Abstract
Transcriptomic studies revealed that hundreds of mRNAs show differential expression in the brains of sleeping relative to awake rats, mice, flies, and sparrows. Although these results have offered clues regarding the molecular consequences of sleep and sleep loss, their functional significance thus far has been limited. This is probably because the previous studies pooled transcripts from all brain cells, including neurons and glia. In Bellesi et al. (2015) [1], we used the translating ribosome affinity purification technology (TRAP) and microarray analysis to obtain a genome-wide mRNA profiling of astrocytes as a function of sleep and wake. We used bacterial artificial chromosome (BAC) transgenic mice expressing eGFP tagged ribosomal protein L10a under the promoter of the Aldh1L1 gene, a highly expressed astrocytic gene. Using this approach, we could extract only the astrocytic mRNAs, and only those already committed to be translated into proteins (L10a is part of the translational machinery).
Here, we report a detailed description of the protocol used in the study (Bellesi et al., 2015 [1]). Array data have been submitted to NCBI GEO under accession number (GSE69079).
Keywords: Sleep, Wake, Sleep deprivation, BacTRAP, Microarray
| Specifications | |
|---|---|
| Organism/cell line/tissue | Adult heterozygous Aldh1L1–eGFP-L10a mice |
| Sex | Either sex |
| Sequencer or array type | Affymetrix GeneChip Mouse Genome 430 2.0 arrays |
| Data format | Raw data: cell files; Normalized data: xls file |
| Experimental factors | Sleep, spontaneous wake, 4 h sleep deprivation |
| Experimental features | Microarray dataset of the effects of sleep and wake on astrocytic gene expression |
| Consent | All animal procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and facilities were reviewed and approved by the IACUC of the University of Wisconsin-Madison |
| Sample source location | Madison, WI, US |
1. Direct link to deposited data
2. Experimental design, materials and methods
2.1. Experimental design
Three groups of adult heterozygous Aldh1L1–eGFP-L10a mice were used (n = 6/group): awake mice (W) were collected during the dark phase (~ 3–5 am) at the end of a long period of wake (> 1 h, interrupted by periods of sleep of < 5 min), and after spending at least 70% of the previous 6–7 h awake. Sleeping mice (S) were collected during the light period (~ 3–5 pm), at the end of a long period of sleep (> 45 min, interrupted by periods of wake of < 4 min), and after spending at least 75% of the previous 6–7 h asleep. Sleep deprived mice (SD) were spontaneously awake during most of the dark phase and then kept awake during the first 4 h of the light period by exposure to novel objects.
2.2. Video monitoring of sleep and wake
To avoid risks of tissue damage and inflammation due to the implant of EEG electrodes, video recordings were performed continuously with infrared cameras and used to determine the behavioral state of mice used in the study. We previously demonstrated that video-monitoring consistently estimates total sleep time with ~ 90% accuracy [2], although it cannot distinguish NREM sleep from REM sleep. Motor activity was quantified by custom-made video-based motion detection algorithms with a time resolution of 1 s. The program detects animal motion every second within a previously set monitored area corresponding to the cage area, by calculating the numbers of pixels whose intensity changed over time. Specifically, it compares the last current image with the previous one and assigns a value in percent of changes in number of pixels occurring every second. These values and the relative time are then saved in a txt report file and subsequently analyzed with custom-made Matlab scripts (MATLAB and Statistics Toolbox Release 2015a, The MathWorks, Inc., Natick, Massachusetts, United States) [3].
2.3. Antibody preparation
To prepare antibody-bound beads Streptavidin MyOne T1 Dynabeads (Invitrogen) were incubated with biotinylated Protein L (Fisher Pierce) for 35 min at RT in PBS 0.1 M using gentle end-over-end rotation. Then, protein L-coated beads were collected on the DynaMag-2 magnet (Invitrogen) and washed five times with PBS 0.1 M containing 3% (w/v) IgG and Protease-free BSA (Jackson ImmunoResearch). Antibody binding was carried out in 0.15 M KCl buffer (20 mM Hepes–KOH, 5 mM MgCl2, 150 mM KCl, 1% NP-40, 0.5 mM DTT, and 100 μg/ml Cycloheximide) for 1 h at room temperature using gentle end-over-end rotation with 50 μg each of two monoclonal anti-GFP antibodies (19C8 and 19F7, Memorial Sloan-Kettering Cancer Center Monoclonal Antibody Core Facility). After antibody binding, beads were washed and resuspended in 0.15 M KCl buffer.
