Abstract
Partial-hand amputees often retain good residual wrist motion, which is essential for functional activities involving use of the hand. Thus, a crucial design criterion for a myoelectric, partial-hand prosthesis control scheme is that it allows the user to retain residual wrist motion. Pattern recognition (PR) of electromyographic (EMG) signals is a well-studied method of controlling myoelectric prostheses. However, wrist motion degrades a PR system’s ability to correctly predict hand-grasp patterns. We studied the effects of (1) window length and number of hand-grasps, (2) static and dynamic wrist motion, and (3) EMG muscle source on the ability of a PR-based control scheme to classify functional hand-grasp patterns. Our results show that training PR classifiers with both extrinsic and intrinsic muscle EMG yields a lower error rate than training with either group by itself (p<0.001); and that training in only variable wrist positions, with only dynamic wrist movements, or with both variable wrist positions and movements results in lower error rates than training in only the neutral wrist position (p<0.001). Finally, our results show that both an increase in window length and a decrease in the number of grasps available to the classifier significantly decrease classification error (p<0.001). These results remained consistent whether the classifier selected or maintained a hand-grasp.
I. Introduction
Partial-hand amputation is the most common form of upper-limb amputation, affecting an estimated 455,000 individuals in the United States as of 2005 [1]. Myoelectric prostheses that use surface electromyographic (EMG) signals to control joint movements [2] have only recently become available to partial-hand amputees with the introduction of small, lightweight motorized fingers. These devices potentially provide the user with functional hand-grasps not previously available, but their benefits are limited by their control method [3]. To date, these devices have been controlled using touch-sensitive resistors or conventional myoelectric control strategies that commonly use the amplitude of EMG signals to control the speed of the prosthesis. The source of EMG signals can either be the intrinsic hand muscles, located in the hand, or the extrinsic hand muscles, located in the forearm. Though conventional control using intrinsic hand muscle EMG allows retention of residual wrist motion, it limits the user to the control of one prosthetic function unless an unintuitive switching mechanism is employed. Alternatively, extrinsic hand muscle EMG can be used, but this compromises normal wrist movement and thus limits function [4].
Pattern recognition (PR) of extrinsic muscle EMG is a method that provides the user with more intuitive control of a greater number of prosthetic functions [5–7]. PR classifiers can discriminate between hand-grasps by comparing EMG patterns acquired while performing a grasp to previously-collected training data. However, the EMG signals generated by wrist muscles can obscure extrinsic hand muscle EMG, thus degrading PR performance. It has been shown that using intrinsic muscle EMG, and including examples of grasp pattern EMG while the wrist moves through various static and dynamic wrist positions, in the PR training algorithm mitigates, though does not negate, this effect [8].
Several other techniques can also be employed to improve PR performance. First, PR performance improves when increasing the quantity of data analyzed via a longer EMG window length, which generates a more robust training set [9]. Second, reducing the number of classes available to the classifier can improve performance. As the number of hand-grasps available to the classifier decreases, the rate of incorrect classification generally decreases. These two techniques have been used to improve the robustness of hand-grasp selection and control for high-level Targeted Muscle Reinnervation (TMR) amputees [10].
The purpose of this study is to determine the PR factors that will maximize classifier performance for a partial-hand prosthesis application. To achieve this goal, the effects of the following three factors on PR performance were investigated: (1) training the classifier with the wrist in multiple static and dynamic positions; (2) training the classifier with extrinsic, intrinsic, or both sets of muscles; and (3) training the classifier with varying numbers of hand-grasps and multiple window lengths.
