Ma L, Chen J, Wang X, et al. Structural modification of honokiol, a biphenyl occurring in Magnolia officinalis: the evaluation of honokiol analogues as inhibitors of angiogenesis and for their cytotoxicity and structure–activity relationship. J. Med. Chem. 2011;54(19):6469–6481. doi: 10.1021/jm200830u. [DOI] [PubMed] [Google Scholar]
- 96.Jeong JJ, Lee JH, Chang KC, Kim HJ. Honokiol exerts an anticancer effect in T98G human glioblastoma cells through the induction of apoptosis and the regulation of adhesion molecules. Int. J. Oncol. 2012;41(4):1358–1364. doi: 10.3892/ijo.2012.1582. [DOI] [PubMed] [Google Scholar]
- 97.Kim BH, Cho JY. Anti-inflammatory effect of honokiol is mediated by PI3K/Akt pathway suppression. Acta Pharmacol. Sin. 2008;29(1):113–122. doi: 10.1111/j.1745-7254.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- 98.Wang X, Duan X, Yang G, et al. Honokiol crosses BBB and BCSFB, and inhibits brain tumor growth in rat 9L intracerebral gliosarcoma model and human U251 xenograft glioma model. PLoS One. 2011;6(4):e18490. doi: 10.1371/journal.pone.0018490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hou W, Chen L, Yang G, et al. Synergistic antitumor effects of liposomal honokiol combined with adriamycin in breast cancer models. Phytother. Res. 2008;22(8):1125–1132. doi: 10.1002/ptr.2472. [DOI] [PubMed] [Google Scholar]
- 100.Jiang QQ, Fan LY, Yang GL, et al. Improved therapeutic effectiveness by combining liposomal honokiol with cisplatin in lung cancer model. BMC Cancer. 2008;8:242. doi: 10.1186/1471-2407-8-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang X, Deng L, Cai L, et al. Preparation, characterization, pharmacokinetics, and bioactivity of honokiol-inhydroxypropyl-beta-cyclodextrin-in-liposome. J. Pharm. Sci. 2011;100(8):3357–3364. doi: 10.1002/jps.22534. [DOI] [PubMed] [Google Scholar]
- 102.Fruman DA, Bismuth G. Fine tuning the immune response with PI3K. Immunol. Rev. 2009;228(1):253–272. doi: 10.1111/j.1600-065X.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- 103.So L, Fruman DA. PI3K signalling in B- and T-lymphocytes: new developments and therapeutic advances. Biochem. J. 2012;442(3):465–481. doi: 10.1042/BJ20112092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Soond DR, Bjorgo E, Moltu K, et al. PI3K p110delta regulates T-cell cytokine production during primary and secondary immune responses in mice and humans. Blood. 2010;115(11):2203–2213. doi: 10.1182/blood-2009-07-232330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hirsch E, Katanaev VL, Garlanda C, et al. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287(5455):1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 106.Schmidt C, Schneble N, Muller JP, et al. Phosphoinositide 3-kinase gamma mediates microglial phagocytosis via lipid kinase-independent control of cAMP. Neuroscience. 2013;233:44–53. doi: 10.1016/j.neuroscience.2012.12.036. [DOI] [PubMed] [Google Scholar]
- 107.Jin R, Yu S, Song Z, et al. Phosphoinositide 3-kinase-gamma expression is upregulated in brain microglia and contributes to ischemia-induced microglial activation in acute experimental stroke. Biochem. Biophys. Res. Comm. 2010;399(3):458–464. doi: 10.1016/j.bbrc.2010.07.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Berenjeno IM, Guillermet-Guibert J, Pearce W, Gray A, Fleming S, Vanhaesebroeck B. Both p110alpha and p110beta isoforms of PI3K can modulate the impact of loss-of-function of the PTEN tumour suppressor. Biochem. J. 2012;442(1):151–159. doi: 10.1042/BJ20111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.So L, Yea SS, Oak JS, et al. Selective inhibition of phosphoinositide 3-kinase p110alpha preserves lymphocyte function. J. Biol. Chem. 2013;288(8):5718–5731. doi: 10.1074/jbc.M112.379446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maxwell MJ, Tsantikos E, Kong AM, Vanhaesebroeck B, Tarlinton DM, Hibbs ML. Attenuation of phosphoinositide 3-kinase delta signaling restrains autoimmune disease. J. Autoimmun. 2012;38(4):381–391. doi: 10.1016/j.jaut.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 111.Schmid MC, Franco I, Kang SW, Hirsch E, Quilliam LA, Varner JA. PI3-kinase gamma promotes Rap1a-mediated activation of myeloid cell integrin alpha4beta1, leading to tumor inflammation and growth. PLoS ONE. 2013;8(4):e60226. doi: 10.1371/journal.pone.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schmid MC, Avraamides CJ, Foubert P, et al. Combined blockade of integrin-alpha4beta1 plus cytokines SDF-1alpha or IL-1beta potently inhibits tumor inflammation and growth. Cancer Res. 2011;71(22):6965–6975. doi: 10.1158/0008-5472.CAN-11-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tzenaki N, Andreou M, Stratigi K, et al. High levels of p110delta PI3K expression in solid tumor cells suppress PTEN activity, generating cellular sensitivity to p110delta inhibitors through PTEN activation. FASEB J. 2012;26(6):2498–2508. doi: 10.1096/fj.11-198192. [DOI] [PubMed] [Google Scholar]
- 114•.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [Demonstrates an alternative approach to reversing the consequences of PD-L1 expression.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115•.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [Demonstrates an alternative approach to reversing the consequences of PD-L1 expression.] [DOI] [PMC free article] [PubMed] [Google Scholar]