2.4. Tissue collection, translating ribosome affinity purification and RNA extraction
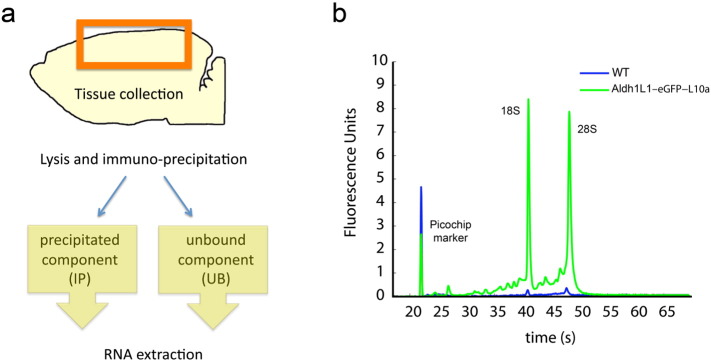
The TRAP protocol has been developed by [4], [5] and it has been also previously described in [6]. Under anesthesia S, SD and W mice (n = 6/group) were decapitated and the forebrain regions (striatum and cerebral cortex) were quickly dissected. Tissue was placed in 2 ml of chilled Lysis Buffer (20 mM Hepes KOH, 5 mM MgCl2, 150 mM KCl, 0.5 mM DTT, 100 μg/ml Cycloheximide, protease inhibitors, 20 μl Rnasin, 20 μl Superasin) and homogenized with a Teflon-Glass homogenizer. Homogenates were then centrifuged at 4 °C for 10 min at 2000 × g to obtain a post-nuclear supernatant. NP-40 (1% final) and DHPC (30 mM final) were added to the supernatant, mixed by gentle inversion and incubated on ice for 5 min. Next, samples were again centrifuged at 4 °C for 10 min at 20,000 × g to obtain a post-mitochondrial supernatant. This fraction was combined with the GFP antibody-coated beads and incubated o/n with gentle end-over-end rotation at 4 °C. Beads were then collected with the magnet and washed four times in high-salt polysome wash buffer (20 mM Hepes–KOH, 5 mM MgCl2, 350 mM KCl, 1% NP-40, 0.5 mM DTT, and 100 μg/ml Cycloheximide). After washes, beads were collected, resuspended and vortexed in 100 μl Lysis Buffer with ß-Mercaptoethanol from the Absolutely RNA Nanoprep kit (Agilent) and incubated for 10 min at RT. As illustrated in Fig. 1a, the RNA was extracted from both the immunoprecipitated (IP) and the supernatant (unbound fraction; UB) fractions. The IP RNA, which represents the mRNA immunoprecipitated from the astrocytes, was separated from the beads with the magnet and purified following the Nanoprep protocol, while the UB RNA, which represents the mRNA of the remaining not precipitated cells, was isolated using the RNeasy Mini kit (Qiagen). Finally, RNA levels in IP and UB samples were assessed by a Qubit Fluorometer (Invitrogen) with the Quant-iT RNA assay kit (Invitrogen, Q32852) and the quality of the RNA was assessed using the Agilent 2100 Bioanalyzer (Agilent) (see Fig. 1b for a representative example).
Fig. 1.
a. Summary scheme of the TRAP method. b. Representative purification of 18S and 28S rRNA from Aldh1L1–eGFPL10a transgenic mice (green) as detected by Bioanalyzer PicoChips (Agilent Technologies). Note that the purification did not occur in a wild type littermate used as a control (blue).
2.5. Microarray: labeling, hybridization and data analysis
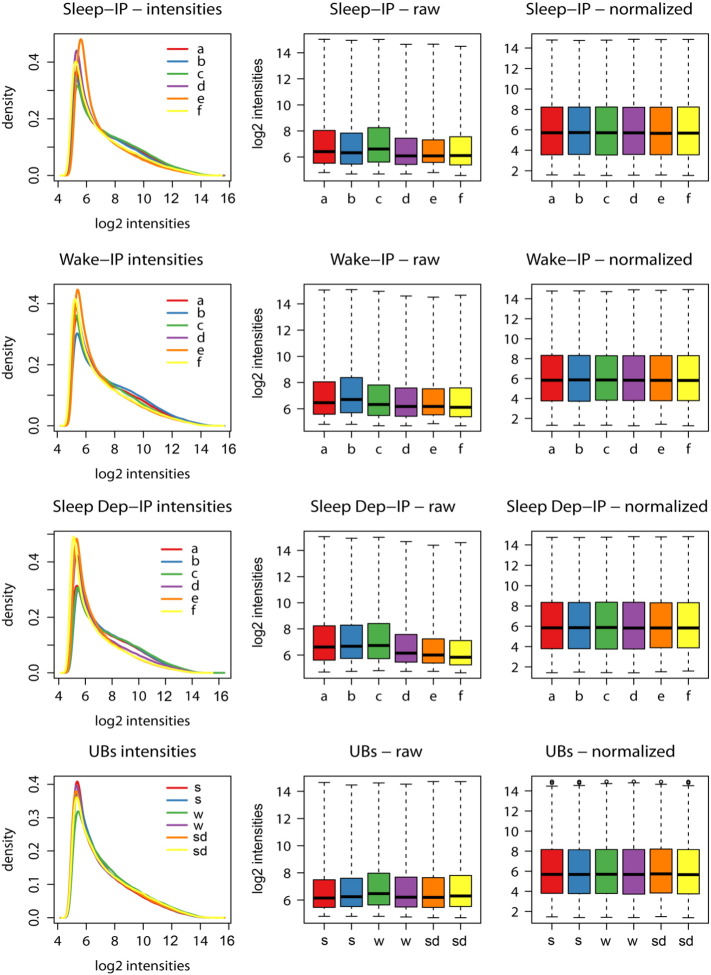
For IP and UB samples (6 IP and 2 UB for each of the 3 experimental groups, S, SD, W), 5 ng of purified mRNA was amplified with the Ovation Pico WTA system (NuGen, #3300). Five micrograms of amplified material was then fragmented, biotin labeled with the Encore Biotin Module (NuGen, #4200), hybridized to Affymetrix GeneChip Mouse Genome 430 2.0 arrays (n = 24, one chip per sample) following Affymetrix standard protocol, and scanned using the GC3000 7G scanner (Affymetrix). Array data analysis was performed using the Bioconductor Limma package [7]. For both IP and UB replicates, GeneChip Cel files were imported into Bioconductor, data were converted to log2 scale, and normalized within each behavioral state group using Robust Multi-array Average (RMA) [8] implemented in the Bioconductor package affy (Fig. 2).