II. Methods
A. Experimental Protocol
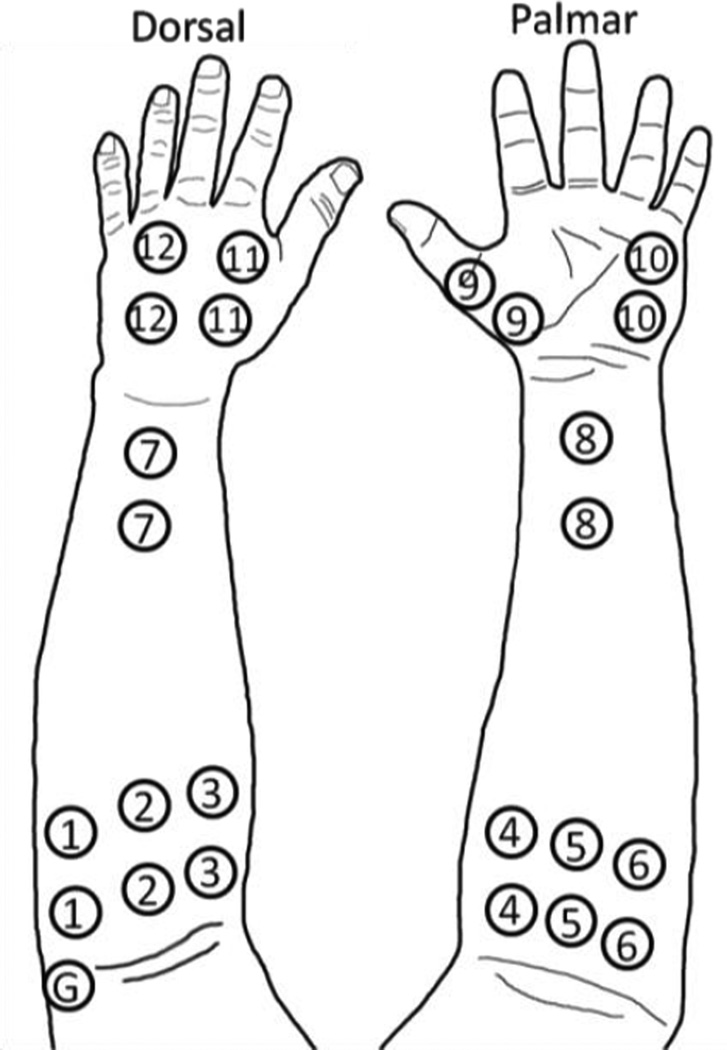
Nine non-amputee subjects were recruited for this study, which was approved by the Northwestern University Institutional Review Board. Eight bipolar, self-adhesive, surface Ag/AgCl EMG electrode pairs were evenly placed around the circumference of each subject’s non-dominant forearm: six around the proximal forearm and two around the distal forearm. Four bipolar electrode pairs were placed on the hand: one on the thenar eminence, one on the hypothenar eminence, one on the dorsal hand between the first and second metacarpals, and one on the dorsal hand between the third and fourth metacarpals. Each pair had an inter-electrode distance of 4cm [11]. A reference electrode was placed over the lateral epicondyle of the humerus [see Fig. 1]. Both the forearm and hand were lightly wrapped with an elastic, cohesive bandage.
Figure 1.
Electrode placement during experiment. Electrodes 1–8 record extrinsic muscle EMG, while electrodes 9–12 record intrinsic muscle EMG. The ground (G) is placed on the lateral epicondyle of the humerus.
Subjects performed six different functional hand-grasps. These grasps, in order of most to least often used for activities of daily living (ADLs) [12], included chuck grip, fine pinch, key grip, power grip, hook grip (as one would hold a briefcase handle), and tool grip (similar to squeezing the trigger of a power drill). Subject also performed a Hand Open (HO) posture and a No Movement (NM) posture. Data were collected under ten conditions and analyzed offline. For the first condition, subjects held a grasp for 4 seconds in a neutral wrist position; this was done four times for each grasp, including HO and NM. This procedure was repeated with a comfortable level of wrist flexion, extension, radial deviation, ulnar deviation, pronation, and supination; these seven conditions made up the static wrist data set. Subjects then performed each grasp four times while moving the wrist from a comfortable flexion position to a comfortable extension position, and back to flexion; and four times while moving the wrist from extension to flexion, and back to extension; each over the span of 4 seconds, or 2 seconds of movement in each direction. This procedure was repeated for radial and ulnar deviation, and pronation and supination; these three conditions made up the dynamic wrist data set. Data were then separated into training and testing sets for the PR algorithm, and analyzed using two-fold cross-validation.