Fig. 2.
Distribution of probeset intensities for S, W, SD IP samples (first three rows; letters (a–f) indicate single mice) and for UB samples (last raw) before and after RMA normalization.
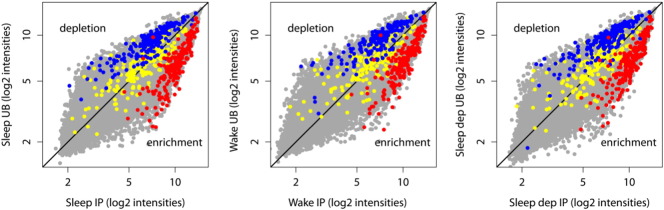
To obtain a measure of the enrichment, the expression intensity of each IP probeset was compared against its UB expression using the Welch's t-test with Benjamini and Hochberg FDR multiple test correction. Probesets with IP/UB ratio > 2 and p < 0.01 were considered enriched, while probesets with IP/UB ratio < 2 and p < 0.01 were considered depleted. Finally, to independently verify the validity of the TRAP method, IP/UB ratios for 200 “top” genes previously found to be enriched in astrocytes, oligodendrocytes, and neurons [8] were calculated (Fig. 3).
Fig. 3.
Scatter plots show normalized mean expression values for IP (x-axis, n = 18, 6/group) and UB (y-axis, n = 6, 2/group) samples of S, W and SD groups. The middle diagonal black line indicates equal expression. In all three experimental groups, the top 200 genes identified by [8] as specific for astrocytes (red) are enriched in IP samples, whereas most of the top 200 genes specific for oligodendrocytes (yellow) and neurons (blue) are enriched in S, W and SD UB samples.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant 1R01MH099231 to CC and GT.
References
- 1.Bellesi M., de Vivo L., Tononi G., Cirelli C. Effects of sleep and wake on astrocytes: clues from molecular and ultrastructural studies. BMC Biol. 2015;13:66. doi: 10.1186/s12915-015-0176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maret S., Faraguna U., Nelson A.B., Cirelli C., Tononi G. Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat. Neurosci. 2011;14:1418–1420. doi: 10.1038/nn.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellesi M., Vyazovskiy V.V., Tononi G., Cirelli C., Conti F. Reduction of EEG theta power and changes in motor activity in rats treated with ceftriaxone. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0034139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doyle J.P., Dougherty J.D., Heiman M., Schmidt E.F., Stevens T.R., Ma G. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135:749–762. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heiman M., Schaefer A., Gong S., Peterson J.D., Day M., Ramsey K.E. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellesi M., Pfister-Genskow M., Maret S., Keles S., Tononi G., Cirelli C. Effects of sleep and wake on oligodendrocytes and their precursors. J. Neurosci. 2013;33:14288–14300. doi: 10.1523/JNEUROSCI.5102-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gentleman R.C., Carey V.J., Bates D.M., Bolstad B., Dettling M., Dudoit S. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irizarry R.A., Hobbs B., Collin F., Beazer-Barclay Y.D., Antonellis K.J., Scherf U. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]