B. Signal Processing
EMG signals were sampled at 1000 Hz, with a band-pass filter between 30–350Hz, using a TI ADS1298 chip. Signal acquisition was guided using custom-built computer software. Pattern recognition was performed via linear discriminant analysis (LDA) classification with four time-domain (mean, zero crossings, turns, waveform length) and six auto-regressive features extracted from each EMG channel [13]. LDA was selected as the pattern recognition algorithm due to its relatively simple calculations and computational efficiency [14]. Additionally, preliminary experiments showed that the LDA classifier performed comparable or superior to both the Gaussian Mixture Model (GMM) and Support Vector Machine (SVM) classifiers. Classification error, defined as the percentage of incorrect classifications, was used to evaluate classifier performance.
C. Data Analysis
Hand-grasp tasks may be separated into two categories: grasp selection and grasp maintenance. During grasp selection, the classifier selects the correct grasp from the available classes consisting of N grasps, HO, and NM, or a total of N+2 classes. After a grasp is selected, grasp maintenance mode activates; in this mode, the N available grasps are mapped to a new class, labeled Hand Close (HC), resulting in only three classes and fewer instances of misclassification. In addition, NM predictions when the desired class is HC are also labeled as correct. For this study, grasp selection was only performed with a static wrist, while grasp maintenance could be performed with either a static or dynamic wrist. From an application perspective, this equates to prepositioning the wrist prior to making a hand-grasp selection. After selection, the user is free to move their wrist while maintaining the selected grasp. Thus, the PR classifier for grasp selection was tested with static wrist data only, and the PR classifier for grasp maintenance was tested with both static and dynamic wrist data.
1) Training Method and Electrode Placement
Four different classifier training methods were evaluated. These training methods were (1) training with the wrist only in the neutral position, (2) training with all seven variable static wrist positions, (3) training while dynamically moving the wrist along each of its three degrees of freedom, and (4) training with all variable static wrist positions and dynamic wrist movements. This analysis was performed with 6 available grasps and a 250ms EMG window with a 25ms frame increment, using extrinsic and intrinsic muscle EMG signals. Electrode placement conditions were separated into three groups consisting of (1) extrinsic muscles, (2) intrinsic muscles, and (3) extrinsic and intrinsic muscles; each was performed with the above-mentioned static and dynamic training scheme, with 6 available grasps, and with a 250ms window and 25ms frame increment.
2) Window Length and Available Grasps
For tests run with the static and dynamic classifier and all twelve extrinsic and intrinsic EMG channels; PR performance was evaluated for window lengths of 100, 200, 300, 400, and 500ms; and for 2, 4, and 6 available grasps, where the grasps analyzed were the N most commonly used hand-grasps in ADLs.
3) Statistical Analysis
Both sets of statistical analyses were calculated with a two-way analysis of variance (ANOVA), and post-hoc pairwise comparisons were made using a Bonferroni correction factor to determine significance.
III. Results
A. Training Method and Electrode Placement
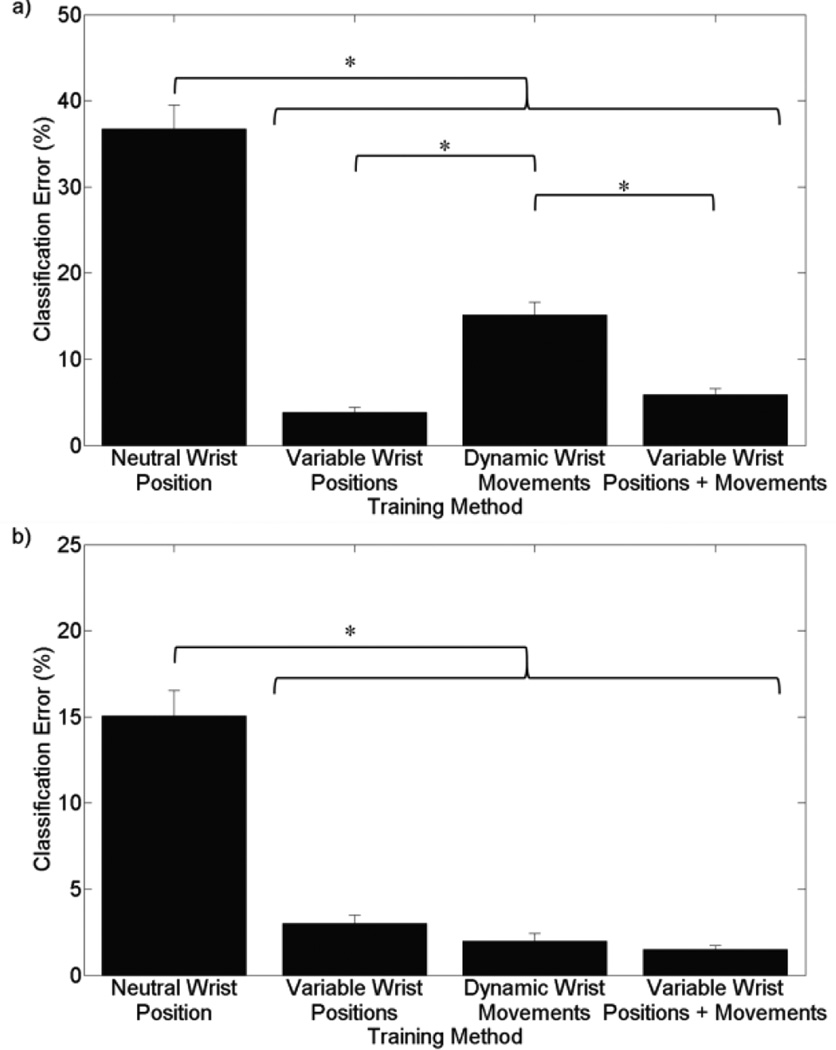
For grasp selection, training with variable static wrist positions or dynamic movements significantly improved PR performance (p<0.001), with training methods utilizing variable wrist positions yielding the lowest error (p<0.001) [see Fig 2(a)]. For grasp maintenance, training with variable static wrist positions or dynamic movements also improved performance (p<0.001) [see Fig 2(b)]. However, there was no significant difference between static, dynamic, or static and dynamic training methods (p>0.126).
Figure 2.
Training method classification error for 6 grasps with 250ms window, collected from extrinsic and intrinsic electrodes. Asterisk (*) represents p<0.01. (a) Classification error during grasp selection. Classifier tested against data in variable wrist positions. (b) Classification error during grasp maintenance. Classifier tested against data in variable wrist positions and movements.
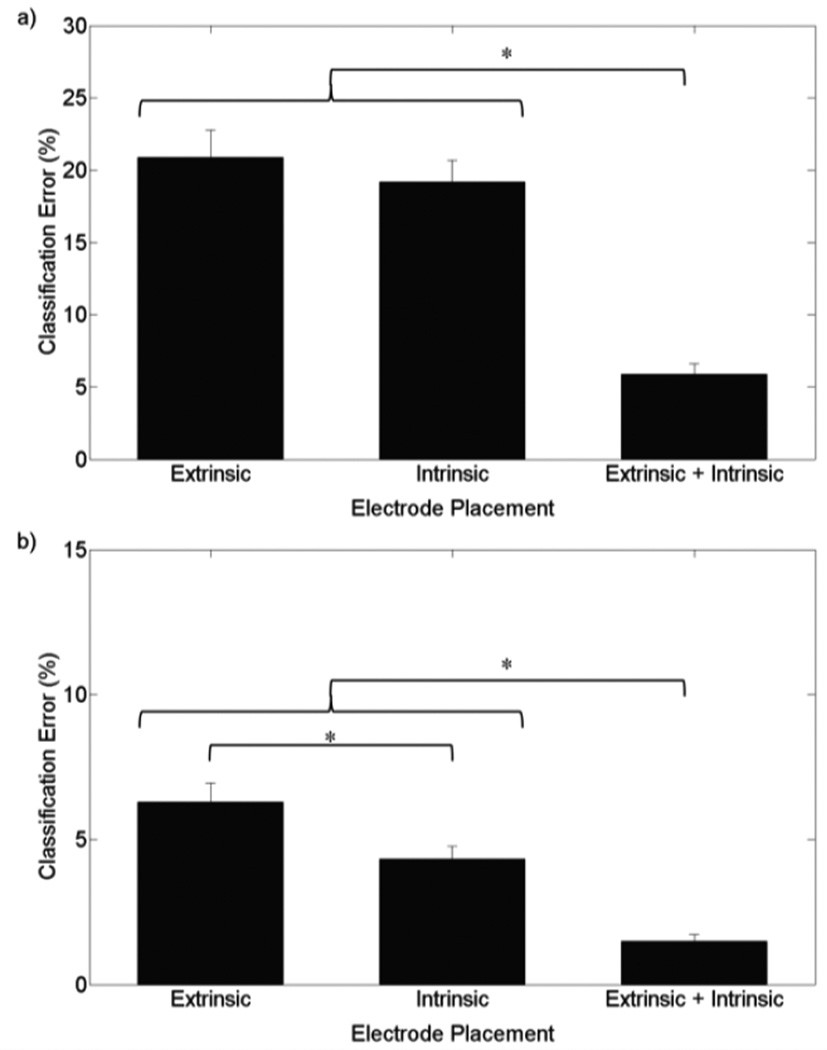
During grasp selection, the error rate for intrinsic channels was similar to extrinsic channels; both had significantly higher error rates than extrinsic and intrinsic channels (p<0.001) [see Fig. 3(a)]. For grasp maintenance, the differences in error rate between extrinsic, intrinsic, and extrinsic and intrinsic channels were all significant (p<0.01) [see Fig. 3(b)]. There was also significant interaction between training method and electrode placement for both grasp selection and maintenance (p<0.001), demonstrating the reduced sensitivity of intrinsic channels to wrist position compared to extrinsic channels.
Figure 3.
Electrode placement classification error for 6 grasps with 250ms window, trained with variable wrist positions and dynamic wrist movements data. Asterisk (*) represents p<0.01. (a) Classification error during grasp selection. Classifier tested against variable wrist position data. (b) Classification error during grasp maintenance. Classifier tested against variable wrist positions and movements data.
B. Window Length and Available Grasps
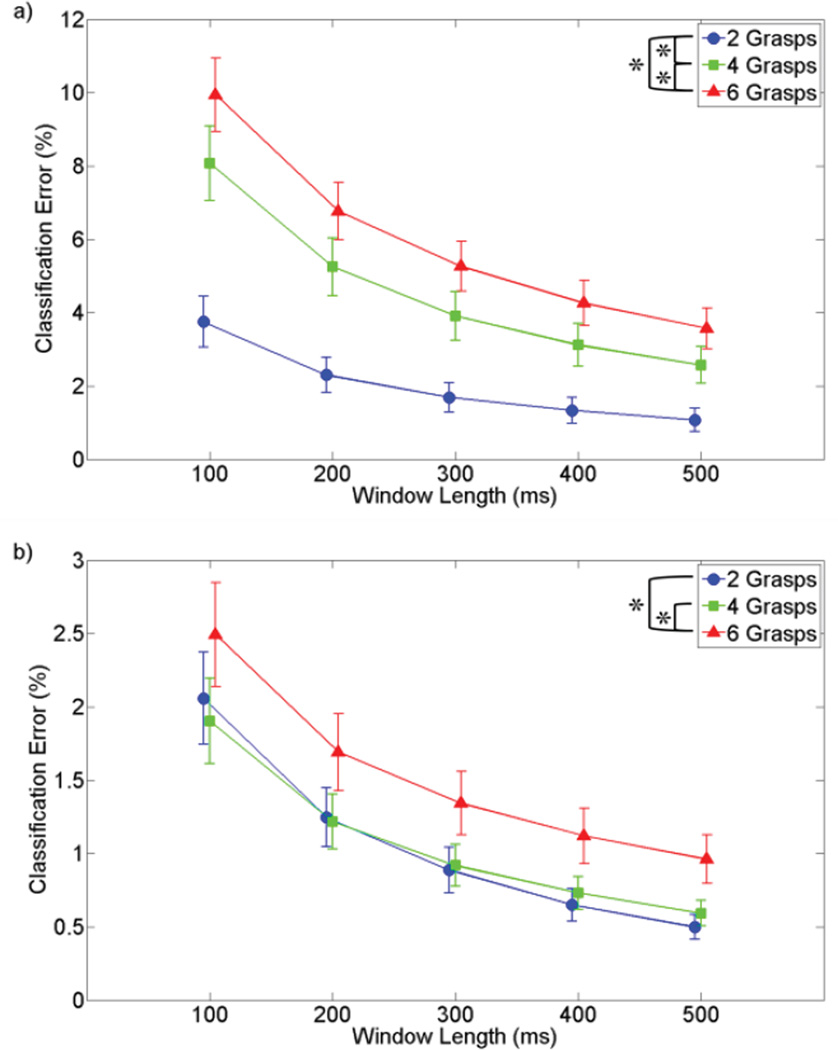
As found in previous studies [9], increasing the classification window length reduced error (p<0.001). In addition, making fewer grasps available to the classifier also reduced error (p<0.001) [see Fig. 4]. During grasp selection, there was significant interaction between window length and available grasps (p<0.001), illustrating the increased importance of longer windows with more available grasps [see Fig. 4(a)]. During grasp maintenance, there was no significant difference between training a classifier with 2 or 4 grasps [see Fig. 4(b)].
Figure 4.
Interaction of window length and available grasps for data trained in variable wrist positions and movements and with extrinsic and intrinsic EMG. Asterisk (*) represents p<0.01. (a) Classification error during grasp selection. Classifier tested against data in variable wrist positions. (b) Classification error during grasp maintenance. Classifier tested against data in variable wrist positions and movements.
IV. Discussion
Because training a classifier with the wrist in the neutral position does not take static or dynamic wrist motions into account, it is understandable that the error rates for this classifier are much higher than classifiers containing training data collected in various wrist positions or movements. For partial-hand prosthesis applications, we recommend training a classifier with data collected from both variable static wrist positions and dynamic wrist movements. This classifier best allows the user to control hand-grasps while retaining wrist function. Intrinsic EMG yields lower error rates than does extrinsic EMG in both grasp selection and grasp maintenance tests, though the difference during grasp selection is not statistically significant. Including both extrinsic and intrinsic EMG in a classifier significantly reduces the error compared to either group by itself, supporting the findings of previous studies [8]. Thus, we recommend using intrinsic muscle EMG for partial-hand prosthesis control, when available.
Longer window lengths provide lower error than shorter window lengths, but at the cost of increased response delay [9]. Consequently, one must balance the speed and accuracy of the system to provide optimal performance. These results show that window length has a minimal effect on accuracy while maintaining a grasp; therefore, though longer windows are important during grasp selection, shorter windows can be used during grasp maintenance while preserving accuracy.
V. Conclusion
For partial-hand prosthesis applications, including wrist motion in a PR classifier and selecting appropriate window lengths are important to ensure a robust system, especially when many grasps are available to the classifier. These techniques would allow users to better operate their prostheses and still maintain the ability to freely position their wrists in space, thus significantly improving function. Also, though acquiring EMG from intrinsic muscles can be difficult, these data supplement extrinsic muscle EMG for significantly improved prosthetic performance.
Acknowledgments
This work was supported by the Department of Education NIDRR RERC grant number H133E130020, NIH grant number T32HD7418, and NICHD grant number 1F31HD078092-01.
Contributor Information
Eric J. Earley, Center for Bionic Medicine at the Rehabilitation Institute of Chicago, Chicago, IL 60611 USA, and with the Department of Biomedical Engineering at Northwestern University (phone: 312-238-2079; ericearley2014@u.northwestern.edu).
Adenike A. Adewuyi, Center for Bionic Medicine at the Rehabilitation Institute of Chicago, Chicago, IL 60611 USA, and with the Department of Biomedical Engineering at Northwestern University (a-adewuyi@fsm.northwestern.edu).
Levi J. Hargrove, Center for Bionic Medicine at the Rehabilitation Institute of Chicago, Chicago, IL 60611 USA, and with the Department of Physical Medicine and Rehabilitation at Northwestern University, Chicago, IL 60611 USA (lhargrove@northwestern.edu).
References
- 1.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008 Mar;89:422–429. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Parker PA, Scott RN. Myoelectric control of prostheses. Crit Rev Biomed Eng. 1986;13:283–310. [PubMed] [Google Scholar]
- 3.Phillips SL, Harris MS, Koss L, Latlief G. Experiences and Outcomes With Powered Partial Hand Prostheses: A Case Series of Subjects With Multiple Limb Amputations. J Prost Ortho. 2012;24:93–97. [Google Scholar]
- 4.Lang M. Conf Proc MyoElectric Controls/Powered Prosthetics Symp. Fredericton, New Brunswick, Canada: 2011. Challenges and Solutions in Control Systems for Electrically Powered Articulating Digits. [Google Scholar]
- 5.Englehart K, Hudgins B. A robust, real-time control scheme for multifunction myoelectric control. IEEE Trans Biomed Eng. 2003 Jul;50:848–854. doi: 10.1109/TBME.2003.813539. [DOI] [PubMed] [Google Scholar]
- 6.Li G, Schultz AE, Kuiken TA. Quantifying pattern recognition-based myoelectric control of multifunctional transradial prostheses. IEEE Trans Neural Syst Rehabil Eng. 2010 Apr;18:185–192. doi: 10.1109/TNSRE.2009.2039619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khushaba RN, Kodagoda S, Takruri M, Dissanayake G. Toward improved control of prosthetic fingers using surface electromyogram (EMG) signals. Expert Syst with Applicat. 2012 Sep 15;39:10731–10738. [Google Scholar]
- 8.Adewuyi AA, Hargrove LJ, Kuiken TA. Towards improved partial-hand prostheses: The effect of wrist kinematics on pattern-recognition-based control; Neural Eng (NER), 2013 6th Int IEEE/EMBS Conf on; 2013. pp. 1489–1492. [Google Scholar]
- 9.Smith LH, Hargrove LJ, Lock BA, Kuiken TA. Determining the optimal window length for pattern recognition-based myoelectric control: balancing the competing effects of classification error and controller delay. IEEE Trans Neural Syst Rehabil Eng. 2011 Apr;19:186–192. doi: 10.1109/TNSRE.2010.2100828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuiken TA, Li G, Lock BA, Lipschutz RD, Miller LA, Stubblefield KA, et al. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA. 2009 Feb 11;301:619–628. doi: 10.1001/jama.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young AJ, Hargrove LJ, Kuiken TA. Improving myoelectric pattern recognition robustness to electrode shift by changing interelectrode distance and electrode configuration. IEEE Trans Biomed Eng. 2012 Mar;59:645–652. doi: 10.1109/TBME.2011.2177662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng JZ, Rosa SDL, Dollar AM. An Investigation of Grasp Type and Frequency in Daily Household and Machine Shop Tasks. Proc IEEE Robot Autom. 2011 May;:4169–4175. [Google Scholar]
- 13.Tkach D, Huang H, Kuiken TA. Study of stability of time-domain features for electromyographic pattern recognition. J Neuroeng Rehabil. 2010;7:21. doi: 10.1186/1743-0003-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hargrove LJ, Englehart K, Hudgins B. A comparison of surface and intramuscular myoelectric signal classification. IEEE Trans Biomed Eng. 2007 May;54:847–853. doi: 10.1109/TBME.2006.889192. [DOI] [PubMed] [Google Scholar]